Abstract
Benzodiazepines are positive allosteric modulators of the GABAA receptor and are prescribed as anxiolytics, hypnotics, and anticonvulsants. While these drugs clearly have clinical value, their use is associated with unwanted side effects such as sedation and motor impairment. Neuroactive steroids are endogenous modulators of GABAA receptors and recent evidence has shown that combinations of the triazolo-benzodiazepine triazolam and the endogenous neuroactive steroid pregnanolone can produce both supra-additive anxiolytic effects and infra-additive reinforcing effects. In the present study, we investigated these same combinations as well as combinations of two clinically-relevant drugs from different chemical classes, the 1, 4 substituted (7-nitro) benzodiazepine clonazepam and the synthetic neuroactive steroid ganaxolone, in rats trained under a 10-response, fixed ratio (FR) schedule of food reinforcement. All four drugs induced a significant and dose-dependent suppression of food-maintained responding. From the dose-response functions, ED50s (i.e., the doses that engendered 50% of the maximum rate-decreasing effect) were generated for each drug. Dose-response functions for combinations of triazolam/pregnanolone, clonazepam/ganaxolone, triazolam/ganaxolone, and clonazepam/pregnanolone were then determined. Isobolographic analysis of the rate-decreasing effects of these combinations revealed that the potencies of the triazolam/pregnanolone combinations were supra-additive while the clonazepam/ganaxolone combinations were additive or infra-additive in relation to predicted values based on dose-additive effects. Furthermore, mixtures of clonazepam/pregnanolone were supra-additive while triazolam/ganaxolone combinations were additive, infra-additive and supra-additive. These results suggest that the ability of benzodiazepine and neuroactive steroid combinations to attenuate rates of food-maintained responding depends critically on both the constituent drugs and the dose of drug in the mixtures.
Keywords: benzodiazepines, neuroactive steroids, schedule-controlled responding
1. Introduction
Benzodiazepine agonists are positive allosteric modulators of the γ-aminobutyric acid type A (GABAA) receptor, potentiating the modulation of chloride conductance by GABA [1–3]. Benzodiazepines and related drugs are prescribed medications for treating anxiety [4–7]. Selective serotonin reuptake inhibitors (SSRI’s) are in general the first line of pharmacotherapy for the treatment of anxiety but have issues with compliance due to a 2–4 week delay in relief of symptoms [7]. Benzodiazepines are effective and relieve symptoms within minutes to hours after their administration, however their use is associated with unwanted side effects such as physical dependence, sedation, motor impairment, and potential for abuse [1, 8]. Considerable effort in drug discovery and design has focused on understanding benzodiazepines and their actions at GABAA receptors, with the goal of increasing the positive therapeutic effects while minimizing or eliminating the unwanted side effects.
Neuroactive steroids are endogenous positive allosteric modulators of the GABAA receptor, produced by neurons and glia in the central nervous system and in the periphery [9]. Neuroactive steroids act at synaptic and extra-synaptic GABAA receptors resulting in both tonic and phasic inhibition of neurons [9, 10]. Behavioral effects due to actions at these sites have yet to be fully elucidated [11]. In animal models of anxiety-like behavior [12, 13] and abuse liability [14–16], neuroactive steroids have shown behavioral effects similar to benzodiazepines. Furthermore, the synthetically-derived version of allopregnanolone, ganaxolone, has shown positive results in mediating symptoms associated with epilepsy in patients, an effect shared with benzodiazepines [17–19].
While the possible therapeutic and abuse-related effects of benzodiazepines and neuroactive steroids when administered alone have been studied extensively, evidence also is accruing that combinations of these GABAA modulators may result in unique interactions. We recently have shown, using isobolographic and dose-addition analyses, that supra-additive effects (i.e., potencies greater than predicted based on the effects of the drugs alone) occur with a mixture of the benzodiazepine triazolam and the neuroactive steroid pregnanolone in a rhesus monkey conflict model of the anxiolytic-like effects of drugs [20]. However, infra-additive effects (i.e., potencies less than predicted based on the effects of the drugs alone) were observed for mixtures of triazolam and pregnanolone in rhesus monkeys responding under a progressive-ratio schedule of i.v. drug injection, an assay that measures the reinforcing effects of drugs [20]. Interestingly, isobolographic and dose-addition analysis of a combination of the benzodiazepine midazolam and pregnanolone in a drug discrimination procedure with rhesus monkeys showed additive effects [21]. Other studies have shown that mixtures of pregnanolone and the benzodiazepine flurazepam elicit supra-additive effects in assays of anesthesia in rats [22]. Altogether, these findings suggest that the type of interaction observed for mixtures of benzodiazepines and neuroactive steroids depends largely on the behavioral effect that is being assessed, notwithstanding the observation that these two types of drug are exerting their effects via the same receptor [23]. Importantly, these initial studies also indicate a possible therapeutic benefit to combinations of benzodiazepines and neuroactive steroids without engendering key unwanted side effects.
To date, studies of benzodiazepine and neuroactive steroid combinations have evaluated a limited number of relatively short-acting drugs, in order to provide insights into mechanism(s) of action without pharmacokinetics as a complicating factor. However, from a drug discovery/development standpoint, combined effects need to be assessed using relatively long-acting benzodiazepines and neuroactive steroids, i.e., drugs with durations of action more relevant for treating anxiety and other psychiatric/neurologic disorders. In the present study, we evaluated the effects of combinations of both short- and long-acting benzodiazepines and neuroactive steroids in rats pressing levers for food pellet delivery (i.e., schedule-controlled responding) in order to determine if the clinically relevant drugs have effects that are similar to the drugs selected based on short durations of action. Schedule-controlled responding was chosen as a relatively simple behavioral measure that is sensitive to GABAA receptor modulators and is common to many of the aforementioned operant-based models, such as conflict, drug discrimination, and self-administration [24]. Benzodiazepines have complex effects on rates of schedule-controlled responding, including evidence for both increases and decreases from baseline responding [25]. Comparatively less is known about the effects of neuroactive steroids on operant responding; therefore, another goal of this study was to evaluate systematically the effects of neuroactive steroids on schedule-controlled responding.
