Abstract
Ethanol consumption during pregnancy produces a wide range of morphological and behavioral alterations known as Fetal Alcohol Spectrum Disorder (FASD). Among the behavioral deficits associated with FASD is an increased probability of developing anxiety disorders. Studies with animal models of FASD have demonstrated that ethanol exposure during the equivalent to the 1st and 2nd trimesters of human pregnancy increases anxiety-like behavior. Here, we examined the impact on this type of behavior of exposure to high doses of ethanol in vapor inhalation chambers during the rat equivalent to the human 3rd trimester of pregnancy (i.e., neonatal period in these animals). We evaluated anxiety-like behavior with the elevated plus maze. Using whole-cell patch-clamp electrophysiological techniques in brain slices, we also characterized glutamatergic and GABAergic synaptic transmission in the basolateral amygdala, a brain region that has been implicated to play a role in emotional behavior. We found that ethanol-exposed adolescent offspring preferred the closed arms over the open arms in the elevated plus maze and displayed lower head dipping activity than controls. Electrophysiological measurements showed an increase in the frequency of spontaneous and miniature excitatory postsynaptic currents in pyramidal neurons from the ethanol group. These findings suggest that high-dose ethanol exposure during the equivalent to the last trimester of human pregnancy can persistently increase excitatory synaptic inputs to principal neurons in the basolateral amygdala, leading to an increase in anxiety-like behaviors.
Keywords: fetal, ethanol, synaptic, behavior, basolateral, amygdala, anxiety
1. Introduction
Consumption of ethanol during pregnancy can produce Fetal Alcohol Syndrome, characterized by growth retardation, facial dysmorphology, and central nervous system alterations (Riley et al., 2011). However, in many cases, in utero ethanol exposure results in only a subset of these abnormalities. The range of effects that can be caused by prenatal ethanol exposure is denoted as Fetal Alcohol Spectrum Disorder (FASD), whose severity depends on several factors, such as the timing, pattern and dose of ethanol consumed, as well as environmental and genetic factors (Jones, 2011). The central nervous system alterations are among the most severe manifestations of FASD, which significantly decrease quality of life and involve a wide range of processes such as learning, memory, attention, fine motor coordination, judgment, social interaction, and emotional behavior (Riley et al., 2011). Regarding the latter, several studies have demonstrated an association between prenatal ethanol exposure and anxiety disorders (O’Connor and Paley, 2009, Hellemans et al., 2010). For instance, O’Leary et al., (2010) found that ethanol exposure during fetal life significantly increases the likelihood of developing anxiety and depression during childhood.
Studies with animal models of FASD have also shown that prenatal ethanol exposure can produce effects on anxiety-like behavior in offspring. Dursun et al., (2006) found that the offspring of rats exposed to high doses of ethanol (6g/kg via intragastric gavage between gestational days 7 and 20; blood ethanol concentration (BEC) 0.35 g/dl 3 hr after gavage) spend less time in the open arms of the elevated plus maze, an indication of increased anxiety-like behavior. Using the open field test, Zhou et al., (2010) showed that high dose prenatal ethanol exposure (6 g/kg of ethanol via intragastric gavage between gestational days 7 and 20; BEC 3 hr after last gavage on gestational day 20 = 0.35 g/dl) increases anxiety in adult rat offspring. Prenatal exposure of rats or mice to lower doses of ethanol has been shown to produce a decrease in anxiety-like behavior (0.5 and 4 g/kg of ethanol via intragastric gavage from delivery until weaning; BEC not determined; Carneiro et al., 2005), or no significant change (voluntary drinking of saccharin water containing 5% ethanol two weeks pre-pregnancy and throughout gestation; BEC = 0.08 g/dl; Savage et al., 2010, Staples et al., 2013; and drinking in the dark paradigm using 20% v/v ethanol in water throughout gestation; BEC = 0.125–0.175 g/dl; Boehm et al., 2008), or an increase (liquid diet containing 6% v/v ethanol throughout gestation; BEC = 0.03 g/dl; Probyn et al., 2012, Cullen et al., 2013) and to have complex effects that are dependent on the sex of the animal and/or its behavioral activation state prior to testing (liquid diet throughout gestation containing approximately 5% v/v ethanol; BEC ≈ 0.13 g/dl; Osborn et al., 1998, Wilcoxon et al., 2005, Gabriel et al., 2006). Collectively, these findings indicate that ethanol exposure during the 1st and 2nd trimesters of pregnancy can have effects on anxiety-like behavior in rodents and that these effects depend on several factors, including the dose and timing of ethanol administration.
Ethen et al., (2009) reported that some pregnant women abstain during the first two trimesters of pregnancy and start drinking during the 3rd trimester, when it may be erroneously assumed that it is safe to drink because key developmental processes are complete. However, neuronal circuits undergo significant differentiation and refinement during this period and this could make them particularly susceptible to the actions of ethanol. To model exposure during this period, ethanol has been administered during the first 1–2 weeks of life in rats, when the brain growth spurt takes place in these animals (Cudd, 2005). An early study with rats exposed to ethanol between postnatal days (P) 4 and 12 using an artificial rearing procedure (BEC = not determined) found evidence consistent with increased anxiety using the open field test (Kaneko et al., 1996). In contrast, more recent studies failed to detect a significant effect of ethanol. Roskam and Koch, (2009) found that injecting rats with a high dose of ethanol at P7 (two doses of 2.5 g/kg i.p. delivered 2 hr apart; BEC = not determined but this paradigm is expected to produce high levels, near 0.5 g/dl; Ikonomidou et al., 2000) did not significantly alter performance in the elevated plus maze test during adulthood. Diaz et al., (2014a) found that moderate ethanol exposure in vapor chambers between P2 and P12 (BEC = 0.1 g/dl) does not significantly affect anxiety-like behavior during adolescence in rats. Therefore, whether ethanol exposure during the equivalent to the last trimester of human pregnancy affects anxiety-like behaviors remains an open question.
