Abstract
Ghrelin-O-Acyltransferase (GOAT) is an 11-transmembrane integral membrane protein that octanoylates the metabolism-regulating peptide hormone ghrelin at Ser3 and may represent an attractive target for the treatment of type II diabetes and the metabolic syndrome. Protein octanoylation is unique to ghrelin in humans, and little is known about the mechanism of GOAT or of related protein-O-acyltransferases HHAT or PORC. In this study, we explored an in vitro microsomal ghrelin octanoylation assay to analyze its enzymologic features. Measurement of Km for 10-mer, 27-mer, and synthetic Tat-peptide-containing ghrelin substrates provided evidence for a role of charge interactions in substrate binding. Ghrelin substrates with amino-alanine in place of Ser3 demonstrated that GOAT can catalyze the formation of an octanoyl-amide bond at a similar rate compared with the natural reaction. A pH-rate comparison of these substrates revealed minimal differences in acyltransferase activity across pH 6.0–9.0, providing evidence that these reactions may be relatively insensitive to the basicity of the substrate nucleophile. The conserved His338 residue was required both for Ser3 and amino-Ala3 ghrelin substrates, suggesting that His338 may have a key catalytic role beyond that of a general base.
Keywords: Ghrelin, Ghrelin-O-Acyltransferase, GOAT, enzymology, mechanism, integral membrane protein, solubilization
Graphical Abstract
Introduction
Ghrelin is a 28 amino-acid secreted peptide hormone that provides a neuroendocrine link between the gut and the brain, modulating metabolism in response to nutrient availability[1–4]. Ghrelin signaling requires octanoylation of Ser3, a unique modification required for activation of the ghrelin receptor, GHSR-1a[3, 5] and catalyzed by the 11-transmembrane integral membrane protein ghrelin-O-acyltransferase (GOAT)[6–9]. Ghrelin may play roles in both energy balance and hunger, depending on the circumstances[10, 11]. The metabolic consequences of GOAT activity have also been observed in the context of surviving starvation, supporting fat storage and glucagon signaling and antagonizing insulin. Accordingly, GOAT-deficient mice generated in two different ways fail to maintain blood glucose in conditions of calorie restriction[9, 12, 13], although the precise details of the experimental systems may influence this result[14].
GOAT is a member of the MBOAT family of acyltransferases, a group of polytopic integral membrane proteins that acylate lipids, sterols, and GPI-anchored proteins[15]. Little is known about their enzyme mechanisms. They contain an invariant lumenal histidine and a highly conserved asparagine[8, 16–18], which are presumed to be involved in catalysis (H338 and N307 in mouse GOAT). However, in the case of HHAT, which N-palmitoylates hedgehog proteins, some activity was preserved upon mutation of the histidine to alanine[19], and for GOAT, it has been demonstrated that the conserved His and Asn are on opposite sides of the ER membrane[8]. These observations in HHAT and GOAT leave open the role of specific MBOAT residues in catalysis.
A number of in vitro assays have been published for GOAT using microsomes prepared from insect cells[7, 8, 20–24] or human cells[10]. These assays have established N-terminal sequence requirements for recognition of ghrelin by GOAT and identified a number of peptide-based inhibitors as well as initial small-molecule scaffolds. However, mechanistic details regarding the mechanism of catalysis and contributions to binding beyond the first few ghrelin residues are lacking.
In this study, we establish and optimize an improved in vitro microsomal GOAT octanoylation assay using biotin-tagged ghrelin. We also describe a novel ghrelin substrate in which the natural Ser is replaced with 2,3-diaminoproprionic acid (dap, amino-Ala) at position 3 and its processing by wild type (WT) and mutant GOATs.
Materials and Methods
All reagents were purchased at the highest quality available from Sigma-Aldrich or Acros Organics unless otherwise indicated. Commercially available reagents were used without further purification.
Cloning
Mouse GOAT with and without a C-terminal 3xFlag tag was cloned into pFastBac1 (Life Technologies, Grand Island, NY) using EcoRI and HindIII and into pFastBacHT modified to contain an N-terminal His10 tag. H338A and H338N GOAT mutants were made using a modified QuikChange protocol (Stratagene). All clones were fully sequence verified and then recombined into baculovirus by transformation of DH10Bac cells (Life Technologies) and plated on appropriate antibiotic plates with a blue-white screen per the manufacturer’s instructions. Recombinant white clones were verified by two colony PCR reactions: Reaction 1 with M13F (-40) and M13Rev primers demonstrated the presence of a full-length insert and the absence of empty virus, and Reaction 2 with M13F (-40) and GOAT-Internal-Rev (5′-GGAGAGCAGGGAAAAAGAGCAAGT-3′) demonstrated the presence of mouse GOAT. Final clones were further confirmed by DNA sequencing of the complete open reading frames. Baculovirus DNA was prepared for transfection by alkaline lysis with isopropanol precipitation and ethanol wash.
Cell Culture and Virus Preparation
Cell culture medium and insect cells were from Life Technologies unless otherwise noted. SF9 (Spodoptera frugiperda) and High Five (Trichoplusia ni) insect cells were maintained in suspension in 25 mL - 3 L spinner flasks (Bellco Glass, Vineland NJ) in SF-900 III and Express Five serum free media, respectively, at 27°C at a density of 0.2–6 × 106 per mL, with aeration at sizes 1L and larger. High Five cells were counted after trituration 30 times through a 200 μl pipet tip. P1 virus was prepared by transfecting 800,000 SF9 cells per well with 5 μg DNA on 6-well plates using Cellfectin II reagent according to the manufacturer’s instructions and harvested after 3 days. P2 and P3 viruses were prepared using sequential passages with multiplicity of infection (MOI)=0.1, with GOAT expression confirmed by immunoblotting at the P2 stage. For Immunoblotting, cell pellets from 1 mL suspension culture were lysed in 250 μl 1x LDS (lithium dodecyl sulfate) loading dye (Life Technologies) containing 150 mM 2-mercaptoethanol, 2 μg/ml aprotinin, 2.5 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mM EDTA, and 1 μL benzonase nuclease (Sigma, St. Louis, MO), incubated at 37°C for 10 min, and cleared by centrifugation for 5 min in at 21,000 × g; 4 μl was loaded per lane.
Microsome Preparation
1–3 L cultures at 2.5 × 106 cells/mL were infected with P3 virus at MOI ~1 for 48 hours and collected by centrifugation. Pellets from 1 L culture (~10 mL) were resuspended in 40 mL HBS (50 mM HEPES pH 7.0, 150 mM NaCl) containing 2 μg/ml aprotinin, 2.5 μg/ml leupeptin, 2 μg/ml pepstatin A, and 1 mM EGTA and lysed using 40 strokes in a 40 mL Dounce homogenizer (loose pestle). Cell debris was pelleted for 10 min at 4,000 × g and then microsomes were collected at 100,000 × g for 1 h, resuspended in 6 mL HBS with a Dounce homogenizer and then passed 10 times through a 22 gauge needle and twice through a 25 gauge needle. Aliquots were flash-frozen in liquid nitrogen and stored at −80°C. Microsomal protein concentration was measured against a BSA standard using the BCA assay (Thermo Fisher Scientific, Waltham, MA), supplementing the working reagent with 0.5% Triton X-100.