For these studies, triazolam and pregnanolone were selected as proof-of-concept drugs from each class in order to continue the evaluation of these combinations initiated with the previous study in monkeys [20]. For comparison, we included the clinically relevant drugs clonazepam and ganaxolone. Clonazepam has been used widely as a treatment for anxiety as well as seizure disorders, whereas ganaxolone is currently in clinical trials as an adjunct for the treatment of epilepsy [17–19, 26]. Information on the behavioral effects of clonazepam and ganaxolone are available for the drugs alone [27–29]; however, mixtures of these drugs have not been evaluated to our knowledge. By comparing the proof-of-concept drugs and the clinically-relevant drugs in this procedure we can determine if rate-altering effects are generalized with respect to combined effects. Moreover, including drugs/drug combinations from different chemical classes should provide information on whether pharmacodynamic interactions are restricted to a particular type of benzodiazepine and/or neuroactive steroid. Isobolographic and dose addition analyses were used in order to quantitatively determine the extent to which the drug mixtures were additive, supra-additive, or infra-additive [30].
2.1. Methods
2.2. Subjects
Seven adult male Sprague Dawley rats (Harlan, Indianapolis, IN) were housed individually under a 12/12-h light/dark cycle (experiments were conducted during the light period) with water available ad libitum. Rats were maintained at 85% of their free feeding weight for the duration of the study and fed standard rodent chow (Harlan Teklad, Indianapolis, IN). All animals were maintained and experiments were conducted in accordance with the policies of the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and the Guide for the Care and Use of Laboratory Animals (National Research Council, 8th edition, 2011).
2.3. Drugs
Triazolam (NIDA, Baltimore, MD) was dissolved in 100% propylene glycol and diluted to a 20/80% propylene glycol/water mixture. Clonazepam (Sigma-Aldrich, St. Louis, MO) was dissolved in 100% propylene glycol and diluted to a 50/50% propylene glycol/water mixture. Pregnanolone (3α-hydroxy-5β-pregnan-20-one) and ganaxolone (3α-hydroxy-3β -methyl-5α-pregnan-20-one) were purchased from Tocris Biosciences (Ellisville, MO) and were dissolved in 45% (w/v) 2-hydroxypropyl-β-cyclodextrin. All drugs were administered intraperitoneally (i.p.) in volumes of 1–2 ml/kg.
2.4. Schedule-Controlled Responding
Behavioral tests were conducted in chambers equipped with two levers (one active, one inactive), stimulus lights, and a food pellet dispenser (Med Associates, St. Albans, VT) placed within sound-attenuating enclosures. Data were collected using a Macintosh computer with custom software and interface that controlled the experiment and recorded data. Rats initially were trained to press either the left or right lever for a food pellet under a continuous reinforcement schedule (i.e., every active lever press resulted in delivery of a food pellet; 45 mg, Bio-Serve, Flemington, NJ, grain-based pellets). Active lever selection was counterbalanced across rats. As performance improved, the response requirement was increased gradually to a 10-response, fixed-ratio (FR) schedule of food pellet delivery.
Each training session began with the rat receiving an i.p. saline injection and being placed in the operant chamber for a period of time in which lever presses to either lever had no programmed consequences (“pretreatment period”). Once the pretreatment period had elapsed, stimulus lights were illuminated and completion of the response requirement on the active lever resulted in delivery of a food pellet. This response period lasted 150 seconds, at which point the lights were turned off and the rat was removed from the chamber. Pharmacological testing began after the rat met the following stability criteria: average response rate (responses/s) varied by less than 20% across the preceding 5 training sessions, with no upward or downward trend. Test sessions were separated by at least two training sessions that satisfied these criteria. The test sessions were identical to training sessions except that rats received an i.p. injection of a vehicle, drug or drug combination, instead of a saline injection. Pretreatment times varied depending on the drug: a 10 min pretreatment was used for triazolam and pregnanolone; a 20 min pretreatment was used for clonazepam and ganaxolone. Combinations with pregnanolone used a 10 min pretreatment and combinations with ganaxolone used a 20 min pretreatment. Prior to evaluating drug combinations, dose-response functions for the two benzodiazepines were generated using both the 10 min and 20 min pretreatment times.
2.5. Analyses of Data
Rates of responding were calculated for each rat and for active lever responding, converted to a percentage of the rate of responding on the previous training session (i.e., after saline injection). The mean (+/− SEM) of the percentages was plotted as a function of dose. Because rates of responding on the inactive lever occurred infrequently, these data were analyzed without transformation. For the drugs alone, the data were analyzed by repeated measures analysis of variance (ANOVA) with Dunnett’s tests comparing each dose to the vehicle control. The dose of each drug and drug combination required to engender a 50% decrease in response rate (ED50) on the active lever was calculated using log-linear regression analysis. ED50 values for each rat were obtained and 95% confidence limits (CL) were calculated from averaging the ED50 values of all rats for each drug or drug combination.
