Abstract
Gas-phase amidation of carboxylic acids in multiply-charged peptides is demonstrated via ion/ion reactions with Woodward’s reagent K (wrk) in both positive and negative mode. Woodward’s reagent K, N-ethyl-3-phenylisoxazolium-3′-sulfonate, is a commonly used reagent that activates carboxylates to form amide bonds with amines in solution. Here, we demonstrate that the analogous gas-phase chemistry occurs upon reaction of the wrk ions and doubly protonated (or doubly deprotonated) peptide ions containing the carboxylic acid functionality. The reaction involves the formation of the enol ester intermediate in the electrostatic complex. Upon collisional activation, the ethyl amine on the reagent is transferred to the activated carbonyl carbon on the peptide, resulting in the formation of an ethyl amide (addition of 27 Da to the peptide) with loss of a neutral ketene derivative. Further collision-induced dissociation (CID) of the products and comparison with solution-phase amidation product confirms the structure of the ethyl amide.
Keywords: Ion/ion reaction, carboxylic acid amidation, Woodward’s reagent K
INTRODUCTION
Peptide sequencing for the identification and structural characterization of proteins is a key application of tandem mass spectrometry in biological research. It is widely appreciated that the nature of an ion plays a key role in determining its fragmentation pattern and consequently the structural information that it can provide.1 In an effort to influence fragmentation behavior or to facilitate spectral interpretation, many chemical derivatization strategies have been developed for the manipulation of proteins and peptides in the solution-phase.2,3,4 For example, solution-phase guanidination converts lysine residues to more basic homoarginine residues, which affects the proton mobility and thus enhancing the aspartic acid effect.5 In contrast, ornithination of arginine residues via deguanidination with hydrazine can promote preferential cleavages C-terminal to the ornithine site.6 These modifications as well as others7,8 alter the fragmentation pathways of peptides, leading to sequence information that is complementary to that of the unmodified ions.
Gas-phase ion/ion reactions have been developed to modify analyte ions for structural characterization in tandem mass spectrometry. While most condensed-phase derivatization reactions are performed in an off-line fashion, gas-phase ion/ion reactions provide a means to manipulate ion type within the mass spectrometer, allowing rapid examination of the reaction products and facile comparison with the unmodified analytes. For example, proton transfer,9 electron transfer10,11 and metal ion transfer12 reactions have been used to decouple the ion type from the ionization method, expanding the range of ion types that can be investigated by MSn. Aside from these charged particle transfer reactions, a number of approaches to covalently modify ions via a long-lived complex in the gas-phase have been developed, aiming at selectively manipulating ion types at specific functionalities. For example, N-hydroxysuccinimide (NHS) and sulfo-NHS ester-based reagents have been used to covalently modify primary amines (N-terminus, lysine side chain)13 and guanidines14,15 on arginine side chains by forming amide bonds via ion/ion reactions. Similarly, the formation of imine bonds (i.e., Schiff base formation) with primary amines using 4-formyl-1,3-benzenedisulfonic acid (FBDSA) dianions has been demonstrated.16,17 Recently, selective oxidation of the methionine side chain was demonstrated via ion/ion reactions with periodate anions. 18 Carboxylate functionalities have been found to be reactive toward the N-hydroxysuccinimide ester of 4-trimethylammonium butyrate (TMAB-NHS) reagent as well. 19 These specific covalent modifications of peptides and proteins via ion/ion reactions have been used to tag peptides and proteins with chromophores, 20 cross-link peptides and proteins, 21,22 and increase sequence information upon collisional activation.23 In addition, some reaction products observed in these gas-phase reactions are not typically observed in solution,14,19 providing exclusive access to novel fragmentation patterns.
The gas-phase modification of carboxylic acid functionalities via ion/ion reactions with the carbodiimide reagents [N-cyclohexyl-N′-(2-morpholinoethyl) carbodiimide (CMC) and [3-(3-Ethylcarbodiimide-1-yl)propyl] trimethylaminium (ECPT)] resulting in the formation of amide bonds has recently been reported.24 In the electrostatic complexes formed with the cationic carbodiimide reagents and doubly deprotonated analytes, the carboxylic acid adds to one of the carbodiimide double bonds, forming an o-acylisourea ester, followed by amide bond formation between the analyte carboxylic acid group and the carbodiimide nitrogen atom upon collision-induced dissociation (CID). A limitation associated with these reagents is that they contain fixed-charged cationic groups, which restricts their use to polypeptide anions. The carboxylic acid groups of peptide anions are often deprotonated and carboxylates are unreactive with CMC. Furthermore, CID of polypeptide anions often yields less informative product ion spectra than the corresponding cations. It is therefore desirable to develop anionic reagents that can react with carboxylic acid groups in polypeptide cations. Here, we demonstrate the use of N-ethyl-3-phenylisoxazolium-3′-sulfonate, commonly referred to as Woodward’s reagent K (wrk), as another ion/ion reagent with specificity toward carboxylic functionalities. Both cationic and anionic versions of the reagent can be generated, which significantly expands the utility of carboxylic acid amidation of polypeptides in the gas-phase. This reaction could be used to selectively probe the C-terminus and acidic side chains in peptides, as is demonstrated here with tryptic peptides. Additionally, the ease of altering the ethyl group on wrk to other groups provides a platform for the design of a variety of gas-phase amidation reagents.