Chemical Synthesis
Peptide synthesis was performed using automated solid phase peptide synthesis and the Fmoc strategy. Biotin-tagged Ghrelin27 (hereafter Ghrelin27) was reported previously [10], and was prepared analogously with a S3A mutation. To prepare Dap3-Ghrelin27, Ser3 was replaced with Alloc(allyloxycarbonyl) protected-1,2-diaminopropionic acid (dap, amino-alanine) and deprotected with Pd(PPh3)4. Biotin-tagged Ghrelin10 and its S3A and Dap3 analogs were prepared analogously, with an aminohexanoic acid linker (Ahx) between the ghrelin sequence and biotinylated lysine (GSSFLSPEHQ(Ahx)K(Biotin)G). Biotin-tagged Ghrelin10-Tat and S3A analog contained the same sequence as Ghrelin10 through K(Biotin), with Tat sequence C-terminal to K(Biotin). Ghrelin sequences synthesized correspond to human ghrelin, and sequences for all ghrelin substrates are shown in Table 1. Synthesized peptides were purified using a reversed-phase C-18 column with a gradient of acetonitrile and water (0.05% trifluoroacetic acid), and structures and purities confirmed with matrix assisted laser desorption mass spectrometry. The final concentrations of the compounds in aqueous solution for assay were based on amino acid analyses (performed at the Harvard or Yale facilities).
Table 1.
Ghrelin substrate sequences and apparent Km measurements.
| Substrate | Sequence | Km (apparent), μM | Vmax (apparent) (nmol/min/mg) |
|---|---|---|---|
| Ghrelin27 | GSSFLSPEHQRVQQRKESKKPPAKLQPK (Biotin)G | 3.5 ± 0.60 | 230 ± 10 |
| Dap3-Ghrelin27 | GS(Dap)FLSPEHQRVQQRKESKKPPAKLQPK (Biotin)G | 3.4 ± 0.61 | 85 ± 5 |
| Ghrelin10 | GSSFLSPEHQ(Ahx)K(Biotin)G | 30.4 ± 5.1 | 160 ± 10 |
| Dap3-Ghrelin10 | GS(Dap)FLSPEHQ(Ahx)K(Biotin)G | 33.9 ± 9.6 | 74 ± 8 |
| Ghrelin10-Tat | GSSFLSPEHQ(Ahx) K(Biotin)YGRKKRRQRRR | 0.68 ± 0.09 | 120 ± 50 |
| Octanoyl-CoA | (vs 10 μM Ghrelin27) | 0.44 ± 0.04 | 250 ± 8 |
| Octanoyl-CoA | (vs 80 μM Ghrelin10) | 0.81 ± 0.04 | 190 ± 4 |
All reactions were carried out for 1 min at 30°C with 25 μg microsome protein (total 2 ng GOAT, quantified against a 50 kDa 3xFlag-tagged standard protein, not shown) and 50 μM palmitoyl-CoA. 1 μM octanoyl-CoA was used in the ghrelin measurements. Ahx=amino-hexanoic acid; K(Biotin) signifies that the biotin is attached to the epsilon amino group.
Microsomal Ghrelin Octanoyltransferase Assay
The assay was performed similarly to previously described methods [8, 25, 26], with modification. Microsomes were thawed on ice, diluted in cold HBS, passed 10 times through a 25 gauge needle, and aliquoted into pre-chilled tubes. Unless otherwise indicated, each 50 μl reaction in HBS (50 mM HEPES pH 7.0, 150 mM NaCl) contained 25 μg microsome protein, was pre-incubated at 30°C for 5 min, and then incubated 1 min at 30°C with 10 μM Ghrelin27, 50 μM palmitoyl-CoA (Avanti Polar Lipids, Alabaster, AL), and 1 μM 3H-octanoyl-CoA (60–90 Ci/mmol (American Radiolabeled Chemicals, St. Louis, MO), diluted 1:20 with nonradioactive octanoyl-CoA (Avanti)) such that the final specific activity of octanoyl-CoA was 3–4.5 Ci/mmol. All components of the assay were pre-incubated at 30°C for at least 5 min. Reactions were quenched and solubilized by adding 1 ml 2% SDS in TBS (50mM Tris pH 7.4, 150mM NaCl) containing 10 μl Pierce Streptavidin Plus UltraLink resin (Thermo) and mixed for at least 15 min. For assays containing ghrelin10-Biotin, 37.5 μl resin was used. Beads were washed with 25 ml TBS + 0.1% SDS on small columns (Bio-Rad, Hercules, CA) using a vacuum manifold and then analyzed by scintillation counting. For detergent compatibility assays, detergents were added to microsomes for the 5 min preincubation step.
Ghrelin Octanoyltransferase Assays at Varying pH
The microsomal ghrelin octanoyltransferase assay above was modified as follows: each microsomal aliquot was diluted and homogenized in 150 mM NaCl, 250 mM bis-Tris propane to achieve the pH of interest (6–9). Each reaction contained 50 μg microsome protein, 10 μM C-terminally biotin-tagged human ghrelin-10 (ghrelin10-Biotin), 50 μM palmitoyl-CoA (Avanti), and 1 μM 3H-octanoyl-CoA (radioactive diluted 1:20 with nonradioactive)). The final amount of 50 mM HEPES pH 7.0 from the frozen microsomal aliquot was 2 μl. Reactions were quenched and solubilized by adding 1 ml 2% SDS in 100 mM Tris pH 7.0, 150 mM NaCl containing 37.5 μl Pierce Streptavidin Plus UltraLink Resin (Thermo) and bound for >15 min. Error in the ratio of rates was calculated using the formula V(xy)=X2V(y)+Y2V(x)+V(x)V(y)[27].
Chemical Reactivity of Acyl Ghrelin Analogs with Hydroxylamine or Hydrazine
Washed ghrelin assay columns were capped and incubated overnight (~12 h) with 1 mL freshly-prepared 1 M hydroxylamine pH 8.0, 1 M hydrazine pH 8.0, or 1 M MES (2-(N-morpholino)ethanesulfonic acid) pH 6.0 as a control at room temperature. The column mixtures were eluted and washed with an additional 1 mL of the same buffer, and the eluents were combined and analyzed by scintillation counting as were the washed beads as described above.
