Abstract
Rationale
Allogeneic bone marrow-derived mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs) have each entered clinical trials but a direct comparison of these cell types has not been performed in a large animal model of hibernating myocardium.
Objective
Using completely blinded methodology, compare the efficacy of global intracoronary allogeneic MSCs (icMSCs, ~35×106) and CDCs (icCDCs, ~35×106) vs. vehicle in cyclosporine-immunosuppressed swine with a chronic LAD stenosis (n=26).
Methods and Results
Studies began 3-months after instrumentation when wall-thickening (%WT) was reduced (LAD%WT 38±11% (mean ± SD) vs. 83±26% in remote, p<0.01) and similar among groups. Four-weeks after treatment, LAD%WT increased similarly following icCDCs and icMSCs, while it remained depressed in vehicle-treated controls (icMSCs: 51±13%; icCDCs: 51±17%; vehicle: 34±3%, treatments p<0.05 vs. vehicle). There was no change in myocardial perfusion. Both icMSCs and icCDCs increased LAD myocyte nuclear density (icMSCs: 1601±279 nuclei/mm2, icCDCs: 1569±294 nuclei/mm2, vehicle: 973±181 nuclei/mm2, treatments p<0.05 vs. vehicle) and reduced myocyte diameter (icMSCs: 16.4±1.5 μm, icCDCs: 16.8±1.2 μm, vehicle: 20.2±3.7 μm, treatments p<0.05 vs. vehicle) to the same extent. Similar changes in myocyte nuclear density and diameter were observed in the remote region of cell-treated animals. Cell fate analysis using Y-FISH demonstrated rare cells from sex-mismatched donors.
Conclusions
Allogeneic icMSCs and icCDCs exhibit comparable therapeutic efficacy in a large animal model of hibernating myocardium. Both cell types produced equivalent increases in regional function and stimulated myocyte regeneration in ischemic and remote myocardium. The activation of endogenous myocyte proliferation and regression of myocyte cellular hypertrophy support a common mechanism of cardiac repair.
Keywords: Cell therapy, ischemic heart disease, hibernating myocardium, cardiac regeneration, allogeneic cell therapy, coronary stenosis
INTRODUCTION
The development of cell-based therapeutic approaches to treat ischemic heart disease has proceeded at a rapid pace since initial clinical trials commenced over a decade ago. Studies have been fueled by optimism that exogenous stem cells could replenish the pool of functional cardiac myocytes that is depleted by acute and chronic injury. There has been an abundance of positive results in preclinical rodent models of myocardial infarction using a variety of cell types with surprisingly few negative studies. Nevertheless, as research has translated to large animal studies and phase I and 2 clinical trials the functional effects of cell-therapy have been variable. Thus, although clinical trials have confirmed the safety of most preclinical cardiac cell-therapies, limited functional improvement has been observed in comparison to animal studies1–3. This has been highlighted by a recent meta-analysis of clinical cell therapy studies utilizing individual patient data, which concluded that intracoronary cell therapy did not reduce clinical events nor improve left ventricular (LV) function in patients with recent myocardial infarction4.
There are several potential explanations for the difficulty in translating preclinical findings to clinical trials. Some are unique to cardiovascular research while others are common to translating preclinical research in other fields of medicine5. First, rodents tolerate myocardial infarctions that are much larger than what a human can survive. This amplifies the deleterious effects of LV remodeling in rodent models. At the same time, remodeling in humans is now markedly attenuated by pharmacological therapy and early reperfusion as compared to untreated animal models. In addition, most models employ young animals that do not have the underlying cardiovascular risk factors present in humans with diseases such as hypertension, diabetes and hypercholesterolemia nor the impact of aging. There is also likely publication bias towards positive results with underreporting of negative preclinical studies.
Perhaps a more substantial barrier common to translating preclinical studies in all fields are the way that most are conducted. In comparison to clinical trial methodology, few preclinical studies employ a blinded randomized controlled approach. The team of preclinical investigators and research scientists is rarely blinded to treatment allocation. While interpretation of some aspects are sometimes blinded (e.g. cardiac imaging results), blinding of investigators involved in pathological analysis and other physiological endpoints is rarely employed. A recent meta-analysis of preclinical cardiovascular cell therapy studies found that only 42% of included studies reported blinded assessment of some data and only 11% reported allocation concealment6. Thus, many studies may have selection bias related to inclusion/exclusion of experiments and variable observer bias in most of the end-point analyses. These problems have recently been highlighted in the field of cardioprotection research by the NIH-sponsored Consortium for Preclinical Assessment of Cardioprotective Therapies (CAESAR)7. Using rigorous blinded treatment and data analysis comparable to randomized clinical trials, the CAESAR consortium has been unable to show the beneficial effects of two agents that had been widely shown to reduce infarct size in prior small, poorly controlled preclinical studies in multiple species8, 9. Thus, the absence of a completely blinded randomized study design may be a significant methodological hurdle to overcome in translation of preclinical research into clinical application.
With this in mind, we determined if a randomized blinded experimental approach could be employed in a single-center preclinical trial comparing two types of cell-therapy vs. vehicle-treated controls. Bone marrow-derived mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs) cultured from explanted heart tissue are promising cell types that have recently entered clinical trials10–13. A particularly attractive feature of both cell types is the feasibility of using allogeneic cells, which would permit off-the-shelf availability of cells for treatment and circumvent the need for personalized cell expansion. Both MSCs and CDCs have been shown to improve ventricular function in preclinical studies by our laboratory and others14–18 but they have not been compared in a head-to-head fashion in a large animal model of myocardial ischemia. Previous studies including our own have been limited by the fact that investigators were not completely blinded to treatment allocation and all functional and pathological analyses. Therefore, the present study addresses these issues by employing a rigorous, clinical trial-based three-way design to directly compare the therapeutic efficacy of intracoronary allogeneic bone marrow-derived mesenchymal stem cells (icMSCs) and intracoronary cardiosphere-derived cells (icCDCs) infused to the entire heart in a swine model of chronic myocardial ischemia. Any potential effects of allogeneic cell rejection were minimized by treating all animals with chronic cyclosporine. The results demonstrate preclinical therapeutic equipoise of allogeneic icMSCs and icCDCs.
METHODS
All procedures and protocols conformed to institutional guidelines for the care and use of animals in research and were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Study design and treatment of data
All personnel involved in data collection and analysis (including echocardiographic imaging and histological assessment of myocyte morphometry and proliferation) were blinded to the treatment status of each animal. Serial data collection and analysis were conducted by an independent biostatistician not involved in the protocols or assays (JAF). Animals were randomized to receive vehicle, icMSCs, or icCDCs in a 1:1:1 ratio. Regional wall thickening of the area supplied by the left anterior descending coronary artery (LAD %WT) 1-month after treatment was selected as the primary endpoint.