EXPERIMENTAL
Materials
Methanol, ammonium hydroxide and glacial acetic acid were purchased from Mallinckrodt (Phillipsburg, NJ). The peptides ARAAARA, AKAAARA, AKAAAKA, AHARAHARA, ARAMAKA, HGAGGHGAGGHL, AAEAAEAA and AADAADAA were custom synthesized by NeoBioLab (Cambridge, MA). The peptides ARACAKA, ARAWAKA were synthesized by Pepnome Ltd. (Shenzhen, China). The peptides GRGDSPR, LKRApYLG, LKRApYGL-NH2 were custom synthesized by AnaSpec Inc. (San Jose, CA). The peptide GLSDGEWQQVLNVWGK was purchased from SynPep (Dublin, CA). Woodward’s reagent K, Angiotensin II (sequence: DRVYIHPF), ubiquitin from bovine erythrocytes, α-casein from bovine milk, TPCK-treated trypsin from bovine pancreas, peptide RPKPQQFFG, acetyl chloride and ethylamine were obtained from Sigma-Aldrich (St. Louis, MO). The reagent 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) was purchased from Thermo Fisher Scientific (Rockford, IL). All materials were used without further purification. All peptide solutions for positive electrospray were prepared in 49.5/49.5/1 (v/v/v) methanol/water/acetic acid (~10 μM), and all peptide solutions for negative electrospray were prepared in 49.5/49.5/1 (v/v/v) methanol/water/ammonium hydroxide (~10 μM). The wrk reagent was dissolved in water (~2 mM), and a new solution was made before each experiment.
Peptide C-terminal methyl esterification
Peptide methyl esterification was performed according to a previously described procedure. 25 A solution of 2 M HCl in methanol was prepared by adding 40 μL acetyl chloride dropwise to 250 μL of chilled, dry methanol. Approximately 1 mg of solid peptide was dissolved in 100 μL of the prepared solution. The reaction was allowed to proceed for 2 h at room temperature and then the samples were lyophilized and reconstituted in 1 mL of water.
Peptide C-terminal amidation
C-terminal ethyl amidation was performed by first dissolving approximately 1 mg of solid peptide in 500 μL of water. The C-terminal carboxylic acid group was activated using an equimolar amount of EDC. To this, 5 μL of 100 nM ethyl amine solution was added, which resulted in the formation of an amide bond between the carboxyl group and the ethyl amine. The reaction mixture was then lyophilized and reconstituted in 1 mL of water.
Tryptic digest
The procedure for the tryptic digestion of ubiquitin has been described previously.25 Separation of the tryptic peptides was performed using a reverse-phase HPLC (Agilent 1100, Palo Alto, CA) equipped with an Aquapore RP-300 (7 μm pore size, 100 mm × 4.6 mm i.d.) column (Perkin-Elmer, Wellesley, MA) operated at 1 mL/min. The gradient for the HPLC separation has been described.25 Following separation, collected fractions were lyophilized and reconstituted in 500 μL of water.
Mass Spectrometry
All experiments were performed using a prototype version of a triple quadrupole/linear ion trap mass spectrometer26 (QTRAP, AB Sciex, Concord, ON, Canada), previously modified for ion/ion reactions.27 Alternately pulsed nano-electrospray (nESI) allows for sequential injections of the analyte and reagent ions into the q2 reaction cell. The reagents and doubly protonated peptide cations (or doubly deprotonated peptide anions) were sequentially isolated in the Q1-mass filter prior to their injection into the q2 reaction cell. After a defined mutual storage reaction time of 100–1000 milliseconds, the product ions were then transferred to Q3 where the electrostatic complexes were mass-selected. Resonant excitation was used to first activate the complexes to induce covalent modifications and later to dissociate the modified peptides in Q3. The product ions were then mass analyzed by mass-selective axial ejection.28
