Abstract
This study examined the extent to which subordinate dimensions of negative emotionality were genetically and environmentally distinct in a sample of 1316 twins (51% female, 85.8% Caucasian, primarily middle class, mean age = 7.87 years, SD = .93), recruited from Wisconsin hospital birth records between 1989 and 2004. Cholesky, independent pathway, and common pathway models were fitted for mother-report, father-report, and in-home observation of temperament. Although findings support the use of negative emotionality, there were heritable aspects of anger and fear not explained by a common genetic factor, and shared environmental influences common to anger and sadness but not fear. Observed fear was independent from observed anger and sadness. Distinctions support specificity in measurement when considering implications for child development.
Keywords: Temperament, negative affectivity, twin
Temperament, defined as individual differences in reactivity and regulation across affective and behavioral domains, is an important contributor to personality and adjustment in childhood and across the lifespan (Rothbart & Bates, 2006), with negative emotionality in particular widely linked to mood and behavior problems and risk for psychopathology (Lemery-Chalfant, Clifford & Swann, in press; Nigg, 2006). Although distinctions between discrete negative emotions are well-supported (Izard, 2007) and temperament research increasingly addresses proneness to discrete negative emotions as well as broad negative emotionality (e.g., Eisenberg et al., 2009), less is known about underlying relations among discrete negative emotions in childhood, including the extent to which facets of negative emotionality are genetically or environmentally related or distinct.
Behavior genetic analyses can clarify the etiological underpinnings of trait negative emotions. By considering correlations between individuals with different degrees of genetic relatedness, quantitative genetic models allow an estimation of variance within a population that can be attributed to genetic or environmental influences, including the extent to which multiple traits are related for genetic or environmental reasons (Plomin, DeFries, Knopik & Neiderhiser, 2013). If proneness to anger, sadness and fear represent different manifestations of an underlying proclivity toward negative emotion, these lower-order traits should show considerable genetic overlap; in contrast, substantial genetic influences unique to each emotion suggest that relying on broad negative emotionality risks overlooking important behavioral distinctions. Thus, we aim to use quantitative genetics to specify the etiological overlap between fear, anger and sadness at the subordinate level, and to test the extent to which genetic and environmental variance in each emotion can be accounted for by a single, common negative emotionality factor.
Temperament in Middle Childhood
Despite debate on the nature and underpinnings of temperament, theorists agree that temperamental differences often emerge early in development, are relatively stable over time and context, and are at least partly biologically-based, with some consensus on major components of temperament (Rothbart & Bates, 2006). Some theorists emphasize neurophysiological systems underlying behavioral differences, and focus on inhibition and activation (Kagan & Fox, 2006); others (e.g., Rothbart & Bates, 2006) focus on broad domains of reactivity and regulation, organized by valence and intensity. Research supports physiological differences in approach and withdrawal, with left frontal EEG asymmetry in infancy related to approach, positive affect and anger, and right frontal asymmetry linked to fear, withdrawal, and later social reticence and anxiety (Carver & Harmon-Jones, 2009; Fox, Henderson, Marshall, Nichols, & Ghera, 2005). However, evidence also exists for individual differences in broad negative reactivity, and factor analytic studies across multiple parent-report measures in middle childhood reveal components related to negative emotionality, self-regulation, and extraversion or approach (Rothbart & Bates, 2006). Further, temperament is hierarchically structured, with higher-order components made up of discrete subordinate dimensions. For instance, the negative emotionality factor of the Children’s Behavior Questionnaire (CBQ) is composed of subordinate dimensions tapping anger, fear, sadness, discomfort, and low soothability (Rothbart, Ahadi, Hershey and Fisher’s (2001).
Discrete emotions have distinct antecedents, adaptive functions, and behavioral consequences (Izard, 2007). Generally, sadness may promote withdrawal and conservation of resources after loss, fear is a response to perceived threat that may lead to avoidance or to aggression if avoidance is not possible, and anger facilitates aggression and resource acquisition (Tooby & Cosmides, 2008). Unlike fear and sadness, anger motivates approach, which may explain moderate positive relations between trait anger and positive emotionality or extraversion in some studies (Carver & Harmon-Jones, 2009). Some studies also suggest distinct cortical and subcortical regions and patterns of activation underlying discrete emotions (Vytal & Hamann, 2010), but findings are mixed (Murphy, Nimmo-Smith, & Lawrence, 2003). Whether emotions represent discrete patterns of physiological and cognitive activation (Izard, 2007) or a more general combination of affect, arousal and attribution (Russell, 1980) is unresolved, but different motivational and adaptive functions highlight the need to differentiate among emotions when predicting outcomes such as internalizing versus externalizing or aggression versus withdrawal.
Both broad negative emotionality and proneness to discrete emotions are stable over time, and broad negative emotionality predicts both internalizing and externalizing problems, although relations with externalizing are stronger and more consistent (Rothbart, Evans & Ahadi, 2000; Rothbart & Bates, 2006). However, when considered separately, anger, sadness and fear show different relations to externalizing and internalizing (Eisenberg et al., 2009). Specifically, sadness, fear, and withdrawal are stronger predictors of pure internalizing (Lemery, Essex, & Smider, 2002; Oldehinkel et al., 2007). Fear and behavioral inhibition in particular are consistently related to risk for anxiety and depression (Degnan & Fox, 2007; Karevold, Coplan, Stoolmiller, & Mathiesen, 2012), but may protect against externalizing (Schwartz, Snidman & Kagan, 1996). In contrast, anger is most strongly related to externalizing (Lemery et al., 2002; Oldehinkel et al., 2007). Along with different predictive utility, there is some evidence that subordinate dimensions of negative emotionality have different genetic and environmental architecture, but this question has yet to be examined in a multivariate framework.
Quantitative Genetics Approach
Quantitative genetic research uses structural equation modeling-based path analysis and model-fitting to decompose the total phenotypic variance in a trait into latent additive genetic (A), dominant genetic (D), shared environmental (C) and nonshared environmental (E) factors, without attempting to isolate measured genes or environmental factors contributing to variation (Plomin et al., 2013). A reflects the average effect of individual genes across the genotype, and D accounts for interaction between alleles at the same (dominance) or different (epistasis) loci. Together, A and D encompass influences that increase phenotypic similarity between individuals who are more closely genetically related. For instance, because monozygotic (MZ) twins are genetically identical, whereas dizygotic (DZ) twins share 50% of their segregating genes on average, if 100% of the resemblance in a trait was explained by additive genetic factors then phenotypic correlations between MZ twins would be expected to be approximately twice as high as DZ twin correlations. MZ correlations higher than twice DZ correlations suggest the action of D, or in some cases, rater bias. In contrast, C encompasses all influences which increase the phenotypic similarity of individuals independent of genetic relatedness. DZ correlations higher than half of MZ correlations suggest shared environmental influences, and if 100% of the resemblance in a trait were due to the shared environment, MZ and DZ correlations would be expected to be approximately equal. Finally, non-genetic factors that make individuals different from one another, including measurement error, are attributed to E. For instance, all differences between MZ twins reared in the same household can be attributed to E. Although conventions vary somewhat across studies, A, C or E components of 30% or less are commonly described as modest, 40–60% as moderate, and 70% as high (Plomin et al., 2013; Saudino, 2005).
Heritability of Temperament
Quantitative and molecular genetic studies support both additive genetic contributions to temperament and gene-environment interplay (Gagne, Vendlinski, & Goldsmith, 2009; Lemery-Chalfant, Kao, Swann, & Goldsmith, 2013). Heritability of temperament is moderate across childhood, generally 20% to 60%, and continuity in temperament is largely genetic (Goldsmith, Buss & Lemery, 1997; Saudino, 2005). However, parent-report and observed temperament show low phenotypic convergence (Rothbart & Bates, 2006) and limited genetic or environmental overlap (Gagne & Saudino, 2010). Further, some parent-report measures of temperament may overestimate heritability due to parents’ tendency to contrast one DZ twin against the other (Saudino, 2003). Only studies that model contrast effects or use measures found to be less vulnerable to such biases (e.g., the IBQ; Goldsmith et al., 1997) are reviewed here.
Parent-report of negative emotionality is moderately to highly heritable in infancy, toddlerhood and middle childhood (generally 40%–70%), with the remaining variance typically explained by nonshared environment (Goldsmith et al., 1997; Goldsmith, Lemery, Buss & Campos, 1999; Singh & Waldman, 2010; Tackett, Waldman, Van Hulle, & Lahey, 2011). Observational measures of broad negative emotion are rare in behavioral genetic studies, but one study finds a latent factor of observed anger and experimenter rated negativity to be heritable at 14 (65%) and 20 months (40%), with shared environment at 20 (38%) and 24 months (51%; Rhee et al., 2012). Heritability is similar for reported fear (56%–83%), anger (66%), and sadness (71–75%; Emde, Robinson, Corley, Nikkari, & Zahn-Waxler, 2001; Goldsmith & Lemery, 2000; Mullineaux et al., 2009), and infants’ anger and fear show substantial, largely independent genetic variance (Goldsmith et al., 1999). Observed infant and toddler anger (Emde et al., 2001; Gagne & Goldsmith, 2011), and experimenter-rated anger between four and eight (Deater-Deckard, Petrill, & Thompson, 2007) are moderately heritable (25%–38%). Some, but not all (e.g., Deater-Deckard et al., 2010) studies also show moderate shared environmental variance in reported and observed anger (Emde et al., 2001; Gagne & Goldsmith, 2011; Goldsmith et al., 1997) and reported social fear in early childhood (Goldsmith et al., 1997). Shared environmental effects for anger are interesting in light of links between anger and positive emotion as approach-related aspects of temperament (Carver & Harmon-Jones, 2009), as some studies show substantial shared environmental variance in positive emotionality (Goldsmith et al., 1997).