Several brain regions have been implicated in the generation of anxiety-like behaviors (Adhikari, 2014). Among these, the amygdala has been shown to play a prominent role. The basolateral nucleus of the amygdala (BLA) processes information from the ventral hippocampus and medial prefrontal cortex, and relays this information to the central nucleus of the amygdala and bed nucleus of the stria terminalis (Adhikari, 2014). These structures project to hypothalamic and brain stem nuclei that mediate physiological changes associated with anxiety (Adhikari, 2014). Several studies have suggested that alterations in the amygdala may contribute to the increase in anxiety-like behavior associated with FASD. Magnetic resonance imaging scans demonstrated amygdala volume reductions in children and adolescents with FASD (Nardelli et al., 2011). Zhou et al., (2010) found that heavy prenatal ethanol exposure enhances excitability of BLA pyramidal neurons by attenuating GABAA receptor-mediated inhibition. Cullen et al., (2013) showed that moderate prenatal ethanol exposure increases dendritic spines in the apical dendrite of BLA pyramidal neurons. However, the effects of developmental ethanol exposure on the BLA are not fully understood, particularly the impact of exposure during the 3rd trimester equivalent when neuronal circuits in this brain region undergo profound remodeling (Ehrlich et al., 2012, Ehrlich et al., 2013).
In this study, we used vapor inhalation chambers to repeatedly expose rats to high doses of ethanol during the 3rd trimester-equivalent. We evaluated the impact of this exposure paradigm on anxiety-like behavior using the elevated plus maze. We also studied the effect of ethanol on excitatory and inhibitory synaptic transmission in the BLA using patch-clamp electrophysiological techniques in brain slices. These studies focused on adolescent rats (Spear, 2000) because human studies have found an association between prenatal ethanol exposure and behavioral problems during adolescence (Olson et al., 1997).
2. Materials and methods
Unless indicated, all chemicals and drugs were from Sigma-Aldrich (St. Louis, MO).
2.1. Animals
Animal procedures were approved by the UNM-Health Sciences Center Institutional Animal Care and Use Committee. Pregnant Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN) and arrived at gestational day 12–15. Dams were individually housed, received food and water ad libitum, and had a plastic hut in the cage to reduce stress. Lights were on between 6 am and 6 pm.
2.2. Binge-like Ethanol Vapor Chamber Exposure
To model binge-like ethanol exposure during the 3rd trimester equivalent, we exposed dams with their pups from P3–5 from 10 am-1 pm daily using vapor inhalation chambers (Morton et al., 2014). We chose this paradigm because exposure to similar levels of ethanol during the same period of development has been previously shown to cause significant neuronal damage (Kane et al., 2011). Ethanol vapor levels were 7.8 ± 0.13 g/dl (n = 37 rounds of exposure), as measured with a breathalyzer (Intoximeters, St. Louis, MO). Controls were exposed to air only. During the 3 days of exposure, animals were handled for weighing purposes and to check for the presence of milk in the stomach. The average number of pups per litter were 10.5 ± 0.5 (n = 12 litters) and 10.13 ± 0.4 (n = 15 litters) for the control and ethanol groups, respectively. Weights were also measured at P6, 10, 15, and 20. After exposure, offspring were allowed to grow until P36–50, when behavioral and electrophysiological experiments were conducted. Blood collection and determination of serum ethanol levels were performed as previously described (Diaz et al., 2014a). With the exception of blood ethanol determinations, only male rats were used for all experiments.
2.3. Maternal Care Assessment
Maternal care was assessed as previously described (Champagne et al., 2003). The behavior of the dam was recorded with a digital videocamera for 15 minutes every hour between 10 am and 5 pm. Dam behavior was independently scored by two investigators every 3 min within each 15 min observation period. The dam behaviors that were recorded were: no contact with pups, licking pups, nursing pups in arched-back position, licking pups while they nursed in arched-back position, nursing while lying over the pups in a blanket posture, and passively nursing the pups while lying on the back or side. The percent of the total observation time that each dam spent on each of these behaviors was determined.
2.4. Elevated Plus-Maze
This behavioral test was performed on P39–42 rats. The apparatus was made of wood painted with a black non-toxic paint. Overhead lighting was set to ~150 lux. The height of the base of the maze was 21″ from the floor. The closed arms were (52.0 × 10.1 × 35.5 cm), and the open arm (52.0 × 10.1 cm). The center square was (10.1 × 10.1 cm). Animal home cages were moved into the testing room at 4 pm, lights were turned off at 5 pm and testing began at 6 pm. The testing room was equipped with a white noise generating system. Each rat was placed on the maze at the juncture of the closed and open arm facing the open arm. We allowed rats to explore the maze for 10 min (Komada et al., 2008), rather than the typical 5 min, to increase the chances of detecting differences between treatment groups, as previously described; during this time the researcher left the room. An overhead camera (ICD-49 B/W digital video camera, Ikegami Electronics, Maywood, NJ) and Ethovision X-T video-tracking software (Noldus, Leesburg, VA) were used to calculate in-zone time, arm entry frequencies, head dips, total distance traveled, and average speed. The calculations were based on the position of the central point of the body for each animal, as previously described (Hefner and Holmes, 2007), with the exception of head dips, which were calculated based on the point of the nose. Between animals, the maze was washed once with 5% bleach-water mix and then a second time with water only, before being thoroughly dried. After testing was completed, rats were placed in a separate cage until all litter mates had been tested, and then returned to their home cage.
2.5. BLA Slice Electrophysiology
BLA slice preparation was done as previously described (Diaz et al., 2014a). Briefly, P36–50 rats were deeply anesthetized with ketamine (250 mg/kg i.p.) and sacrificed by rapid decapitation. Brains were quickly removed and submerged for 2 min in cold cutting solution containing (in mM): 220 sucrose, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 12 MgSO4, 10 glucose, 0.2 CaCl2, and 0.43 ketamine, pre-equilibrated with 95% O2/5% CO2. Coronal brain slices containing the BLA (250 μm) were prepared using a vibrating slicer (Leica Microsystems, Bannockburn, IL, USA). Immediately following this procedure, slices were placed in a chamber containing normal artificial cerebrospinal fluid (aCSF) and allowed to recover for 40 min at 34–36 °C followed by storage at 22 °C. Normal aCSF contained (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgSO4, 2 CaCl2, and 0.4 ascorbic acid and was continuously equilibrated with 95% O2/5% CO2.