Detergent Solubilization of GOAT
To test for detergents compatible with GOAT octanoylation, we screened initially at 1.5 × critical micelle concentration and at 1 mM for the following detergents: Anzergent 3–14, APO 10, Big Chap, Brij35, C13E8, CHAPS, CHAPSO, Cholate, CycloFos 6, CycloFos 7, Cymal 6, Cymal 7, Cy-TripGlu[28], Deoxy Big Chap, Deoxycholate, Digitonin, Dodecyl Maltoside, Fos-Choline-12, Fos-Choline-13, Fos-Choline-16, GDN [29], GNG-3 (Glucose Neopentyl Glycol)[30], Hexadecyl Maltoside, LysoFos-Choline 14, LysoFos-Choline 18, MNG-3 (Maltose Neopentyl Glycol)[31], Octyl Glucoside, Ph-TripGlu[28], Sucrose Monododecanoate, Taurocholate, TDAO (Tetradecyl Dimethylamine Oxide), Tetradecyl Dimethyl Glycine, Tetradecyl Maltoside, TRIPAO[32], and Triton X-100. Mixed micelles of cholesteryl hemisuccinate (CHS) plus CHAPS, MNG-3 (Lauryl Maltose Neopentyl Glycol), or Fos-Choline-16 were made by mixing detergent:CHS at ratios of 5:1, 10:1, and 20:1 (w/w), respectively, and added analogously at varying concentrations. Detergents found to be compatible with octanoyltransfer above the CMC were next tested by solubilization of microsomes prior to assay; solubilization was accomplished for 1 h with end-over-end mixing at 4°C and clearing by centrifugation for 30 min at 100,000 × g. No conditions were found in which solubilized mixtures retained detectable activity. GOAT-TEV-3xFlag was purified from SF9 microsomes in Fos-Choline-16 (FC-16), as described [8], with final elution in 0.0008% FC-16 (1.5 × critical micelle concentration (CMC)), or purified analogously using 1% n-dodecyl-β-D-maltoside (DDM). Purified GOAT was inactive, with or without addition of control microsomes. Purified GOAT solubilized in FC-16 was exchanged during Flag affinity into a number of the above detergents at 1.5 × CMC and was inactive in all cases. All available detergents were purchased from Anatrace (Maumee, OH), digitonin was from Calbiochem (now EMD Millipore, Billerica MA), and GDN was a generous gift from Pil-Seok Chae.
Results
In Vitro Ghrelin Octanoyltransferase Assay Optimization
A previously published ghrelin octanoylation assay [10] from members of our group used a microsomal preparation from human embryonic kidney cells expressing mouse GOAT. This preparation was suboptimal for detailed enzymologic characterization due to low signal to noise ratio and low conversion of 3H-octanoyl-CoA to 3H-octanoyl ghrelin. In comparison, using an analogous preparation made from insect cells infected with baculovirus expressing mouse GOAT, Yang et al. [26] achieved approximately 100-fold more signal with no apparent increase in background. Therefore, we prepared recombinant baculovirus expressing mouse GOAT in three varieties: untagged, C-terminal 3xFlag tag, and C-terminal 3xFlag tag with N-terminal His10 tag, infected SF9 cells, and prepared microsomes 48 hours later (Figure 1A). Control microsomes were prepared from cells infected with virus produced from empty-vector alone.
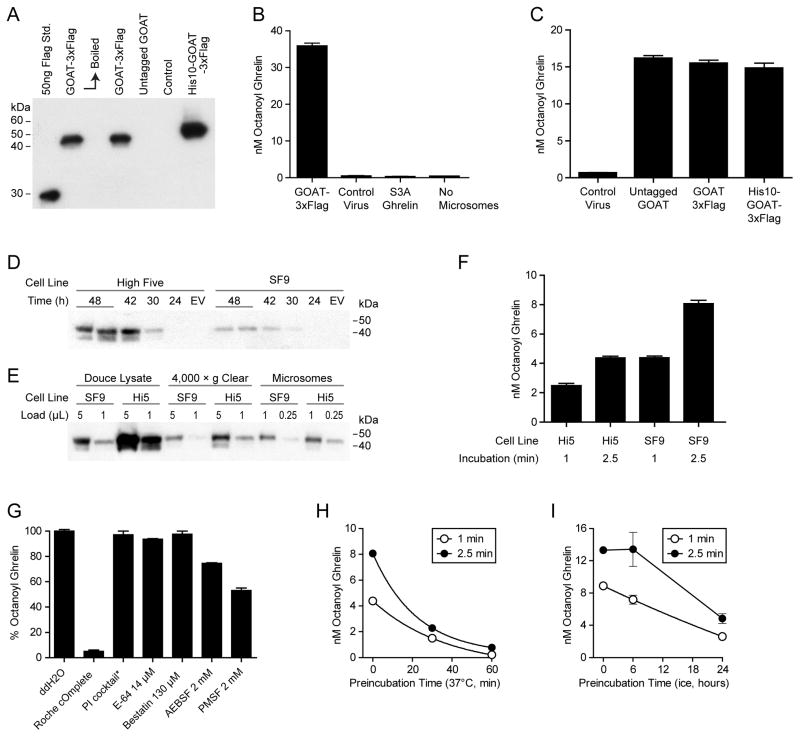
Figure 1. GOAT octanoylation assay establishment.
(A) Anti-Flag immunoblot of SF9 cells expressing various tagged GOAT constructs. As previously reported with GOAT expressed in human cells, boiling (10 min at 100°C) caused aggregation of GOAT and loss of signal. Control cells are infected with virus made from empty vector.
(B) GOAT octanoylation assay. Each 50 μl reaction was incubated for 5 min at 37°C with 50 μg microsome protein, 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA, 10 μM Ghrelin27. Microsomes made with control virus made from empty vector and Ghrelin27-S3A are shown as controls.
(C) Activity of microsomes containing untagged GOAT, N-GOAT-3xFlag-C, and N-His10-GOAT-3xFlag-C under the same conditions as (B).
(D) Anti-Flag immunoblot of High Five and SF9 cells infected with GOAT-3xFlag virus for the indicated times. EV=virus made with empty vector, 48 hours. Each lane contains the equivalent of 20 μl suspension culture.
(E) Microsome preparation from 1 L cultures of SF9 and High Five (Hi5) cells. Loading shows two different amounts at each step and an equivalent fraction of the total is shown at each step.
(F) 25 μg microsome protein from High Five or SF9 cells were incubated for the indicated time at 37°C with 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA, 10 μM Ghrelin27.
(G) The indicated protease inhibitors were pre-incubated with GOAT microsomes for 5 min before 1 min assay. PI cocktail: 2 μg/mL Leupeptin, Aprotinin, Pepstatin A, 2mM EGTA.
(H, I) GOAT microsomes stability. Microsomes were pre-incubated at the 37°C or on ice, respectively, at 95% of final assay concentration for the indicated times and then assayed for 1 or 2.5 min.
Acyltransferase assays with this SF9 microsomal GOAT preparation (Figure 1B) [10, 22] showed robust conversion to product, approximately 500-fold greater than that with the HEK-293 microsomal preparation and ~100-fold signal over background. This assay uses biotinylated human ghrelin (Ghrelin27-biotin) in which the biotin is attached to a C-terminal Lys and 3H-octanoyl-CoA and is comparable to that reported for proghrelin-His8 [33]. Radioactive acyl-ghrelin is isolated used streptavidin beads to separate it from unreacted octanoyl-CoA and hydrolyzed octanoate. Ghrelin containing a Ser3Ala mutation showed undetectable (background) levels of acyl transfer, despite the presence of serines at positions 2 and 6, recapitulating the known specificity of GOAT [7, 33]. Untagged and two different tagged GOAT preparations showed similar acyltransferase activity (Figure 1C).
High Five cells are an alternate lepidopteran cell line used with baculoviral expression systems that can sometimes express more recombinant protein than SF9 cells [34]. We therefore expressed GOAT-3xFlag in both High Five and SF9 cells. Optimal protein expression occurred 48 hours after infection, and higher in High Five than SF9 cells (Figure 1D). Microsomes made from both cell lines (Figure 1E) were assayed for 1 min and 2.5 min (Figure 1F), and although SF9 microsomes contained less GOAT, they were more active. Therefore, SF9 cells were selected for further use.