Shyness, behavioral inhibition and stranger fear are often assessed with laboratory observations of children’s interactions with unfamiliar adults or peers, and relatively consistently show high to moderate heritability and no significant role for the shared environment. Stranger fear in infancy is moderately to highly heritable according to observation (68%) and parent-report of distress to novelty (58%; Goldsmith et al., 1999). Behavioral inhibition in infancy is moderately to highly heritable in interaction with unfamiliar peers (70%) and adult strangers (42%–56%) with heritability of narrow facets of inhibition ranging from 30% to 70% ( DiLalla, Kagan, & Reznick, 1994; Emde et al., 1992; Robinson, Kagan, Reznick, & Corley, 1992). Finally, a study of behavioral inhibition in middle childhood finds a broad-sense heritability of 59% for exploratory behavior across nonsocial, adult and peer interaction contexts, with context-specific heritability ranging from 51% (adult) to 71% (peer; McGuire et al., 2003).
Genetic influences on negative emotionality are well established, but there is a need to extend quantitative genetic research on discrete emotion beyond the univariate framework, especially as subordinate facets of the same construct may have distinct patterns of genetic and environmental variance. In addition, both the stability of temperament and the range of environmental challenges (e.g. in school and peer settings; Rothbart & Bates, 2006) increase in middle childhood, but despite widespread use of negative emotionality in phenotypic research, no quantitative genetic study has examined fear, anger and sadness together in multivariate models or considered structured observation of negative emotion past infancy or early childhood.
The Current Study
The overarching aim was to examine the genetic and environmental influences on three subordinate-level aspects of negative emotionality (anger, sadness and fear) in middle childhood. We fit models separately for mother-report, father-report, and in-home observation in order to maximize comparisons across studies that only utilize one method. It is common to composite mother and father-report when possible, but reporter discrepancies can reflect true differences in children’s behavior (De Los Reyes, Henry, Tolan, & Wakschlag, 2009). Further, mother and father-report of behavior problems show unique genetic variance, with discrepancies explained by differences in perspective as well as error (Bartels, Hudziak, Boomsma, Rietveld, van Beijsterveldt, & van den Oord, 2003), and mother and father-report of temperament can differ greatly in heritability (Mullineaux et al., 2009). Given the prevalence of studies only using mother-report, and the lack of research examining genetic and environmental influences on father-report of temperament, examining mother and father-report separately both increases comparability to other studies and provides information on reporter differences in heritability.
The first aim was to estimate the heritability of each emotion. Regarding reporter differences, moderate to high heritability was expected for mother-report of anger, sadness and fear, whereas in-home observation was expected to be largely explained by nonshared environment, with modest to moderate genetic influences. Given limited research examining father-report of temperament, no specific predictions were made. Regarding differences in heritability across emotion, fear was expected to be the most heritable emotion regardless of reporter, and modest but significant shared environment was expected for anger on the basis of past findings of shared environmental effects for anger and other approach-related traits (Emde et al., 2001; Gagne & Goldsmith, 2011; Goldsmith et al., 1997).
The second goal was to examine the genetic and environmental covariance between anger, sadness and fear, using three increasingly restrictive models (the Cholesky decomposition, independent pathway and common pathway models) to assess the extent to which these emotions can be explained by a common set of latent genetic and environmental factors. If the common factor provides a good fit for the data and accounts for the majority of genetic and shared environmental variance in all emotions, with residual variance largely explained by E, it supports the conceptualization of negative emotionality as a broad genetically and environmentally influenced dimension of temperament. In contrast, a poorly fitting common factor model, or substantial genetic or shared environmental factors unique to one emotion, suggest meaningful variance which is not accounted for by a negative emotionality factor. Finally, if the best-fitting model is the Cholesky decomposition, it indicates that the genetic and environmental covariance among negative emotions is more complex, e.g., if two distinct additive genetic factors influence sadness, one of which is shared with anger and the other with fear, the Cholesky model would be expected to provide the best fit to the data. We expected that a common, moderately heritable factor would represent the data, but also that each emotion would show important genetic and, in some cases, shared environmental variance which could not be explained by the common factor.
Method
Participants
Participants were twin children and their mothers and fathers drawn from the Wisconsin Twin Project, an ongoing longitudinal study following twins (recruited from hospital birth records in the state of Wisconsin between the years of 1989 and 2004) from toddlerhood through adolescence (Lemery-Chalfant, 2006). When twins were approximately eight years old (M = 7.87 years, SD = .93), 933 families (1866 twins) were selected for a follow-up assessment. Of the twins with completed zygosity assessments, 658 twin pairs (1316 twins; 33.6% MZ, 31.0% same sex DZ, 35.4% opposite sex DZ; 49% male) had mother-report and 1106 had father-report of temperament, with the sample size for observed emotion composites ranging from 1166 (fear) to 1246 (anger and sadness). The sample is largely Caucasian (85.8%). The remaining participants are African American (6.2%), Native American (1.5%), Asian (.2%), biracial (1.8%) and other (3.5%), which is representative of the population of Wisconsin. Families are largely educated and middle class, with annual family income ranging from below $10,000 to over $200,000 (mean family income between $50,001 and $60,000) and formal education ranging from 6 years to a graduate degree (M = 14.88 years for mothers and 14.43 years for fathers), with the majority of participants reporting either some college, trade or technical school, or a college degree.
Procedure
Families completed two phone interviews, mailed questionnaire packets, and a 4-hour home visit (Lemery-Chalfant, 2006). As part of the first phone interview, mothers and fathers reported on twins’ temperament, along with demographic information and measures of children’s behavior and environment. At the home visit, twins took part in sixteen videotaped and coded episodes of the middle childhood version of the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley & Prescott, 2001). Twins were tested separately in different rooms of the house, with the same child tester administering the same episode in the same room for each twin. Time was provided between episodes for twins to rest and return to a neutral baseline state (Goldsmith et al., 2001). When tapes were coded, cotwins were coded by different raters in order to reduce rater bias.
Measures
Zygosity
Zygosity was assessed using mother-report on the Zygosity Questionnaire for Young Twins (Goldsmith, 1991). This detailed 32-item parent-report instrument assesses physical similarities (e.g., differences in hair color, texture and shade), and demonstrates over 95% agreement with zygosity assessed by genotyping (Price, Freeman, Craig, Petrill, Ebersole, & Plomin, 2000). Physical similarity was also observed directly, and pairs with ambiguous zygosity were genotyped (Lemery-Chalfant, 2006).
Parent-report temperament
Fear, anger and sadness were assessed using mother and father-report on the Children’s Behavior Questionnaire (CBQ; Rothbart et al., 2001). In prior studies, the CBQ has shown high longitudinal stability, and good construct and convergent validity (Rothbart et al., 2001; Rothbart et al., 2000). Ten-item scales assessing fear, anger and sadness included questions like, “is afraid of loud noises,” “gets angry when told s/he has to go to bed,” and “cries sadly when a favorite toy gets lost or broken,” respectively. Items are answered on a 1–7 Likert scale, with 1 being “extremely untrue of your child” and 7 being “extremely true of your child” in the past six months. Internal consistency at the scale level was good to adequate for both mother and father-report of anger (alpha = .83, .82), sadness (alpha = .69, .67), and fear (alpha = .75, .75), respectively.
Observed temperament
Observed temperament was assessed using a home-based version of the Lab-TAB designed for use with 6–8 year-old children (Goldsmith et al., 2001). The Lab-TAB is a standardized assessment of temperament including episodes intended to tap discrete emotional reactions under naturalistic conditions. Episodes are videotaped, range from three to ten minutes, and are divided into coding epochs that range from five to 30 seconds. The intensity of facial, vocal, and postural displays of emotion is coded in each epoch, along with other relevant behaviors (e.g., duration of gaze aversion), as specified in the Lab-TAB manual (Goldsmith et al., 2001).
A similar preschool version of the Lab-TAB has been validated in laboratory and home settings (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011), showing moderate convergence between Lab-TAB composites and observer post-visit ratings (correlations ranging from .21 to .76), although correlations with parent-report on the CBQ are more modest. At least ten percent of episodes were double-coded by a master coder to monitor interrater agreement, and Kappas for coded variables ranged from .79 to .89 for fear, .70 to .93 for sadness, and .63 to .84 for anger. Although kappa-indexed agreement was good, we also standardized scores within coder, then rescaled them back onto the raw scores of a referent coder (usually the coder who coded the greatest number of episodes) to further reduce any coder differences (see first-author for details).
Selected episodes: Anger and sadness
Observed anger and sadness were assessed using four Lab-TAB episodes that regularly evoke both sadness and anger responses from children. In ‘I’m Not Sharing,’ the child tester unequally shares a bag of candy with the child. In ‘Impossibly Perfect Stars,’ the child is asked to repeatedly draw stars by a child tester, who responds by critiquing each star and asking the child to try again. In ‘Transparent Box,’ the child is presented with several appealing toys in a locked transparent box, along with a set of keys that cannot open the box, and in ‘Wrong Gift’, instead of a preferred gift, the child is given a box containing an unappealing gift that the child has previously ranked as his or her least favorite. Stars, Not Sharing, and Transparent Box are coded in 10-second epochs, and Wrong Gift in 5-second epochs, with two 4-minute episodes (Stars and Transparent Box) and two variable-length episodes (Not Sharing and Wrong Gift). Latency in seconds, and intensities of facial, bodily, and vocal expressions of anger and sadness on 0–2 or 0–3 scales, were coded in accordance with the Lab-TAB manual (Goldsmith et al., 2001), and lower-order parameters included in each episode-level composite are summarized in Table 1. In each episode, mean intensity scales were coded for facial and bodily anger and sadness. Latency to facial or bodily anger and sadness was coded in all episodes except Wrong Gift, which included a discrete emotion-eliciting event. Other parameters coded in each episode differed. Specifically, vocal expression was coded in Stars and Not Sharing. In addition, during the coding for the current study, we combined facial and bodily scales for anger and sadness in Not Sharing into scales including both facial and bodily emotion due to low frequency of these responses. Thus, Stars included speed and vocal, bodily and facial intensity, Not Sharing included speed to first bodily or facial response, speed to first vocalization, bodily and facial intensity, and vocal intensity, Transparent Box included speed, bodily intensity and facial intensity, and Wrong Gift included bodily and facial intensity.