Isolation and characterization of MSCs and CDCs
Bone marrow (n=2) and left ventricular tissue (n=3) samples were collected from healthy donor pigs (age 8±3 weeks) for the isolation and cultivation of MSCs and CDCs, as previously described16, 17, 19 and summarized in the Online Supplement. Cells were cultured for 4 to 7 passages, at which time they were collected for characterization by flow cytometry and immunohistochemistry or intracoronary infusion to recipient pigs with hibernating myocardium. Flow cytometric and immunohistochemical characterization included assessment of hematopoietic (CD45, cKit), mesenchymal (CD90, CD105) and cardiac (GATA4, Nkx2.5) markers. Prior to administration, both cell types were filtered through a 30 μm pore filter to circumvent administering cell aggregates (MACS pre-separation filters, Miltenyi Biotec) and suspended in heparinized HBSS solution (3000U heparin in 30ml in total) for intracoronary infusion.
Effects of icMSCs and icCDCs on flow and function in hibernating myocardium
Experimental groups and the study timeline are summarized in Supplemental Figure I. Pigs (n=34) were chronically instrumented with a 1.5 mm Delrin occluder on the proximal LAD to produce hibernating myocardium as previously described20. Briefly, juvenile pigs were sedated (Telazol 100mg/ml / xylazine 100 mg/ml, 0.022 mg/kg i.m.), intubated and ventilated with a 0.5–2% isoflurane-oxygen mixture. Through a small pericardiotomy, the Delrin occluder was placed on the proximal LAD. Antibiotics (cefazolin, 25 mg/kg and gentamicin, 3 mg/kg i.m.) were given 1-hour before surgery and repeated after closing the chest. Analgesia included an intercostal nerve block (0.5% Marcaine) and intramuscular doses of butorphanol (2.2 mg/kg q6h) and flunixin (1–2 mg/kg q.d.).
Serial physiological studies
Studies began 3-months after instrumentation when stable reductions in LAD wall thickening and perfusion indicative of hibernating myocardium were present21. Under propofol sedation (5–10 mg/kg/hr i.v.), a catheter (Millar) was inserted to measure LV pressure and inject microspheres. Regional wall-thickening was assessed with transthoracic echocardiography from a right parasternal approach16. All pigs showed anterior dysfunction without dyskinesis. Systolic wall-thickening (ΔWT=ESWT−EDWT; %WT=ΔWT/EDWT×100) was quantified in dysfunctional LAD regions as well as remote, normally-perfused regions of the same heart.
Microsphere perfusion
Microsphere flow was measured at rest and following adenosine vasodilation to characterize hibernating myocardium 3-months after instrumentation and repeated 4 weeks after therapy as previously described22. Briefly, we injected 15μm microspheres (~3×106) labeled with fluorescing dyes into the left ventricle while a reference arterial sample was withdrawn at 6 ml/min for 90-seconds. At the end of the study, samples were taken from mid-ventricular circumferential rings divided into twelve wedges with each cut into 3 transmural layers. Fluorescent dyes were extracted using standard techniques and quantified at selected excitation wavelengths23.
Cell administration and follow-up
After baseline physiological measurements, pigs were randomly assigned to receive coded treatments of either vehicle (n=8), ~35×106 icMSCs (n=9), or ~35×106 icCDCs (n=9) with all investigators and research personnel blinded to treatment allocation. Each treatment was divided into three aliquots and infused into each of the three major coronary artery distributions over 10-minutes at a rate of 1×106 cells/minute. All animals were treated with cyclosporine A beginning 3 days before the baseline study and continuing throughout terminal experiments (200 mg/day p.o., Watson Pharma). Animals returned to the laboratory 4-weeks later for post-treatment physiological studies under propofol anesthesia, after which the heart was arrested with potassium chloride and quickly excised for histopathological analysis.
Myocardial histopathology
Quantitative immunohistochemistry and morphometry were evaluated in all vehicle-, icMSC-, and icCDC-treated animals with hibernating myocardium. Y-FISH analysis was performed in the subset of female animals that received male donor-derived cells to track cell fate, as described below.
Arteriolar and capillary density
Regional tissue sections were incubated with anti-α smooth muscle actin and anti-von Willebrand factor antibodies with appropriate fluorescent-labeled secondary antibodies to identify coronary arterioles and capillaries, respectively. Vessels were counted in at least 10 random fields from the subendocardial and subepicardial layers of each tissue sample (LAD and remote) and expressed per tissue area (mm2).
Myocyte nuclear density and morphometry
Paraffin-embedded samples from LAD and non-ischemic remote regions were fixed for morphometry as previously described16, 17, 24. PAS-stained sections were used to quantify myocyte diameter by counting at least 100 cells from the inner and outer half of the LAD and remote regions. Myocytes were included regardless of size as long as myofilaments could be identified surrounding the nucleus. Myocyte nuclear density was also assessed in PAS-stained sections as previously described16. To evaluate whether changes in nuclear density reflected increases in the number of nuclei per myocyte, longitudinal myocytes (n=20 in each histological sample) that had boundaries that were clearly visible (intercalated discs and the lateral borders of the myocyte) were used to quantify the number of nuclei per cell in each heart using previously published methodology25. Point-counting of trichrome-stained sections was used to quantify connective tissue26.
Quantification of myocyte proliferation
Myocardial samples were incubated with anti-Ki67 and anti-phospho-Histone-H3 antibodies to identify myocytes in the growth phase of the cell cycle and undergoing mitosis as previously described17. Paraffin-fixed sections (4μm thickness) were incubated with either anti-Ki67 (mouse monoclonal antibody, clone MIB-1, Dako, 1:200) or anti-phospho-histone-H3 (rabbit polyclonal antibody, Upstate Biotech, 1:1000) to detect proliferating cells and anti-cTnI (rabbit polyclonal antibody, Santa Cruz, 1:200) to detect myofilaments. Nuclei were stained with DAPI (Vectasheild).
Fluorescent in situ hybridization (Y-FISH)
Y-FISH (IDLabs, Ontario, Canada) was used to determine the fate of male donor icMSCs (n=4) and icCDCs (n=5) injected into female recipients17. Tissue samples were hybridized with FITC-conjugated porcine Y-chromosome probe according to the manufacturer’s instructions (IDLabs, Ontario, Canada). Y-FISH staining in male control cardiac tissue was used to correct for the fact that myocyte nuclear diameter was greater than histological slice thickness. The “efficiency” of Y chromosome identification was 37±3% and similar to previously published data17. Sections were also incubated with antibodies to cardiac Troponin I to detect co-localization of Y-FISH in myocytes derived from donor cells.
Statistical analysis
Data are expressed as mean ± standard deviation. Differences among treatment groups were assessed by two-way analysis of variance (ANOVA) with repeated measures and the post-hoc Holm-Sidak test. For all comparisons, p<0.05 was considered significant.
RESULTS
A total of 34 animals were instrumented with a chronic LAD stenosis. Consistent with previous data demonstrating an elevated risk of sudden cardiac death in this model22, 7 animals died prior to completion of the study. Of these, 5 died prior to the 3-month study and 2 died between the 3- and 4-month studies (1 CDC-treated animal and 1 vehicle-treated animal, >10 days after the intracoronary infusion). One animal was removed from further study at the 3-month time point when angiography demonstrated that there was no stenosis of the LAD and the Delrin occluder had been displaced from the vessel. All remaining animals (n=26) were included in the final data analysis with no exclusions after randomization to treatment.