We also systematically screened protease inhibitor conditions to determine how they impacted GOAT activity (Figure 1G). The commercial protease inhibitor tablet (Roche) greatly inhibited GOAT activity compared with our standard cocktail (leupeptin, aprotinin, peptstatin A, EGTA). There was modest inhibition from the serine protease inhibitors AEBSF and PMSF. We next assayed the stability of microsomal GOAT activity on standing. With preincubation at 37°C, there was an apparent exponential decay in signal with t1/2 of approximately 20 min (Figure 1H). On ice, there was little change in signal after 6 hours, and approximately 30% of activity remained after 24 hours (Figure 1I). Efforts to obtain detergent-solubilized GOAT activity were unsuccessful (see Materials and Methods).
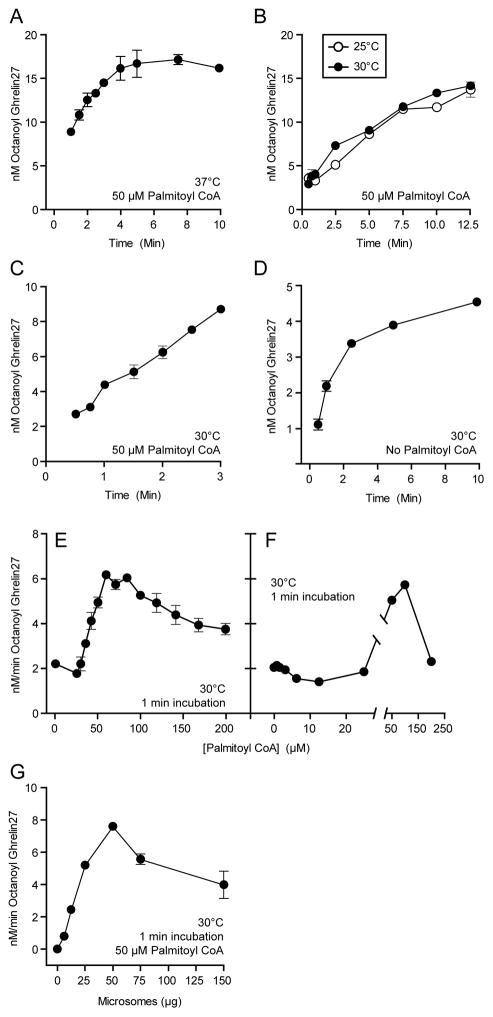
Prior studies revealed that GOAT activity rapidly diminishes over time [33]. This was somewhat ameliorated by the addition of 50 μM palmitoyl-CoA to limit microsomal octanoyl-CoA hydrolysis (<25% under the conditions of the assay [33]). Our standard assay, which includes 50 μM palmitoyl-CoA, shows a reduction in activity as a function of time in less than 2 minutes (Figure 2). This non-linearity persisted despite attempts to stabilize the enzyme by reducing the temperature or adjusting palmitoyl-CoA concentration. We employed the shortest practical time-point of 1 min, to approximate initial conditions for further studies. Under these conditions, there is a linear relationship between octanoyltransferase activity and the amount of microsomes added up to 25 μg (0.5ug/μL) as shown in Figure 2G. We therefore used these conditions to approximate steady-state kinetic parameters as discussed below.
Figure 2. GOAT octanoylation assay optimization.
(A) Reaction mixtures containing 10 μM Ghrelin27, 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA, and 25 μg microsome protein were incubated at 37°C for the indicated time and then quenched in 2% SDS.
(B) Activity over time for 10 μM Ghrelin27 at 25°C and 30°C.
(C) Activity over time for 10 μM Ghrelin27 at 30°C, with 50 μM palmitoyl-CoA in the reaction mixture.
(D) Activity over time for 10 μM Ghrelin27 at 30°C, without 50 μM palmitoyl-CoA in the reaction mixture.
(E,F) Activity in 1 min assay at 30°C with indicated concentration of palmitoyl-CoA. Error bars in F (range of duplicates) are smaller than the data points.
(G) Activity in 1 min at 30°C assay containing 50 μM palmitoyl-CoA with the indicated amount of microsome protein added to the reaction mixture.
Kinetic Measurements and Substrate Structure-Activity Relationships
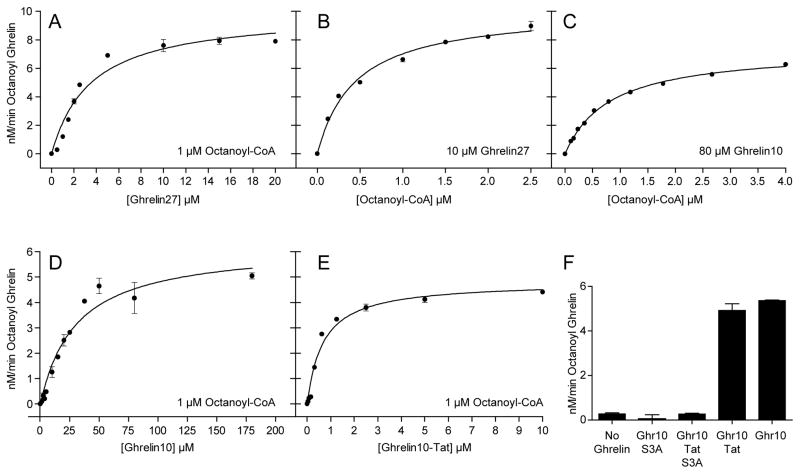
The GOAT reaction demonstrated apparent Michaelis-Menten kinetics with respect to the substrates ghrelin and octanoyl-CoA (Figure 3A, 3B). The apparent Km values for octanoyl-CoA and Ghrelin27 were 0.44 and 3.5 μM, respectively, comparable to published values 0.6 and 6 μM [33] (the latter is for proghrelin-His8; proghrelin is likely the natural substrate for GOAT [22]). The apparent Km for octanoyl-CoA was in the range of 0.4–0.8 μM depending on the ghrelin substrate.
Figure 3. Kinetic measurements for ghrelin substrates.
Each assay mixture was incubated at 30°C for 1 min in the presence of 50 μM palmitoyl-CoA with 25 μg microsome protein. Solid lines are best-fit to the Michaelis-Menten equation, and Km values are shown in Table 1. (A) Ghrelin27 with 1 μM octanoyl-CoA. (B) Octanoyl-CoA with 10 μM Ghrelin27. (C) Octanoyl-CoA with 80 μM Ghrelin10. (D) Ghrelin10 with 1 μM octanoyl-CoA. (E) Ghrelin10-Tat with 1 μM octanoyl-CoA. (F) Each reaction contained 50 μM of the indicated substrate; Ghr10, Ghrelin10.
The shorter substrate Ghrelin10 was efficiently octanoylated, but displayed ~ 10-fold higher apparent Km than Ghrelin27 (Figure 3D and Table 1). Ghrelin10-Tat showed ~ 5-fold lower apparent Km than Ghrelin27 (Figure 3E and Table 1). As expected, S3A ghrelin substrate mutants were not processed (Figure 3F).