Table 1.
Summary of Episode-level Composites
| Emotion | Episode | Parameters included in final composite | Scale | Skew | Kurtosis | α | r |
|---|---|---|---|---|---|---|---|
| Anger | Stars | Speed (facial/bodily) | Time (s) | −1.11 | −0.07 | .68 | |
| Mean facial intensity | 0–2 | 1.30 | 1.93 | ||||
| Mean bodily intensity | 0–2 | 2.02 | 5.25 | ||||
| Mean vocal intensity | 0–2 | 2.46 | 6.87 | ||||
| Not Sharing | Speed (facial/bodily) | Time (s) | −1.16 | −0.26 | .76 | ||
| Speed (vocal) | Time (s) | 0.46 | −1.63 | ||||
| Mean facial/bodily intensity | 0–3 | 1.50 | 2.80 | ||||
| Mean vocal intensity | 0–3 | 2.05 | 0.07 | ||||
| Transparent Box | Speed (facial/bodily) | Time (s) | −0.12 | −1.45 | .79 | ||
| Mean facial intensity | 0–2 | 2.43 | 7.32 | ||||
| Mean bodily intensity | 0–2 | 2.73 | 7.43 | ||||
| Wrong Gift | Mean facial intensity | 0–2 | 2.01 | 5.39 | .60 | ||
| Mean bodily intensity | 0–2 | 2.60 | 9.81 | ||||
|
| |||||||
| Sadness | Stars | Speed (facial/bodily) | Time (s) | −0.18 | −1.11 | .71 | |
| Mean facial intensity | 0–2 | 3.01 | 11.93 | ||||
| Mean bodily intensity | 0–2 | 2.14 | 5.78 | ||||
| Mean vocal intensity | 0–2 | 1.58 | 2.03 | ||||
| Not Sharing | Speed (facial/bodily) | Time (s) | −1.31 | 0.72 | .49 | ||
| Mean facial/bodily intensity | 0–3 | 1.86 | 4.48 | ||||
| Wrong Gift | Mean facial intensity | 0–2 | 1.72 | 2.72 | .61 | ||
| Mean bodily intensity | 0–2 | 1.72 | 2.93 | ||||
|
| |||||||
| Fear | Scary Mask | Speed (facial/bodily) | Time (s) | −2.94 | 7.07 | .63 | |
| Mean facial intensity | 0–3 | 0.91 | 0.11 | ||||
| Mean bodily intensity | 0–3 | 0.23 | −0.58 | ||||
| Storytelling | Speed (facial/bodily) | Time (s) | −0.72 | −1.30 | .42 | ||
| Mean bodily intensity | 0–3 | 1.02 | −0.18 | ||||
Note. Intensity of approach, avoidance and vocal expression in Scary Mask, intensity of avoidance in Storytelling, and speed and intensity of vocal sadness in Not Sharing, were coded but not included in episode-level composites due to low correlations with other parameters. Facial intensity was coded in Storytelling but not included due to low frequency of responses above 0. No sadness composite was formed for Transparent Box due to low frequency of responses above 0. Square-root transformations were applied to parameters with skewness > 2.00 or kurtosis > 7.00. Parameters were standardized using z-score transformations, and unit-weighted mean composites were formed. s = seconds.
Selected episodes: Fear
Fear was assessed with two episodes, ‘Scary Mask,’ and ‘Storytelling’. In Scary Mask, coded in 15-sec epochs, the child interacts with a friendly stranger wearing a frightening mask, beginning when the child first notices the stranger’s face and continuing as the stranger takes off the mask, begins a conversation with the child, and finally asks the child to touch and wear the mask. In Storytelling, coded in 10-sec epochs, the child is asked to give a speech about the previous day’s events in front of an audience of multiple child testers. The episode includes at least one prompt by the child tester (e.g., ‘is there anything else you would like to tell us?’). In both episodes, variables are coded on scales of 0–3 in accordance with the Lab-TAB manual (Goldsmith et al., 2001) and include latency to first fear in seconds, facial and bodily intensity, and intensity of avoidance (summarized in Table 1). Intensity of approach and vocal intensity were also coded in Scary Mask but not Storytelling.
Lab-TAB composite formation
Lower-order composites
Latency scores were winsorized to three standard deviations, then reverse-coded to transform them to speed scores. Mean intensity scores were computed across all epochs for facial, bodily and vocal emotion in each episode. Three parameters (mean intensity of facial fear in Storytelling, and bodily and facial sadness in Transparent Box) were discarded due to low frequency of scores above zero (< 30%). Square-root transformations were used for lower-order parameters exceeding recommended cutoffs for skewness and kurtosis (2.00 and 7.00, respectively; Muthén & Kaplan, 1985) prior to observed composite formation (see Table 1 for skewness and kurtosis). Z-score transformations were used to standardize lower-order speed and intensity parameters prior to higher-order composite formation. Mean composites for anger, sadness and fear were formed from speed and mean intensity scores on the basis of zero-order correlations, with variables only included in an episode-level composite if they were at least moderately and significantly correlated with at least one other lower-order parameter (r > .30). For anger, episode-level composites included all available emotion parameters for Stars (speed, vocal intensity, facial intensity, bodily intensity), Not Sharing (bodily and facial speed, vocal speed, bodily and facial intensity, vocal intensity), Transparent Box (speed, bodily intensity, facial intensity), and Wrong Gift (bodily and expressed intensity). Correlations across all lower-order anger parameters within episode ranged from low to moderate (.217 to .677), but each parameter was correlated above r = .30 with at least one other parameter. For sadness, all available emotion parameters were used for Stars and Wrong Gift (r from .203 to .698), but for Not Sharing, vocal parameters were not highly correlated with speed and intensity of facial and bodily sadness, and the final sadness composite for Not Sharing was formed from speed and intensity of bodily and facial sadness (r = .492). The final fear composite for Scary Mask included speed, facial, and bodily fear (r from .340 to .366), but vocal intensity and intensity of avoidance were excluded due to low correlations with other parameters. The final composite for Storytelling included only speed and bodily intensity (r = .420), with facial fear excluded due to low variability and intensity of avoidance excluded due to low correlations.
Higher-order cross-episode composites
Episode-level anger composites for Stars, Not Sharing, and Transparent Box, and sadness composites for Stars and Not Sharing, were standardized using z-score transformations, and used to form cross-episode mean composites of anger and sadness, respectively. Correlations among episode-level anger composites were moderate, with r ranging from .249 (Not Sharing and Transparent Box) to .311 (Stars and Not Sharing), whereas for sadness, only the cross-episode composites for Stars and Not Sharing were aggregated (r = .174). Fear assessed in Storytelling and Scary Mask were moderately correlated and composited (r = .262).
Data Analysis Plan
Analyses for aim 1: univariate ACE models
The latent A, C and E factors in the classic univariate biometrical ACE model are estimated from observed variances and covariances of MZ and DZ twins’ scores on a single trait. The ACE model is a multigroup structural equation model, with covariances modeled differently for MZ (who share 100% of their segregating DNA) and DZ twins (who share on average 50%). C is fully shared between cotwins; thus, the correlation between latent C factors is fixed to 1.0. Due to model identification limitations with twin data, it is not possible to estimate both C and D factors within the same model, but it is possible to fit an alternate ADE model, which accounts for nonadditive genetic effects (MZ twins share 100% of nonadditive genetic effects, whereas DZ twins inherit the same alleles at a locus 25% of the time). The E factor is uncorrelated for both MZ and DZ twins.
We fit univariate ACE or ADE models estimating genetic and environmental influences on sadness, anger, and fear separately for mother-report, father-report, and in-home observation using the statistical program OpenMX (Boker et al., 2011), an R-based program that uses Maximum Likelihood estimation. After the full models were fit, nested models were tested by systematically dropping parameters, and the fit of reduced models was compared with that of the full model using the −2 log likelihood chi-square test of fit to find the most parsimonious solution. A nonsignificant difference in fit implies that the reduced model accounts for the observed data as well as the full model, whereas a significant loss of fit indicates the dropped parameter is required to adequately reproduce the data. Because E contains measurement error, it is always retained, and D is not estimated without A, as it is unlikely that all genetic influences are interactive, with no additive effects. Because variance components are interdependent and bounded at zero, standard error based confidence limits may be inaccurate. We obtained 95% confidence limits for variance components using the likelihood-based confidence interval option in OpenMx, which computes non-symmetric confidence limits by changing the value of the selected parameter until the model fit is reduced by a specified amount, while the other estimated parameters are allowed to vary freely (OpenMx Development Team, 2011). We used the default reduction in fit, a change in −2 log likelihood of 3.841459, corresponding to 95% confidence on a chi-square distribution with one degree of freedom (OpenMx Development Team, 2011).
Analyses for aim 2: multivariate ACE models
Cholesky decompositions
Just as univariate models decompose the variance in a single trait into genetic and environmental factors, Cholesky decompositions (Figure 1, panel A) parse the covariance between traits into latent A, C or D, and E factors, allowing an estimation of the extent to which genetic and environmental influences on one trait are shared with other traits, regardless of trait heritability. In a trivariate Cholesky decomposition, the first set of latent factors (A1, C1 and E1) encompasses genetic, shared and nonshared environmental influences on the first trait, which may be shared with the second and third traits. The second set of latent factors (A2, C2, and E2) represents influences on the second trait that are independent of the first, but may be shared with the third. The third set of latent factors represents influences unique to the third trait. Thus, the order of traits is relevant to the interpretation of each set of factors, but does not affect model fit or the significance of shared A, C, or E variance between any two traits. We selected anger as the first trait and fear as the third because anger and fear were not expected to be strongly related, and we expected sadness to share covariance with both traits.