RESULTS AND DISCUSSION
Reactivity with carboxylic acids
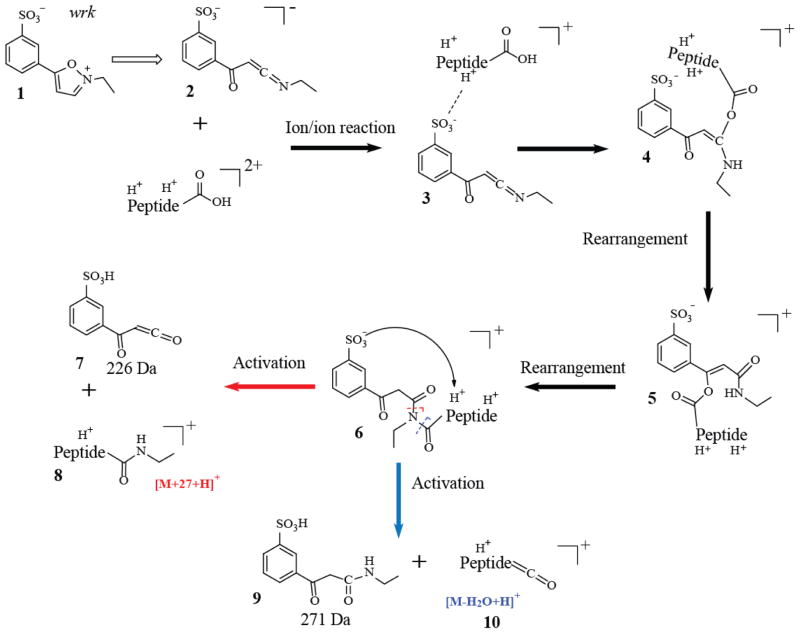
Woodward’s reagent K29, 30 and some other isoxazolium salts constitute a class of commonly used reagents that activate carboxylates to form amide bonds with amines in solution. These reagents have been used to modify carboxylic acid groups in the active centers of enzymes31,32,33 and to determine of the total carboxylate content in proteins.34 The reaction mechanism includes the irreversible conversion of the reagent to a reactive keto-ketenimine by proton abstraction from the 3 position of the isoxazole. This intermediate then reacts with a carboxylic acid to create an enol ester, which is highly susceptible to nucleophilic attack. The condensed-phase reaction with an amine proceeds to amide bond formation with loss of the inactive diketo derivative.2 However, in the gas-phase, the absence of local nucleophilic groups allows the enol ester intermediate to rearrange to an imide (Scheme 1), which gives rise to a characteristic loss of a neutral ketene derivative from one side of the imide upon collisional activation. The net result is an ethyl amidation of the carboxylic acid functionality on the analyte, represented as [M+27+H]+ in Scheme 1. Here we present the use of wrk in both positive mode and negative mode, i.e., [wrk-H]− and [wrk+H]+ reacting with doubly-protonated or doubly-deprotonated analytes via gas-phase ion/ion reactions.
Scheme 1.
Formation of the ethyl amide via the ion/ion reaction between a doubly protonated carboxylic acid-containing peptide and [wrk-H]−.2,31
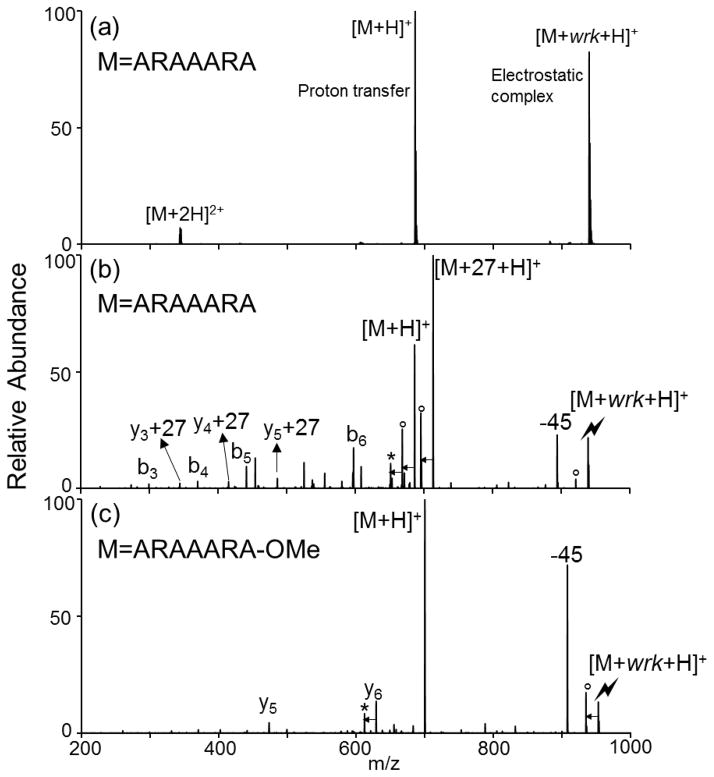
Several model peptides have been used to illustrate the chemistry shown in Scheme 1. The doubly protonated peptide ARAAARA was allowed to react with the deprotonated reagent [wrk-H]− (Figure 1(a)), which is believed to be in the form of the reactive keto-ketenimine 2, based on its mass (m/z 252). The spectrum produced via the ion/ion reaction between [ARAAARA+2H]2+ and [wrk-H]− is shown in Figure 1(a), in which the long-lived electrostatic complex 3, [M+wrk+H]+, is observed. Subsequent CID of the complex (Figure 1(b)) gives rise to three dissociation pathways. The first pathway leads to proton transfer from the peptide to the reagent, resulting in loss of the neutral wrk reagent, as reflected by [M+H]+ signal. The second and third pathways are shown in Scheme 1, wherein an addition reaction first takes place between the reactive keto-ketenimine and the carboxylic acid group within the electrostatic complex 3, resulting in the formation of intermediate 4. After several tautomerizations and six-member ring rearrangements, an imide 6 is formed. In previous studies, 6, N-acyl-N-ethylbenzoylacetamide, has been proposed as a side product in the solution-phase activation of acetic acid with wrk.31 In the solution-phase with the presence of a primary amine, the reaction proceeds to form an amide bond with the loss of the inactive diketo derivative (same as 9). However, in the gas-phase when a local primary amine group is absent, the rearrangement to form 6 dominates. Upon CID, either one of the amide bonds of the imide 6 can be cleaved. The second pathway involves the cleavage at the reagent side, which gives rise to the signature loss of a ketene to form 7 and the amidation product 8, indicated by [M+27+H]+. The third pathway involves the cleavage at the peptide side of the amide nitrogen, resulting in a loss of a 271 Da diketo derivative 9 and the formation of the [M-H2O+H]+ (10) peak.