The effects of the drug combinations were represented visually using isobolograms, in which the ED50 of the neuroactive steroid was plotted on the y axis and the ED50 of the benzodiazepine plotted on the x axis and connected by a line, i.e., the line of additivity. Drug combinations were administered in fixed-proportions based upon their relative potencies. Rats were tested with fixed-proportion mixtures of 1:30, 1:100, and 1:300 triazolam:pregnanolone because these ratios result in fractional multipliers of triazolam and pregnanolone ED50 values that range from 0–1 (Tallarida 2001). Furthermore, drug mixtures can elicit differing results depending on the proportion tested. For clonazepam:ganaxolone mixtures, rats were administered in fixed-proportion mixtures of 1:10, 1:30, and 1:100 because these ratios result in fractional multipliers of clonazepam and ganaxolone ED50 values that range from 0–1. Triazolam:ganaxolone and clonazepam:pregnanolone mixtures were administered in fixed-proportion mixtures of 1:10, 1:30, and 1:100. Drug interactions were analyzed by comparing the total dose of the two drugs in the combination that engendered 50% of the maximum effect (ED50mix) to the predicted additive total dose (ED50add). The latter is calculated using the following equation: ED50add = fA + (1 − f)B where A and B are the ED50’s of the two individual drugs alone, f is the fractional multiplier associated with a specific combination mixture and is calculated by f = FP ÷ (FP + RPA), where FP = fixed-proportion of a particular mixture, and RPA = relative potency ratio of the two drugs = ED50A ÷ ED50B. Statistical significance was determined by conducting t-tests comparing the ED50mix and ED50add. In the isobologram, ED50 values that are plotted below the additively line imply supra-additivity while ED50 values plotted above the line suggest infra-additivity [30]. The interaction index (γ) also was calculated (ED50mix/ED50add) to quantify deviation from additivity [31]. A value of 1 suggests additivity while values less than one imply supra-additivity and greater than 1 suggest infra-additivity. All data were analyzed using Graphpad Prism Version 6.0 for Windows.
3. Results
3.1. Triazolam and Pregnanolone Alone and Mixtures
Under training and vehicle control conditions, rats maintained average rates of responding of approximately 0.73 responses/s (Table 1). Once stable responding was achieved, responses on the inactive lever were extremely infrequent (data not shown). Rates of responding on the active lever were decreased by all four drugs, with maximally effective doses decreasing responding to 0–20% of control rates. Although the pretreatment time initially was varied between 10 and 20 min; with the exception of ganaxolone, the effects of the drugs were not different between these two pretreatment times (Table 1). For ganaxolone, the 10-min pretreatment time did not result in reduced rates of responding, whereas a robust suppression of response rates was observed with the 20-min pretreatment time. Rates of responding returned to at or near control levels by 60 min after treatment except for ganaxolone, in which rates of responding remained robustly attenuated (Table 1).
TABLE 1.
Rates of responding (responses/s, ±SEM) for maximally-effective doses of each drug, as a function of pretreatment periods (N=7). Drug vehicle served as a control.
| Time point (min) | Control | Triazolam 0.3 mg/kg | Clonazepam 0.3 mg/kg | Pregnanolone 10 mg/kg | Ganaxolone 10 mg/kg |
|---|---|---|---|---|---|
| 10 | 0.73 (0.08) | 0.02 (0.02) | 0.02 (0.01) | 0.09 (0.05) | 0.79 (0.08) |
| 20 | 0.74 (0.07) | 0.05 (0.02) | 0.17(0.08) | 0.01(0.01) | 0.05(0.03) |
| 40 | 0.73 (0.09) | 0.19 (0.09) | 0.24 (0.01) | 0.07(0.06) | 0.00 |
| 60 | 0.75 (0.06) | 0.60 (0.13) | 0.46 (0.32) | 0.52 (0.1) | 0.16 (0.09) |
Triazolam and pregnanolone dose-dependently and reliably reduced responding under the FR schedule of food pellet delivery (Figure 1, top panel; triazolam: F (4,24) = 36.68, p < 0.05; pregnanolone: F (4,24) = 32.77, p< 0.05). As with baseline conditions, there were virtually no responses on the inactive lever (data not shown). When individual doses of the drugs were compared to their respective control conditions (vehicle administration), 0.1 mg/kg and 0.3 mg/kg triazolam and 10 mg/kg pregnanolone significantly decreased response rates compared with vehicle (Dunnett’s tests, p’s < 0.05). Based on calculations of ED50 values (95% confidence intervals), triazolam was approximately 60-fold more potent than pregnanolone, with ED50s of 0.14 (0.01–0.18) and 8.0 (6.4–9.6) mg/kg for triazolam and pregnanolone, respectively.
Figure 1.
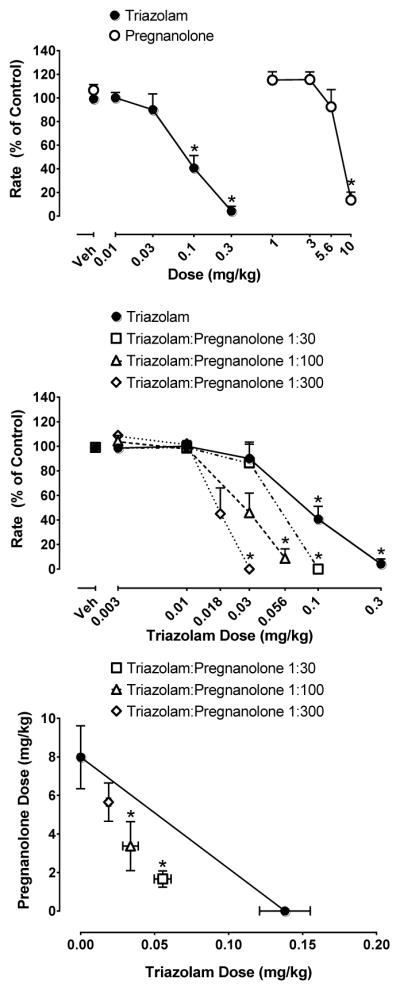
Top, effects of triazolam and pregnanolone alone on response rate under the fixed-ratio schedule of food pellet delivery. X-axis, dose of triazolam and pregnanolone in mg/kg. Y-axis, response rate as a percent of control. Points above “Veh” represent vehicle administration. Middle, effects of triazolam and triazolam+pregnanolone mixtures under the fixed-ratio schedule of food pellet delivery. Bottom, isobologram of triazolam + pregnanolone mixtures. X-axis dose of triazolam in mg/kg. Y-axis, dose of pregnanolone in mg/kg. Each data point represents the mean (±SEM) from seven rats.
