Abstract
Circadian clocks optimize the timing of physiological processes in synchrony with daily recurring and therefore predictable changes in the environment. Until the late 1990s, circadian clocks were thought to exist only in the central nervous systems of animals; elegant studies in cultured fibroblasts and using genetically encoded reporters in Drosophila melanogaster and in mice showed that clocks are ubiquitous and cell autonomous. These findings inspired investigations of the advantages construed by enabling each organ to independently adjust its function to the time of day. Studies of rhythmic gene expression in several organs suggested that peripheral organ clocks might play an important role in optimizing metabolic physiology by synchronizing tissue-intrinsic metabolic processes to cycles of nutrient availability and energy requirements. The effects of clock disruption in liver, pancreas, muscle, and adipose tissues support that hypothesis. Adipose tissues coordinate energy storage and utilization and modulate behavior and the physiology of other organs by secreting hormones known as “adipokines.” Due to behavior- and environment-driven diurnal variations in supply and demand for chemical and thermal energy, adipose tissues might represent an important peripheral location for coordinating circadian energy balance (intake, storage, and utilization) over the whole organism. Given the complexity of adipose cell types and depots, the sensitivity of adipose tissue biology to age and diet composition, and the plethora of known and yet-to-be-discovered adipokines and lipokines, we have just begun to scratch the surface of understanding the role of circadian clocks in adipose tissues.
Keywords: circadian, clock, adipose, adipocyte, PPARγ, Reverbα
Mammalian circadian clocks involve a core cell-autonomous feedback loop in which the E-box specific DNA binding transcription factors Clock and Bmal1 (also known as Arntl) drive expression of their own repressors, periods (Per1,2,3) and cryptochromes (Cry1,2) (Partch et al., 2014). Clock and Bmal1 also drive rhythmic expression of the Nr1d (Reverbα, Reverbβ) and Nr1f (Rorα,β,γ) families of nuclear hormone receptors (NRs), which compete to repress or activate transcription respectively from cognate response elements, including the Bmal1 promoter. NRs are master regulators of metabolic physiology (Mangelsdorf et al., 1995; Mullican et al., 2013), and several of them are expressed rhythmically in liver, muscle, and adipose tissues, which may be important for coordinating their metabolic functions with circadian time (Yang et al., 2006; Yang et al., 2007). Notably, the only single gene deletion that abolishes circadian rhythms in all tissues is ablation of Bmal1. Mice with tissue-specific disruption of Bmal1 have been important tools in deciphering the role of circadian clocks in individual organs without disrupting central clock-driven behavioral rhythms (Storch et al., 2007; Westgate et al., 2008).
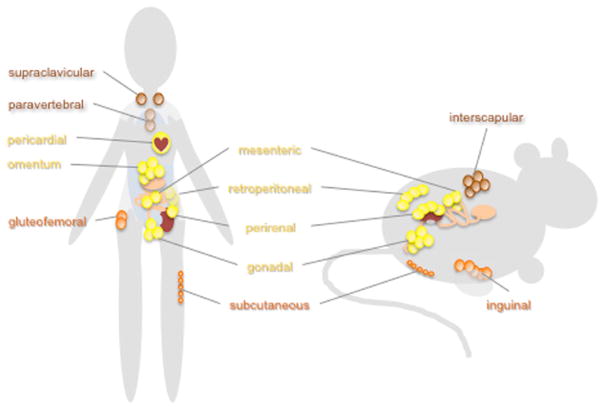
Adipose tissues are the main sites of long-term energy storage and participate in whole-body energy homeostasis and thermoregulation (Rosen and Spiegelman, 2014). Far from being a single homogeneous organ, fat resides in several major deposits in humans, classified into 3 types: subcutaneous white adipose tissues, visceral white adipose tissues, and brown adipose tissues. Adipocytes are also present in smaller deposits, including those that provide mechanical cushioning for joints and feet, energy reserves for mammary gland remodeling, and expansion during pregnancy and lactation, and in the bone marrow. Rodents also have white and brown adipose tissues, and some of the major fat pads in mice are similar to specific human fat depots, while others may be species specific (Figure 1) (Tran and Kahn, 2010). The cellular makeup and physiology of fat in different locations are distinct, as evidenced by profound metabolic changes induced by transplanting fat from one location to another (Lee et al., 2013). Like other peripheral organs, human and mouse adipose tissues (Ando et al., 2005; Zvonic et al., 2006; Gomez-Santos et al., 2009) and cultured adipocytes (Ramanathan et al., 2014) harbor circadian clocks that are set by metabolic signals downstream of feeding (Zvonic et al., 2006). Diurnally expressed transcripts seem to be similarly widespread in adipose tissues as they are in other peripheral organs and include many metabolically important genes (Zvonic et al., 2006; Sukumaran et al., 2010; Shostak et al., 2013).