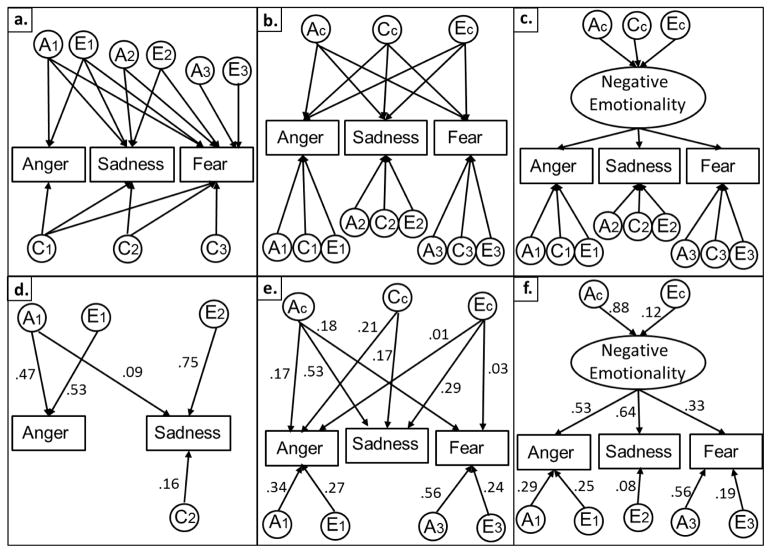
Figure 1.
Top: example Trivariate Cholesky Decomposition (a.), Independent Pathway Model (b.), and Common Pathway Model (c.), showing one twin only for simplicity. Latent A1, C1, and E1 factors in the cholesky decomposition represent additive genetic, shared environmental, and nonshared environmental influences on anger, which may also be shared with sadness and fear. A2, C2, and E2 factors represent A, C and E influences on sadness that are independent of anger but may be shared with fear, and A3, C3, and E3 factors represent influences unique to fear. In the Independent Pathway Model, covariance among emotions is fully accounted for by a single set of common additive genetic (AC) shared environmental (CC), and nonshared environmental (EC) factors, but the relative loadings on each phenotype may vary. Unique additive genetic, shared environmental, and nonshared environmental influences are also estimated (e.g. A1, C1 and E1 are unique to anger). In the Common Pathway Model, covariance is fully accounted for by a single common factor (i.e., negative emotionality), decomposed into additive genetic (AC), shared environmental (CC), and nonshared environmental (EC) factors. Bottom: final best-fitting models for observed (d.), mother-report (e.), and father-report (f.). Standardized parameter estimates are reported.
Independent and Common pathway models
The independent pathway model (Figure 1, panel B; Martin & Eaves,1977), assumes that the same A, C and E factors account for the covariance among anger, sadness and fear, and allows the magnitude of paths from these shared factors to each emotion to differ, such that, e.g., covariance between anger and sadness may be explained largely by C, and covariance between sadness and fear by A. Because the independent pathway model is not nested within the Cholesky decomposition, we used Akaike’s Information Criterion (AIC; Akaike, 1987) to compare the fit of these two models, with lower values indicating a model with more support. The common pathway model (Figure 1, panel C; McArdle & Goldsmith, 1990) is nested within the independent pathway model and assumes a common negative emotionality factor, which itself has single estimates of A, C and E, with each emotion loading on this factor. Thus, unless residuals are allowed to correlate, the common pathway model requires that any A, C and E factors that explain covariance between two emotions must also be shared with the third. Genetic and environmental variance in each trait that is independent of the common factor is also estimated. To the extent that covariance between anger, sadness and fear is explained by a common set of A, C and E factors, and the structure of these factors is similar for each emotion, the common pathway model provides a good fit to the data.
Results
Preliminary Analyses
Means and standard deviations are given in Table 2; all parent-report scales and observed episode-level and cross-episode composites were normally distributed. Phenotypic correlations between mother and father-report and observed anger, sadness, and fear are given in Table 2. Because twins are clustered in families, correlations were run in MPlus using the type=complex and cluster options (Muthén & Muthén, 1998–2011). As expected, correlations between emotions were high-to-moderate for both parent reports, whereas correlations between observed emotions were more modest, and convergence between parent-report and observed emotions was low. Age and family socioeconomic status residualized scores were used for twin modeling to account for these effects on ACE estimates, as is standard practice to reduce potential biases when it is infeasible to incorporate additional covariates (McGue & Bouchard, 1984).
Table 2.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | MZ | SSDZ | OSDZ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Anger (M) | – | .56** | .26** | .62** | .36** | .15** | .78** | .49** | .05 | .06* | −.03 | .69 | .48 | .53 |
| 2. Sadness (M) | – | .42** | .33** | .48** | .24** | .82** | .45** | .00 | .03 | .01 | .73 | .54 | .40 | |
| 3. Fear (M) | – | .10** | .20** | .58** | .74** | .40** | −.07* | .01 | −.04 | .76 | .32 | .31 | ||
| 4. Anger (F) | – | .58** | .21** | .45** | .78** | .09* | .07* | −.01 | .71 | .32 | .40 | |||
| 5. Sadness (F) | – | .35** | .43** | .81** | .04 | .08* | −.03 | .73 | .47 | .40 | ||||
| 6. Fear (F) | – | .43** | .71** | −.04 | .05 | −.03 | .81 | .44 | .31 | |||||
| 7. NE (M) | – | .57** | −.01 | .05 | −.02 | .77 | .53 | .53 | ||||||
| 8. NE (F) | – | −.04 | .09* | −.03 | .81 | .46 | .43 | |||||||
| 9. Anger (O) | – | .21* | .13* | .47 | .31 | .21 | ||||||||
| 10. Sadness (O) | – | .01 | .26 | .34 | .12 | |||||||||
| 11. Fear (O) | – | .50 | .45 | .45 | ||||||||||
|
| ||||||||||||||
| Male
| ||||||||||||||
| M | 4.63† | 3.84 | 3.79 | 4.56† | 3.77 | 3.71 | 4.08 | 4.01 | 0.12† | 0.02 | −0.08+ | |||
| SD | 0.92 | 0.76 | 0.98 | 0.85 | 0.71 | 0.92 | 0.67 | 0.62 | 0.77 | 0.80 | .80 | |||
| Minimum | 1.50 | 1.70 | 1.40 | 1.70 | 1.70 | 1.30 | 1.93 | 1.93 | −1.65 | −1.90 | −1.96 | |||
| Maximum | 7.00 | 5.90 | 6.60 | 6.71 | 5.89 | 6.22 | 6.16 | 5.67 | 2.33 | 2.36 | 2.02 | |||
|
| ||||||||||||||
| Female
| ||||||||||||||
| M | 4.38 | 3.99 | 3.98 | 4.31† | 3.89 | 3.96 | 4.12 | 4.05 | −0.11† | −0.02 | 0.07+ | |||
| SD | 0.97 | 0.80 | 0.99 | 0.89 | 0.71 | 0.90 | 0.74 | 0.65 | 0.72 | 0.77 | 0.77 | |||
| Minimum | 1.00 | 1.40 | 1.38 | 1.40 | 1.40 | 1.80 | 1.38 | 1.74 | −1.83 | −1.65 | −1.89 | |||
| Maximum | 6.90 | 6.27 | 6.70 | 6.80 | 5.97 | 6.59 | 6.22 | 5.94 | 2.33 | 2.36 | 1.88 | |||
Note.
p < .05,
p < .001.
M = mother-report, F = father-report, O = in-home observation. NE = negative emotionality, a mean composite of anger, sadness and fear scales that does not include any independent assessment. Observational assessments are mean composites of standardized scales and thus have means close to zero. Twin intraclass correlations are reported on the right. MZ = monozygotic, SSDZ = same sex dizygotic, OSDZ = opposite sex dizygotic twin pairs.
Males had significantly higher mother report, father report, and observed anger.
Females had significantly higher observed fear. All other sex differences in means were nonsignificant.
Twin Intraclass Correlations
Table 2 reports twin intraclass correlations (ICCs). Mother-report of anger and sadness and father-report of sadness show DZ correlations higher than half the MZ correlations, suggesting both additive genetic and shared environmental factors, whereas MZ correlations approximately twice as high as DZ correlations for mother-report of fear and father-report of anger and fear suggest additive genetic and nonshared environmental variance. ICCs for observed emotion show evidence of heritability for anger, with shared environmental factors likely to be important for observed sadness and fear. ADE models were tested for mother and father-report of fear, as DZ correlations lower than half MZ correlations suggest non-additive effects are plausible.
Quantitative Genetic Analyses
Saturated Models
Saturated models were fit to test for sex differences and rater contrast and assimilation effects. Fully saturated multigroup models freely estimating means, variances and covariances for male MZ, female MZ, male DZ, female DZ, and opposite sex DZ twins were tested against a series of models constraining means and variances to be equal across twin pairs and zygosity groups, and constraining means, variances and covariances to be equal across sex. Higher phenotypic variance in DZ relative to MZ zygosity groups indicates that parents may be contrasting the DZ twins against each other or inflating the similarity of MZ twins (assimilation effects), whereas lower DZ variance indicates possible sibling cooperation or imitation effects (Saudino, 2003; Neale & Cardon, 1992). In each case except for anger and observed fear, means, variances and covariances could be equated across sex, and means and variances across zygosity. For mother-report, father-report and observed anger, males had higher means than females, and for observed fear, females had higher means than males, but there were no significant sex differences in variances or covariances between same sex male, same sex female, and opposite sex groups for any variable. In all models tested, variances could be constrained to be equal across MZ and DZ twins, providing no evidence of rater contrast effects.
Univariate ACE and ADE Models
Standardized estimates of A, C (or D), and E factors for each emotion, and fit statistics of full and best fitting reduced models, are given in Table 3. According to the best-fitting models for both mother and father-report, all emotions were moderately-to-highly heritable, with A influences lowest for mother-report anger (.45) and sadness (.45) and highest for father-report fear (.80). Significant C was found for mother-report anger (.26) and sadness (.27), but not fear, which was best explained by an AE model with high A (.74). For father-report, anger (.74) and fear (.80) were highly heritable, with no significant C, but although father-report sadness was moderately heritable (.53), the C factor (.19) could not be dropped without a significant loss of fit. Similar to parent-report, observed anger had moderate additive genetic (.47) and nonshared environmental (.53) variance. For observed sadness and fear, the CE model fit the data best, with modest but significant C for sadness (.22) and moderate C for fear (.46). For all observed emotions, the majority of variance was explained by E.