For whole-cell patch-clamp electrophysiological recordings, neurons were visualized using infrared-differential interference contrast microscopy and recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). BLA pyramidal neurons were identified based on their morphology (large/pyramidal-shaped) and initial membrane resistance < 150 MΩ, as previously described (Kroner et al., 2005, Lack et al., 2007, Diaz et al., 2011). For recordings of glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs), we used an internal solution containing (in mM): 120 K-gluconate, 15 KCl, 0.1 EGTA, 10 HEPES, 4 MgCl2, 4 Mg-ATP, 0.3 Na-GTP, 7 phosphocreatine, and 1 QX-314, pH 7.3 and 300 mOsm. EPSCs were recorded in the presence of the GABAA receptor blocker gabazine (10 μM; Tocris, Ellisville, MO). For recordings of GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs), a KCl-based internal solution was used as previously described (Diaz et al., 2011). IPSCs were isolated by blocking AMPA and NMDA receptors using kynurenic acid (1 mM) and D,L-APV (50 μM; Tocris). For all experiments, tetrodotoxin (1 μM, Tocris) was added to the aCSF to block action potential-dependent events and allow the recording of miniature EPSCs (mEPSCs) or IPSCs (mIPSCs). The membrane potential was held at −70 mV for all experiments. During application of antagonists, neurons were allowed to equilibrate for at least 5 min prior to beginning an experiment. Data were acquired in gap-free mode at 10 kHz and filtered at 2 kHz. Only recordings in which the resistance changed less than 20% were kept for analysis.
2.6. Data Analyses
Electrophysiological recordings were initially analyzed with MiniAnalysis (Synaptosoft, Decatur, GA). All data were statistically analyzed with Prizm 5 (Graphpad, San Diego, CA). Data were initially analyzed with the Pearson omnibus or the Kolmogorov-Smirnov normality tests. Data that followed a normal distribution were analyzed using parametric tests, while data that did not follow a normal distribution were analyzed using non-parametric tests. A P < 0.05 was considered to be statistically significant. Unless indicated, the experimental unit used for all statistical analyses was an animal.
3. Results
At the end of the 3 hr ethanol exposure, the vapor chamber paradigm resulted in serum ethanol levels near 70 mM in P4 pups and 5 mM in the dam (Fig 1A; as a reference, the legal intoxication limit in the U.S.A. is 17.4 mM = 0.08 g/dl). The vapor chamber paradigm was well tolerated by the pups, which did not completely lose consciousness at the end of each round of exposure but displayed motor responses to tactile stimulation that were slower than those observed in air exposed rats. These findings are consistent with the literature indicating that significantly greater concentrations of ethanol are required to anesthetize neonatal vs. adult rats (Fang et al., 1997). Weight gain was not significantly affected by ethanol treatment (Fig 1B; two-way ANOVA: interaction F (6, 116) = 0.52, P = 0.788; postnatal day F (6, 116) = 320, P < 0.0001; ethanol treatment F (1, 116) = 2.767, P = 0.09). There were no pup deaths during chamber exposure in the control group (total number of pups = 126 from 12 litters) and only 1 pup died in the ethanol group (total number of pups = 152 from 15 litters). The percent of pups with milk in their stomachs was comparable between the control (98.97 ± 1.02 %; n = 7 litters) and ethanol (98.86 ± 1.14 %; n = 8 litters) groups. Most indexes of maternal care were not significantly affected by ethanol exposure with the exception of blanket nursing, which was significantly increased in the ethanol group (U = 0; P = 0.004 by Mann-Whitney test; Table 1).
Figure 1. Characterization of vapor chamber ethanol exposure paradigm.
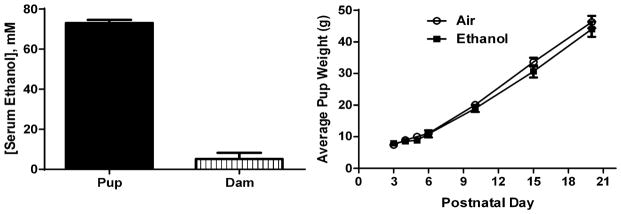
A. Serum ethanol concentrations measured at the end of the 3 hr ethanol vapor exposure on postnatal day 4 pups (n = 7 pups from 7 litters) and dams (n = 9). B. Average weight gain of pups exposed to air (control; n = 4–12 litters) or ethanol (4–14 litters). Vapor chamber exposure took place between postnatal days 3 and 5.
Table 1.
Effect of Ethanol Exposure on Maternal Care.
| Parameter | Control (n=6) | Ethanol (n=5) |
|---|---|---|
| No Contact | 35.2 ± 8.2 | 15.4 ± 5.9 |
| Lick Pups | 1.5 ± 0.7 | 2.9 ± 1.0 |
| Arched-Back Nursing | 40.4 ± 8.6 | 35 ± 3.5 |
| Lick Pups & Arched-Back Nursing | 7.6 ± 1.2 | 5 ± 1.4 |
| Blanket Nursing | 10.5 ± 1.6 | 31.8 ± 3.3** |
| Passive Nursing | 4.6 ± 2.1 | 9.7 ± 2.9 |
p<0.01 by Mann-Whitney Test.