Figure 1.
Major sites of adipocyte accumulation in humans and rodents. Adipocytes accumulate in characteristic fatty deposits in humans, mice, and rats. White adipose tissues are subdivided into subcutaneous (shown in orange) and visceral (shown in yellow) adipose tissues. Visceral fat accumulation is associated with undesirable metabolic consequences while unhealthy lipodystrophy tends to cause preferential loss of subcutaneous fat. Brown adipose tissues (shown in brown) produce heat by burning fat stores and tend to be metabolically beneficial.
Adipose tissues contain a heterogeneous mixture of cell types, including adipocytes, vasculature, and macrophages. In this review, we focus on the role of circadian rhythms and clock components in adipocytes. White adipocytes balance the synthesis, storage, and breakdown of triglycerides during times of energy surplus or demand. They are also the primary sites of synthesis and secretion of several adipokines, adipocyte-derived hormones that signal long-term energy storage to the central nervous system and regulate food intake and other aspects of physiology. Brown adipocytes contain a high density of mitochondria and generate heat by uncoupling lipid oxidation from mitochrondrial adenosine triphosphate (ATP) generation via expression of uncoupling protein 1 (Ucp1) (Cannon and Nedergaard, 2004). Through this process, they play a critical role in body temperature modulation. Recently, so-called beige adipocytes have been described, which are developmentally related to white adipocytes but seem to be functionally flexible and can adapt to function more like brown adipocytes (Harms and Seale, 2013).
ADIPOGENESIS
The detailed mechanisms of adipose tissue specification and development are beyond the scope of this review and remain incompletely understood (Rosen and Spiegelman, 2014). Brown adipocytes are derived from a different precursor than white adipocytes, and their developmental lineage is closer to that of muscle cells, which are also rich in mitochondria and can produce heat. Indeed, it is possible to “transdifferentiate” myocytes into brown adipocytes in culture by expressing the transcriptional cofactor Prdm16 (Seale et al., 2008). Conversely, depleting Prdm16 from primary brown adipocytes converts them to myocytes. White and beige adipocytes are derived from a common distinct stem cell population.
The differentiation of mature lipid-storing white adipocytes from committed adipocyte precursors has been extensively studied in cell culture models and may be influenced by circadian clocks. Bmal1 has been reported to influence adipogenesis in cultured preadipocytes, but there is no consensus among the published findings. The first study to address the role of Bmal1 in adipogenesis reported that small interfering RNA (siRNA) knockdown of Bmal1 reduces adipogenesis in 3T3-L1 cells (Shimba et al., 2005), while a subsequent study reported that knockdown of Bmal1 enhanced adipogenesis in 10T1/2 cells and in mouse embryonic fibroblasts (Guo et al., 2012). It is difficult to know how these cell culture systems relate to in vivo adipose tissue development. Several groups have reported increased adiposity in mice lacking Bmal1 expression either in all cells (Lamia et al., 2008; Guo et al., 2012; Kennaway et al., 2013) or specifically in adipocytes (Paschos et al., 2012) and in Clockm/m mutant mice (Rudic et al., 2004; Shostak et al., 2013), in which exon 19 of the Clock transcript is skipped, resulting in reduced DNA binding activity (King et al., 1997). This is consistent with the increased adipogenesis observed in some studies of Bmal1−/− cells (Guo et al., 2012) but could also reflect differences in feeding patterns of Bmal1−/− and Clockm/m mice, which eat a similar amount of food to that eaten by wild-type mice but consume a higher proportion of calories during the day (Turek et al., 2005; Lamia et al., 2008). It is now well established that the temporal pattern of food consumption can profoundly affect weight gain and adiposity independent of total caloric intake (Arble et al., 2009; Bray et al., 2010; Hatori et al., 2012; Chaix et al., 2014). The increased adiposity in circadian mutant mice could also reflect increased lipid accumulation due to increased lipogenesis or decreased lipolysis, which will be discussed further below.