Figure 1.
Product ion spectra derived from (a) ion/ion reaction between [ARAAARA+2H]2+ and [wrk-H]−, (b) CID of [ARAAARA+wrk+H]+ complex, and (c) CID of [ARAAARA-OMe+wrk+H]+ complex. (Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia loss whereas circles (°) denote water losses.)
When the C-terminal free carboxylic acid is methyl esterified, the ion/ion reaction between [ARAAARA-OMe+2H]2+ and [wrk-H]− results in complex formation. However, as seen in Figure 1(c), subsequent CID of the isolated complex does not lead to the 226 Da signature loss. In this instance, proton transfer and a loss of 45 Da from the complex dominate. The 45 Da loss is likely to be due to ethyl amine loss arising from the N-C bond cleavage on the reagent ion. Similarly, the signature 226 Da loss is not observed upon the ion/ion reaction of wrk anion with the doubly sodiated peptide (Supplemental Figure 1), [ARAAARA+2Na]2+, where the sodium is thought to be associated with the C-terminal carboxyl group.35 These results suggest that wrk is selective for free, neutral carboxylic acid groups.
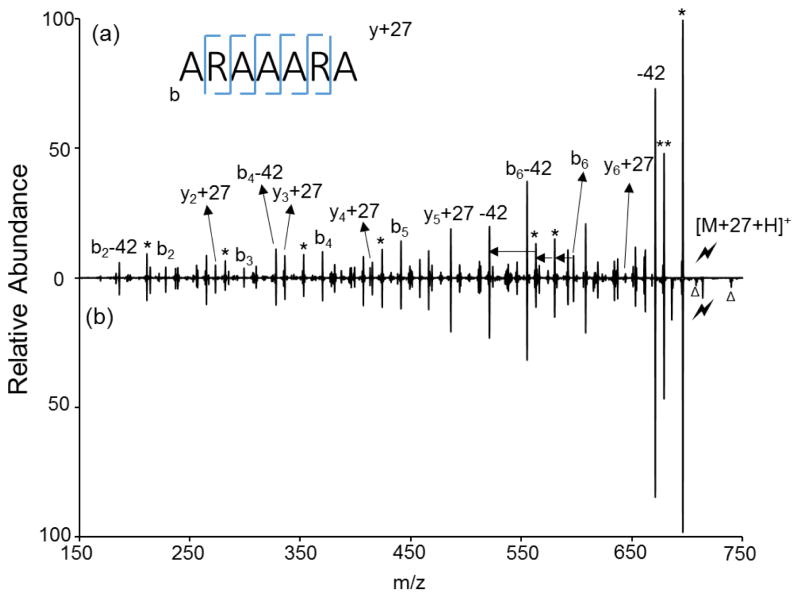
Further isolation and CID was performed to investigate the structure of the amidated product noted in Figure 1(b). As can be seen in Figure 2(a), CID of the [M+27+H]+ ion arising from the gas-phase reaction gives a series of b- and y+27 ions, suggesting the 27 addition is located at the C-terminus. These sequence fragments are also present in the CID spectrum of the electrostatic complex, as shown in Figure 1(b). The 42 Da losses are NH=C=NH losses originating from the deguanidination of the arginine side chain.6 A solution-phase ethyl amidation of ARAAARA was performed to generate [ARAAARA-CONHEt + H]+ as a comparison. The CID spectrum of the solution-phase product (Figure 2b) was compared with that of [M+27+H]+ generated via the gas-phase reaction. These spectra are essentially identical, supporting the conclusion that the gas-phase C-terminal amidation reaction is occurring upon ion/ion reaction with wrk. This process was also observed with several other model peptides, including ARAAAKA and AKAAAKA (Supplemental Figure 2). The same chemistry works for more highly protonated peptides as well. Supplemental Figure 3 demonstrates an example of the reaction between [RARARAA+3H]3+ and [wrk-H]−, which results in a doubly protonated product [RARARAA+27+2H]2+.
Figure 2.