Allogeneic MSC and CDC cell characterization
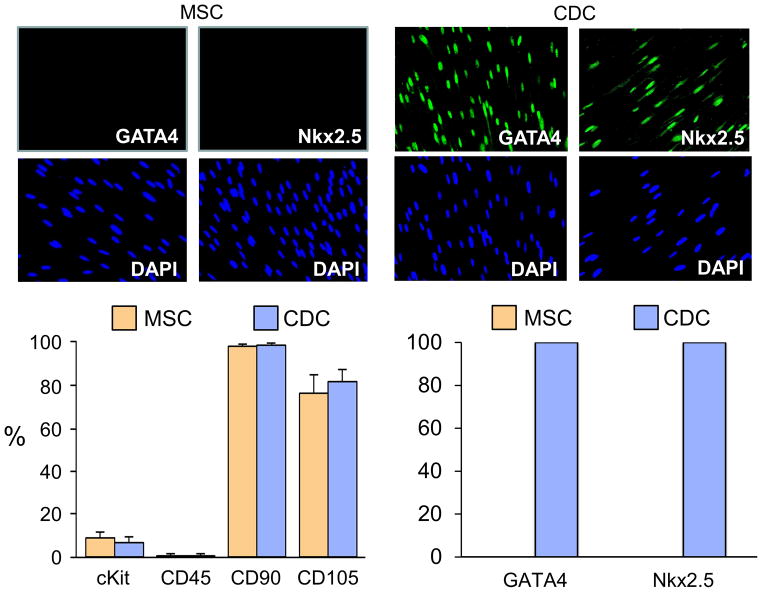
Cell culture resulted in comparable total cell injections from each treatment (32.4±7.3 ×106 icMSCs and 35.3±5.9 ×106 icCDCs, p=0.40). Flow cytometry confirmed that cell surface marker expression profiles were consistent with previous reports16, 17, 27. At the time of infusion, MSCs and CDCs expressed similar frequencies of CD90+ and CD105+. Both were also CD45− with a small number expressing cKit+ (Figure 1). Despite these similarities, they had markedly different expression of cardiac transcription factors. Both GATA4 and Nkx2.5 were absent in MSCs but ubiquitous in CDCs with nearly all demonstrating that they were cardiac lineage committed at the time of administration.
Figure 1. Characterization of Porcine Allogeneic MSCs and CDCs.
Summary of MSC and CDC characterization by flow cytometry and immunohistochemistry (n=9 each cell type; corresponding with cell donor and passage at the time of each cell injection). The antigenic profile was similar between cell types, as MSCs and CDCs were each predominately CD90+, CD105+ and CD45−, with a small number of cKit+ cells (lower left figure). In contrast, CDCs exhibited uniform expression of the cardiac transcription factors GATA4 and Nkx2.5, demonstrating their commitment to a cardiac lineage, while MSCs did not express GATA4 or Nkx2.5 (top image and lower right figure).
Comparative physiological effects of icMSCs and icCDCs in swine with hibernating myocardium
There were no differences in hemodynamic variables at rest or during adenosine between treatment groups (Supplemental Table I). Baseline studies 3-months after instrumentation confirmed the presence of viable dysfunctional hibernating myocardium. Regional LAD wall thickening (LAD %WT) was reduced compared with normal remote regions (38±11% vs. 83±26%, p<0.01) along with reductions in resting perfusion (LAD 0.63±0.21 vs. 0.80±0.34 ml/min/g in remote, p<0.05). Adenosine-dilated flow was also attenuated (LAD 1.62±0.90 vs. 4.00±2.90 ml/min/g in remote, p<0.001). Indices of LV function and flow were comparable among all treatment groups at baseline.
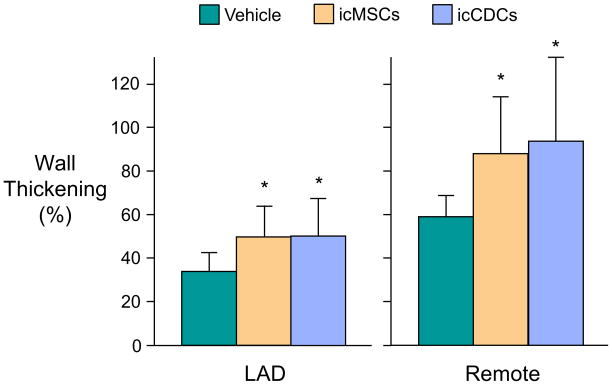
The effects of icMSCs and icCDCs on regional function are summarized in Figure 2 and Supplemental Table II. One-month after treatment, LAD%WT increased in both icMSC-treated (50.7±13.4%) and icCDC-treated (50.9±16.6%) animals vs. vehicle-treated controls (33.7±9.2%; p<0.05 vs. each cell-therapy). Functional effects of cell therapy were not limited to the ischemic LAD area, as both cell types prevented the deterioration of remote zone wall thickening that was observed in vehicle-treated animals. As a result, remote zone %WT was significantly higher in animals treated with icMSCs (88.4±26.0%,) and icCDCs (94.3±39.3%) compared with vehicle-treated controls (59.5±18.2%; p<0.05 vs. each cell-therapy) 1-month after treatment. Global LV ejection fraction was minimally reduced at baseline and was not significantly affected by any treatment (Supplemental Table II). Importantly, there were no significant differences in functional improvement comparing icMSCs vs. icCDCs.
Figure 2. Global icMSCs and icCDCs Elicit Comparable Improvements in Regional Wall Thickening Throughout the Left Ventricle.
One-month after treatment, icMSC- and icCDC-treated animals exhibited significantly greater wall thickening in the ischemic LAD and non-ischemic remote regions of the LV compared vs. vehicle-treated controls. *p<0.05 vs. Vehicle.
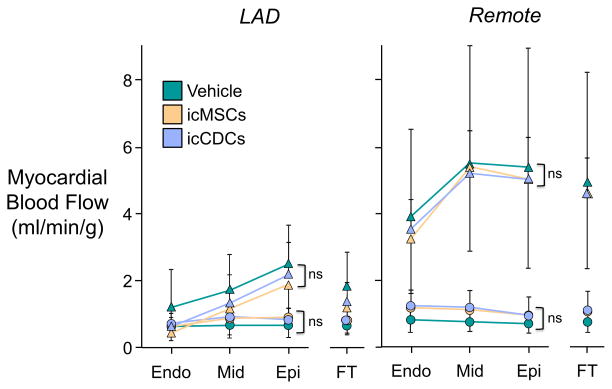
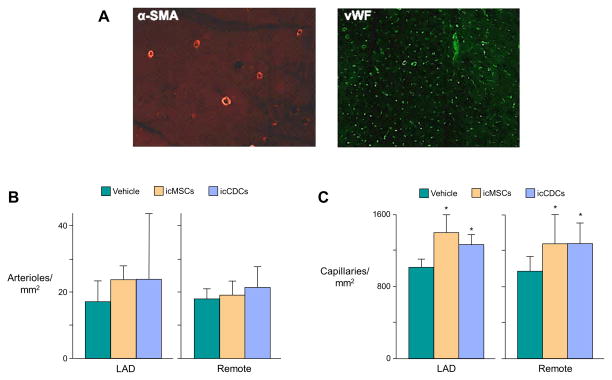
Consistent with our previous results16, there were no significant effects of either cell-therapy on serial measurements of coronary flow at rest or during adenosine vasodilation (Figure 3). Although icMSC- and icCDC-treated animals exhibited a significant increase in capillary density in both LAD and remote regions of the left ventricle (Figure 4), neither treatment promoted arteriogenesis since there was no change in arteriolar density. Thus, the functional improvement afforded by icMSCs and icCDCs was accompanied by capillary angiogenesis without arteriogenesis or increased collateral blood flow.