3.1. Ethanol exposure increases anxiety-like behavior
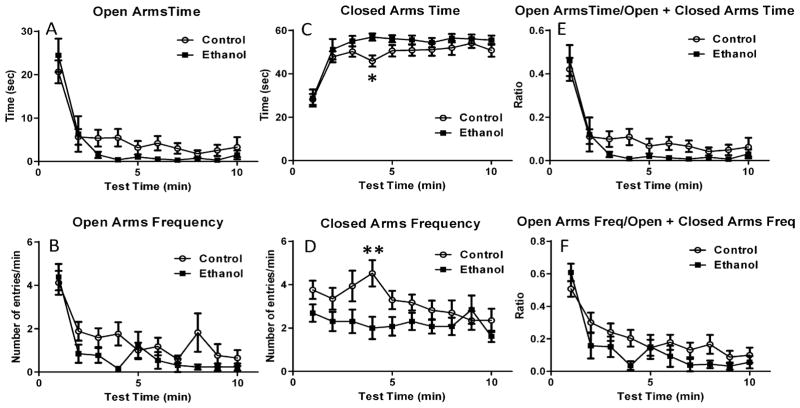
We evaluated the effect of 3rd trimester-equivalent ethanol exposure on anxiety-like behavior using the elevated plus maze. We used minute-by-minute scoring to better characterize the pattern of response to this test (Carobrez and Bertoglio, 2005). Although ethanol exposure did not significantly affect the time spent in the open arms (Fig 2A; repeated-measures two-way ANOVA: interaction F (9, 252) = 1.082, P = 0.37; time F (9, 252) = 28.10, P < 0.001; ethanol treatment F (1, 28) = 2.116, P = 0.15), it induced an overall significant reduction in the frequency of open arm entries (Fig 2B; repeated-measures two-way ANOVA: interaction F (9, 252) = 1.138, P = 0.33; time F (9, 252) = 13.37, P < 0.0001; ethanol treatment F (1, 28) = 4.25, P = 0.04; Bonferroni’s multiple comparison test: P>0.05 for control vs. ethanol at all postnatal days). Ethanol exposure significantly increased the time spent in the closed arms (Fig 2C; repeated-measures two-way ANOVA: interaction F (9, 252) = 0.65, P = 0.74; time F (9, 252) = 21.07, P < 0.0001; ethanol treatment F (1, 28) = 4.8, P = 0.03; Bonferroni’s multiple comparison test: P<0.05 at 4 min) and decreased the frequency of closed arm entries (Fig 2D; repeated-measures two-way ANOVA: interaction F (9, 252) = 1.52, P = 0.14; time F (9, 252) = 1.68, P = 0.09; ethanol treatment F (1, 28) = 6.53, P = 0.01; Bonferroni’s multiple comparison test: P<0.05 at 4 min). The ratio of the time spent in the open arm over the sum of the time spent in the open and the closed arms was not significantly affected by ethanol exposure (Fig 2E; repeated-measures two-way ANOVA: interaction F (9, 252) = 0.77, P = 0.63; time F (9, 252) = 29.34, P < 0.001; ethanol treatment F (1, 28) = 2.52, P = 0.12). Ethanol induced an overall decrease in the ratio of the frequency of open arm entries over the sum of the frequency of open and the closed arm entries (Fig 2F; repeated-measures two-way ANOVA: interaction F (9, 252) = 1.26, P = 0.25; time F (9, 252) = 17.61, P < 0.001; ethanol treatment F (1, 28) = 4.37, P = 0.04; Bonferroni’s multiple comparison test: P>0.05 for control vs. ethanol at all postnatal days).
Figure 2. Impact of ethanol exposure on open and closed arm entries in the elevated plus maze test.
Ethanol exposure did not significantly reduce the time spent in the open arms (A), but induced an overall significant reduction in the frequency of entrances into the open arms (B). Ethanol exposure significantly increased the time spent in the closed arms (C) and significantly reduced the frequency of closed arms entries (D). Ethanol exposure did not significantly affect the ratio of open arms time/open + closed arm time (E), but induced an overall significant reduction in the ratio of the frequency of open arm entries/frequency of entries into the open + closed arms (F) (n = 17 rats from 4 litters for control and 13 rats from 4 litters for ethanol; * P < 0.05; ** P < 0.01 by two-way ANOVA followed by Bonferroni’s posthoc test; for more details on results of statistical analyses, please see text).
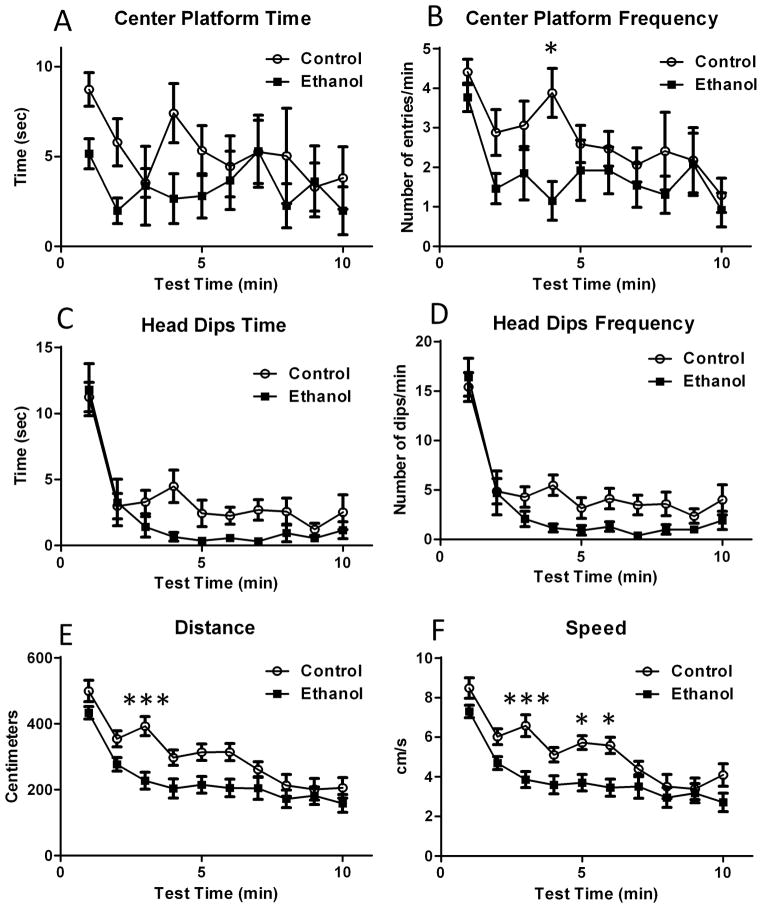
Fig 3A shows that ethanol exposure was associated with a non-significant reduction in the time spent in the center platform (repeated-measures two-way ANOVA: interaction F (9, 252) = 0.68, P = 0.72; time F (9, 252) = 1.19, P = 0.29; ethanol treatment F (1, 28) = 4.19, P = 0.0501); however, ethanol exposure significantly reduced the frequency of entries into the center platform at the 4 min time point (Fig 3B; repeated-measures two-way ANOVA: interaction F (9, 252) = 0.96, P = 0.47; time: F (9, 252) = 3.95, P < 0.0001; ethanol treatment F (1, 28) = 4.3, P = 0.04; Bonferroni’s multiple comparison test: P<0.05 at 4 min).