REVERBα AND REVERBβ
The nuclear hormone receptor Reverbα, which is among the highest amplitude diurnally expressed proteins and participates in circadian rhythm maintenance, has also been suggested to play a role in adipogenesis. Reverbα expression is dynamically regulated during adipogenesis (Chawla and Lazar, 1993; Canaple et al., 2006), and Reverbα activating ligands can promote adipogenesis (Kojetin and Burris, 2011). However, Reverbα−/− mice have normal or even increased amounts of adipose tissue (Chomez et al., 2000; Delezie et al., 2012), suggesting that Reverbα is not required for adipogenesis in vivo. This could simply reflect redundancy with the highly related transcription factor Reverbβ; examination of mice lacking both Reverbα and Reverbβ in adipose tissues would help determine whether they are important players in this process.
In contrast, Reverbα seems to be uniquely important for the circadian regulation of body temperature in brown adipose tissue (Gerhart-Hines et al., 2013). Wild-type mice display robust diurnal rhythms in body temperature, with core body temperature varying between approximately 35 °C during the day and 37 °C at night. In Reverbα−/− mice, this oscillation is severely dampened, and core body temperature remains high throughout the day. This is likely due to loss of Reverbα-dependent suppression of Ucp1 expression during the day in brown adipose tissue (Gerhart-Hines et al., 2013), a striking example of a cascade of circadian transcriptional regulation modulating a specific physiological output in a time-of-day–dependent manner. Importantly, human body temperature also exhibits diurnal oscillation (Cermakian and Boivin, 2009), and circadian rhythms of human brown adipose tissue activity have been described (van der Veen et al., 2012). Thus, this mechanism may be conserved in humans and could potentially be modulated by synthetic Reverbα ligands (Kojetin and Burris, 2011).
PPARγ
Another nuclear hormone receptor that has been linked to circadian rhythms, peroxisome proliferator-activated receptor γ (PPARγ), is a master regulator of adipocyte differentiation and function (Ahmadian et al., 2013). PPARγ is highly expressed in adipose tissue and is required for adipogenesis (Barak et al., 1999). Indeed, expression and activation of PPARγ is sufficient to drive the differentiation of fibroblasts into adipocytes (Tontonoz et al., 1994). Circadian clocks regulate PPARγ in several ways. Pparγ expression in mouse epididymal white adipose tissue is approximately 4 times higher during the middle of the night than it is in the morning (Yang et al., 2006), although it is unclear whether this rhythmic expression is driven by systemic factors or local adipose clocks. PPARγ activity is controlled by interaction with a plethora of transcriptional coregulators (Feige and Auwerx, 2007; Powell et al., 2007), among which the best studied are PPARγ coactivator 1 alpha (Pgc1α) and beta (Pgc1β). Pgc1α and Pgc1β messenger RNA (mRNAs) exhibit diurnal rhythms in liver, muscle, and white and brown adipose tissues (Yang et al., 2006; Liu et al., 2007; Paschos et al., 2012). The oscillating expression patterns of Pparγ and Pgc1α peak synchronously near dawn in mouse WAT (Yang et al., 2006), suggesting that rhythmic coactivator expression could amplify circadian PPARγ activity in WAT during the day. Interestingly, Pgc1α and Pgc1β are likely direct targets of several circadian transcription factors because multiple sites in chromatin near their loci are among the small number of sites that are bound by all 7 circadian factors examined in a genome-wide study in mouse liver (Koike et al., 2012). This, coupled with their rhythmic expression in a wide variety of tissues, suggests that Pgc1α/β may be intimately linked to circadian clock function. Pgc1α−/− mice have slightly altered circadian behavior patterns, and circadian gene expression is altered in Pgc1α−/− cells (Liu et al., 2007). While these effects are small, it is possible that Pgc1β is redundant with Pgc1α in regulating rhythms and that loss of both isoforms would have a greater impact on clock function. Pgc1β−/− mice have altered diurnal activity, but circadian rhythm was not specifically examined (Sonoda et al., 2007). It has also been suggested that PPARγ may support circadian rhythms. Notably, deletion of Pparγ reduced the amplitude of circadian expression of several transcripts in adipose tissue (Yang et al., 2012). Cell or tissue-specific deletion of Pparγ also decreased circadian gene expression in cultured adipocytes and in the vasculature, respectively (Wang et al., 2008; Yang et al., 2012). Genomic profiling of PPARγ chromatin association and target gene activation has defined thousands of transcripts that are directly regulated by PPARγ (Lefterova et al., 2008; Nielsen et al., 2008). The PPARγ target gene network includes important drivers of adipocyte biology, and several of those are transcribed rhythmically in adipose tissues in vivo (Figure 2). It seems likely that PPARγ is part of a coordinated transcriptional activation cascade in WAT analogous to the role of Reverbα in BAT exemplified by circadian thermoregulation driven by rhythmic Reverbα-dependent repression of Ucp1.