Figure 3. Myocardial Perfusion at Rest and During Adenosine Vasodilation is Not Affected by Global icMSCs or icCDCs.
Swine with hibernating myocardium exhibited a significant reduction in myocardial blood flow at rest (circles) and during adenosine vasodilation (triangles) in the ischemic LAD region (left panel) compared with non-ischemic remote myocardium (right panel). There was no significant change in animals treated with icMSCs or icCDCs. Endo: subendocardium; Mid: midmyocardium; Epi: subepicardium; FT: full-thickness
Figure 4. Global icMSCs and icCDCs Promote Capillary Angiogenesis but Do Not Affect Arteriolar Density.
(A) Representative images of α-smooth muscle actin-positive arterioles (left panel) and von-Willebrand factor-positive capillaries (right panel). (B) Arteriolar density was not significantly different among icMSC, icCDC, or vehicle-treated animals. (C) Global intracoronary cell infusion increased capillary density throughout the LV compared with vehicle-treated controls. There was no difference between icMSCs and icCDCs. *p<0.05 vs. Vehicle
Comparative effects of icMSCs and icCDCs on cardiomyocyte number and size
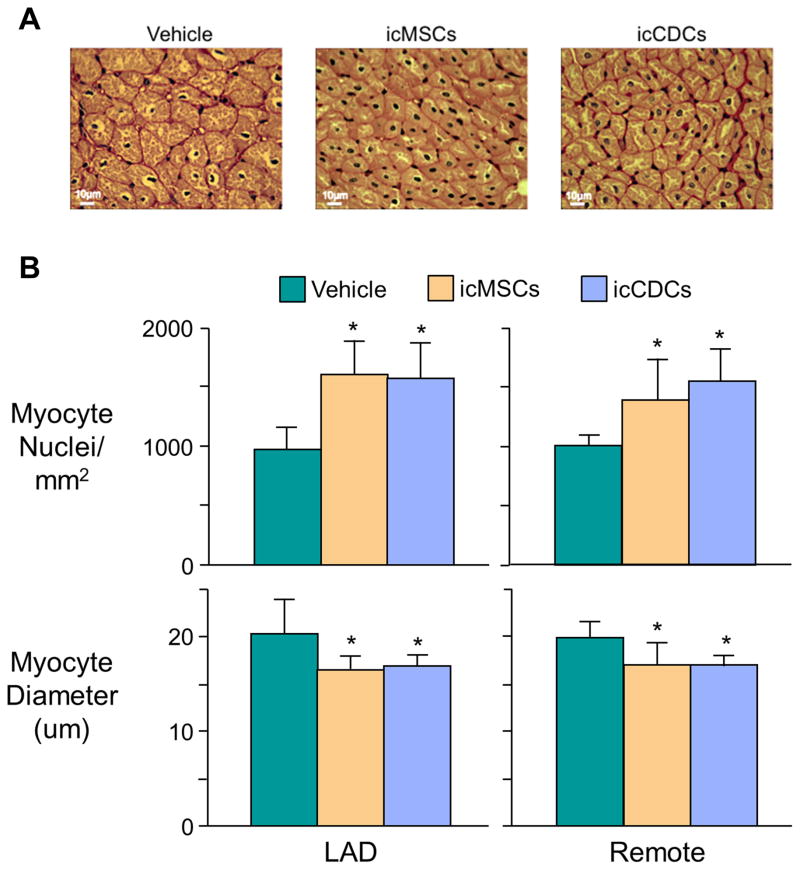
Blinded analysis of myocyte nuclear density and diameter are summarized in Figure 5. Nuclear density in vehicle-treated animals was reduced throughout the left ventricle (LAD: 973±181 nuclei/mm2, Remote: 1,005±88 nuclei/mm2) compared to previous measurements from normal sham pigs of similar size (~1200–1400 nuclei/mm2)17, 26. icMSC treatment (LAD: 1,601±279 nuclei/mm2, Remote: 1,387±348 nuclei/mm2; both p<0.05 vs. vehicle) and icCDC treatment (LAD:1,569±294 nuclei/mm2, Remote: 1,545±276 nuclei/mm2; both p<0.05 vs. vehicle) each significantly increased myocyte nuclear density throughout the left ventricle. Increases in nuclear density were not the result of karyokinesis without cytokinesis since the number of nuclei per myocyte was not different among all treatment groups (Vehicle: 4.4±0.3; icMSCs: 4.5±0.6; icCDCs: 4.2±0.3, p-ns). Increases in myocyte nuclear density were also accompanied by reductions in myocyte diameter. Vehicle-treated controls (LAD: 20.2±3.7 μm, Remote: 19.8±1.7 μm) were larger than icMSC-treated (LAD: 16.4±1.5 μm, Remote: 16.9±2.4 μm; both p<0.05 vs. vehicle) and icCDC-treated animals (LAD: 16.8±1.2 μm, Remote: 16.9±1.0 μm; both p<0.05 vs. vehicle). Interestingly, these changes were not associated with anatomic LV hypertrophy, as postmortem LV mass/body mass ratio was not different among groups (Vehicle: 2.5±0.3, icMSCs: 2.3±0.3, icCDCs: 2.3±0.3 g/kg).
Figure 5. Global icMSCs and icCDCs Increase Myocyte Nuclear Density and Reduce Myocyte Diameter in LAD and Remote Myocardium.
(A) PAS-stained sections from the ischemic LAD region. (B) Both icMSCs and icCDCs produced a significant increase in myocyte nuclear density in the ischemic LAD and normally-perfused remote regions of the left ventricle. These changes were accompanied with reductions in myocyte diameter (bottom panel). *p<0.05 vs. Vehicle.