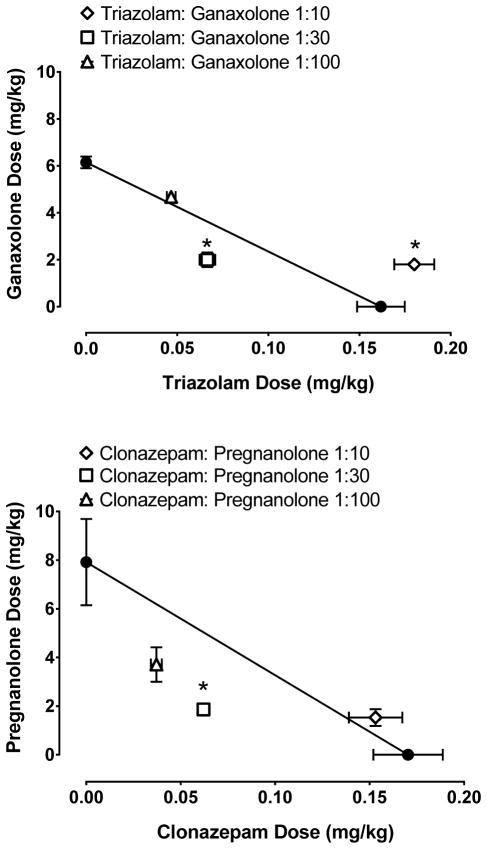
Figure 1 (middle panel) shows the effects of triazolam:pregnanolone mixtures on rates of responding. As with triazolam alone, all mixtures dose-dependently and reliably suppressed responding (1:30: F (3,18) = 40.49, p <0.05; 1:100: F (4,24) = 35.48, p <0.05; 1:300: F (4,24) = 23.77). Similar to the results seen using the drugs alone, these drug combinations did not elicit responding on the inactive lever (data not shown). When doses of the drug mixtures were compared to their respective control conditions (vehicle administration) 0.1 mg/kg triazolam: 3 mg/kg pregnanolone of the 1:30, 0.056 mg/kg triazolam: 5.6 mg/kg pregnanolone of the 1:100 mixture, and 0.03 mg/kg triazolam: 9 mg/kg pregnanolone of the 1:300 mixture significantly decreased response rates compared with vehicle (Dunnett’s tests, p’s < 0.05). Compared to triazolam alone, however, the mixtures produced leftward shifts in the dose-response function that corresponded to increasing proportions of pregnanolone to triazolam. Isobolographic representation (Figure 1, bottom panel) of the 1:30 and 1:100 mixtures suggests supra-additivity as the ED50 values of these mixtures lie below the line of additivity. The 1:300 mixture fell close to the line suggesting additivity. Statistical analysis comparing the experimentally derived (ED50mix) and predicted (ED50add) values confirmed these results and are shown in Table 2.
TABLE 2.
Experimentally determined (ED50mix) and predicted additive (ED50add) values of triazolam + pregnanolone, clonazepam+ganaxolone, triazolam+ganaxolone and clonazepam+pregnanolone mixtures (N=7)
| Drug combination | ED50mix(±95% Confidence Interval) | ED50add(±95% Confidence Interval) | Interaction index^ |
|---|---|---|---|
| Triazolam/pregnanolone (1:30) | 1.718 (1.284–2.152)* | 2.784 (2.066–3.502) | 0.63 |
| Triazolam/pregnanolone (1:100) | 3.405 (2.118–4.692)* | 5.04 (3.889–6.182) | 0.69 |
| Triazolam/pregnanolone (1:300) | 5.676 (4.68–6.672) | 6.66 (5.257–8.063) | 0.87 |
| Clonazepam/ganaxolone (1:10) | 1.765 (1.276–2.253) | 1.691 (1.212–2.17) | 1.07 |
| Clonazepam/ganaxolone (1:30) | 5.102 (4.253–5.95)* | 3.141 (2.495–3.787) | 1.65 |
| Clonazepam/ganaxolone (1:100) | 4.629 (3.407–5.85) | 4.725 (4.081–5.369) | 0.99 |
| Triazolam/ganaxolone (1:10) | 1.981 (1.686–2.275)* | 1.403 (1.159–1.647) | 1.47 |
| Triazolam/ganaxolone (1:30) | 2.062 (1.738–2.387)* | 2.785 (2.371–3.198) | 0.75 |
| Triazolam/ganaxolone (1:100) | 4.718 (4.126–5.311) | 4.475 (3.94–5.010) | 1.06 |
| Clonazepam/pregnanolone (1:10) | 1.685 (1.304–2.065) | 1.519 (1.131–1.907) | 1.3 |
| Clonazepam/pregnanolone (1:30) | 1.924 (1.862–1.986)* | 3.125 (2.358–3.892) | 0.67 |
| Clonazepam/pregnanolone (1:100) | 3.747 (3.034–4.460) | 5.307 (4.090–6.524) | 0.77 |
experimentally determined value significantly different than the predictive additive value (p <0.05).
interaction index (i.e. ED50mix/ED50add).