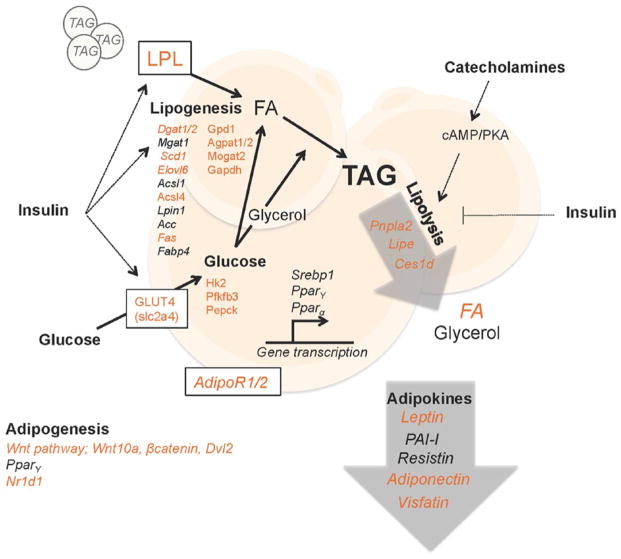
Figure 2.
The circadian clock regulates lipid metabolism in white adipose tissue. Adipocytes are cells that specialize in storing triacylglycerols (TAGs). TAGs are hydrolyzed in a process called lipolysis to produce soluble energy in the form of glycerol and fatty acids (FAs) when other tissues demand energy. Lipolysis is stimulated by catecholamines, which are mainly produced in the adrenal medulla. TAGs from the circulation constitute the majority of the stored lipids in adipocytes. TAGs are produced by esterification of FAs that are imported from the circulation, derived from circulating lipids that are hydrolyzed by lipoprotein lipase (LPL), or are produced from other substrates, such as glucose, in a process called de novo lipogenesis. Insulin acts on adipocyte metabolism in several ways, as indicated in the figure. In addition, adipose tissue is an endocrine organ, secreting essential endocrine factors called adipokines. The circadian clock coordinates lipid energy metabolism, partly through regulating the expression of enzymes, transporters, and adipokines. Genes and adipokines marked in italics are reported to display diurnal oscillation in expression. Those that are shown in orange font are direct targets of BMAL1/CLOCK and/or found altered in mice with a disrupted intrinsic clock. Lpl, lipoprotein lipase; Dgat1/2, diglyceride acyltransferase 1/2; Mgat1, monoglyceride acyltransferase 1; Scd1, stearoyl-CoA desaturase-1; Elovl6, elongation of very long-chain fatty acids protein 6; Acsl1/4, acyl-CoA synthetase long-chain family member 1/4; Lpin1, phosphatidate phosphatase LPIN1; Ac1/2c, acetyl-CoA carboxylase 1/2; Fas, fatty acid synthase; Fabp4, fatty acid binding protein 4; Gpd1, glycerol-3-phosphate dehydrogenase 1; Agpat1/2, 1-acylglycerol-3-phosphate o-acyltransferase 4; Mogat2, monoacylglycerol acyltransferase 2; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; Hk2, hexokinase 2; Pfkfb3, 6-phosphofructo-2-kinase/fructose-2,6-biphospatase 3; Pepck, phosphoenolpyruvate carboxykinase; Srebp1, sterol regulatory element-binding protein 1; Ppar α/γ, peroxisome proliferator-activated receptors α/γ; AdipoR1/2, adiponectin receptor 1/2; Pnpla2 (Atgl), patatin-like phospholipase domain containing/adipose triglyceride lipase; Lipe (Hsl), hormone-sensitive lipase; Ces1d (Tgh), carboxylesterase 1D/triacylglycerol hydrolase; FA, fatty acid; Wnt10a, wingless-type MMTB integration site family member 10a; Dvl2, disheveled segment polarity protein 2; Nr1d1 (RevErbα), nuclear receptor subfamily 1 group D member 1; slc2a4 (GLUT4), solute carrier family 2/glucose transporter type 4.