Table 3.
Univariate ACE/ADE Model Fit and Parameter Estimates
| Mother-Report | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scale | Model | −2LL | df | Δ −2LL | Δ df | p | AIC | A | C/D | E |
| Anger (M) | ACE | 3200.83 | 1296 | – | – | – | 608.83 | .45 (.28–.62) | .26 (.11–.41) | .29 (.24–.35) |
| AE | 3212.3 | 1297 | 11.47 | 1 | < .001 | 618.3 | ||||
| CE | 3224.38 | 1297 | 23.55 | 1 | < .001 | 630.38 | ||||
| E | 3467.2 | 1298 | 266.37 | 2 | < .001 | 871.2 | ||||
|
| ||||||||||
| Sadness (M) | ACE | 2735.37 | 1296 | – | – | – | 143.37 | .45 (.28–.62) | .27 (.11–.42) | .28 (.24–.35) |
| AE | 2746.55 | 1297 | 11.18 | 1 | < .001 | 152.55 | ||||
| CE | 2759.46 | 1297 | 24.09 | 1 | < .001 | 165.46 | ||||
| E | 3013.49 | 1298 | 278.12 | 2 | < .001 | 417.49 | ||||
|
| ||||||||||
| Fear (M) | ADE | 3338.77 | 1296 | – | – | – | 746.77 | .44 (.09–.79) | .31 (.00–.67) | .25 (.09–.30) |
| AE | 3342.11 | 1297 | 3.34 | 1 | 0.07 | 748.11 | .74 (.65–.84) | – | .26 (.22–.31) | |
| E | 3561.64 | 1298 | 222.87 | 2 | < .001 | 965.64 | ||||
|
| ||||||||||
| Father-Report | ||||||||||
|
| ||||||||||
| Anger (F) | ADE | 2589.2 | 1088 | – | – | – | 413.2 | .71 (.34–.84) | .02 (.00–.39) | .27 (.22–.33) |
| AE | 2589.21 | 1089 | 0.01 | 1 | 0.92 | 411.21 | .73 (.62–.84) | – | .27 (.23–.33) | |
| E | 2770.14 | 1090 | 180.94 | 2 | < .001 | 590.14 | ||||
|
| ||||||||||
| Sadness (F) | ACE | 2101.4 | 1088 | – | – | – | −74.6 | .53 (.34–.73) | .19 (.01–.36) | .28 (.23–.36) |
| AE | 2105.87 | 1089 | 4.47 | 1 | 0.03 | −72.13 | ||||
| CE | 2128.32 | 1089 | 26.92 | 1 | < .001 | −49.68 | ||||
| E | 2321.43 | 1090 | 220.03 | 2 | < .001 | 141.43 | ||||
|
| ||||||||||
| Fear (F) | ADE | 2624.4 | 1088 | – | – | – | 448.4 | .77 (.40–.90) | .03 (.00–.38) | .20 (.17–.25) |
| AE | 2624.42 | 1089 | 0.02 | 1 | 0.89 | 446.42 | .80 (.70–.90) | – | .20 (.17–.25) | |
| E | 2873.52 | 1090 | 249.12 | 2 | < .001 | 693.52 | ||||
|
| ||||||||||
| Observed | ||||||||||
|
| ||||||||||
| Anger (O) | ACE | 2652.21 | 1231 | – | – | – | 190.21 | .39 (.12–.57) | .06 (.00–.27) | .55 (.46–.66) |
| AE | 2652.58 | 1232 | 0.37 | 1 | 0.54 | 188.58 | .47 (.36–.57) | – | .53 (.46–.63) | |
| CE | 2660.1 | 1232 | 7.89 | 1 | < .001 | 196.1 | ||||
| E | 2730.44 | 1233 | 78.23 | 2 | < .001 | 264.44 | ||||
|
| ||||||||||
| Sadness (O) | ACE | 2831.78 | 1230 | – | – | – | 371.78 | .06 (.00–.36) | .18 (.00–.30) | .76 (.64–.87) |
| AE | 2834.29 | 1231 | 2.51 | 1 | 0.11 | 372.29 | ||||
| CE | 2831.94 | 1231 | 0.16 | 1 | 0.69 | 369.94 | – | .22 (.14–.31) | .78 (.70–.87) | |
| E | 2862.82 | 1232 | 31.04 | 2 | < .001 | 398.82 | ||||
|
| ||||||||||
| Fear (O) | ACE | 2589.27 | 1164 | – | – | – | 261.27 | .00 (.00–.23) | .46 (.27–.56) | .54 (.45–.61) |
| AE | 2610.58 | 1165 | 21.32 | 1 | < .001 | 280.58 | ||||
| CE | 2589.27 | 1165 | 0 | 1 | .99 | 259.27 | – | .46 (.37–.56) | .54 (.48–.61) | |
| E | 2722.58 | 1166 | 133.31 | 2 | < .001 | 390.58 | ||||
Note. −2LL = −2 log likelihood; df = degrees of freedom; Δ = change; p = probability; AIC = Akaike’s Information Criterion; A, C, D and E are standardized squared parameter estimates for additive genetic, common environment, and nonshared environment factors, respectively. Standardized confidence intervals are reported in parentheses. The most parsimonious final model is indicated in bold.
Genetic and Environmental Covariance Across Emotion
Two trivariate Cholesky decompositions were fit for anger, sadness, and fear, one for mother-report and one for father-report. It was not possible to fit a trivariate Cholesky decomposition using observed emotion, because fear was not sufficiently correlated with anger or sadness (r < .10), but a bivariate Cholesky decompositions was fit examining the covariance between anger and sadness. Fit statistics for the full and best fitting reduced models, as well as unstandardized and standardized A, C, and E squared parameter estimates, are given in Table 4.
Table 4.
Multivariate Model Fit and Parameter Estimates
| Best-Fitting Full and Final Models (Observed)
| ||||||
|---|---|---|---|---|---|---|
| Model | −2LL | df | Δ−2LL | Δ df | p | AIC |
| Bivariate Cholesky (Full) | 5444.50 | 2460 | – | – | – | 524.50 |
| Bivariate Cholesky (Final) | 5445.51 | 2464 | 1.17 | 4 | .88 | 517.67 |
|
| ||||||
| Model Comparisons (Mother-Report)
| ||||||
| Model | −2LL | df | Δ−2LL | Δ df | p | AIC |
|
| ||||||
| Trivariate Cholesky (Full) | 8700.50 | 3882 | – | – | – | 936.50 |
| Trivariate Cholesky (Final) | 8709.62 | 3887 | 9.11 | 5 | .10 | 935.62 |
| IPM (Full) | 8700.64 | 3882 | – | 0 | – | 936.64 |
| IPM (Final) | 8709.93 | 3888 | 9.29 | 6 | .16 | 933.93 |
| CPM (Full) | 8722.56 | 3886 | 21.92 | 4 | < .001 | 950.56 |
| CPM (Final) | 8722.56 | 3890 | 0 | 4 | .99 | 942.56 |
|
| ||||||
| Model Comparisons (Father-Report) | ||||||
|
| ||||||
| Model | −2LL | df | Δ−2LL | Δ df | p | AIC |
|
| ||||||
| Trivariate Cholesky (Full) | 6873.51 | 3258 | – | – | – | 357.51 |
| Trivariate Cholesky (Final) | 6885.82 | 3266 | 12.31 | 8 | .14 | 353.82 |
| IPM (Full) | 6876.27 | 3258 | – | 0 | – | 360.27 |
| IPM (Final) | 6889.34 | 3267 | 13.07 | 9 | .16 | 355.34 |
| CPM (Full) | 6881.93 | 3262 | 5.67 | 4 | .23 | 357.93 |
| CPM (Final) | 6885.13 | 2367 | 8.87 | 9 | .45 | 351.13 |
| Parameter Estimates for Best-fitting Final Models
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Observed
| ||||||||
| Model | Scale | A1 | C1 | E1 | A2 | C2 | E2 | |
| Bivariate Cholesky | Anger | .26/.47 (.37–.58) | – | .28/.53 (.45–.62) | ||||
| Sadness | .06/.09 (.05–.15) | – | – | – | .09/.16 (.08–.23) | .45/.75 (.67–.85) | ||
|
| ||||||||
| Mother-report
| ||||||||
| Model | Scale | Ac | Cc | Ec | Au | Cu | Eu | |
|
| ||||||||
| Independent Pathway Model | Anger | .15/.17 (.09–.28) | .17/.21 (.10–.33) | .01/.01 (.003–.03) | .28/.34 (.23–.42) | – | .23/.27 (.23–.33) | |
| Sadness | .31/.53 (.38–.68) | .10/.17 (.06–.30) | .17/.29 (.25–.35) | – | – | – | ||
| Fear | .17/.18 (.11–.29) | – | .02/.03 (.01–.05) | .56/.56 (.45–.66) | – | .22/.24 (.20–.29) | ||
|
| ||||||||
| Father-report
| ||||||||
| Model | Scale | Ac | Cc | Ec | Au | Cu | Eu | λ |
|
| ||||||||
| Common Pathway Model | NE | .88 (.81–.94) | – | .12 (.06–.19) | ||||
| Anger | .29/.38 (.30–.46) | – | .18/.25 (.20–.30) | .53 | ||||
| Sadness | – | – | .08/.16 (.10–.22) | .64 | ||||
| Fear | .56/.68 (.59–.78) | – | .16/.19 (.16–.24) | .33 | ||||
Note. −2LL = −2 log likelihood; df = degrees of freedom; Δ= change; p = probability; AIC = Akaike’s Information Criterion; A = additive genetic; C = common environment; D = dominant (non-additive) genetic; E = nonshared environment. −2 log likelihood values and p values are not reported for comparisons between the Cholesky decomposition and the independent pathway model because these models are not nested. A, C and E values aq2re first reported as unstandardized squared parameter estimates, followed by standardized parameter estimates, with confidence intervals for standardized parameters in parentheses. IPM = independent pathways model; CPM = common pathways model; NE = latent negative emotionality; λ = factor loadings of each phenotype on NE. See Figure 1 for clarification of A, C and E common (e.g., A1, Ac) and unique (e.g., A3, Au) latent factors. The most parsimonious final model is indicated in bold.