3.2. Clonazepam and Ganaxolone Alone and Mixtures
Figure 2 (top panel) shows the rate-suppressing effects of the benzodiazepine clonazepam and the neuroactive steroid ganaxolone. Both drugs dose-dependently and reliably reduced rates of responding (clonazepam: F (4,24) = 20.35, p< 0.05; ganaxolone: F (4,24) = 104.2, p < 0.05). When compared to control conditions (vehicle administration), 0.3 mg/kg of clonazepam and 10 mg/kg ganaxolone engendered reliably lower rates than vehicle (Dunnett’s test, p’s < 0.05). The ED50 values (95% confidence interval) for clonazepam and ganaxolone were 0.21 (0.13–0.28) and 6.15 (5.54–6.76) mg/kg, respectively; showing an approximately 30-fold difference in potency between these two drugs. Furthermore, there were virtually no responses on the inactive lever (data not shown). Figure 2 (middle panel) shows the dose-dependent effects on rates of responding for the clonazepam and ganaxolone mixtures. As with clonazepam alone, all mixtures dose-dependently and reliably suppressed responding (1:10: F (3,18) = 29.00, p <0.05; 1:30: F (3,18) = 48.94, p <0.05; 1:100: F (3,18) = 22.11). Similar to the results seen using the drugs alone, these drug combinations did not elicit responding on the inactive lever (data not shown). When individual doses of the drugs were compared to their respective control conditions (vehicle administration), 0.3 mg/kg clonazepam: 3 mg/kg ganaxolone of the 1:10 and the 1:30 mixtures, and 0.03 mg/kg clonazepam: 3mg/kg ganaxolone of the 1:100 mixture significantly decreased response rates compared with vehicle (Dunnett’s tests, p’s < 0.05). Figure 2 (bottom panel) shows the isobologram depicting these data. The 1:10 and 1:100 mixtures lie on the line of additivity, while the 1:30 mixture falls above the additivity line, consistent with an infra-additive interaction for the latter mixture. Statistical analysis comparing the experimentally derived (ED50mix) and predicted (ED50add) values confirmed these results and are shown in Table 2.
Figure 2.
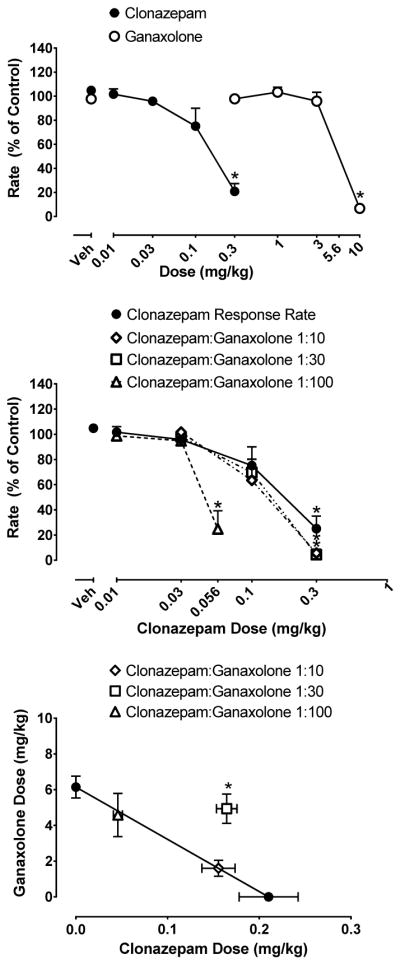
Top, effects of clonazepam and ganaxolone alone on response rate under the fixed-ratio schedule of food pellet delivery. X-axis, dose of clonazepam and ganaxolone in mg/kg. Y-axis, response rate as a percent of control. Points above “Veh” represent vehicle administration. Middle, effects of clonazepam and clonazepam+ganaxolone mixtures under the fixed-ratio schedule of food pellet delivery. Bottom, isobologram of clonazepam + ganaxolone mixtures. X-axis dose of clonazepam in mg/kg. Y-axis, dose of ganaxolone in mg/kg. Each data point represents the mean (±SEM) from seven rats.
3.3. Triazolam:Ganaxolone and Clonazepam: Pregnanolone Mixtures
Because the type and degree of interaction varied to such a large extent, we investigated whether the differential effects observed with triazolam:pregnanolone mixtures vs. clonazepam:ganaxolone mixtures were unique to the particular combinations or were due to a particular drug. To summarize these data, only the isobolograms are shown in Figure 3. The isobologram of triazolam and ganaxolone mixtures indicated that the 1:10 mixture was infra-additive because the ED50mix was above the additivity line, while the 1:30 mixture was found to be supra-additive as evident by the ED50mix lying below the additive line (Figure 3, top panel). The 1:100 mixture was additive. Figure 3 (bottom panel) shows the isobologram of clonazepam and pregnanolone mixtures, and only one drug mixture (1:30) was found to be supra-additive while the other mixtures were additive. Statistical analysis comparing the experimentally derived (ED50mix) and predicted (ED50add) values confirmed these results and are shown in Table 2.
Figure 3.
Effects of triazolam+ganaxolone and clonazepam+pregnanolone mixtures on response rate under the fixed-ratio schedule of food pellet delivery. Top, isobologram of triazolam+ganaxolone mixtures. Bottom, isobologram of clonazepam+pregnanolone mixtures. Each data point represents the mean (±SEM) from seven rats.
4. Discussion
The present study evaluated combinations of different benzodiazepines and neuroactive steroids on schedule- controlled responding in rats. Triazolam was chosen as a representative of the benzodiazepine class following our previous study in monkeys [20], and as it has been extensively studied using operant-based procedures [32]. However its kinetic properties, specifically short duration of action, hinder its use as a therapeutic. The effects of clonazepam on response rates also has been evaluated [33, 34] although not as frequently as triazolam even though it has a greater clinical utility. In our study, all of the drugs reliably reduced response rates under the FR schedule of food pellet delivery. These findings corroborate earlier work investigating the acute effects of triazolam and clonazepam administration alone in preclinical behavioral models. Our results also confirm the findings of other studies showing that neuroactive steroids have response-altering effects similar to benzodiazepines, presumably due to their actions as modulators of the GABAA receptor [35–37]. Furthermore, neuroactive steroids also have been shown to elicit similar discriminative stimulus effects as benzodiazepines [14, 38] and can alter effects of cue-induced reinstatement in models of ethanol and cocaine seeking [39–41] in a manner similar to benzodiazepines [42, 43]. However, unlike benzodiazepines, neuroactive steroids do not engender tolerance to rate-altering effects or anticonvulsant effects [44–47], findings that further support their clinical use.