REGULATION OF REVERBα AND PPARγ BY CIRCADIAN REPRESSORS
Circadian repressors also regulate Reverbα and PPARγ. Per2 directly associates with several nuclear hormone receptors, including Reverbα, PPARα, and PPARγ (Grimaldi et al., 2010; Schmutz et al., 2010). Per3 binds PPARγ (Costa et al., 2011); its association with other NRs has not been examined. Cry1 and Cry2 interact with several nuclear hormone receptors, including PPARγ (K. Lamia, S. Jordan, and E. Henriksson, unpublished observation) but not Reverbα (Lamia et al., 2011). Per2, Per3, Cry1, and Cry2 each interacts with NRs via the NRs’ ligand binding domains (Schmutz et al., 2010; Costa et al., 2011; Lamia et al., 2011) and represses the transcriptional activity of associated NRs (Grimaldi et al., 2010; Schmutz et al., 2010; Costa et al., 2011; Lamia et al., 2011), but the mechanisms by which they do so remain unclear. NR ligand binding domain interactions with coactivators and corepressors are mediated by short hydrophobic motifs called “NR boxes” and “CoRNR boxes” (Heery et al., 1997; Hu and Lazar, 1999). The interaction of Per2 with NRs seems to require an NR box in the N-terminus of Per2 that is not conserved in Per1 or Per3 (Schmutz et al., 2010), and the interaction of Per3 with PPARγ also requires the N-terminal domain of Per3 (Costa et al., 2011), in which a similar sequence nearby may serve the same function.
Consistent with a role of circadian repressors in modulating adipocyte biology by repressing NRs, overexpression of Per3 prevented and deletion of Per3 enhanced the differentiation of mesenchymal stem cells into adipocytes (Costa et al., 2011), and Per3−/− mice have increased adipose tissue mass in the absence of altered feeding patterns (Dallmann and Weaver, 2010). Per2-deficient fibroblasts also differentiate more readily into adipocytes (Grimaldi et al., 2010). Per2−/− mice accumulate excess adipose tissue (Yang et al., 2009; Grimaldi et al., 2010), but because they also exhibit altered food consumption (Yang et al., 2009; Dallmann and Weaver, 2010), further study will be required to understand the cell-autonomous role of Per2 in adipocytes. The large number of chromatin sites bound uniquely by Pers and Crys suggests that they likely repress several nonclock transcription factors, including some NRs (Koike et al., 2012). A better understanding of the overlapping and distinct roles of Crys and Pers in regulating adipose biology and other circadian output pathways awaits further study.
ADIPOSE-SPECIFIC CLOCK DISRUPTION
The current gold-standard tool for analyzing the physiological effects of disrupting clock function in specific peripheral organs without altering the SCN master clock was recently applied to adipose tissues (Paschos et al., 2012). The resulting analysis of mice in which Bmal1 is depleted in adipocytes (Ad-Bmal1−/−) is illustrative both of the importance of circadian modulation of adipose function and of the limitations of this technique. Tissue-specific ablation of a gene harboring loxP sites by expression of Cre recombinase is only as good as the promoter driving Cre expression. Adipose-specific promoters are notoriously inefficient (resulting in less than 100% deletion) and somewhat promiscuous (expressed in some undesired locations) (Jeffery et al., 2014). To address promiscuity, 2 strains of Ad-Bmal1−/− mice were made, using either aP2-Cre or adiponectin-Cre to delete Bmal1 in adipocytes. This increases confidence that the phenotypes observed in both strains are due to the loss of Bmal1 in adipocytes, although it is formally possible that both aP2-Cre and adiponectin-Cre are also coexpressed in a small population of other cells. Regarding the efficiency of deletion, both strains of Ad-Bmal1−/− mice had incomplete loss of clock gene oscillation in WAT and BAT, as well as incomplete deletion of Bmal1 in isolated adipocytes, suggesting that some functions controlled by local adipocyte clocks may not be lost in these animals. Given that both aP2 and adiponectin are maximally expressed in mature adipocytes, it is especially likely that Bmal1 is expressed in mesenchymal stem cells and preadipocytes within adipose depots of Ad-Bmal1−/− mice.