For observed anger and sadness, the best-fitting model was an AE-ACE model (Figure 1, panel D). The trait correlation between anger and sadness was fully explained by A, but this A factor explained 47% of the variance in anger and only 9% of the variance in sadness. Sadness had significant C (.16), but both emotions were largely explained by emotion-specific E. For mother-report, the best fitting model was an ACE-ACE-AE model with both shared and emotion-specific A. One set of A factors explained 47% of the variance in anger, 17% of the variance in sadness, and 9% of the variance in fear. A second set of A factors independent of anger explained 36% of the variance in sadness and 10% of the variance in fear, but fear was largely explained by emotion-specific A (.55). Further, C was significant for anger (.24) and sadness (.18) but not fear, and all C influences could be explained by a single factor fully shared across emotion. A small proportion of E was shared between anger and sadness (.01) and between sadness and fear (.02), but E was largely emotion-specific. For father-report, the best-fitting model was an AE-AE-AE model, with both shared and emotion-specific A. Anger was highly heritable (.72), and A influences on anger also explained substantial variance in sadness (.35) and modest variance in fear (.05). A second set of A factors explained 38% of the variance in sadness and 12% of the variance in fear, but fear was largely explained by emotion-specific A (.63). As with mother-report, E was largely emotion-specific, with anger and sadness sharing a small E factor (.02).
Independent and Common Pathway Models
Independent pathway and common pathway models were tested for mother and father-report, but not for observed emotion. This is because observed fear was insufficiently correlated with either observed anger (r = .134) or sadness (r = .014) to support a three-indicator model, as is recommended for model identification (McArdle & Goldsmith, 1990), and fixing one path for model identification can distort estimates of genetic and environmental variance. Table 4 summarizes the fit of the full and most reduced independent pathway models for mother and father-report in comparison to the corresponding full and most reduced Cholesky decompositions, as well as parameter estimates and comparisons of fit between the full and reduced independent pathway models and the nested common pathway models. For mother-report, the independent pathway model did not fit significantly worse than the Cholesky decomposition (Δ AIC = .14, Δ df = 0 with the same number of parameters estimated), suggesting that the genetic and environmental covariance between these emotions can be represented by a single set of ACE factors. However, the common pathway model led to a significant loss of fit relative to the independent pathway model (Δ χ2(4) = 21.92, p < .001, Δ AIC = 13.92). In the final independent pathway model (Figure 1, panel E), it was necessary to retain shared A and E factors for all emotions, and a shared C factor for anger and sadness, but the path from the shared C factor to fear could be dropped. Interestingly, for sadness, all A (.53), C (.17), and E (.29) variance was fully shared with other emotions, and for anger, all C (.21) was explained by the shared C factor. However, anger and fear had both shared and emotion-specific A, with 17% of the variance in anger and 18% of the variance in fear explained by the shared A factor, and 34% of the variance in anger and 56% of the variance in fear explained by emotion-specific A. It was necessary to retain paths from the common E factor to both anger and fear, but the variance explained by this factor was small (.01–.03), with 27% of the variance in anger and 24% of the variance in fear explained by emotion-specific E factors.
For father-report, neither the independent pathway model (Δ AIC = 2.76, Δ df = 0) nor the common pathway model led to significant loss of fit relative to the full Cholesky decomposition (Δ AIC = .42, Δ df = 4), and the common pathway model did not fit significantly worse than the full independent pathway model (Δ χ2(4) = 5.67, p = .23, Δ AIC = −2.34), suggesting that a single negative emotionality factor provides an adequate representation of the data (see Table 4). This factor (Figure 1, panel F) was highly heritable (.88), with the remainder of variance accounted for by E. However, substantial emotion-specific A was needed to explain both anger (.38) and fear (.68), and all emotions had significant emotion-specific E ranging from 16% for sadness to 25% for anger. Factor loadings on the common factor were higher for sadness (.64) and anger (.53) than fear (.33), which seemed to be relatively more independent of the other emotions.
Discussion
We used quantitative genetic modeling to characterize the genetic and environmental underpinnings of anger, sadness and fear in middle childhood, with the aim of examining the extent to which these emotions represent a broad, genetically and environmentally influenced predisposition toward negative emotionality. Results support negative emotionality as a coherent, genetically influenced trait, but provide clear evidence for genetic influences on anger and fear that are not shared with other emotions, and thus also support an emotion-specific approach.
Heritability of Negative Emotions in Middle Childhood
Because genetically informed temperament research has focused on infancy and early childhood, one aim of this study was to describe the univariate heritability of anger, sadness and fear in middle childhood. Consistent with past research (Gagne et al., 2009; Saudino, 2005), mother and father-report of all emotions were moderately to highly heritable, with the highest heritability for mother and father-report of fear, and the lowest for mother-report of anger and sadness and father-report of sadness. Some unanticipated findings did emerge, most notably the presence of shared environmental variance in dimensions of emotion that were largely explained by genetic factors in past studies (Goldsmith et al., 1997; Mullineaux et al., 2009). Specifically, we observed shared environmental effects at the univariate level for mother-report, father-report, and observed sadness, as well as negligible heritability and moderate shared environment for observed fear. However, although past studies find modest shared environment for observed and mother-report anger (Emde et al., 2001; Gagne & Goldsmith, 2011; Goldsmith et al., 1997), we only found shared environmental variance in mother-report anger, with father-report highly and observer-report moderately heritable. Anger is theoretically and empirically linked to approach-related aspects of temperament such as positive emotionality (e.g., Carver & Harmon-Jones, 2009; Deater-Deckard et al., 2010), which is also less heritable (Goldmith et al., 1997, 1999; Saudino, 2005). However, our findings are consistent with some literature, as not all studies of anger find shared environmental effects (Deater-Deckard et al., 2010; Mullineaux et al., 2009).
Aspects of temperament related to fear, shyness or inhibition are highly heritable when assessed in laboratory interaction with peers or adults (e.g., DiLalla et al., 1994; Goldsmith et al., 1999), perhaps because unfamiliar situations and stranger interaction facilitate rather than suppress a child’s predisposition towards shyness or withdrawal. Our use of in-home rather than laboratory observation may have contributed to differences between this and prior studies regarding observed fear, as the home is likely a more comfortable context for some shy or fearful children. Because behavior genetic research consistently finds that heritability of temperament tends to increase rather than decrease with age (Saudino, 2005), the higher shared environmental variance in the current study relative to past research in younger samples is unlikely to be due to age differences. However, past research in middle childhood has either examined lower-order dimensions of temperament in a small sample, with limited power to detect shared environmental effects (Mullineaux et al., 2009), or examined broad negative emotionality over wide age ranges from childhood to adolescence (Singh & Waldman, 2010; Tackett et al., 2011), which may underestimate emotion-specific shared environmental influences such as we found for sadness.
Finally, we found high nonshared environmental effects for observed temperament, particularly sadness. Nonshared environmental effects often range from 40–80% for observed temperament (Saudino, 2005), and our findings are comparable (54–76%). Although this variance may index meaningful contributions of the child’s environment, it may also index measurement error, as well as the sensitivity of behavioral observation to daily variation and context. Further, due to the use of separate coders for each twin in the current study, rater differences may lead to higher estimates of nonshared environmental variance.
Genetic and Environmental Influences on Covariance Across Emotions
The second aim was to test whether genetic and environmental influences shared across emotions could be accounted for by a common factor, or whether more specificity would be required to explain relations among emotions. Despite evidence for distinct neural and biological systems involved in discrete emotions (Vytal & Hamann, 2010), many developmental theories assume that genetic and environmental influences on anger, sadness and fear act through a biologically-based, higher order negative emotionality dimension (Rothbart & Bates, 2006). We tested two nested models, the independent pathway model and the common pathway model, both of which posit that a single set of shared genetic and environmental factors is able to explain covariance among emotions. The independent pathway model allows the loadings of the three emotion measures to vary freely (from zero to 1.0) on each of the three common, latent A, C and E factors, and the common pathway model introduces a scaling of the phenotypic variance (i.e., a psychometric factor interpreted as negative emotionality) through which the latent variables act on all three emotions. Findings differed for mother and father-report, as covariance between father-report of anger, sadness and fear was adequately represented by the common pathway model, but the less-restrictive independent pathway model was required to explain relations among these emotions according to mother-report.
Consistent with past studies of mother-report negative emotionality (e.g., Mullineaux et al., 2009; Tackett et al., 2011), we found that a common set of (largely genetic) factors does influence each emotion, supporting the validity of dispositional negative emotionality. However, all three emotions were not equally influenced by this shared A. According to both mother and father-report, genetic influences on sadness were fully shared with anger and fear, but a majority of the variance in fear, and a smaller but still substantial amount of variance in anger, were explained by emotion-specific A factors. Further, we found a small C factor that was shared between mother-report anger and sadness but not fear, likely explaining why a common factor was insufficient to represent the genetic and environmental structure of mother-report negative emotionality. Thus, we extend past research by suggesting that a less restrictive model enables the detection of shared environmental influences not evident at the broad factor level.