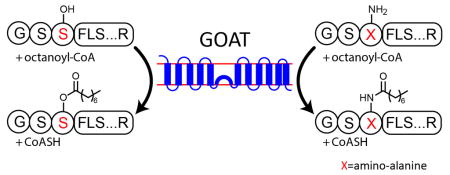
Octanoylation of Dap3-Ghrelin Analogs
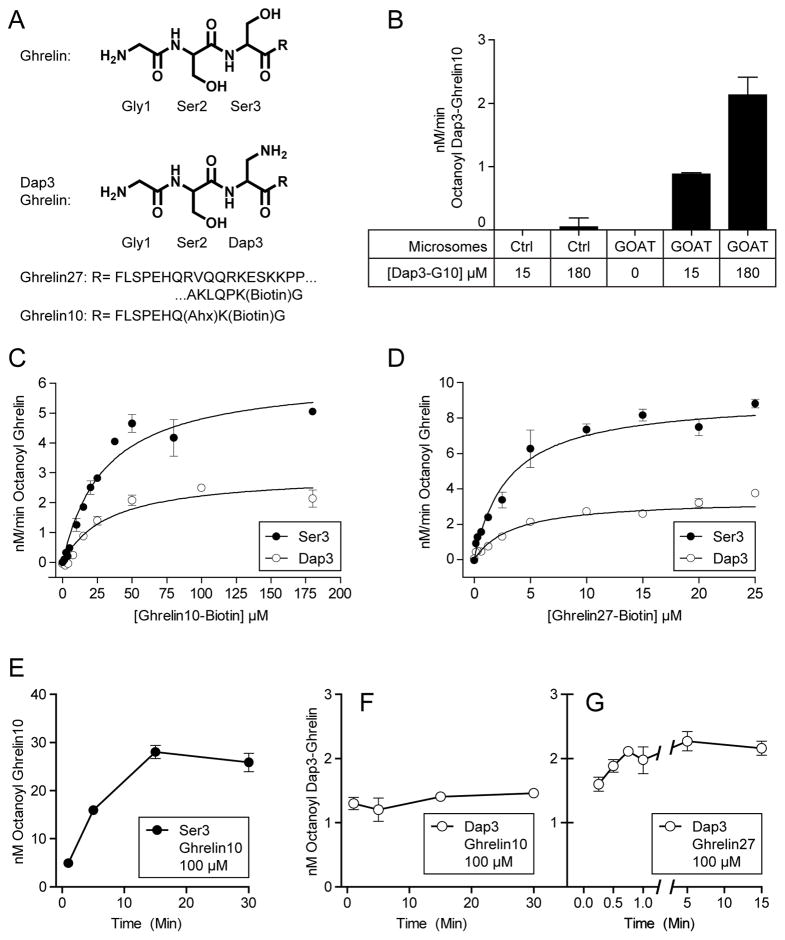
To explore the chemical flexibility of GOAT, We replaced Ser3 in Ghrelin27 and Ghrelin10 with amino-alanine (Dap), which substitutes the serine sidechain –OH with –NH2. The structure of the first 3 residues of ghrelin and the Dap3 analogs are shown in Figure 4A. Dap3-Ghrelin10 can be octanoylated in the octanoyltransferase assay in a GOAT-dependent fashion (Figure 4B). Reactions for Dap3-Ghrelin10 (Figure 4C) and Dap3-Ghrelin27 (Figure 4D) display saturation kinetics, with Km for both substrates indistinguishable from their natural Ser3 analogs (Table 1). Maximum reaction rates for both substrates was approximately 2-fold lower than for the natural Ser analogs.
Figure 4. GOAT octanoylates Dap3 substrates.
(A) Structure of Ghrelin and Dap3 (amino-alanine) analog.
(B) Octanoylation of Dap3-Ghrelin10 (Dap3-G10) requires GOAT. 25 μg GOAT or empty-vector virus control microsomes were incubated for 1 min at 30°C with 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA, and the indicated concentration of Dap3-Ghrelin10.
(C) Kinetic measurements for Ghrelin10 and Dap3-Ghrelin10 and D, kinetic measurements for Ghrelin27 and Dap3-Ghrelin27 with 1 μM octanoyl-CoA. Solid lines are best-fit to the Michaelis-Menten equation, and Km values are shown in Table 1.
(E) Octanoylation of 100 μM Ser3 Ghrelin10 over time. Each mixture contained 25 μg membrane protein, 1 μM octanoyl-CoA, and 50 μM palmitoyl-CoA.
(F,G) Octanoylation of Dap3-Ghrelin10 (100 μM) and Dap3-Ghrelin27 (10 μM) over time, respectively.
Acyl ghrelin analog inhibition has previously been reported for GOAT, with more potent inhibition by amide-linked octanoyl ghrelin pentapeptides than for ester-linked peptides[33]. Therefore, we compared the formation of product over time for Ghrelin10 (Figure 4E), Dap3-Ghrelin10 (Figure 4F), and Dap3-Ghrelin27 (Figure 4G) under the standard assay conditions (30°C, 25 μg microsome protein, 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA). The Ghrelin10 (Ser3) reaction continues to increase in signal for 15 min, with linearity for at least the first 5 min; similar results were seen with Ghrelin27 (Figure 2B, C). In contrast, no additional signal is seen for Dap3-Ghrelin10 and Dap3-Ghrelin27 after 1 min (Figure 4 F,G), and therefore it appears that there is severe product inhibition for amine but less so for hydroxyl substrates.
Chemical reactivity of Dap3-Ghrelin Octanoylation Product
While the radioactive incorporation assay was consistent with GOAT-catalyzed amide bond formation of the Dap3-ghrelin substrates, it was formally possible that the presence of the amino group at the 3-position leads to site-switching to afford Ser ester products. To assess this possibility, we employed the nucleophilic amines hydroxylamine and hydrazine that can readily cleave ester but not amide functionalities [7, 27, 35]. As a positive control, strepavidin-immobilized biotin-tagged 3H-octanoylated Ghrelin10 and Ghrelin27 were treated with 1M hydroxylamine or 1M hydrazine. This induced substantial loss of the radioactivity into the supernatant, expected because of aminolysis of the ester linkages. In comparison, no detectable cleavage occured in the 1M MES-treated control reaction (Figure 5A). Because we had incomplete cleavage with hydroxylamine for octanoylated Ghrelin27, we selected hydrazine for the experiment with Ghrelin10 (Ser3) and Dap3-Ghrelin10 (Figure 5B). Nearly all the [3H]-octanoate was released into the supernatant for octanoylated-Ghrelin10, whereas octanoylated-Dap3-Ghrelin resisted hydrazine treatment. These results confirm that the octanoylated Dap3-adducts are indeed amide rather than ester modified [34].
Figure 5. Dap3 ghrelin substrates Form an Octanoyl-Amide.