Figure 3. Impact of ethanol exposure on additional elevated plus maze test parameters.
(A) The time spent in the center platform was not significantly affected by ethanol exposure. (B) The frequency of center platform entrances was significantly reduced by ethanol exposure. Ethanol induced an overall significant reduction in the time spent head dipping in the open arms (C) and the frequency of head dipping while in these arms (D). The total distance covered (E) and the speed at which the animals moved (F) while in the maze were significantly reduced in ethanol-exposed animals (n = 17 rats from 4 litters for control and 13 rats from 4 litters for ethanol; * P < 0.05; *** P < 0.001 by two-way ANOVA followed by Bonferroni’s posthoc test. For more details on results of statistical analyses, please see text).
In addition to the spatiotemporal parameters described above, we analyzed the time spent by the rats dipping their heads over the open arms. Repeated-measures two-way ANOVA revealed that ethanol treatment induced an overall significant decrease in the head dip time (Fig 3C; interaction F (9, 252) = 1.0, P = 0.43; time F (9, 252) = 23.91, P < 0.0001; ethanol treatment F (1, 28) = 4.69, P = 0.03). Similar results were obtained regarding the frequency of head dips (Fig 3D; repeated measures two-way ANOVA: interaction F (9, 252) = 1.0, P = 0.40; time F (9, 252) = 33.45, P < 0.0001; ethanol treatment F (1, 28) = 5.93, P = 0.02); however, posthoc analyses with the Bonferroni’s multiple comparison test did not detect significant differences for either head dip time or frequency at any time point. We also measured the effect of ethanol exposure on the overall distance covered by the rats while in the elevated plus maze, as well as their average speed. As shown in Fig 3E, ethanol-exposed rats covered significantly less distance than controls (repeated-measures two-way ANOVA: interaction F (9, 252) = 1.68, P = 0.09; time F (9, 252) = 27.54, P < 0.0001; ethanol treatment F (1, 28) = 8.92, P = 0.005; Bonferroni’s multiple comparison test: P<0.05 at 3 min). In addition, the ethanol-exposed rats moved more slowly than controls (Fig 3F; repeated-measures two-way ANOVA: interaction F (9, 252) = 1.69, P = 0.09; time F (9, 252) = 22.89, P < 0.0001; ethanol treatment F (1, 28) = 12.59, P = 0.001; Bonferroni’s multiple comparison test: P<0.05 at 3 and 5–6 min).
3.2. Ethanol exposure increases BLA glutamatergic transmission without altering GABAergic transmission
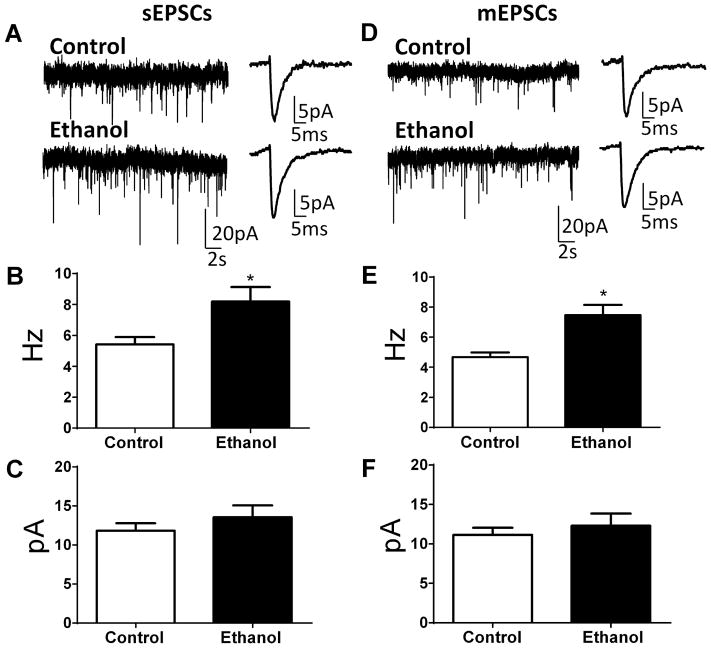
Whole-cell patch-clamp electrophysiological recordings revealed that neither the membrane capacitance (control = 247 ± 12 pF, n = 13; ethanol = 266 ± 9 pF, n = 12; t (23) = 1.22, P = 0.23 by unpaired t-test) nor the membrane resistance (control = 135 ± 22 MΩ, n = 12; ethanol = 110 ± 8 pF, n = 12; U = 68, P = 0.82 by Mann-Whitney test) were significantly affected in BLA pyramidal neurons by ethanol exposure. In slices from ethanol-exposed offspring, there was a significant increase in sEPSC frequency (Fig. 4A, B; t (10) = 2.64, n = 6, P = 0.024 by unpaired t-test), but no change in sEPSC amplitude (Fig. 4A, C; t (10) = 0.97; n = 6, P = 0.35). Assessment of action potential-independent glutamatergic transmission revealed a significant increase in mEPSC frequency (Fig. 4D, E; U = 0, n = 6, P = 0.002 by Mann-Whitney test) with no difference in mEPSC amplitude (Fig. 4D, F; U = 17, n = 6, P = 0.89 by Mann-Whitney test) between control and ethanol-exposed offspring. Comparison of Figs 4B and E shows that the frequency of mEPSCs was only slightly lower than the frequency of sEPSCs. Therefore, spontaneous action potential-dependent events have a relatively small contribution to the events recorded in the absence of tetrodotoxin. This is likely due to the fact that the cell bodies of neurons that provide the majority of the glutamatergic afferents to the BLA are not present in the coronal slice preparation.