Effects of icMSCs and icCDCs on myocyte proliferative markers
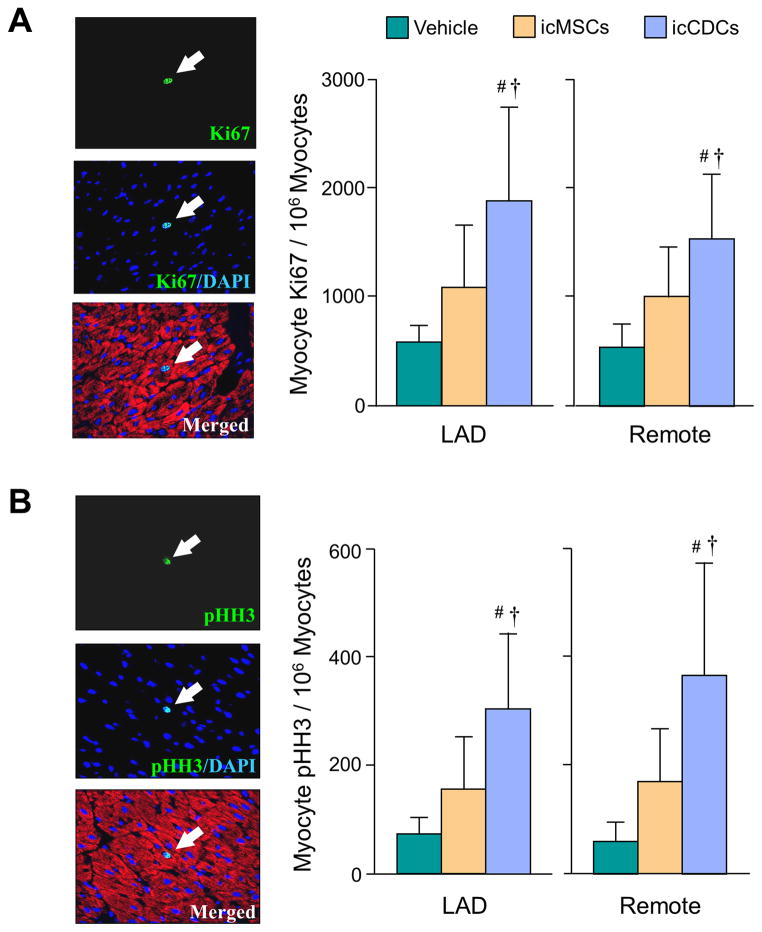
Ki67 expression (Vehicle-treated LAD: 573±158 nuclei/106 myocyte nuclei; Remote: 540±209 nuclei/106 myocyte nuclei) tended to increase throughout the LV after icMSCs (LAD: 1068±585 nuclei/106 myocyte nuclei, p=0.11 vs. vehicle; Remote: 994±471 nuclei/106 myocyte nuclei, p=0.06 vs. vehicle). After icCDCs, Ki67 expression was greater (LAD: 1866±858 nuclei/106 myocyte nuclei, Remote: 1530±588 nuclei/106 myocyte nuclei; both p<0.05 vs. vehicle and icMSCs) (Figure 6a). There were similar differences in pHH3 expression (Figure 6b) with icMSC-treated animals being borderline significant (LAD: 156±96 nuclei/106 myocyte nuclei, p=0.10 vs. vehicle; Remote: 167±99 nuclei/106 myocyte nuclei, p=0.11 vs. vehicle) vs. vehicle-treated animals (LAD: 73±31 nuclei/106 myocyte nuclei, Remote: 57±37 nuclei/106 myocyte nuclei). In contrast, the magnitude of pHH3 expression increased significantly after icCDCs (LAD: 302±141 nuclei/106 myocyte nuclei, Remote: 362±210 nuclei/106 myocyte nuclei; both p<0.05 vs. vehicle and icMSCs).
Figure 6. Myocyte Proliferative Indices.
(A) Myocytes in the growth phase of the cell cycle one-month after treatment were quantified with Ki67 (arrow). Ki67-positive myocytes were higher after icCDC treatment as compared to icMSCs and Vehicle-controls. (B) Mitotic myocytes were quantified with phospho-histone H3 (pHH3, arrow). Mitotic myocytes were higher after icCDCs vs icMSCs and Vehicle-controls. *p<0.05 vs. Vehicle; †p<0.05 vs. icMSCs.
Engraftment and fate of allogeneic icMSCs and icCDCs
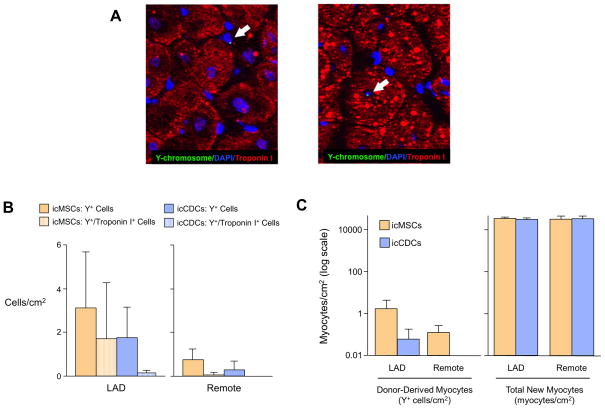
To estimate the number of new myocytes derived from icMSCs and icCDCs, Y+ cells were assessed in the LAD and remote regions of the hearts of female recipients who had received male donor-derived cells (icMSCs: n=4, icCDCs: n=5, Figure 7a). A similar number of Y+ cells were observed in icMSC-treated (LAD: 3.1±2.6 Y+ cells/cm2; Remote: 0.7±0.5 Y+ cells/cm2) vs. icCDC-treated animals (LAD: 1.8±1.4 Y+ cells/cm2, p=0.36 vs. icMSC-treated; Remote: 0.3±0.4 Y+ cells/cm2, p=0.20 vs. icMSC-treated; Figure 7b). This amounted to only ~1 in 10,000/cm2 of the new myocytes formed from allogeneic CDCs or MSCs based on our estimates of ~40,000 to 60,000/ cm2 new myocytes following intracoronary cell therapy (after accounting for myocyte multi-nucleation; Figure 7c). The paucity of Y+ myocytes despite large increases in total myocyte number provides evidence that both icCDCs and icMSCs stimulated endogenous myocyte proliferation with rare cardiac myocytes derived from injected MSCs or CDCs.
Figure 7. The Majority of New Myocytes Derived from Global icMSCs and icCDCs Arise From Endogenous Sources.
(A) Y-chromosomes were detected by Y-FISH. Y-FISH positive cells were primarily found in the interstitial space (left panel) with rare examples of Y+ cardiac myocytes (right panel). (B) The frequency of Y+ cells was low but similar in icMSC-treated and icCDC-treated animals. Rare Y+ cells also expressed Troponin I (hatched bars), indicative of cardiomyocyte differentiation. (C) The number of new myocytes differentiating from icMSCs and icCDCs was very small in comparison to the estimated increase in myocytes based on increases in myocyte nuclear density, implicating endogenous recipient cells as the primary source of new myocyte formation.
DISCUSSION
There are several important findings from the present study. First, using a blinded, randomized vehicle-controlled study design, we have shown that global infusion of allogeneic icMSCs and icCDCs produces comparable increases in myocardial function and myocyte proliferation. Improvements in function were associated with myocyte proliferation in both ischemic and remote myocardium. Second, while there was no functional arteriogenesis, cell therapy globally increased capillary density and myocyte nuclear density with a reduction in myocyte diameter. This, along with the observation that the number of nuclei per myocyte is unchanged, is consistent with significant myocyte proliferation throughout the left ventricle that is quantitatively similar between the two cell-therapies. Finally, the increase in myocyte nuclear number despite minimal engraftment of allogeneic cells in sex mismatched donor-recipients indicates that new myocytes are formed via endogenous mechanisms rather than direct differentiation of injected cells and is supported by the elevated expression of myocyte proliferative markers. Collectively, our results demonstrate that global intracoronary infusion of allogeneic MSCs and CDCs promotes a similar degree of myocardial repair via the stimulation of endogenous myocyte proliferation.