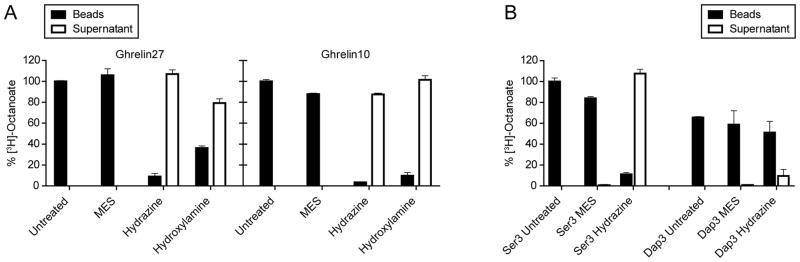
(A) Hydrazine and hydroxylamine cleavage of octanoylated ghrelin. Reaction mixtures were incubated 1 min at 30°C with 10 μM Ghrelin27 or 100 μM Ghrelin10, quenched in 2% SDS, bound to streptavidin resin, and washed. Capped columns were then incubated overnight with 1 mL of 1M hydrazine or hydroxylamine; 1 M MES buffer was used as a control. Beads and supernatants were then scintillation counted.
(B) Ghrelin10 (Ser3) and Dap3-Ghrelin10 were treated with hydrazine or MES as in (A).
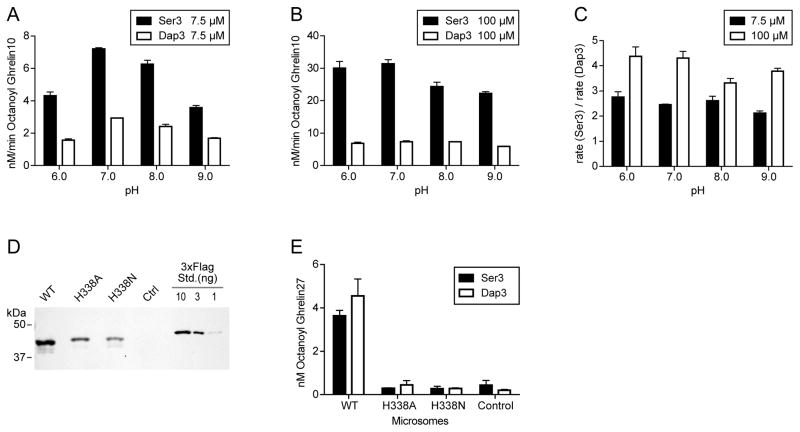
pH effects of Ser3 and Dap3 Ghrelin Octanoylation
Because the pKa of the sidechain of peptide-incorporated amino-Ala (RNH3+ to RNH2 pKa is ~8 [36]) is dramatically different from that of the sidechain of Ser (ROH to RO- is ~13 [37]), we hypothesized that the GOAT-catalyzed acyl transfer reactions with the two substrates might show sharply different pH sensitivities if proton transfer of the nucleophile were rate-determining. We measured the rate of ghrelin octanoylation for Ser3-containing Ghrelin10 and Dap3-Ghrelin10 at 7.5 μM (sub-saturating), and 100 μM (saturating) as shown in Figure 6A–C. As shown, the rates for both substrates are relatively insensitive to pH throughought the range 6–9, with no more than a 2-fold variation for each substrate. These results suggest that deprotonation of the nucleophile does not appear to be rate-limiting for the overall GOAT reaction.
Figure 6. GOAT-catalyzed reactions of Ser3 and Dap3 Ghrelin10 substrates at pH 6–9.
(A–C), Reactions were carried out for 1 min in 100 mM Bis-Tris Propane at the indicated pH using Ghrelin10 (Ser3) and Dap3-Ghrelin10. Rates for Ser3 and Dap3 at 7.5 μM are shown in (A) and 100 μM in (B). 100 μM is saturating conditions (see Figure 4C). The ratio of the rate of the two substrates across pH 6–9 is shown in (C). (D) Expression of mouse GOAT in SF9 cells with C-terminal 3xFlag tag and the indicated mutations. 10 μg microsomes (BCA) were loaded in each lane. Ctrl, control microsomes made with empty baculovirus. 3xFlag Std. is purified CK2α-3xFlag. (E) Reactions were carried out for 5 min in 100 mM Bis-Tris Propane at pH 8.0 with 10 μM Ghrelin27 (Ser3) or Dap3-Ghrelin27 with 50 μg total microsome protein.
His338 and GOAT assays with Ser3 and Dap3-Ghrelin27
GOATs bearing alanine mutants of MBOAT-invariant histidine-338 are inactive with Ser3-ghrelin substrates, and His338 has been proposed to function as a catalytic base [6, 7, 33]. Given the increased chemical reactivity of the amino-Ala and its moderate sidechain pKa, we hypothesized that it might circumvent the need for the catalytic base. While H338A and H338N mutants expressed at similar levels to WT (Figure 6D) and were inactive with Ser3 ghrelin, neither H338A nor H338N mutant showed detectable acyl transfer with amino-Ala Dap3-Ghrelin27 substrate at pH 8.0 (Figure 6E) or pH 9.0 (not shown). This argues somewhat against a role for H338 as a catalytic base.
Discussion
In this study we provide new insights into GOAT’s catalytic mechanism, building on a number of contemporary studies [10, 20, 21, 33]. Using recombinant baculovirus expressing mouse GOAT in insect cells and synthetic biotin-tagged ghrelin analogs ranging from 10–27 residues in length, we identified conditions which have allowed us to approximate initial steady-state conditions. However, the inability to have a solubilized, purified catalytically active system and likely the complexity of the GOAT-catalyzed process itself still hamper a more precise enzymologic analysis.
That Ghrelin27 shows a 10-fold lower apparent Km relative to that of Ghrelin10, suggests that the C-terminal peptide sequence contributes to binding with GOAT, the lipid bilayer, or both. These 17 additional residues include 3 Lys, 2 Arg, and 1 Glu for a net +4 positive charge, which may contribute to enhanced apparent affinity through electrostatic interactions. Supporting this hypothesis, when these 17 residues were replaced with the Tat sequence (which has a net positive charge of +8), the apparent Km is lowered an additional 5-fold. This was also fortuitous for the design of our inhibitor GO-CoA-Tat[10].
The ability to produce ghrlein substrates using solid phase peptide synthesis allowed us to explore the chemistry of acylation by comparing peptides bearing the natural Ser and artificial amino-alanine (Dap) at position 3, substituting the serine hydroxyl for an amine. We demonstrate that GOAT has the ability to octanoylate either hydroxyl or amine acceptor, forming an ester or amide bond (see below). The Km for Ser3 and Dap3 versions of both ghrelin substrates were indistinguishable, but the Dap3 reaction was nonlinear within the first 30 seconds, resulting in a lower apparent rate due to severe apparent product inhibiton.
We compared the rates of Ser3 and Dap3 substrates from pH 6.0–9.0. The serine hydroxyl has a pKa of approximately 13 and will not change protonation over this range. In contrast, the pKa of the β-NH2 group in Dap is approximately 8 [36, 37]. We note that sidechain pKa may be shifted by a number of pH units in the microenvironment of an enzyme active site [38] and may also be reduced in the hydrophobic context of microsomes. Thus, the combination of the rate similarity for amine and hydroxy nucleophilic substrates and the insensitivity to pH suggests that the chemical step is unlikely to be rate-limiting in the catalytic mechanism. As discussed previously, transporting and positioning octanoyl-CoA through the lipid bilayer may be the slow step for the overall GOAT reaction and it is also possible that an enzyme conformational change or product release is the slow step.