Figure 4. Ethanol exposure increased spontaneous (sEPSCs) and miniature (mEPSCs) excitatory postsynaptic currents in pyramidal neurons from the basolateral amygdala.
A. Sample traces of sEPSC slice recordings from control and ethanol-exposed animals. Shown on the right panel are average traces displayed at an expanded time scale. Ethanol significantly increased sEPSC frequency (B) without affecting sEPSC amplitude (C) (* P < 0.05 by unpaired t-test; n = 6 rats (from 3 air- and 5 ethanol-exposed litters). D. Sample traces of mEPSC slice recordings from control and ethanol-exposed animals. Shown on the right panel are average traces displayed at an expanded time scale. Ethanol significantly increased mEPSC frequency (E) without affecting mEPSC amplitude (F) (* P < 0.05 by Mann-Whitney test; n = 6 rats (from 3 air- and 5 ethanol-exposed litters).
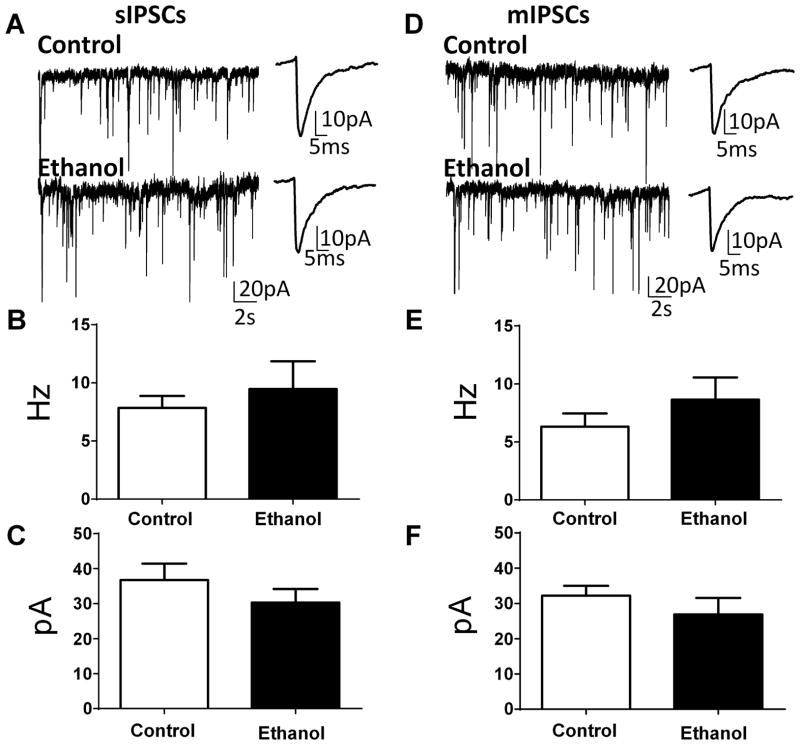
Examination of spontaneous GABAergic transmission showed no difference in sIPSC frequency (Fig. 5A, B; t (11) = 0.66, n = 6, P = 0.5) or sIPSC amplitude (Fig. 5A, C; U = 14; n =6, P = 0.34). Likewise, there was no effect of ethanol exposure on mIPSC frequency (Fig. 5D, E; t (11) = 1.07, n = 6, P = 0.3) or mIPSC amplitude (Fig. 5D, F; t (11) = 1.02, n = 6, P = 0.33). Comparison of Figs 5B and E shows that the frequency of mIPSCs was only slightly lower than the frequency of sIPSCs, suggesting that interneurons that provide GABAergic inputs to BLA pyramidal neurons have relatively low spontaneous action potential firing activity under our experimental conditions.
Figure 5. Ethanol exposure did not affect spontaneous (sIPSCs) and miniature (mIPSCs) inhibitory postsynaptic currents in pyramidal neurons from the basolateral amygdala.
A. Sample traces of sIPSC slice recordings from control and ethanol-exposed animals. Shown on the right panel are average traces displayed at an expanded time scale. Ethanol did not significantly affect sIPSC frequency (B) or sIPSC amplitude (C) (n = 6–7 rats from 4 air- and 4 ethanol-exposed litters). D. Sample traces of mIPSC slice recordings from control and ethanol-exposed animals. Shown on the right panel are average traces displayed at an expanded time scale. Ethanol did not significantly affect mIPSC frequency (E) or mIPSC amplitude (F) (n = 6–7 rats from 3 air- and 4 ethanol-exposed litters).
4. Discussion
In this study, we exposed neonatal rats to ethanol using vapor chambers to model high-dose ethanol exposure during the last trimester of human pregnancy. This paradigm yielded pup serum ethanol levels that were approximately 4 times the legal intoxication limit in the U.S.A. High dose exposure to ethanol during late pregnancy has been documented in several studies, for example, by measuring maternal blood alcohol levels near the time of delivery. These studies have reported blood alcohol levels as high as 44–57 mM (0.2–0.27 g/dl) (Burd et al., 2012, Kvigne et al., 2012). We chose to expose to higher levels of ethanol (i.e., ~70 mM) because the developing rodent brain is significantly less sensitive to ethanol than the developing human brain (Gohlke et al., 2008) and neonatal rats are relatively resistant to the depressant effects of ethanol (Hollstedt et al., 1980, Fang et al., 1997). In addition, we previously found that exposure to lower levels of ethanol (i.e., 0.1 g/dl) during the 3rd trimester equivalent have relatively subtle effects on synaptic transmission in the amygdala (Diaz et al., 2014a) and wished to test a higher dose of ethanol that was comparable to that used in other studies of the effect of exposure during this period on anxiety-like behavior in mature rats (Roskam and Koch, 2009). In general agreement with previous studies (Puglia and Valenzuela, 2010, Everett et al., 2012, Diaz et al., 2014b), the paradigm minimally affected pup weight gain or nursing ability. Maternal care was also minimally altered, consistent with the finding that serum ethanol concentrations in the dam were less than 10% of the levels found in the pup, which is likely due to increased ability to metabolize ethanol in the dams. Importantly, blanket nursing was significantly increased in ethanol exposed dams (perhaps because of increased in sleep in the dams), which likely compensated for any hypothermic effects of ethanol present in the neonatal rats. Moreover, a previous study with P6–16 rats gavaged with two 2 g/kg doses of ethanol administered three hours apart did not detect a significant effect of treatment on pup temperature (Kruckeberg et al., 1984), suggesting that hypothermic responses were not a significant confound in our study.