Reporter differences in the heritability and structure of temperament have many potential explanations. We found no evidence of contrast or cooperation effects (which inflate genetic and shared environmental effects, respectively), but the finding of shared environment for mother but not father report may reflect biases leading mothers to rate all children more similarly (Bartels et al., 2003). However, reporter differences also likely reflect true behavioral differences, perhaps due to different contexts in which parents interact with children. Mothers still spend more time in child-rearing activities, and are more likely to be primary caregivers (Pleck & Masciadrelli, 2004), and thus may be more familiar with aspects of twins’ behavior influenced by the home environment and daily context, potentially increasing shared environmental variance.
Another question is which aspects of the shared environment might influence the development or expression of anger and sadness. Past research in this twin sample finds that the quality of the physical home environment (e.g., safety, cleanliness) moderates the heritability of negative emotionality, with both heritability and total variance higher under adverse conditions (Lemery et al., 2013). This suggests that a safe, structured home environment may constrain the expression of genetic influences on negatively reactive temperament. Further, the emotional climate in the home or the use of similar parenting behaviors with both twins (particularly in frustrating or upsetting situations) may elicit in-the-moment similarities in anger or sadness. To the degree that mothers are parenting in such situations, this is one explanation for the higher shared environmental variance in mother report relative to father report of these emotions.
The finding that fear was largely genetically distinct from anger and sadness regardless of reporter is unsurprising. Developmental literature suggests that observed anger and fear show some stability over time, but are not longitudinally related to each other from infancy to middle childhood (Rothbart et al., 2000), and studies of the physiological correlates of approach and withdrawal emotion and behavior (Fox et al., 2005) also support distinctions between fear and anger. Anger in the current study was more strongly related to sadness than fear for both genetic and shared environmental reasons, but also had moderate emotion-specific A. This A factor may index genetic influences shared with approach but not withdrawal-related aspects of temperament, consistent with research showing that anger shares A and E variance with approach and anticipatory positive affect (Deater-Deckard et al., 2010). This finding is also consistent with a recent study examining the phenotypic structure of observed temperament in preschoolers, which extracted separate factors for dysphoria (anger, sadness and hostility) and fear and behavioral inhibition (Dyson, Olino, Durbin, Goldsmith, & Klein, 2012). Dyson and colleagues (2012) suggest that the age at which fear and dysphoria begin to show a structure more similar to adult negative emotionality is an important developmental question. We provide evidence for the continuing independence of fear in middle childhood, even according to parent-report.
A substantial literature examines relations between temperament and psychopathology, with evidence for both broad and specific mechanisms (e.g., low effortful control as a risk for multiple disorders versus anger as a risk for conduct problems), but much of this research has taken place at the level of broad negative emotionality or neuroticism (Nigg, 2006). Considering temperament at the subordinate level may help clarify multiple genetically and environmentally influenced pathways to disorder. For instance, a combination of fear and anger-proneness may be important for reactive aggression, to a greater degree than fear or anger alone, whereas low fear but not necessarily high anger may increase risk of instrumental aggression (Nigg, 2006). Further, our finding that genetic variance in fear is less well represented than anger and sadness at the level of broad negative emotionality may be one reason why negative emotionality is often more predictive of externalizing than internalizing symptoms (Rothbart & Bates, 2006), which are more related to fear (Lemery et al., 2002; Oldehinkel et al., 2007). Negative emotionality may capture aspects of reactive distress which are relevant to both externalizing and internalizing (Rothbart & Bates, 2006), but less relevant for withdrawal-specific pathways to internalizing (e.g., via social withdrawal), whereas early behavioral inhibition relatively consistently predicts internalizing problems across childhood and adolescence (Degnan & Fox, 2007). Measurement of both fearfulness or inhibition and proneness to anger and sadness or general distress may be valuable for studies intending to capture multiple pathways from temperament to disorder.
In contrast to parent-report, observed anger and sadness were largely distinct, with anger moderately heritable, sadness more influenced by the shared environment, and genetic effects explaining the modest correlation between them. Correlations between observed fear and both observed anger and sadness were too low to allow an examination of genetic and environmental covariance. These modest relations are consistent with observed phenotypic data in preschoolers (e.g., Dyson et al., 2012), but relations between observed anger and sadness in the present study may be due to episode context shared between anger and sadness but not fear. Further, the low correlations between episodes, both within and across emotion, hold implications for the study of temperament using observational measures. The facets of children’s emotional reactivity captured by each episode are not the same, and measuring a construct defined by consistency across situations, such as negative emotionality, will likely require multiple and repeated observed episodes. At the same time, children’s reactivity within specific episodes may also be meaningful, due to aspects of adjustment captured by responses to unfair sharing, criticism, or other scenarios that may not be tapped by broader measures of negative emotionality.
Limitations
This study is subject to several limitations. First, due to a limited number of observed episodes and relatively low correlations between observed emotions across episodes, aggregating data across multiple episodes was not always possible. Aggregation increases reliability and convergence with parent-report (Rothbart & Bates, 2006; Forman et al., 2003). In addition, we derived anger and sadness composites from the same episodes, leading to the possibility that covariance between these emotions was partially or fully due to shared episode context. During the development of the lab-TAB, episodes intended to tap pure frustration or loss consistently elicited anger in some children and sadness in others. In the current study, we were unable to form anger and sadness composites by aggregating across separate episodes due to low cross-episode correlations, complicating the interpretation of findings for anger and sadness. Anger and sadness were not highly correlated across or within episode (.08–.24), suggesting the relative independence of these emotions even given shared episode context, but these correlations may have been reduced by scoring constraints (i.e., a child cannot be scored as both angry and sad on a single parameter within the same epoch). It will be important for future studies to address these issues using more extensive measurement of observed anger and sadness.
The generalizability of our results to diverse populations is also limited, as participants were largely Caucasian and middle class, and a majority had parents with at least some college education. Although the demographic characteristics of the sample are representative for Wisconsin, heritability is specific to the population under consideration (Plomin et al., 2013). Two other questions specific to twin research are whether findings generalize to singletons and whether environments experienced by MZ twins are more similar than those experienced by DZ twins in a way that is relevant to a trait of interest (i.e., Equal Environments Assumption). Infant twins do not differ from singletons on temperament (Goldsmith & Campos, 1990), and adult twins and singleton siblings do not differ on personality (Johnson, Krueger, Bouchard, & McGue, 2002). Other research also suggests that the Equal Environments Assumption holds for personality and temperament, as greater similarity in childhood experiences appears unrelated to similarity in personality, and differences in infant temperament relate to actual rather than perceived zygosity (Borkenau et al., 2002; Goldsmith et al., 1999).
Finally, we did not model gene-environment interplay, although both gene-environment interaction and gene-environment correlation are likely common (Lemery-Chalfant, 2010). Gene-environment interaction with family level factors (e.g. SES) may increase A, because this interaction increases differences among genetically non-identical individuals who share the same environment, whereas gene-environment interaction with factors unique to individuals decreases the resemblance of both MZ and DZ twins and may increase E. Thus, while the A factor provides a relatively good estimate of the broad genetic influence on a trait, including gene-environment interplay, small or nonsignificant C in classic twin designs should not be taken as evidence for the irrelevance of family-level influences on child development (Lemery-Chalfant et al., 2013).
Future Directions
It is important to know what aspects of discrete negative emotions may be influenced by a single set of shared genetic factors. One possibility is general reactivity to aversive, threatening or surprising stimuli, which may manifest as different discrete emotions depending on the context, or a lower physiological threshold of responsivity (Rothbart & Bates, 2006). Genetic influences on self-regulation may also modulate reactive temperament across a range of emotional and behavioral tendencies. Negative emotionality and effortful control are conceptually distinct but empirically negatively correlated in U.S. samples (Rothbart et al., 2001), and may be expected to share genetic variance (e.g., Gagne & Goldsmith, 2011).
Findings also highlight the need for a greater consideration of narrow as well as broad dimensions of temperament, perhaps especially when examining relations between temperament and psychopathology. For instance, irritability (an aversive reaction to stimulation) may share genetic influences with sadness or fear but not frustration (a response to blocked resources or goals), which may be more related to approach and impulsivity. Differentiating between approach-related anger and anger in response to threat or overstimulation may be informative for examining different pathways to risk for externalizing problems, particularly if these aspects of anger are etiologically distinct. Finally, future studies should more closely examine reporter similarities and differences in the genetic and environmental underpinnings of temperament, and potential differences in prediction of child psychopathology and adaption, as mothers, fathers, and trained laboratory observers may each be capable of detecting, or even eliciting, distinct aspects of children’s emotional responses. In addition, although child-report versions of the CBQ have not yet been developed, the development of a corresponding child-report measure of emotion and comparison with parent-report is one important avenue for future research.
Taken as a whole, the primary implication of our findings is that the genetic and environmental underpinnings of temperament may differ greatly depending on the level of analysis and the context. Findings support the use of broad negative emotionality, but there was evidence for heritable aspects of anger and fear that cannot be accounted for by a shared set of genetic influences, and shared environmental influences on mother-report anger and sadness that were not shared with fear. Thus, what is common to all three emotions is meaningful, but unable to encompass the full extent of systematic, biologically based individual differences in discrete emotions. Fear, in particular, is not well captured by broad measures of negative emotionality. In light of these distinctions, future research should consider the unique contributions of narrow facets of temperament to children’s psychological and socio-emotional functioning, both independently and in interaction with other aspects of children’s temperament and environmental context.
Acknowledgments
This research was supported by National Institute of Mental Health grants R01-MH59785 and R01-MH101504 and the Waisman Center grant P30-HD003352 to Goldsmith. Special thanks to the staff and students at the Wisconsin Twin Project, especially to Nicole Schmidt for coordinating the project all these years, and to Cory Schmidt for his master coding of the observed temperament, and the participating families who generously shared their experiences..