MBOAT-invariant His338-mutant GOAT is unable to acylate Ser3-Ghrelin [6, 7, 33]. However, because some Dap3 amine sidechains are likely to be substantially deprotonated at pH 8–9 upon entering GOAT’s active site (subject to local environmental effects), we hypothesized if the H338 functioned as a catalytic base, H338A or H338N GOAT might retain catalytic activity with Dap3-Ghrelin. However, there was no acyl transfer within the limits of detection of this assay, arguing somewhat against a role for H338 as a catalytic base. It is still possible that His338 could be important in hydrogen bonding the peptide substrate and orienting it for catalysis. It is also plausible that the amine substrate could use His338 for proton transfer in a late stage of acyl transfer to prevent the reverse reaction. Further structural studies are needed to shed light on this issue.
The ability of GOAT to funcation as both an O- and N-acyltransferase is a property shared with a number of other O-acyltransferases, but uncommon among N-acyltransferases. The O-acyltransferases carnitine acetyltransferase, carnitine palmitoyltransferase I, and carntine octanoyltransferase can all process amino-substrates, albeit at reduced rate; carntine palmitoyltransferase II (CPTII) showed no reactivity. As seen with GOAT, amide-linked product analogs are potent inhibitors of these enzymes [33, 39, 40]. LpxA from gram negative bacteria in which Lipid A contains only O-linked fatty acids can acylate either UDP-GlcNac or its amine analog UDP-GlcNac3N. In contrast, LpxA from bacteria containing only N-linked fatty acids cannot accept the hydroxyl substrate UDP-GlcNac [41]. Aminoglycoside N-acetyltransferase from mycobacterium tuberculosis AAC(2′)-Ic acylates aminoglycoside antibiotics with 2′ amino or 2′ hydroxyl groups, with higher efficiency for 2′-amino substrates. In spite of the name, the natural substrate of this enzyme is unknown, and therefore could be either hydroxyl or amine[42]. The structure of AAC(2′)-Ic supports a reaction mechanism where the 2′ amino or hydroxyl is positioned for direct nucleophilic attack on acetyl-CoA[43].
In contrast with the versatility in reaction chemistry of the majority of O-acyltransferases, a number of N-acyltransferases cannot acylate the hydroxyl analogs of their natural substrates, including serotonin N-acetyltransferase from sheep and Drosophila, related Drosophila enzyme AANATL7, tetrahydrodipicolinate N-succinyltransferase, and mouse glycine N-acyltransferase [44–48]. In all of these cases, hydroxyl analogs were found to be dead-end inhibitors. It was hypothesized previously that these differences in reactivity might be due to mechanistic requirements for increased nucleophilicity of the amine substrate[45]. A corollary to this is that O-selectivity may be more difficult to achieve than N-selectivity, and therefore a deeper understanding of the mechanistic differences of these enzymes will have implications for engineering selective reactivity. This selectivity appears to be present in CPTII and was also demonstrated for the polyketide associated acyltransferase PapA5, which acylates the hydroxyl substrate octanol but not octylamine [49].
Highlights.
We measure enzyme kinetic parameters for ghrelin O-acyltransferase (GOAT)
We show that GOAT can acylate ghrelin substrate containing amino-Ala replacing Ser3
GOAT catalysis is relatively pH insensitive for natural and amino-Ala substrates
His-338, while important, may not be a catalytic base for GOAT reactions
Acknowledgments
We thank Pil-Seok Chae, Samuel Gellman, Dan Leahy, Jennifer Kavran, Lily Raines, and Matthew Ward for gifts of detergents and helpful discussion. We thank the NIH, Pfeiffer Foundation, Kaufman Foundation, and Keck Foundation for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschöp MH. GOAT links dietary lipids with the endocrine control of energy balance. Nature medicine. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castaneda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Dieguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell metabolism. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa A, Speaker RB, 3rd, Lindberg I. Enzymatic characterization of a human acyltransferase activity. PLoS One. 2009;4:e5426. doi: 10.1371/journal.pone.0005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MS, Ruch TR, Hsiao PY, Hwang Y, Zhang P, Dai L, Huang CR, Berndsen CE, Kim MS, Pandey A, Wolberger C, Marmorstein R, Machamer C, Boeke JD, Cole PA. Architectural organization of the metabolic regulatory enzyme ghrelin O-acyltransferase. J Biol Chem. 2013;288:32211–32228. doi: 10.1074/jbc.M113.510313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012 doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschöp MH, Boeke JD, Cole PA. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teubner BJ, Garretson JT, Hwang Y, Cole PA, Bartness TJ. Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior. Hormones and behavior. 2013;63:667–673. doi: 10.1016/j.yhbeh.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci U S A. 2015;112:1226–1231. doi: 10.1073/pnas.1423643112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi CX, Heppner KM, Kirchner H, Tong J, Bielohuby M, Gaylinn BD, Muller TD, Bartley E, Davis HW, Zhao Y, Joseph A, Kruthaupt T, Ottaway N, Kabra D, Habegger KM, Benoit SC, Bidlingmaier M, Thorner MO, Perez-Tilve D, Tschöp MH, Pfluger PT. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends in biochemical sciences. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 16.Matevossian A, Resh MD. Membrane topology of hedgehog acyltransferase. J Biol Chem. 2015;290:2235–2243. doi: 10.1074/jbc.M114.625764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo ZY, Lin S, Heinen JA, Chang CC, Chang TY. The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J Biol Chem. 2005;280:37814–37826. doi: 10.1074/jbc.M508384200. [DOI] [PubMed] [Google Scholar]
- 18.Pagac M, de la Mora HV, Duperrex C, Roubaty C, Vionnet C, Conzelmann A. Topology of 1-acyl-sn-glycerol-3-phosphate acyltransferases SLC1 and ALE1 and related membrane-bound O-acyltransferases (MBOATs) of Saccharomyces cerevisiae. J Biol Chem. 2011;286:36438–36447. doi: 10.1074/jbc.M111.256511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buglino JA, Resh MD. Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog. PLoS One. 2010;5:e11195. doi: 10.1371/journal.pone.0011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darling JE, Prybolsky EP, Sieburg M, Hougland JL. A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin O-acyltransferase. Anal Biochem. 2013;437:68–76. doi: 10.1016/j.ab.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Darling JE, Zhao F, Loftus RJ, Patton LM, Gibbs RA, Hougland JL. Structure-activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry. 2015;54:1100–1110. doi: 10.1021/bi5010359. [DOI] [PubMed] [Google Scholar]