The present study is the first, to our knowledge, to assess the rate-altering effects of these drugs as mixtures using rats. Other studies using non-human primates have found additive discriminative stimulus effects [21, 48], and our previous work using rhesus monkeys found infra-additive reinforcing and supra-additive anti-conflict effects [20] with these combinations. These findings formed the basis of our current research whereby we extended evaluation of the behavioral effects of these combinations to rats as well as to mixtures of the more clinically-relevant clonazepam and ganaxolone. Contrary to our results that found supra-additivity of triazolam and pregnanolone mixtures, our earlier study found additive effects on response rates after administration[20]. This may be due to species differences and as well as the behavioral endpoint that was measured. Here we evaluated drug effects in a simpler model of food reinforcement whereas in the earlier study in monkeys, rates of suppressed responding was the final behavioral measure.
In the current study, we also evaluated the effects of the more clinically relevant drugs, clonazepam and ganaxolone. To our knowledge no study to date has evaluated the rate-altering effects of these drugs alone or in a mixture in rats. Interestingly, we found infra-additive rate-altering effects with this mixture. This was a surprising finding due to having previously observed primarily supra-additivity with triazolam and pregnanolone combinations: Given the similarities among these drugs, we expected the same results with clonazepam and ganaxolone mixtures. Due to these seemingly contradictory results we evaluated mixtures of triazolam/ganaxolone and clonazepam/pregnanolone in order to determine if either the benzodiazepine or the neuroactive steroid in the mixture was “driving” the results. The results were not straightforward: The triazolam/ganaxolone mixtures were both supra and infra-additive depending upon the proportion tested, whereas the clonazepam/pregnanolone mixtures mimicked the results seen with triazolam/pregnanolone (i.e., supra-additivity). Together with the triazolam/pregnanolone and clonazepam/ganaxolone results, these observations do not provide support for the idea that a single drug is associated with one type of interaction except for pregnanolone, in which the majority of mixtures involving this drug were supra-additive only.
Based on what is known about triazolam, pregnanolone, clonazepam, and ganaxolone, it is unclear why such strikingly different interactions would be observed with the different drug combinations. While these drugs were selected as representatives of each class based on their duration of action, it is difficult to discern a key role for pharmacokinetics since the session length was only 2.5 minutes. Any differences in duration would need to be evaluated across a longer period of time based upon the intrinsic kinetic properties. There were differences, however, in onset of action. A 10 minute pretreatment period was sufficient in reducing rates of responding for all drugs but ganaxolone, which was effective at the 20 minute pretreatment time, suggesting that the rate-altering effects of ganaxolone were delayed compared to the other three drugs tested. Ganaxolone is a synthetic derivative of the endogenously generated neuroactive steroid allopregnanolone which is the 3α-reduced epimer of pregnanolone. Methyl group additions to the compound slows metabolism by 3α-hydroxysteroid dehydrogenase and therefore increases the bioavailability of the drug [49, 50]. It is unclear if these chemical changes impart delayed onset in operant procedures, however in a study evaluating its anticonvulsant effects [44], a 15 minute pretreatment period was sufficient to block pentylenetetrazol-induced seizures with doses similar to those from the current study. Furthermore, peak plasma concentrations and anticonvulsant activity was found between 30–60 minutes. Regardless, the pretreatment times for drugs tested in combination with ganaxolone were adjusted to match this compound’s slower onset of action, and as with duration of action, it is difficult to understand how onset of action could play a key role in the varied results obtained in the present study.
In recent years, it has been proposed that neuroactive steroids act at both synaptic and extrasynaptic GABAA receptors [9, 51, 52]. Stimulation of receptors at these two distinct sites by GABA elicits different inhibitory effects. Synaptic GABAA receptors mediate phasic inhibition through presynaptic GABA release, while the extrasynaptic receptors are thought to play a role in tonic inhibition due to ambient concentrations of GABA providing a low but steady activation of the receptor. The neuroactive steroid binding site located at both the synaptic and extrasynaptic GABAA receptors can potentiate the site specific inhibitory actions [51]. Moreover, synaptic and extrasynaptic GABAA receptors are thought to have separate subunit compositions which can influence neuronal inhibition and thus may include possible binding sites for GABAA receptor modulators. Extrasynaptic receptors appear to contain predominantly delta subunits which may be absent from the synaptic receptor composition [23]. Extrasynaptic receptors containing delta subunits are also highly sensitive to modulation by neurosteroids [2]. Although we do not yet have data to address this, we hypothesize that differential interactions between benzodiazepines and neuroactive steroids reflect unique effects of the two classes of compounds on synaptic- vs. extrasynaptic-mediated (i.e., delta subunit-mediated) inhibitory tone.
An important observation from the effects seen here is the utility of evaluating multiple fixed-proportions instead of a single mixture based on the constituent drugs’ ED50s. Early studies have validated the importance of testing separate fixed-proportion mixtures in order to critically evaluate if deviation from additivity exists [53]. Our results highlight the need to continue this strategy due to the effects seen in all of the combination studies we measured. While two clonazepam/ganaxolone mixtures were additive (1:10 and 1:100), the 1:30 mixture was found to be infra-additive. As mentioned previously, we tested relative proportions of the drugs based on the individual drugs’ ED50s that have fractional multipliers spanning 0 to 1. This f value is used in the ED50add equation to determine the theoretical dose-pair that would elicit 50% reduction in response rate for the specific mixed-ratio tested. The 1:30 mixture of clonazepam/ganaxolone is closest to an f value of 0.5; in other words, closest to the actual relative potencies of the two drugs in the mixture. Our results with triazolam/ganaxolone mixtures were more varied in that all three proportions had different results. In this case, the dose of each drug in the mixture was a key determinant in mediating the rate-altering effects of the combinations.