This is the first study to directly compare allogeneic intracoronary infusion of CDCs and MSCs in a large animal model of chronic myocardial ischemia. A few studies have compared these cell types after direct intramyocardial injection into infarcted tissue. For example, following xenogeneic transplantation of human cells to immunocompromised mice with myocardial infarction, CDCs were shown to be therapeutically superior to bone marrow-derived MSCs, adipose tissue-derived MSCs, and bone marrow-derived mononuclear cells 27. Xenogeneic cell transfer of human cKit+ cardiac stem cells (CSCs) also promoted greater myocardial repair than human bone marrow-derived MSCs after injection in mice with permanent coronary artery ligation28. In a subsequent study using a porcine reperfused myocardial infarct model, myocardial injection of human CSCs and bone marrow-derived MSCs produced equivalent functional improvement29. Interestingly, the combination of human MSCs and CSCs produced greater functional improvement than either cell type alone. While not examined in our study, it is plausible that combining MSCs and CDCs could yield greater functional repair than either cell alone and should be considered in future studies.
While intracoronary infusion of both allogeneic MSCs and CDCs were efficacious, our preclinical results suggest that it may be difficult to demonstrate that either cell type would be superior to the other in a head-to-head clinical trial. The similarity of functional repair afforded by each cell type is perhaps not surprising given several similarities between MSCs and CDCs revealed by in vitro analyses. For example, using a microarray-based approach, Dey and colleagues30 demonstrated significant overlap in the transcriptional profiles of bone marrow-derived MSCs and CDCs. Consistent with this, we observed nearly identical cell surface antigenic profiles in MSCs and CDCs with high levels of CD105+ and CD90+, a small population of cKit+ cells, and the absence of CD45 expression at the time of infusion. Nevertheless, since Ki67 and pHH3 expression in icCDC-treated animals remained elevated in animals receiving icMSCs at 4-weeks, it is possible that icCDCs could lead to more prolonged stimulation of myocyte proliferation and superior functional improvement if the comparison was performed beyond one-month.
Taken in the context of previous findings, our results add support to the emerging notion that both allogeneic MSCs and CDCs are safe and promote functional improvement. Previous studies have shown that MSCs and CDCs stimulate endogenous myocyte proliferation15, 31, 32 and subsequent myocardial regeneration despite limited long-term engraftment of injected cells. In the present study, histological analyses indicated that only ~1 of every 10,000 new myocytes was derived from donor-derived MSCs or CDCs, yet cell-treated animals exhibited elevated markers of myocyte proliferation. Taken together, these data are consistent with the idea that exogenous MSCs and CDCs stimulate endogenous myocyte proliferation, resulting in a significant increase in myocyte number. The cellular mechanism by which endogenous myocyte proliferation is stimulated remains unclear and could not be evaluated in our large animal model. Recent data in rats indicate that a potential candidate for the reparative actions of CDCs is the secretion of microRNA-rich exosomes that, in vitro, enhance angiogenesis, promote myocyte proliferation, and decrease apoptosis33. There is also evidence supporting an important role of cell-to-cell contact-dependent CDC stimulation of myocyte proliferation that is mediated by β1 integrin signaling34. The requirement of cell-to-cell contact to promote myocardial repair extends to MSCs as well. Hatzistergos et al.15 demonstrated that intramyocardial injection of MSC-conditioned media (containing secreted growth factors) was not sufficient to replicate the beneficial effects of MSC injection in a porcine infarct model. Based on the limited in vivo data at hand, MSCs and CDCs are likely to facilitate endogenous myocyte repair either via cell-to-cell communication or the prolonged release of paracrine factors or exosomes from cells that are retained in the heart.
Our results also demonstrate that icMSCs and icCDCs can promote functional improvement in the absence of changes in myocardial perfusion in the setting of a chronic coronary stenosis. Indeed, the significant improvement in LAD regional wall thickening in icMSC- and icCDC-treated animals was not accompanied by changes in resting or adenosine-vasodilated perfusion. These findings are consistent with previous results in this model16, 17 and provide further evidence that interventions that stimulate myocyte proliferation can dissociate the myocardial flow-function relationship by improving function independently of perfusion35. The failure of either cell type to produce measurable improvements in myocardial blood flow may relate to their inability to significantly increase arteriolar density. At the same time, both cell types stimulated capillary angiogenesis, which has been used by some as an indirect index of improved tissue perfusion. Increases in capillary number did not lead to measurable changes in resting or vasodilated perfusion in the LAD region supplied by the chronic stenosis since capillaries have a negligible contribution to total coronary vascular resistance. Interestingly, however, increases in capillary density matched increases in myocyte nuclear density with the capillary-to-myocyte ratio maintained after icMSCs and icCDCs. This matching has been described by others and may facilitate tissue oxygen exchange. The concomitant regression of cellular hypertrophy would also improve oxygen transport across the sarcolemma via reversion of the so-called “ischemic core” phenomenon. This postulates that increases in myocyte cross-sectional area limit oxygen delivery to the center of the myocyte36. Thus, the decrease in myocyte size as well as increases in capillary density could improve oxygen transport at the cellular level.
The majority of cardiac cell therapy studies have attempted to regenerate myocytes in areas of infarction by administering cells down an infarct-related artery using “stop-flow” intracoronary infusion or to infarct border areas via transendocardial injection. However, our laboratory has demonstrated therapeutic success with global intracoronary infusion under continuous flow. This allows cells to be administered throughout the dysfunctional left ventricle, thus promoting beneficial effects in non-ischemic remote areas of the heart. From a practical standpoint, cell infusion without transient coronary occlusion or the use of transendocardial injection devices could be performed easily in any standard catheterization laboratory, thereby facilitating rapid, widespread clinical implementation. This approach is also safe with a variety of cell types, as demonstrated in the present study, as long as cells are infused prior to extended passage, filtering is performed, and an appropriate dose and rate of infusion are selected. Finally, from a clinical perspective, the amount of cyclosporine used (~3 mg/kg/day) is well below that used in organ transplantation. Although previous studies have demonstrated safety and efficacy of allogeneic MSCs and CDCs without immunosuppression11, 37, it is possible that short-term, low-dose cyclosporine could delay the immune response to allogeneic cells and enhance functional improvement.
Since the swine model of hibernating myocardium used in the present study is characterized by regional dysfunction of viable myocardium in the absence of scar, our results indicate that global icCDCs and icMSCs promote functional repair independent of any potential effects on infarct size. This finding may be particularly relevant in the clinical translation of intracoronary cell therapy, since many patients exhibit large areas of viable, dysfunctional myocardium with residual perfusion due to contemporary reperfusion strategies that successfully limit infarct size and preserved LV EF38, 39. Despite improvements in regional wall thickening, neither cell type produced significant increases in global LV function. This likely reflects the mild impairment in ejection fraction present at baseline as well as the limitations of M-mode echocardiographic assessment. Importantly, recent data from our laboratory demonstrate that global intracoronary CDC delivery can also improve global function when myocardial scar is present and global EF is reduced40, 41.