Similar to mice in which Bmal1 is deleted in all cells, Ad-Bmal1−/− mice fed a high-fat diet develop obesity, suggesting that this can be attributed to the cell-autonomous loss of circadian rhythms in fat. High-fat fed Ad-Bmal1−/− mice also have altered feeding patterns and reduced respiratory oxygen consumption, each of which could contribute to obesity. Adipocytes secrete hormones and fatty acids that modulate feeding behavior and energy expenditure through hypothalamic circuits (Lam et al., 2005; Friedman, 2014; Bluher and Mantzoros, 2015), and many of them undergo circadian oscillation. Interestingly, several polyunsaturated fatty acids (PUFAs) were found to oscillate with peak concentration in the blood and hypothalamus during the day in control mice, and this rhythm was attenuated in Ad-Bmal1−/− mice, which could explain their increased daytime food intake, ultimately leading to obesity. The reduced release of PUFAs at CT6 was attributed to decreased expression of Ces1d, encoding triacylglycerol hydrolase (Tgh), which promotes lipid release from adipocytes (Wei et al., 2010; Dominguez et al., 2014). Notably, the lipid class most affected by acute treatment of mice with Tgh inhibitors was polyunsaturated lipids (Dominguez et al., 2014), supporting the hypothesis that altered Ces1d expression in Ad-Bmal1−/− mice could specifically affect this lipid class.
ADIPOKINES
In addition to fatty acids, adipocytes secrete peptide hormones that influence feeding behavior and other aspects of physiology. Among the best studied of these is leptin. Leptin serves as a satiety signal by shifting the balance of anorexogenic and orexigenic hypothalamic peptides; mice lacking either leptin (Ob/Ob) or its receptor (Db/Db) are morbidly obese due to severe overeating. While leptin levels are correlated with overall adiposity, its concentration also oscillates diurnally in human plasma and cerebrospinal fluid (Sinha et al., 1996; Alzoghaibi et al., 2014; Sanchez-de-la-Torre et al., 2014; Wardlaw et al., 2014). Leptin also oscillates in mice (Ando et al., 2005; Turek et al., 2005); several studies have measured elevated leptin in Clockm/m or Bmal1−/− animals, but it is unclear whether that indicates circadian control of leptin production or a secondary effect of increased adiposity. mRNAs encoding other adipokines, including adiponectin and resistin, have been reported to oscillate in mouse WAT as well (Ando et al., 2005; Oliver et al., 2006).
LIPOGENESIS AND LIPOLYSIS
While their modulation of feeding behavior by secreting lipids and peptide-based hormones has garnered recent attention, adipocytes are historically most recognized for their critical role in energy homeostasis as the major site of long-term energy storage in the form of lipids. During times of energy surplus (e.g., feeding), adipocytes import glucose and fatty acids from the circulation and convert them to triglycerides (3 fatty acids connected to a glycerol backbone via ester linkages), which are stored in lipid droplets. When energy is in demand (e.g., fasting and/or strenuous activity), adipose tissue converts triglycerides into free fatty acids and glycerol, which are released into the circulation and provide rich sources of ATP to other organs. The processes of triglyceride synthesis (“lipogenesis”) and conversion of triglycerides into glycerol and free fatty acids (“lipolysis”) proceed via a choreographed sequence of enzymatic reactions, many of which may be subject to circadian regulation (Figure 2).