Finally it is important to note that while assessing mixtures of drugs is highly dependent on the behavioral endpoint [20, 37, 48, 54], the present findings clearly indicate that the nature of a drug interaction can vary dramatically within a single endpoint. In fact, schedule-controlled behavior was chosen in the present study because operant lever pressing is common to many other procedures, such as drug discrimination, self-administration, and conflict procedures. It is clear, however, that interactions in schedule-controlled responding cannot be generalized in a simple manner to other operant-based procedures. Instead, these findings underscore the need to evaluate different drugs and a range of mixtures in order to avoid too narrow conclusions for a given model of behavior.
Highlights.
We tested rate effects of benzodiazepines and neuroactive steroids in rats
Combinations of benzodiazepine and neuroactive steroids elicit reductions in response rate and were dependent upon the combination of drugs tested and proportions of drugs in the mixtures
These results provide valuable insight into the possible sedative/motor effects of clinically relevant benzodiazepine and neuroactive steroid combinations
Acknowledgments
This work was supported by DA011792, DA033795, and AA016179. The authors gratefully acknowledge the technical assistance of Camille Washington in conducting these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barak W. Gunter, University of Mississippi Medical Center, Department of Psychiatry and Human Behavior, Department of Neurobiology and Anatomical Sciences, Program in Neuroscience, 2500 North State Street, Jackson, Mississippi 39216
Donna M. Platt, University of Mississippi Medical Center, Department of Psychiatry and Human Behavior, Department of Neurobiology and Anatomical Sciences, Program in Neuroscience, 2500 North State Street, Jackson, Mississippi 39216
James K. Rowlett, University of Mississippi Medical Center, Department of Psychiatry and Human Behavior, Department of Neurobiology and Anatomical Sciences, Program in Neuroscience, 2500 North State Street, Jackson, Mississippi 39216. Tulane National Primate Research Center, Tulane University School of Medicine, 18703 Three Rivers Road, Covington, Louisiana 70433.
References
- 1.Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10(9):685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson JR, Feltner DE, Dugar A. Management of generalized anxiety disorder in primary care: identifying the challenges and unmet needs. Prim Care Companion J Clin Psychiatry. 2010;12(2) doi: 10.4088/PCC.09r00772blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin DS, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567–96. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- 6.Bandelow B, et al. The diagnosis of and treatment recommendations for anxiety disorders. Dtsch Arztebl Int. 2014;111(27–28):473–80. doi: 10.3238/arztebl.2014.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandelow B, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals--implications for problems of long-term use and abuse. Psychopharmacology (Berl) 1997;134(1):1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JJ, et al. Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Rupprecht R, et al. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol. 1996;56(1–6 Spec No):163–8. doi: 10.1016/0960-0760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- 11.Dawson GR, Collinson N, Atack JR. Development of subtype selective GABAA modulators. CNS Spectr. 2005;10(1):21–7. doi: 10.1017/s1092852900009871. [DOI] [PubMed] [Google Scholar]
- 12.Gomez C, et al. Rapid anxiolytic activity of progesterone and pregnanolone in male rats. Pharmacol Biochem Behav. 2002;72(3):543–50. doi: 10.1016/s0091-3057(02)00722-0. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers RJ, Johnson NJ. Behaviorally selective effects of neuroactive steroids on plus-maze anxiety in mice. Pharmacol Biochem Behav. 1998;59(1):221–32. doi: 10.1016/s0091-3057(97)00339-0. [DOI] [PubMed] [Google Scholar]
- 14.Bai X, Gerak LR. Comparing the discriminative stimuli produced by either the neuroactive steroid pregnanolone or the benzodiazepine midazolam in rats. Psychopharmacology (Berl) 2011;214(2):427–35. doi: 10.1007/s00213-010-2047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowlett JK, et al. Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8-7071 in rhesus monkeys. Psychopharmacology (Berl) 1999;145(2):205–12. doi: 10.1007/s002130051050. [DOI] [PubMed] [Google Scholar]
- 16.Sinnott RS, Mark GP, Finn DA. Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol Biochem Behav. 2002;72(4):923–9. doi: 10.1016/s0091-3057(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 17.Monaghan EP, McAuley JW, Data JL. Ganaxolone: a novel positive allosteric modulator of the GABA(A) receptor complex for the treatment of epilepsy. Expert Opin Investig Drugs. 1999;8(10):1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- 18.Mula M. Emerging drugs for focal epilepsy. Expert Opin Emerg Drugs. 2013;18(1):87–95. doi: 10.1517/14728214.2013.750294. [DOI] [PubMed] [Google Scholar]
- 19.Pieribone VA, et al. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48(10):1870–4. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Fischer BD, Rowlett JK. Anticonflict and reinforcing effects of triazolam + pregnanolone combinations in rhesus monkeys. J Pharmacol Exp Ther. 2011;337(3):805–11. doi: 10.1124/jpet.111.180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon LR, France CP. Combined discriminative stimulus effects of midazolam with other positive GABAA modulators and GABAA receptor agonists in rhesus monkeys. Psychopharmacology (Berl) 2005;178(4):400–9. doi: 10.1007/s00213-004-2022-4. [DOI] [PubMed] [Google Scholar]
- 22.Norberg L, Backstrom T, Wahlstrom G. Anaesthetic effects of pregnanolone in combination with allopregnanolone, thiopental, hexobarbital and flurazepam: an EEG study in the rat. Br J Anaesth. 1999;82(5):731–7. doi: 10.1093/bja/82.5.731. [DOI] [PubMed] [Google Scholar]