The cardiac cell therapy field has grown rapidly over the past decade, but attempts to translate promising preclinical findings to patients have been met with disappointment. Unfortunately, concerns over experimental design and investigator blinding, coupled with publication bias towards positive results, may deserve blame for some of this. A recent review by Rosen and Myerburg42 contrasted the positive results of numerous unblinded or uncontrolled studies with neutral findings in randomized, blinded, and appropriately controlled trials. These same issues extend to pre-clinical studies conducted in large animal models of heart disease that serve as the pivotal step in the translation of novel cell-based therapeutic approaches6. The present study circumvented these limitations by employing an approach where animals were randomized to one of three treatment arms with all data coded and each investigator, research scientist and technician blinded throughout data collection and all analyses. The codified data used a common repository that was only accessible to the investigator overseeing the statistical analysis. Improvements in myocardial function, as well as the quantitative extent of new myocyte formation, were nearly identical between cell types, indicating that MSCs and CDCs exhibit comparable therapeutic potential. Fortunately, these blinded results confirm our previous findings in unblinded studies demonstrating functional repair of hibernating myocardium following global intracoronary infusion of autologous MSCs and CDCs16, 17.
Methodological considerations
We used a single infusion with a comparable number of cells for each cell type that has previously demonstrated efficacy in our previous studies but differences in therapeutic efficacy could exist at alternative doses43. Our study design did not determine the specific component of the heterogeneous CDC population responsible for their effects. Few studies have administered CDCs fractionated using surface markers but one has suggested CD105+/CD90−/cKit− cells to constitute the active fraction44. Thus, we may have found enhanced functional improvement if this sub-population of CDCs had been administered. Like nearly all experimental studies, the donors and recipients of MSCs and CDCs in the present study were relatively young, healthy animals. Thus, clinical translation to patients may still be complicated by the fact that the target population is of advanced age with multiple cardiovascular risk factors that could impact therapeutic efficacy. Although there were not significant differences between groups in any measured variable prior to treatment, remote zone wall thickening tended to be higher (p=0.08) in animals randomized to receive icMSCs, which may have influenced our ability to compare the effects of each cell type on this parameter. In contrast to autologous MSCs16, MSC-treated animals in the present study did not exhibit an increase in LV mass. This could reflect differences between autologous and allogeneic cells or the impact of blinding, randomization, and the use of concurrently-treated animals. Finally, we found different relative increases in Ki67 labeling vs. pHH3 labeling 4-weeks after cell infusion vs. vehicle-treated controls. This could reflect the time course of these markers prior to tissue harvesting as well as variations in the time over which cells are Ki67 or pHH3 positive with each treatment. Recent studies have demonstrated that physiological stimuli such as revascularization itself can also stimulate cardiac repair and alter these myocyte proliferation indices45. Regardless of the proliferative indices measured at a single time point (which reflect a rate of myocyte division at that point in time), the increase in nuclear density and reduction in myocyte size assessed 4-weeks after treatment reflects the cumulative myocyte increase which was the same with icMSCs and icCDCs.
Conclusions
The present study demonstrates therapeutic equipoise of two contemporary cell therapy platforms in a large animal model of chronic ischemia. Global icMSCs and icCDCs each significantly improved regional contractile function and increased myocyte number throughout the LV without affecting myocardial perfusion. These results occurred despite evidence of limited cell engraftment 1-month after treatment, implicating cell therapy-mediated stimulation of endogenous myocyte regeneration as a primary mechanism of repair for both cell types. Our preclinical study demonstrates the feasibility of implementing a rigorous blinded experimental design that is similar to that employed in single center randomized clinical trials. While the results suggest that it may be difficult to identify therapeutic superiority of either cell type over the other in clinical studies, they support their ability to produce myocyte cellular remodeling before global LV dysfunction and heart failure develop.
Supplementary Material
Novelty and Significance.
What Is Known?
Despite extensive preclinical evidence, clinical efficacy of cardiac cell-based therapeutic approaches remains unclear.
Most patients with coronary artery disease as well as heart failure have relatively preserved left ventricular systolic function as assessed by ejection fraction.
In ischemic heart disease, significant myocyte loss and compensatory hypertrophy can develop before global LV dysfunction and heart failure.
What New Information Does This Article Contribute?
Using a blinded, randomized, vehicle-controlled study design, we found that global intracoronary infusion of allogeneic MSCs and CDCs produced comparable improvements in regional function in swine with chronic myocardial ischemia and hibernating myocardium.
Both icCDCs and icCDCs increased capillary density, myocyte nuclear density and reduced myocyte cellular hypertrophy.
Few cells were derived from allogeneic cell transplants, clearly implicating activation of endogenous repair mechanisms in icCDCs and icMSCs treated animals.
The beneficial effects of both cell therapies occur in ischemic as well as remote regions of the heart at a time when reductions in ejection fraction and heart failure are absent.
In this study, using a rigorous, clinical trial-based study design, we demonstrate therapeutic equipoise of two therapies in a large animal model of chronic ischemia associated with myocyte cellular loss and hypertrophy in the absence of infarction. We found that improvements in regional function were accompanied by substantial reverse myocyte remodeling with increases in myocyte number and reductions in myocyte size. The ability of both of these therapies to replace myocytes lost from the stress of chronic repetitive ischemia raises the possibility that treating the entire heart to prevent the development of LV dysfunction and ischemic cardiomyopathy may be feasible. Strategies to prevent the development of heart failure earlier in the course of the disease may potentially have more impact than reversing dysfunction in the advanced failing heart.
Acknowledgments
These studies could not have been completed without the assistance of Elaine Granica, Rebeccah Young, Beth Palka, Anne Coe and Marsha Barber.
SOURCES OF FUNDING
Supported by the National Heart Lung and Blood Institute (HL-055324, HL-061610, F32HL-114335), New York State Department of Health (NYSTEM CO24351) and the Albert and Elizabeth Rekate Fund in Cardiovascular Medicine.
Nonstandard Abbreviations and Acronyms
- MSCs
Mesenchymal Stem Cells
- icMSCs
Intracoronary Mesenchymal Stem Cells
- CDCs
Cardiosphere-Derived Cells
- icCDCs
Intracoronary Cardiosphere-Derived Cells
- LAD
Left Anterior Descending Coronary Artery
- LADWT
LAD Wall Thickening
- LV
Left Ventricular
- Y-FISH
Y-chromosome Fluorescent In Situ Hybridization
- pHH3
Phosphorylated Histone-H3
Footnotes
DISCLOSURES
There are no relationships or financial associations with industry for any of the authors that would pose a conflict of interest in connection with the work.