As discussed earlier in relation to adipogenesis, several genetic models of local or systemic clock disruption have altered fat accumulation (Rudic et al., 2004; Lamia et al., 2008; Guo et al., 2012; Paschos et al., 2012; Barclay et al., 2013; Kennaway et al., 2013; Griebel et al., 2014). Rather than alterations in adipogenesis, these findings may reflect changes in lipid synthesis (lipogenesis) or breakdown (lipolysis). Several studies support the hypothesis that adipose tissue clocks regulate these processes via transcriptional control of rate-limiting enzymes. For example, mice treated with Reverbα and Reverbβ activating ligands lose weight secondary to increased energy expenditure and suppression of lipogenic genes (Solt et al., 2012). A diurnal rhythm of lipolysis in rodent white adipose tissue explants was first measured more than 30 years ago (Suzuki et al., 1983), and recent studies suggest that lipolysis could be driven by local adipose circadian clocks (Shostak et al., 2013). Among the most critical enzymes in lipolysis are adipocyte triglyceride lipase (Atgl) encoded by the Pnpla2 gene and hormone-sensitive lipase (Hsl) encoded by Lipe. Both Pnpla2 and Lipe mRNA expression exhibit circadian oscillation in white adipose tissue (Shostak et al., 2013). Each of the promoters is associated with Bmal1 in chromatin immunoprecipitation assays (Koike et al., 2012; Shostak et al., 2013), suggesting that they could be direct output genes of the local WAT clock. Further supporting that model, Pnpla2 and Lipe mRNAs oscillate in explanted fat pads following circadian synchronization (Shostak et al., 2013). Triglyceride hydrolase (Tgh), encoded by Ces1d, was recently found to contribute significantly to lipid release from adipocytes (Wei et al., 2010) and also seems to be a direct target of Bmal1 (Koike et al., 2012; Paschos et al., 2012), regulated by local adipose clocks.
OUTLOOK
In the past decade, we have learned not only that peripheral tissue clocks are critical for optimizing physiology in synchrony with environmental and behavioral demands; we have begun to uncover molecular mechanisms by which peripheral clocks interact with other tissue-specific pathways to regulate a wide range of physiological systems. Several outstanding questions remain. For example, the mechanism by which clocks are disrupted seems to have a major impact on the effects of the manipulation both on gene expression and on physiology. For example, abolishing circadian rhythms by deleting Bmal1, overexpressing Reverbα, or deleting both Cry1 and Cry2 results in different diurnal transcriptome profiles in mouse livers (Kornmann et al., 2007; Lamia et al., 2008; Vollmers et al., 2009), leading to questions about the relative importance of local clocks or rhythmic feeding patterns for driving widespread diurnal transcription (Vollmers et al., 2009). Conditional deletion of Bmal1 has been a critical tool for establishing the proof of concept that tissue-autonomous clocks are important modulators of the physiology of several organs, and now we need new tools to improve our understanding of the roles of other core clock components and circadian output factors in physiology. The importance of circadian regulation in adipose tissues is clear and likely affects tissue development, as well as cell-autonomous lipid synthesis and mobilization, and the secretion of adipokines and lipokines that regulate feeding and possibly other behaviors. The recent development of small molecules targeting core clock components (Hirota et al., 2012; Solt et al., 2012) raises the possibility of novel therapeutic approaches to treating metabolic disease by pharmacologically modulating circadian clocks. Further studies will help define the specific actions of various clock components in white, beige, and brown adipocytes located in myriad fat storage locations and how well these properties are conserved between rodents and humans. Better understanding these details could also help determine the mechanisms by which altering the timing of food consumption has such a profound impact on fat accumulation, an important question in these times of increasing circadian desynchronization caused by modern lifestyles.
References
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: The good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoghaibi MA, Pandi-Perumal SR, Sharif MM, BaHammam AS. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS One. 2014;9:e92214. doi: 10.1371/journal.pone.0092214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barclay JL, Shostak A, Leliavski A, Tsang AH, Johren O, Muller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304:E1053–1063. doi: 10.1152/ajpendo.00512.2012. [DOI] [PubMed] [Google Scholar]
- Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–145. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Kueht M, Young ME. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes. 2010;34:1589–1598. doi: 10.1038/ijo.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes Rev. 2009;10(Suppl 2):25–36. doi: 10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Lazar MA. Induction of Rev-ErbA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem. 1993;268:16265–16269. [PubMed] [Google Scholar]
- Chomez P, Neveu I, Mansen A, Kiesler E, Larsson L, Vennstrom B, Arenas E. Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development. 2000;127:1489–1498. doi: 10.1242/dev.127.7.1489. [DOI] [PubMed] [Google Scholar]