Product ion spectra derived from CID of (a) [ARAAARA+27+H]+ generated via gas phase ion/ion reaction and (b) [ARAAARA-CONHEt + H]+ from solution phase modification. (Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia losses whereas triangles (△) denote impurities that can be seen in isolation)
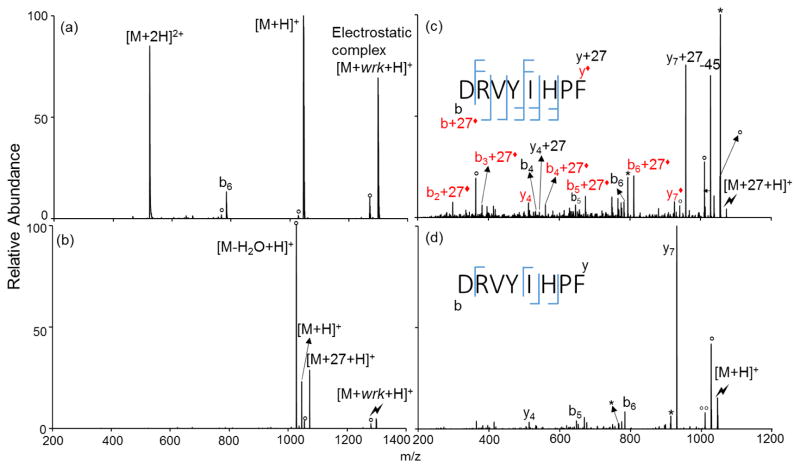
In order to explore the reactivity of side-chain carboxylic acids as well as the C-terminus, Angiotensin II (DRVYIHPF) was used as Asp-containing a model peptide. As shown in Figure 3, the reaction between the doubly protonated peptide and [wrk-H]− produces an electrostatic complex, and CID of the complex gives [M-H2O+H]+ and [M+H]+ products in addition to the signature [M+27+H]+ ion. Subsequent CID of the [M+27+H]+ ion (Figure 3(c)) produces b- and y- ions, together with y- and b- ions that contain an addition of 27 Da, suggesting the amidation occurs on both the C-terminus and the aspartic acid side-chain. In this case, the covalent modification can alter fragmentation pathways to yield more information about the peptides. This is demonstrated in the comparison between Figure 2(c) and Figure 3(d), which shows the CID spectrum of [DRVYIHPF+H]+ generated via positive nESI. The y7 ion arising from the aspartic acid effect 36 is dominant in Figure 3(d), whereas in Figure 3(c) higher sequence coverage is observed on the amidated species. For the side-chain amidated isomer, the abundance of the y7◆ is relatively at the same scale as other fragments originating from this isomer, whereas for the C-terminal amidated isomer, again the y7+27 arising from the aspartic effect is greatly more abundant (Figure 3(c)). The amidation suppresses the aspartic acid effect, probably due to the fact that the amidated aspartic side chain is less capable of initiating a nucleophilic attack at the amide bond C-terminal to the aspartic acid residue.
Figure 3.
Product ion spectra derived from (a) the ion/ion reaction between [DRVYIHPF+2H]2+ and [wrk-H]+, (b) CID of [DRVYIHPF+H+wrk]+ complex, (c) CID of [DRVYIHPF+27+H]+, and (d) CID of [DRVYIHPF+H]+(Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia losses whereas circles (°) denote water losses. The red labels with a solid diamond represent fragments corresponding to a modification on an aspartic acid side chain.)
Specificity for carboxylic acids
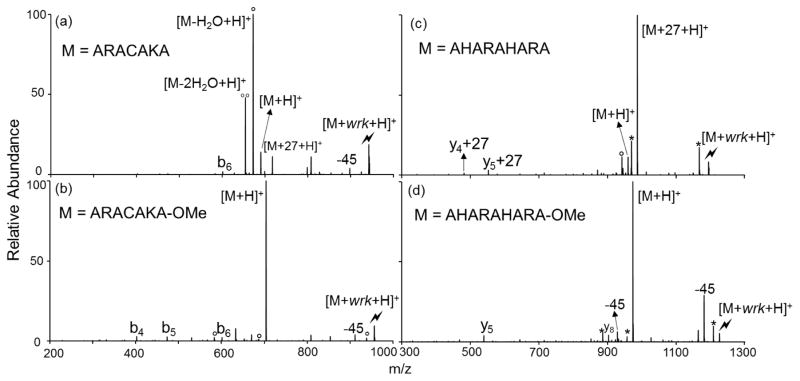
Although wrk has been used successfully for solution-phase conjugation applications with proteins and other molecules to form amide linkages, its specificity has been called into question.37,38 The relevant studies observed that wrk also reacted with cysteine and histidine residues in E. coli L-threonine dehydrogenase and phosphoenolpyruvate carboxykinase. To address this possible concern, we tested the reactivity of wrk with other side-chain functionalities. The peptides ARACAKA, AHARAHARA, HGAGGHGAGGHL and their methyl esters were used to test the reactivity of cysteine and histidine residues, for example. Ion/ion reactions between the doubly protonated peptides and [wrk-H]− were performed, and the generated complexes were activated. Activation of the [ARACAKA+wrk+H]+ species produces the [M+27+H]+ peak (Figure 4(a)), which is absent in the CID spectrum of [ARACAKA-OMe+wrk+H]+ (Figure 4(b)) where the C-terminus has been protected. This suggests that in the gas phase, cysteine does not react with wrk. Similarly, histidine residues do not show reactivity with wrk (Figures 4(c) and (d)). A number of other model peptides were used to examine the reactivity of other amino acid residues, including ARAMAKA, ARAWAKA, GRGDSPR, RPKPQQFFG, etc. As before, only the carboxylic acid functionalities on these peptides show reactivity with wrk. Additionally, reactions have been performed with the phosphorylated peptides LKRApYLG and C-terminal protected LKRApYGL-CONH2 and show that the phosphorylation does not affect the wrk reactivity with the C-terminus (Supplemental Figure 4). These results suggest that wrk reacts selectively with carboxylic acids.