References
- Akaike H. Factor analysis and AIC. Psykometrika. 1987;52:317–332. doi: 10.1007/BF02294359. [DOI] [Google Scholar]
- Bartels M, Hudziak JJ, Boomsma DI, Rietveld MJ, van Beijsterveldt TC, van den Oord EJ. A study of parent ratings of internalizing and externalizing problem behavior in 12-year-old twins. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1351–1359. doi: 10.1097/01.CHI.0000085755.71002.5d. [DOI] [PubMed] [Google Scholar]
- Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkenau P, Riemann R, Angleitner A, Spinath FM. Similarity of childhood experiences and personality resemblance in monozygotic and dizygotic twins: A test of the equal environments assumption. Personality and Individual Differences. 2002;33:261–269. doi: 10.1016/S0191-8869(01)00150-7. [DOI] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Henry D, Tolan P, Wakschlag L. Linking informant discrepancies to observed variations in young children’s disruptive behavior. Journal of Abnormal Child Psychology. 2009;37:637–652. doi: 10.1007/s10802-009-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Beekman C, Wang Z, Kim J, Petrill SA, Thompson LA, DeThorne LS. Approach/positive anticipation, frustration/anger, and overt aggression in childhood. Journal of Personality. 2010;78:991–1010. doi: 10.1111/j.1467-6494.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Petrill SA, Thompson LA. Anger/frustration, task persistence, and conduct problems in childhood: A behavioral genetic analysis. Journal of Childhood Psychology and Psychiatry. 2007;48:80–87. doi: 10.1111/j.1469-7610.2006.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan K, Fox N. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- DiLalla LF, Kagan J, Reznick JS. Genetic etiology of behavioral inhibition among 2-year-old children. Infant Behavior and Development. 1994;17:405–412. doi: 10.1016/0163-6383(94)90032-9. [DOI] [Google Scholar]
- Dyson MW, Olino TM, Durbin EC, Goldsmith HH, Klein DN. The structure of temperament in preschoolers: A two-stage factor analytic approach. Emotion. 2012;12:44–57. doi: 10.1037/a0025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, Losoya SH. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emde RN, Robinson JL, Corley RP, Nikkari D, Zahn-Waxler C. Reactions to restraint and anger-related expressions during the second year. In: Emde RN, Hewitt JK, editors. Infancy to early childhood: Genetic and environmental influences on developmental change. New York: Oxford University Press; 2001. pp. 127–140. [Google Scholar]
- Emde RN, Plomin R, Robinson J, Corley R, DeFries J, Fulker DW, Zahn-Waxler C. Temperament, emotion, and cognition at fourteen months: The MacArthur Longitudinal Twin Study. Child Development. 1992;63:1437–1455. doi: 10.1111/j.1467-8624.1992.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Larsen K, Coy KC, Gorman LL, Stuart S. Infant emotionality: Observational methods and the validity of maternal reports. Infancy. 2003;4:541–565. doi: 10.1207/S15327078IN0404_08. [DOI] [Google Scholar]
- Fox N, Henderson H, Marshall P, Nichols K, Ghera M. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Gagne JR, Goldsmith HH. A longitudinal analysis of anger and inhibitory control in twins from 12 to 36 months of age. Developmental Science. 2011;14:112–124. doi: 10.1111/j.1467-7687.2010.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Saudino KJ. Wait for it! A twin study of inhibitory control in early childhood. Behavior Genetics. 2010;40:327–337. doi: 10.1007/s10519-009-9316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, Goldsmith HH. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment. 2011;23:337–353. doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Vendlinski MK, Goldsmith HH. The genetics of childhood temperament. In: Yong-Kyu K, editor. The handbook of behavior genetics. New York: Springer; 2009. pp. 251–267. [DOI] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: A research note. Behavior Genetics. 1991;21:257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: Expanded content, stronger genetic evidence, new evidence for the importance of environment. Developmental Psychology. 1997;33:891–905. doi: 10.1037//0012-1649.33.6.891. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Campos JJ. The structure of temperamental fear and pleasure in infants: A psychometric perspective. Child Development. 1990;61:1944–1964. doi: 10.1111/j.1467-8624.1990.tb03577.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Gottesman II. Origins of variation in behavioral style: A longitudinal study of temperament in young twins. Child Development. 1981;52:91–103. [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KL. Linking temperamental fearfulness and anxiety symptoms: A behavior-genetic perspective. Biological Psychiatry. 2000;48:1199–1209. doi: 10.1016/S0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Developmental Psychology. 1999;35:972–985. doi: 10.1037//0012-1649.35.4.972. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly HH, Lemery KL, Longley S, Prescott A. The Laboratory Temperament Assessment Battery: Middle Childhood Version. University of Wisconsin-Madison; 2001. Unpublished manuscript. [Google Scholar]
- Izard CE. Basic emotions, natural kinds, emotion schemas, and a new paradigm. Perspectives on Psychological Science. 2007;2:260–280. doi: 10.1111/j.1745-6916.2007.00044.x. [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF, Bouchard TJ, McGue M. The personalities of twins: Just ordinary folks. Twin Research. 2002;5:125–131. doi: 10.1375/1369052022992. [DOI] [PubMed] [Google Scholar]
- Kagan J, Fox NA. Biology, culture and temperamental biases. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. Vol. 6. New York: Wiley; 2006. pp. 167–223. [Google Scholar]
- Karevold E, Coplan R, Stoolmiller M, Mathiesen K. A longitudinal study of the links between temperamental shyness, activity, and trajectories of internalising problems from infancy to middle childhood. Australian Journal of Psychology. 2012;63:36–43. [Google Scholar]
- Lemery KS, Essex MJ, Smider NA. Revealing the relation between temperament and behavior problem symptoms by eliminating measurement confounding: Expert ratings and factor analyses. Child Development. 2002;73:867–882. doi: 10.1111/1467-8624.00444. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K. Wisconsin Twin Panel: Current directions and findings. Twin Research and Human Genetics. 2006;9:1030–1037. doi: 10.1375/twin.9.6.1030. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant KL. Genes and environments: How they work together to promote resilience. In: Reich J, Zautra AJ, Hall JS, editors. Handbook of adult resilience. New York: Guildford; 2010. pp. 55–78. [Google Scholar]
- Lemery-Chalfant K, Kao K, Swann G, Goldsmith H. Childhood temperament: Passive gene-environment correlation, gene-environment interaction, and the hidden importance of the family environment. Development and Psychopathology. 2013;25:51–63. doi: 10.1017/S0954579412000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Clifford S, Swann G. Temperament and child psychopathology: Specificity in shared genetic effects. In: Kim’s Y, Saudino K, Ganiban J, editors. Behavior genetics book series: Behavior genetics of temperament and personality. New York: Springer; in press. [Google Scholar]
- Martin NG, Eaves LJ. The genetical analysis of covariance structure. Heredity. 1977;38:79–95. doi: 10.1038/hdy.1977.9. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behavior Genetics. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McGuire S, Clifford J, Fink J, Basho S, McDonnell AM. Children’s reactions to the unfamiliar in middle childhood and adolescence: An observational twin/sibling study. Personality and Individual Differences. 2003;35:339–354. doi: 10.1016/S0191-8869(02)00194-0. [DOI] [Google Scholar]
- Mullineaux P, Deater-Deckard K, Petrill S, Thompson L, DeThorne L. Temperament in middle childhood: A behavioral genetic analysis of fathers’ and mothers’ reports. Journal of Research in Personality. 2009;43:737–746. doi: 10.1016/j.jrp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotion: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/CABN.3.3.207. [DOI] [PubMed] [Google Scholar]
- Muthén B, Kaplan D. A comparison of some methodologies for the factor analysis of non-normal Likert variables. British Journal of Mathematical and Statistical Psychology. 1985;38:171–189. doi: 10.1111/j.2044-8317.1985.tb00832.x. [DOI] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, Ferdinand RF, Verhulst FC, Ormel J. Effortful control as moderator of the association between negative emotionality and adolescents’ mental health problems. Development and Psychopathology. 2007;19:523–539. doi: 10.1017/S0954579407070253. [DOI] [PubMed] [Google Scholar]
- OpenMx Development Team. OpenMx Official Documentation. 2011 Retrieved from http://openmx.psyc.virginia.edu/docs/openmx/latest/_static/rdoc/mxCI.html.
- Pleck JH, Masciadrelli BP. Parental involvement in U.S. Residential fathers: Levels, sources, and consequences. In: Lamb ME, editor. The role of the father in child development. 4. New York: Wiley; 2004. pp. 222–271. [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Behavioral genetics. 6. New York: Worth Publishers; 2013. [Google Scholar]
- Price TS, Freeman B, Craig IW, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Corley RP, Friedman NP, Hewitt JK, Hink LK, Johnson DP, Young SE. The etiology of observed negative emotionality from 14 to 24 months. Frontiers in Genetics. 2012:3. doi: 10.3389/fgene.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Kagan J, Reznick JS, Corley R. The heritability of inhibited and uninhibited behavior: A twin study. Developmental Psychology. 1992;28:1030–1037. doi: 10.1037//0012-1649.28.6.1030. [DOI] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at 3 to 7 years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. Vol. 6. New York: Wiley; 2006. pp. 105–176. [DOI] [Google Scholar]
- Rothbart MK, Evans DE, Ahadi SA. Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037/h0077714. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Parent ratings of infant temperament lessons from twin studies. Infant Behavior and Development. 2003;26:100–107. doi: 10.1016/S0163-6383(02)0017-6. [DOI] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. Journal of Developmental and Behavioral Pediatrics. 2005;26:214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Early childhood temperament as a determinant of externalising behaviour in adolescence. Development and Psychopathology. 1996;8:527–537. doi: 10.1017/S0954579400007252. [DOI] [Google Scholar]
- Singh AL, Waldman ID. The etiology of associations between negative emotionality and childhood externalizing disorders. Journal of Abnormal Psychology. 2010;119:376–388. doi: 10.1037/a0019342. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Waldman ID, Van Hulle CA, Lahey B. Shared genetic influences on negative emotionality and major depression/conduct disorder comorbidity. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:818–827. doi: 10.1016/j.jaac.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. The evolutionary psychology of emotions and their relationship to internal regulatory variables. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3. New York: Guildford; 2008. pp. 114–137. [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: A voxel-based meta-analysis. Journal of Cognitive Neuroscience. 2010;22:2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]