- Costa MJ, So AY, Kaasik K, Krueger KC, Pillsbury ML, Fu YH, Ptacek LJ, Yamamoto KR, Feldman BJ. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J Biol Chem. 2011;286:9063–9070. doi: 10.1074/jbc.M110.164558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann R, Weaver DR. Altered body mass regulation in male mPeriod mutant mice on high-fat diet. Chronobiol Int. 2010;27:1317–1328. doi: 10.3109/07420528.2010.489166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delezie J, Dumont S, Dardente H, Oudart H, Grechez-Cassiau A, Klosen P, Teboul M, Delaunay F, Pevet P, Challet E. The nuclear receptor REV-ERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26:3321–3335. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Galmozzi A, Chang JW, Hsu KL, Pawlak J, Li W, Godio C, Thomas J, Partida D, Niessen S, et al. Integrated phenotypic and activity-based profiling links Ces3 to obesity and diabetes. Nat Chem Biol. 2014;10:113–121. doi: 10.1038/nchembio.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223:T1–8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature. 2013;503:410–413. doi: 10.1038/nature12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Santos C, Gomez-Abellan P, Madrid JA, Hernandez-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity. 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Ravinet-Trillou C, Beeske S, Avenet P, Pichat P. Mice deficient in cryptochrome 1 (cry1 (−/−)) exhibit resistance to obesity induced by a high-fat diet. Front Endocrinol. 2014;5:49. doi: 10.3389/fendo.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, Minze LJ, Hsueh W, Ma K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26:3453–3463. doi: 10.1096/fj.12-205781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- Jeffery E, Berry R, Church CD, Yu S, Shook BA, Horsley V, Rosen ED, Rodeheffer MS. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3:206–211. doi: 10.4161/adip.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Varcoe TJ, Voultsios A, Boden MJ. Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One. 2013;8:e65255. doi: 10.1371/journal.pone.0065255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin DJ, Burris TP. A role for rev-erbalpha ligands in regulation of adipogenesis. Curr Pharm Design. 2011;17:320–324. doi: 10.2174/138161211795164211. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullican SE, Dispirito JR, Lazar MA. The orphan nuclear receptors at their 25-year reunion. J Mol Endocrinol. 2013;51:T115–T140. doi: 10.1530/JME-13-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, et al. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver P, Ribot J, Rodriguez AM, Sanchez J, Pico C, Palou A. Resistin as a putative modulator of insulin action in the daily feeding/fasting rhythm. Pflugers Arch. 2006;452:260–267. doi: 10.1007/s00424-005-0034-5. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E, Kuhn P, Xu W. Nuclear receptor cofactors in PPARgamma-mediated adipogenesis and adipocyte energy metabolism. PPAR Res. 2007;2007:53843. doi: 10.1155/2007/53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Xu H, Khan SK, Shen Y, Gitis PJ, Welsh DK, Hogenesch JB, Liu AC. Cell type-specific functions of period genes revealed by novel adipocyte and hepatocyte circadian clock models. PLoS Genet. 2014;10:e1004244. doi: 10.1371/journal.pgen.1004244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-de-la-Torre M, Barcelo A, Pierola J, de la Pena M, Valls J, Barbe F. Impact of obstructive sleep apnea on the 24-h metabolic hormone profile. Sleep Med. 2014;15:625–630. doi: 10.1016/j.sleep.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010;24:345–357. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195–2203. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Xue B, Jusko WJ, Dubois DC, Almon RR. Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology. Physiol Genomics. 2010;42A:141–152. doi: 10.1152/physiolgenomics.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Shimomura Y, Satoh Y. Diurnal changes in lipolytic activity of isolated fat cells and their increased responsiveness to epinephrine and theophylline with meal feeding in rats. J Nutr Sci Vitaminol. 1983;29:399–411. doi: 10.3177/jnsv.29.399. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity. 2012;20:1527–1529. doi: 10.1038/oby.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, et al. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw SL, Burant CF, Klein S, Meece K, White A, Kasten T, Lucey BP, Bateman RJ. Continuous 24-hour leptin, proopiomelanocortin, and amino acid measurements in human cerebrospinal fluid: Correlations with plasma leptin, soluble leptin receptor, and amino acid levels. J Clin Endocrinol Metab. 2014;99:2540–2548. doi: 10.1210/jc.2013-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, Mitchell G, Korbutt GS, Lehner R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- Yang G, Jia Z, Aoyagi T, McClain D, Mortensen RM, Yang T. Systemic PPARgamma deletion impairs circadian rhythms of behavior and metabolism. PLoS One. 2012;7:e38117. doi: 10.1371/journal.pone.0038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yang X, Lamia KA, Evans RM. Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:387–394. doi: 10.1101/sqb.2007.72.058. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]