- 22.Taylor MS, Hwang Y, Hsiao PY, Boeke JD, Cole PA. Ghrelin O-acyltransferase assays and inhibition. Methods in enzymology. 2012;514:205–228. doi: 10.1016/B978-0-12-381272-8.00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner AL, Janda KD. A small molecule antagonist of ghrelin O-acyltransferase (GOAT) Chem Commun (Camb) 2011;47:7512–7514. doi: 10.1039/c1cc11817j. [DOI] [PubMed] [Google Scholar]
- 24.Garner AL, Janda KD. cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT) Angewandte Chemie. 2010;49:9630–9634. doi: 10.1002/anie.201003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor MS, Hwang Y, Hsiao PY, Boeke JD, Cole PA. Ghrelin O-acyltransferase assays and inhibition. Methods in enzymology. 2012;514:205–228. doi: 10.1016/B978-0-12-381272-8.00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci U S A. 2008;105:10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bizzozero OA. Chemical analysis of acylation sites and species. Methods in enzymology. 1995;250:361–379. doi: 10.1016/0076-6879(95)50085-5. [DOI] [PubMed] [Google Scholar]
- 28.Chae PS, Wander MJ, Bowling AP, Laible PD, Gellman SH. Glycotripod amphiphiles for solubilization and stabilization of a membrane-protein superassembly: importance of branching in the hydrophilic portion. Chembiochem: a European journal of chemical biology. 2008;9:1706–1709. doi: 10.1002/cbic.200800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, Gellman SH. A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chemistry. 2012;18:9485–9490. doi: 10.1002/chem.201200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae PS, Rana RR, Gotfryd K, Rasmussen SG, Kruse AC, Cho KH, Capaldi S, Carlsson E, Kobilka B, Loland CJ, Gether U, Banerjee S, Byrne B, Lee JK, Gellman SH. Glucose-neopentyl glycol (GNG) amphiphiles for membrane protein study. Chem Commun (Camb) 2013;49:2287–2289. doi: 10.1039/c2cc36844g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B, Gellman SH. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nature methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chae PS, Guzei IA, Gellman SH. Crystallographic characterization of N-oxide tripod amphiphiles. Journal of the American Chemical Society. 2010;132:1953–1959. doi: 10.1021/ja9085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci U S A. 2008;105:10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honegger A, Hughes GJ, Wilson KJ. Chemical modification of peptides by hydrazine. The Biochemical journal. 1981;199:53–59. doi: 10.1042/bj1990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigst TA, Antipova A, Mayr H. Nucleophilic reactivities of hydrazines and amines: the futile search for the alpha-effect in hydrazine reactivities. The Journal of organic chemistry. 2012;77:8142–8155. doi: 10.1021/jo301497g. [DOI] [PubMed] [Google Scholar]
- 36.Lan Y, Langlet-Bertin B, Abbate V, Vermeer LS, Kong X, Sullivan KE, Leborgne C, Scherman D, Hider RC, Drake AF, Bansal SS, Kichler A, Mason AJ. Incorporation of 2,3-diaminopropionic acid into linear cationic amphipathic peptides produces pH-sensitive vectors. Chembiochem: a European journal of chemical biology. 2010;11:1266–1272. doi: 10.1002/cbic.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruice TC, Fife TH, Bruno JJ, Brandon NE. Hydroxyl group catalysis. II. The reactivity of the hydroxyl group of serine. The nucleophilicity of alcohols and the ease of hydrolysis of their acetyl esters as related to their pKa. Biochemistry. 1962;1:7–12. doi: 10.1021/bi00907a002. [DOI] [PubMed] [Google Scholar]
- 38.Harris TK, Turner GJ. Structural basis of perturbed pKa values of catalytic groups in enzyme active sites. IUBMB life. 2002;53:85–98. doi: 10.1080/15216540211468. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins DL, Griffith OW. DL-aminocarnitine and acetyl-DL-aminocarnitine. Potent inhibitors of carnitine acyltransferases and hepatic triglyceride catabolism. J Biol Chem. 1985;260:14748–14755. [PubMed] [Google Scholar]
- 40.Murthy MS, Ramsay RR, Pande SV. Acyl-CoA chain length affects the specificity of various carnitine palmitoyltransferases with respect to carnitine analogues. Possible application in the discrimination of different carnitine palmitoyltransferase activities. The Biochemical journal. 1990;267:273–276. doi: 10.1042/bj2670273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweet CR, Williams AH, Karbarz MJ, Werts C, Kalb SR, Cotter RJ, Raetz CR. Enzymatic synthesis of lipid A molecules with four amide-linked acyl chains. LpxA acyltransferases selective for an analog of UDP-N-acetylglucosamine in which an amine replaces the 3″-hydroxyl group. J Biol Chem. 2004;279:25411–25419. doi: 10.1074/jbc.M400597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde SS, Javid-Majd F, Blanchard JS. Overexpression and mechanistic analysis of chromosomally encoded aminoglycoside 2′-N-acetyltransferase (AAC(2′)-Ic) from Mycobacterium tuberculosis. J Biol Chem. 2001;276:45876–45881. doi: 10.1074/jbc.M108810200. [DOI] [PubMed] [Google Scholar]
- 43.Vetting MW, Hegde SS, Javid-Majd F, Blanchard JS, Roderick SL. Aminoglycoside 2′-N-acetyltransferase from Mycobacterium tuberculosis in complex with coenzyme A and aminoglycoside substrates. Nature structural biology. 2002;9:653–658. doi: 10.1038/nsb830. [DOI] [PubMed] [Google Scholar]
- 44.Berges DA, DeWolf WE, Jr, Dunn GL, Newman DJ, Schmidt SJ, Taggart JJ, Gilvarg C. Studies on the active site of succinyl-CoA:tetrahydrodipicolinate N-succinyltransferase. Characterization using analogs of tetrahydrodipicolinate. J Biol Chem. 1986;261:6160–6167. [PubMed] [Google Scholar]
- 45.De Angelis J, Gastel J, Klein DC, Cole PA. Kinetic analysis of the catalytic mechanism of serotonin N-acetyltransferase (EC 2.3.1.87) J Biol Chem. 1998;273:3045–3050. doi: 10.1074/jbc.273.5.3045. [DOI] [PubMed] [Google Scholar]
- 46.Dempsey DR, Jeffries KA, Bond JD, Carpenter AM, Rodriguez-Ospina S, Breydo L, Caswell KK, Merkler DJ. Mechanistic and structural analysis of Drosophila melanogaster arylalkylamine N-acetyltransferases. Biochemistry. 2014;53:7777–7793. doi: 10.1021/bi5006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dempsey DR, Jeffries KA, Handa S, Carpenter AM, Rodriguez-Ospina S, Breydo L, Merkler DJ. Mechanistic and Structural Analysis of a Drosophila melanogaster Enzyme, Arylalkylamine N-Acetyltransferase Like 7, an Enzyme That Catalyzes the Formation of N-Acetylarylalkylamides and N-Acetylhistamine. Biochemistry. 2015;54:2644–2658. doi: 10.1021/acs.biochem.5b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dempsey DR, Bond JD, Carpenter AM, Rodriguez Ospina S, Merkler DJ. Expression, purification, and characterization of mouse glycine N-acyltransferase in Escherichia coli. Protein expression and purification. 2014;97:23–28. doi: 10.1016/j.pep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onwueme KC, Ferreras JA, Buglino J, Lima CD, Quadri LE. Mycobacterial polyketide-associated proteins are acyltransferases: proof of principle with Mycobacterium tuberculosis PapA5. Proc Natl Acad Sci U S A. 2004;101:4608–4613. doi: 10.1073/pnas.0306928101. [DOI] [PMC free article] [PubMed] [Google Scholar]