The vapor chamber paradigm altered performance in the elevated plus maze test at P39–42, a period that is considered to be equivalent to human adolescence (Spear, 2000). Ethanol-exposed rats entered the open arms significantly less frequently and exhibited significantly lower head dipping activity than controls. These findings are consistent with an increase in anxiety-like behavior in ethanol-exposed offspring (Rodgers and Dalvi, 1997, Carobrez and Bertoglio, 2005). In addition, rats from the ethanol group spent significantly more time in the closed arms and entered the closed arms and center platform less frequently than did controls. These findings suggest that the ethanol-exposed rats went into the closed arms and stayed there. The ratio of the frequency of open arm entries/frequency of open + closed arms entries was also significantly reduced in ethanol-exposed rats. Both the distance covered by the rats while in the maze and the speed at which they moved were reduced in the ethanol-exposed group. Collectively, these findings indicate that ethanol-exposed offspring exhibit higher avoidance of the open arms and less exploratory behavior, consistent with an anxiety-like phenotype. An alternative interpretation is that the ethanol-exposed adolescent rats have impairments in locomotor activity and that this altered performance in the elevated plus maze. However, we did not detect any deficits in average body speed, step cadence, swing speed, or stride length using the Catwalk test in the same rats at P48–71 (Baculis and Valenzuela, unpublished observation), suggesting that locomotor activity deficits are not responsible for the alterations in elevated plus maze performance.
Pyramidal neurons are the primary output of the BLA and their activity is controlled by the balance between excitatory glutamatergic inputs and inhibitory GABAergic inputs (McCool et al., 2010). Several studies suggest that a shift in the balance of BLA synaptic transmission in favor of excitation promotes anxiety-like behaviors (Sajdyk and Shekhar, 1997, Aroniadou-Anderjaska et al., 2012, Boyle, 2013, Tian et al., 2013, Masneuf et al., 2014). For instance, chronic intermittent exposure to ethanol vapor was shown to increase the amplitude and frequency of AMPA receptor-mediated sEPSCs in BLA pyramidal neurons from adolescent rats (Lack et al., 2007). Withdrawal from this exposure also increased sEPSC and mEPSC frequency (Lack et al., 2007). Microinjection of an AMPA receptor antagonist into the BLA decreased anxiety-like behavior in the light/dark box in ethanol withdrawn rats (Lack et al., 2007). In general agreement with these results, we found that 3rd trimester-equivalent ethanol exposure induces a long-lasting increase in the frequency of both sEPSCs and mEPSCs in BLA pyramidal neurons. Given that this effect was not associated with a significant change in either spontaneous or quantal GABAergic synaptic transmission, these results suggest that developmental ethanol exposure tilted the balance of neurotransmission toward excitation in BLA pyramidal neurons. The increase in mEPSC frequency in the absence of a change in the amplitude of these events indicates that ethanol exposure induced a persistent increase in the probability of glutamate release without affecting postsynaptic AMPA receptor density or conductance. An alternative explanation is that ethanol exposure induced activation of silent synapses (Mameli et al., 2005). Because sEPSCs represent a mixed population of spontaneous action potential-dependent and -independent events, the increase in mEPSC frequency could explain the increase in sEPSC frequency. Indeed, under our experimental conditions, sEPSC frequency was minimally reduced by tetrodotoxin, indicating that action potential-independent currents are the dominant population of events observed during sEPSC recordings. It is also possible that ethanol exposure induced a long-lasting increase in spontaneous action potential firing of glutamatergic afferent axons that make synaptic connections with BLA pyramidal neurons, which could, in part, explain the increase in sEPSC frequency. Future studies should determine if ethanol exposure potentiates a specific subset of BLA glutamatergic inputs.
In conclusion, this study demonstrates that heavy ethanol exposure during the 3rd trimester-equivalent of human pregnancy induces a long-lasting increase in anxiety-like behavior in adolescent rats. At the cellular level, this effect was correlated with potentiation of glutamatergic synaptic transmission in BLA pyramidal neurons. The mechanisms responsible for these persistent effects of developmental ethanol exposure should be further investigated, including the potential role of stress hormones, considering that increased activity of the hypothalamic-pituitary-adrenal axis has been shown to play a role in the pathophysiology of FASD (Hellemans et al., 2010, Mead and Sarkar, 2014).
Highlights.