References
- 1.Simari RD, Pepine CJ, Traverse JH, Henry TD, Bolli R, Spoon DB, Yeh E, Hare JM, Schulman IH, Anderson RD, Lambert C, Sayre SL, Taylor DA, Ebert RF, Moye LA. Bone marrow mononuclear cell therapy for acute myocardial infarction: a perspective from the cardiovascular cell therapy research network. Circ Res. 2014;114:1564–8. doi: 10.1161/CIRCRESAHA.114.303720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marban E, Malliaras K. Mixed results for bone marrow-derived cell therapy for ischemic heart disease. JAMA. 2012;308:2405–6. doi: 10.1001/jama.2012.64751. [DOI] [PubMed] [Google Scholar]
- 4.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye L, Suerder D, Corti R, Huikuri HV, Miettinen JA, Woehrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma D, Diederichsen AC, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer GM. Meta-Analysis of cell-based CaRdiac stUdiEs (ACCRUE) in patients with Acute Myocardial Infarction Based on Individual Patient Data. Circ Res. 2015;116:1346–60. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. The elusive heart fix. Science. 2014;345:252–7. doi: 10.1126/science.345.6194.252. [DOI] [PubMed] [Google Scholar]
- 6.Jansen Of Lorkeers SJ, Eding JE, Vesterinen HM, van der Spoel TI, Sena ES, Duckers HJ, Doevendans PA, Macleod MR, Chamuleau SA. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circ Res. 2015;116:80–6. doi: 10.1161/CIRCRESAHA.116.304872. [DOI] [PubMed] [Google Scholar]
- 7.Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-Sponsored Consortium for preclinicAl assESsment of cARdioprotective Therapies (CAESAR): A New Paradigm for Rigorous, Accurate, and Reproducible Evaluation of Putative Infarct-Sparing Interventions in Mice, Rabbits, and Pigs. Circ Res. 2015;116:572–86. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefer DJ, JS, Steenbergen C, Kukreja R, Guo Y, Tang X, Li Q, Ockaili R, Salloum F, Kong M, Polhemus DJ, Bhushan S, Goodchild T, Chang C, Book MJ, Du J, Bolli R. Sodium nitrite fails to limit myocardial infarct size: results from the CAESAR cardioprotection consortium. FASEB J. 2014;28:LB645. [Google Scholar]
- 9.Kukreja R, TX, Lefer DJ, Steenbergen C, Jones S, Guo Y, Li Q, Kong M, Stowers H, Hunt G, Tokita Y, Wu W, Ockaili R, Salloum F, Book MJ, Du J, Bhushan S, Goodchild T, Chang C, Bolli R. Adminstration of sildenafil at reperfusion fails to reduce infarct size: results from the CAESAR cardioprotection consortium. FASEB J. 2014;28:LB650. [Google Scholar]
- 10.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous Mesenchymal Stem Cells Mobilize cKit+ and CD133+ Bone Marrow Progenitor Cells and Improve Regional Function in Hibernating Myocardium. CircRes. 2011;109:1044–54. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki G, Weil BR, Leiker MM, Ribbeck AE, Young RF, Cimato TR, Canty JM., Jr Global Intracoronary Infusion of Allogeneic Cardiosphere-Derived Cells Improves Ventricular Function and Stimulates Endogenous Myocyte Regeneration throughout the Heart in Swine with Hibernating Myocardium. PLoS One. 2014;9:e113009. doi: 10.1371/journal.pone.0113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marban L, Marban E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–75. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 20.Fallavollita JA, Perry BJ, Canty JM., Jr 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium: Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95:1900–1909. doi: 10.1161/01.cir.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 21.Fallavollita JA, Logue M, Canty JM., Jr Stability of hibernating myocardium in pigs with a chronic left anterior descending coronary artery stenosis: Absence of progressive fibrosis in the setting of stable reductions in flow, function and coronary flow reserve. J Am Coll Cardiol. 2001;37:1989–1995. doi: 10.1016/s0735-1097(01)01250-5. [DOI] [PubMed] [Google Scholar]
- 22.Canty JM, Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: Chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94:1142–1149. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 23.Schimmel C, Frazer D, Glenny RW. Extending fluorescent microsphere methods for regional organ blood flow to 13 simultaneous colors. Am J Physiol. 2001;280:H2496–H2506. doi: 10.1152/ajpheart.2001.280.6.H2496. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki G, Iyer V, Cimato T, Canty JM., Jr Pravastatin improves function in hibernating myocardium by mobilizing CD133+ and cKit+ hematopoietic progenitor cells and promoting myocytes to reenter the growth phase of the cardiac cell cycle. Circ Res. 2009;104:255–264. doi: 10.1161/CIRCRESAHA.108.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruel A, Nyengaard JR. Design-based stereological estimation of the total number of cardiac myocytes in histological sections. Basic Res Cardiol. 2005;100:311–9. doi: 10.1007/s00395-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 26.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100:2380–2386. doi: 10.1161/01.cir.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 27.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–53. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oskouei BN, Lamirault G, Joseph C, Treuer AV, Landa S, Da Silva J, Hatzistergos K, Dauer M, Balkan W, McNiece I, Hare JM. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Translational Medicine. 2012;1:116–24. doi: 10.5966/sctm.2011-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey D, Han L, Bauer M, Sanada F, Oikonomopoulos A, Hosoda T, Unno K, De Almeida P, Leri A, Wu JC. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–62. doi: 10.1161/CIRCRESAHA.112.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malliaras K, Ibrahim A, Tseliou E, Liu W, Sun B, Middleton RC, Seinfeld J, Wang L, Sharifi BG, Marban E. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med. 2014;6:760–77. doi: 10.1002/emmm.201303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, Li T, Lu L, Lu G, Marban E. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014;32:2397–406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canty JM, Jr, Suzuki G. Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol. 2012;52:822–31. doi: 10.1016/j.yjmcc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–73. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–12. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 39.Narula J, Dawson MS, Singh BK, Amanullah A, Acio ER, Chaudhry FA, Arani RB, Iskandrian AE. Noninvasive characterization of stunned, hibernating, remodeled and nonviable myocardium in ischemic cardiomyopathy. J Am Coll Cardiol. 2000;36:1913–1919. doi: 10.1016/s0735-1097(00)00959-1. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki G, Weil BR, Leiker MM, Goelz A, Fallavollita JA, Canty JM., Jr Global intracoronary infusion of allogeneic cardiosphere-derived cells (CDCs) immediately after reperfusion stimulates myocyte regeneration in remote viable myocardium in swine with acute myocardial infarction. Circulation. 2014;130:A15656. [Google Scholar]
- 41.Suzuki G, Weil BR, Leiker MM, Canty J, JM Global intracoronary infusion of allogeneic atrial cardiosphere-derived cells (icCDCs) stimulates myocyte regeneration and reverses LV remodeling in miniature swine with chronic myocardial infarction. Global intracoronary infusion of allogeneic atrial cardiosphere-derived cells (icCDCs) stimulates myocyte regeneration and reverses LV remodeling in miniature swine with chronic myocardial infarction. Circulation. 2013;128:A9912. [Google Scholar]
- 42.Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marban E. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. J Am Coll Cardiol. 2014;64:922–37. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, Liu W, Smith RR, Marban E. Relative Roles of CD90 and c-Kit to the Regenerative Efficacy of Cardiosphere-Derived Cells in Humans and in a Mouse Model of Myocardial Infarction. J Am Heart Assoc. 2014;3:e001260. doi: 10.1161/JAHA.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page BJ, Banas MD, Suzuki G, Weil BR, Young RF, Fallavollita JA, Palka BA, Canty JM., Jr Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol. 2015;65:684–97. doi: 10.1016/j.jacc.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.