Figure 4.
Product ion spectra derived from CID of (a) [ARACAKA+wrk+H]+ complex, (b) [ARACAKA-OMe+wrk+H]+ complex, (c) [AHARAHARA+wrk+H]+ complex and (d) [AHARAHARA-OMe+wrk+H]+ complex. (Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia losses whereas circles (°) denote water losses)
Application to tryptic peptides
Ion/ion reactions with wrk and tryptic peptides were performed to evaluate the amidation reaction in a common peptide sequencing scenario. A tryptic digestion was carried out on bovine ubiquitin, yielding the peptides LIFAGK, TLSDYNIQK, ESTLHLVLR, EGIPPDQQR and TITLEVEPSDTIENVK. The doubly protonated peptides were reacted with [wrk-H]−. In each case, the ion/ion reactions generated [M+27+H]+ ions upon collisional activation of the ion/ion complex. In each case, the CID spectrum of the [M+27+H]+ ion is compared with the corresponding CID spectrum of the [M+H]+ ion (see below).
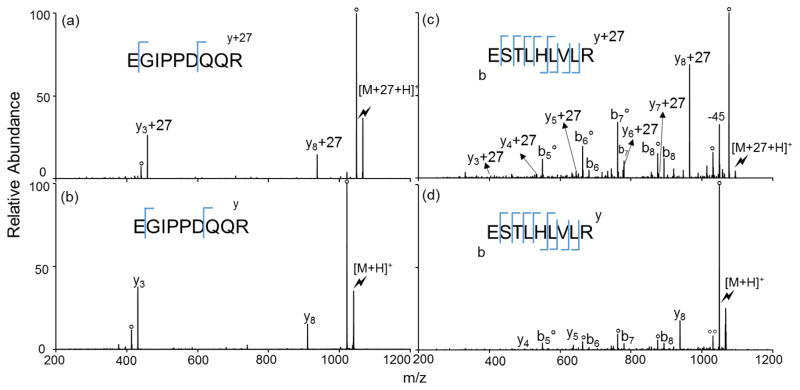
Of particular interest here is the possible influence of the basic side chain of the C-terminal residue on the reactivity of the C-terminus toward wrk. The C-terminal side-chain is expected to be protonated in most doubly protonated tryptic peptides. The presence of the proximal charge might be expected to alter the reactivity of the carboxylic acid of the C-terminus, particularly if attachment of the sulfonate group places the reactive portion of the reagent close to the C-terminal carboxylic acid. CID spectra of the modified and unmodified singly-protonated versions of the two tryptic peptides containing a C-terminal arginine, EGIPPDQQR and ESTLHLVLR, are shown in Figure 5. For both the modified and unmodified forms of EGIPPDQQR, the spectra are dominated by a water loss fragment and y-ions generated directly C-terminal to the glutamic acid and aspartic acid residues (i.e., y8- and y3-ions, respectively). In the case of the modified peptide, essentially all of the y-ions are modified (i.e., they are observed as y8+27 and y3+27). The lack of unmodified y-ions in the case of the modified peptide suggests that the modification predominantly occurs at the C-terminus with little modification at either of the acidic side-chains. No b-ions are observed, presumably due to the presence of the arginine residue at the C-terminus. For ESTLHLVLR, b-ions are observed if they contain the histidine residue. No b-ions generated from the modified peptide are observed to be modified. Additionally, all y-ions derived from the modified peptide are observed as y+27 species. Though there is a glutamic acid residue in the sequence, these data indicate that the C-terminus preferentially reacts with wrk in this case as well, possibly due to the localization of the reagent near to the C-terminus. This differs from the DRVYIHPF case, as noted in the discussion of Figure 3(c), which shows mixtures of modified and unmodified b- and y-ions. In the case of DRVYIHPF, the arginine residue is adjacent to the N-terminal aspartic acid residue and the spectrum clearly shows that there is a mixture of the two possible modification sites.
Figure 5.