- 23.Stell BM, et al. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100(24):14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morse WH. Schedule-controlled behaviors as determinants of drug response. Fed Proc. 1975;34(9):1868–9. [PubMed] [Google Scholar]
- 25.Wettstein JG, Spealman RD. GABA-related drugs modulate the behavioral effects of lorazepam. Psychopharmacology (Berl) 1988;95(1):38–42. doi: 10.1007/BF00212763. [DOI] [PubMed] [Google Scholar]
- 26.Tan C, et al. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv245. [DOI] [PubMed] [Google Scholar]
- 27.Ator NA. High-dose discrimination training with midazolam: context determines generalization profile. Pharmacol Biochem Behav. 1999;64(2):237–43. doi: 10.1016/s0091-3057(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 28.Hogenkamp DJ, et al. Pharmacological profile of a 17beta-heteroaryl-substituted neuroactive steroid. Psychopharmacology (Berl) 2014;231(17):3517–24. doi: 10.1007/s00213-014-3494-5. [DOI] [PubMed] [Google Scholar]
- 29.Rowlett JK, et al. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology (Berl) 2006;184(2):201–11. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- 30.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–72. [PubMed] [Google Scholar]
- 31.Tallarida RJ. The interaction index: a measure of drug synergism. Pain. 2002;98(1–2):163–8. doi: 10.1016/s0304-3959(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 32.Rowlett JK, Woolverton WL. Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: I. Comparison with triazolam in rhesus monkeys and rats. J Pharmacol Exp Ther. 1997;280(1):162–73. [PubMed] [Google Scholar]
- 33.Picker M, et al. Effects of clonazepam and ethosuximide on the responding of pigeons under a fixed-consecutive-number schedule with and without an external discriminative stimulus. Psychopharmacology (Berl) 1986;88(3):325–30. doi: 10.1007/BF00180833. [DOI] [PubMed] [Google Scholar]
- 34.Carter RB, Leander JD. Comparison of the effects of naloxone and picrotoxin on schedule-controlled responding in the pigeon: possible GABA-antagonistic effects of naloxone. J Pharmacol Exp Ther. 1984;230(1):40–6. [PubMed] [Google Scholar]
- 35.Vanover KE, et al. Response-rate suppression in operant paradigm as predictor of soporific potency in rats and identification of three novel sedative-hypnotic neuroactive steroids. J Pharmacol Exp Ther. 1999;291(3):1317–23. [PubMed] [Google Scholar]
- 36.McMahon LR, France CP. Acute and chronic effects of the neuroactive steroid pregnanolone on schedule-controlled responding in rhesus monkeys. Behav Pharmacol. 2002;13(7):545–55. doi: 10.1097/00008877-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Gerak LR, et al. Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 2004;173(1–2):195–202. doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- 38.McMahon LR, France CP. Discriminative stimulus effects of positive GABAA modulators and other anxiolytics, sedatives, and anticonvulsants in untreated and diazepam-treated monkeys. J Pharmacol Exp Ther. 2003;304(1):109–20. doi: 10.1124/jpet.102.040931. [DOI] [PubMed] [Google Scholar]
- 39.Schmoutz CD, Runyon SP, Goeders NE. Effects of inhibitory GABA-active neurosteroids on cocaine seeking and cocaine taking in rats. Psychopharmacology (Berl) 2014;231(17):3391–400. doi: 10.1007/s00213-013-3404-2. [DOI] [PubMed] [Google Scholar]
- 40.Ramaker MJ, et al. Differences in the reinstatement of ethanol seeking with ganaxolone and gaboxadol. Neuroscience. 2014;272:180–7. doi: 10.1016/j.neuroscience.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1–2):222–8. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- 42.Keller CM, et al. Combinations of oxazepam and metyrapone attenuate cocaine and methamphetamine cue reactivity. Drug Alcohol Depend. 2013;133(2):405–12. doi: 10.1016/j.drugalcdep.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Goeders NE, et al. Alprazolam and oxazepam block the cue-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2009;201(4):581–8. doi: 10.1007/s00213-008-1326-1. [DOI] [PubMed] [Google Scholar]
- 44.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295(3):1241–8. [PubMed] [Google Scholar]
- 45.Kokate TG, et al. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287(2):553–8. [PubMed] [Google Scholar]
- 46.Eppolito AK, Gerak LR. Tolerance to the rate-increasing and not rate-decreasing effects of pregnanolone in rats. Behav Pharmacol. 2010;21(8):736–44. doi: 10.1097/FBP.0b013e32833fa79d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanover KE, et al. Characterization of the anxiolytic properties of a novel neuroactive steroid, Co 2–6749 (GMA-839; WAY-141839; 3alpha, 21-dihydroxy-3beta-trifluoromethyl-19-nor-5beta-pregnan-20-one), a selective modulator of gamma-aminobutyric acid(A) receptors. J Pharmacol Exp Ther. 2000;295(1):337–45. [PubMed] [Google Scholar]
- 48.Gerak LR, France CP. Quantitative analyses of antagonism: combinations of midazolam and either flunitrazepam or pregnanolone in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther. 2012;340(3):742–9. doi: 10.1124/jpet.111.188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter RB, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 1997;280(3):1284–95. [PubMed] [Google Scholar]
- 50.Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9(2–3):207–27. [PubMed] [Google Scholar]
- 51.Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116(1):20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell EA, et al. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52(4–5):588–95. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Gessner PK, Cabana BE. A study of the interaction of the hypnotic effects and of the toxic effects of chloral hydrate and ethanol. J Pharmacol Exp Ther. 1970;174(2):247–59. [PubMed] [Google Scholar]
- 54.Gerak LR, France CP. Chronic benzodiazepine treatment does not alter interactions between positive GABA(A) modulators and flumazenil or pentylenetetrazole in monkeys. Behav Pharmacol. 2011;22(1):49–57. doi: 10.1097/FBP.0b013e3283425aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]