Fetal alcohol spectrum disorder is associated with anxiety disorders
Impact of ethanol exposure during the 3rd trimester equivalent was studied
Neonatal rats were exposed to high doses of ethanol in vapor chambers
Ethanol-exposed rats exhibited anxiety-like behavior during adolescence
Excitatory synaptic transmission was increased in the basolateral amygdala
Acknowledgments
We thank Dr. Donald Partridge for critically reading the manuscript and Dr. Jonathan Brigman for assistance with the elevated plus maze experiments. This work was supported by NIH Grant R37-AA015614 and P50-AA022534.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience. 2012;221:157–169. doi: 10.1016/j.neuroscience.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Moore EM, Walsh CD, Gross CD, Cavelli AM, Gigante E, Linsenbardt DN. Using drinking in the dark to model prenatal binge-like exposure to ethanol in C57BL/6J mice. Dev Psychobiol. 2008;50:566–578. doi: 10.1002/dev.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle LM. A neuroplasticity hypothesis of chronic stress in the basolateral amygdala. Yale J Biol Med. 2013;86:117–125. [PMC free article] [PubMed] [Google Scholar]
- Burd L, Blair J, Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol. 2012;32:652–659. doi: 10.1038/jp.2012.57. [DOI] [PubMed] [Google Scholar]
- Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS One. 2013;8:e54924. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Jotty K, Locke JL, Jones SR, Valenzuela CF. Moderate Alcohol Exposure during the Rat Equivalent to the Third Trimester of Human Pregnancy Alters Regulation of GABAA Receptor-Mediated Synaptic Transmission by Dopamine in the Basolateral Amygdala. Front Pediatr. 2014a;2:46. doi: 10.3389/fped.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Vollmer CC, Zamudio-Bulcock PA, Vollmer W, Blomquist SL, Morton RA, Everett JC, Zurek AA, Yu J, Orser BA, Valenzuela CF. Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor delta subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology. 2014b;79:262–274. doi: 10.1016/j.neuropharm.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Dogru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110:926–941. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590:4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett JC, Licon-Munoz Y, Valenzuela CF. Effects of third trimester-equivalent ethanol exposure on Cl(-) co-transporter expression, network activity, and GABAergic transmission in the CA3 hippocampal region of neonatal rats. Alcohol. 2012;46:595–601. doi: 10.1016/j.alcohol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Gong D, Ionescu P, Laster MJ, Eger EI, 2nd, Kendig J. Maturation decreases ethanol minimum alveolar anesthetic concentration (MAC) more than desflurane MAC in rats. Anesth Analg. 1997;84:852–858. doi: 10.1097/00000539-199704000-00028. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Yu CL, Osborn JA, Weinberg J. Prenatal ethanol exposure alters sensitivity to the effects of corticotropin-releasing factor (CRF) on behavior in the elevated plus-maze. Psychoneuroendocrinology. 2006;31:1046–1056. doi: 10.1016/j.psyneuen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Gohlke JM, Griffith WC, Faustman EM. Computational models of ethanol-induced neurodevelopmental toxicity across species: Implications for risk assessment. Birth Defects Res B Dev Reprod Toxicol. 2008;83:1–11. doi: 10.1002/bdrb.20137. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jones KL. The effects of alcohol on fetal development. Birth Defects Res C Embryo Today. 2011;93:3–11. doi: 10.1002/bdrc.20200. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, Drew PD. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun. 2011;25(Suppl 1):S137–145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko WM, Riley EP, Ehlers CL. Effects of artificial rearing on electrophysiology and behavior in adult rats. Depress Anxiety. 1996;4:279–288. doi: 10.1002/(SICI)1520-6394(1996)4:6<279::AID-DA4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Kruckeberg TW, Gaetano PK, Burns EM, Stibler H, Cerven E, Borg S. Ethanol in preweanling rats with dams: body temperature unaffected. Neurobehav Toxicol Teratol. 1984;6:307–311. [PubMed] [Google Scholar]
- Kvigne VL, Randall B, Simanton EG, Brenneman G, Welty TK. Blood alcohol levels for American Indian mothers and newborns. Pediatrics. 2012;130:e1015–1018. doi: 10.1542/peds.2011-1400. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, Crowley N, Flynn S, Holmes A, Kash T. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EA, Sarkar DK. Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms. Front Genet. 2014;5:154. doi: 10.3389/fgene.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Diaz MR, Topper LA, Valenzuela CF. Construction of vapor chambers used to expose mice to alcohol during the equivalent of all three trimesters of human development. J Vis Exp. 2014 doi: 10.3791/51839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2011;35:1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15:225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- O’Leary CM, Nassar N, Zubrick SR, Kurinczuk JJ, Stanley F, Bower C. Evidence of a complex association between dose, pattern and timing of prenatal alcohol exposure and child behaviour problems. Addiction. 2010;105:74–86. doi: 10.1111/j.1360-0443.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- Olson HC, Streissguth AP, Sampson PD, Barr HM, Bookstein FL, Thiede K. Association of prenatal alcohol exposure with behavioral and learning problems in early adolescence. J Am Acad Child Adolesc Psychiatry. 1997;36:1187–1194. doi: 10.1097/00004583-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998;22:685–696. [PubMed] [Google Scholar]
- Probyn ME, Zanini S, Ward LC, Bertram JF, Moritz KM. A rodent model of low- to moderate-dose ethanol consumption during pregnancy: patterns of ethanol consumption and effects on fetal and offspring growth. Reprod Fertil Dev. 2012;24:859–870. doi: 10.1071/RD11200. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Roskam S, Koch M. Effects of neonatal and peripubertal ethanol treatment on various aspects of adult rat behavior and brain anatomy. Int J Dev Neurosci. 2009;27:249–256. doi: 10.1016/j.ijdevneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. J Pharmacol Exp Ther. 1997;283:969–977. [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, Varaschin RK, Wright CA, Seidel JL, Caldwell KK, Hamilton DA. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res. 2010;34:1793–1802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Staples MC, Rosenberg MJ, Allen NA, Porch MW, Savage DD. Impact of combined prenatal ethanol and prenatal stress exposure on anxiety and hippocampal-sensitive learning in adult offspring. Alcohol Clin Exp Res. 2013;37:2039–2047. doi: 10.1111/acer.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Wang Y, Zhang N, Guo YY, Feng B, Liu SB, Zhao MG. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. 2013;38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Zhou R, Wang S, Zhu X. Prenatal ethanol exposure attenuates GABAergic inhibition in basolateral amygdala leading to neuronal hyperexcitability and anxiety-like behavior of adult rat offspring. Neuroscience. 2010;170:749–757. doi: 10.1016/j.neuroscience.2010.07.055. [DOI] [PubMed] [Google Scholar]