Product ion spectra derived from CID of (a) [EGIPPDQQR+27+H]+, (b) [EGIPPDQQR+H]+ (c) [ESTLHLVLR+27+H]+ and (d) [ESTLHLVLR+H]+(Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia losses whereas circles (°) denote water losses)
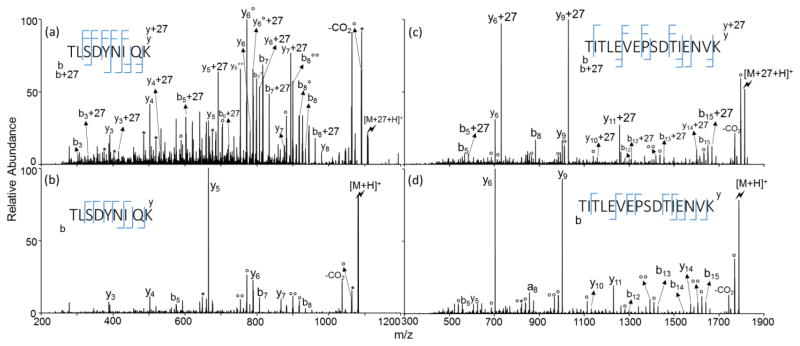
Examples of ion/ion reactions with peptides containing a C-terminal lysine are shown in Figure 6. For TLSDYNIQK, both b+27 and y+27 ions are observed in the CID spectrum of [M+27+H]+ as well as b- and y- ions, which is consistent with a mixture of modification sites. The modified ion population is less dominated by the aspartic acid cleavage leading to the y5-ion noted with the unmodified species, which might reflect the inhibition of this channel for the ions modified on the aspartic acid residue. A similar effect on the dominant aspartic acid cleavage was also noted with modification of angiotensin (DRVYIHPF, Figure 3), which showed some amidation of the aspartic acid residue. Data for the modified and unmodified TITLEVEPSDTIENVK ions (Figures 6(c) and 6(d)) suggest that the modification takes place mostly at the C-terminus but there are also mixtures of modified and unmodified y- and b-ions that indicate contributions from ions modified at different sites. The lysine-terminated tryptic peptides certainly show significant reactivity at the C-terminus but they appear to be less strongly directed to the C-terminus than the arginine-terminated peptides, based on this admittedly small data set. Additional example showing the differences between tryptic peptides that contain C-terminal lysine or arginine is show in Supplemental Figure 5 using EPMIGVNEQLAYFYPELFR from bovine α-casein, and GLSDGEWQQVLNVWGK from horse myoglobin.
Figure 6.
Product ion spectra derived from CID of (a) [TLSDYNIQK+27+H]+, (b) [TLSDYNIQK+H]+, (c) [TITLEVEPSDTIENVK+27+H]+ and (d) [TITLEVEPSDTIENVK+H]+ (Lightning bolt denotes ions subjected to collisional activation. Asterisks (*) denote ammonia losses whereas circle (°) denotes water losses)
A much larger data set of tryptic peptides is needed in order to draw firm conclusions regarding the effect of gas-phase amidation on tandem MS results. However, for these tryptic peptide ions, amidation appears to have relatively little effect on fragmentation with the notable exception of inhibiting the well-known cleavage C-terminal to aspartic acid residues when the relevant aspartic acid side-chain is amidated. Otherwise, ethyl amidation has little effect on proton mobility and does not appear to open any new highly favorable channels. The observed mass shifts associated with the modification can provide information regarding the sites of acidic residues and, when modification is at the C-terminus, can be used to differentiate b- and y-ions.
Woodward’s reagent K cations and doubly deprotonated analytes
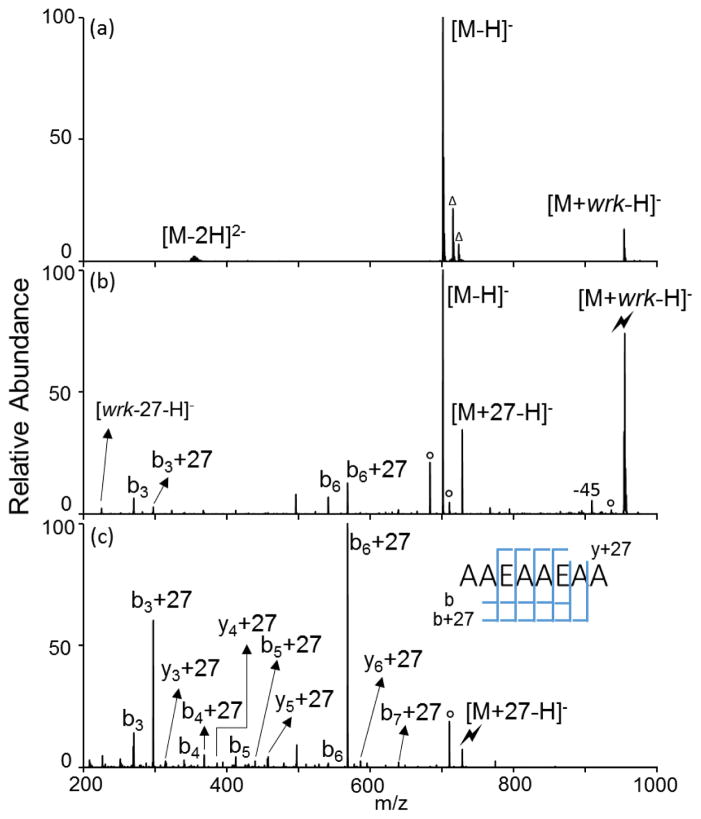
The reagent species examined here can be generated as the protonated molecule, [wrk+H]+ (m/z 254), via positive nESI. This reagent cation is found to react with carboxylic acid groups present in analyte dianions in a similar fashion as the wrk anion. This is illustrated using the ion/ion reaction between wrk cations and the doubly deprotonated form of AAEAAEAA that contains one neutral carboxylic acid group, as shown in Figure 7. The complex is presumed to be stabilized by the interaction between the sulfonic acid group, –SO3H, on the reagent and a carboxylate group on the peptide anion. In Figure 7(b), a signature [M+27-H]− peak is produced from the complex upon activation. The complementary ion, [wrk-27-H]−, is also observed. The proton transfer pathway gives rise to the [M-H]− peak. Some sequence fragments of the peptide with or without the 27 Da addition are also present. Subsequent CID of the [M+27-H]− gives rise to a series of both modified and unmodified b- ions, indicating the amidation reaction occurs at one of the glutamic side chains or the C-terminus. Similar reactivity can be observed on doubly deprotonated AADAADAA as well (Supplemental Figure 6).
Figure 7.
Product ion spectra derived from (a) ion/ion reaction between [AAEAAEAA-2H]2−[wrk+H]+, (b) CID of [AAEAAEAA+wrk-H]− complex and (c) CID of [AAEAAEAA+27-H]− (Lightning bolt indicates ions subjected to collisional activation. Circles (°) denote water losses whereas triangles (△) denote peaks originated from impurities that can be seen in isolation)
CONCLUSIONS
We demonstrate that wrk reagent ions are able to modify carboxylic acid sites of doubly protonated (wrk anion) or doubly deprotonated (wrk cation) peptides in the gas-phase. The sulfonate or sulfonic acid group anchors the reagent to a protonated or deprotonated site, respectively, on the reaction partner, stabilizing a long-lived complex. Covalent chemistry involving the carboxylic acid functionality can proceed via a mechanism proposed in Scheme 1 and the formation of an ethyl amide is indicated by the signature addition of 27 Da to the analyte ion. Comparison of the CID spectra of modified peptides generated in the gas-phase and in solution indicates that the two pathways (i.e., gas-phase and solution-phase) form essentially the same products. In the absence of a carboxylic acid reactive site (i.e., the carboxylic acid is converted to a methyl ester, or to a sodium salt), the covalent chemistry cannot proceed and no ethyl amide is observed. Other amino acid side-chains such as cysteine, histidine, methionine, tryptophan, etc. are found to be unreactive with wrk in the gas-phase. This gas-phase amidation using the wrk anion, in particular, is of interest in that it affords means for the selective covalent modification of carboxylic acid groups in polypeptide cations as illustrated here by application to tryptic peptide cations derived from bovine ubiquitin.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under Grant GM 45372.
References
- 1.McLuckey SA, Mentinova M. Ion/neutral, ion/electron, ion/photon, and ion/ion interactions in tandem mass spectrometry: Do we need them all? Are they enough? J Am Soc Mass Spectrom. 2011;22:3–12. doi: 10.1007/s13361-010-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermanson GT. Bioconjugation Techniques. 2. Academic Press; Amsterdam: 2008. [Google Scholar]
- 3.Yang WC, Mirzaei H, Liu XP, Regnier FE. Enhancement of amino acid detection and quantification by electrospray ionization mass spectrometry. Anal Chem. 2006;78:4702–4708. doi: 10.1021/ac0600510. [DOI] [PubMed] [Google Scholar]
- 4.Beardsley RL, Sharon LA, Reilly JP. Peptide de novo sequencing facilitated by a dual-labeling strategy. Anal Chem. 2005;77:6300–6309. doi: 10.1021/ac050540k. [DOI] [PubMed] [Google Scholar]
- 5.Pitteri SJ, Reid GE, McLuckey SA. Affecting proton mobility in activated whole protein ions via lysine guanidination. J Proteome Res. 2004;3:46–54. doi: 10.1021/pr034054u. [DOI] [PubMed] [Google Scholar]
- 6.McGee WM, McLuckey SA. The ornithine effect in peptide cation dissociation. J Mass Spectrom. 2013;48:856–861. doi: 10.1002/jms.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen JA, Brodbelt JS. Simplifying fragmentation patterns of multiply charged peptides by N-terminal derivatization and electron transfer collision activated dissociation. Anal Chem. 2009;81:3645–3653. doi: 10.1021/ac9000942. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza VL, Vachet RW. Probing protein structure by amino acid-specific covalent labeling and mass spectrometry. Mass Spectrom Rev. 2009;28:785–815. doi: 10.1002/mas.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chrisman PA, Erickson DE, McLuckey SA. Relative information content top-down proteomics by mass spectrometry: the utility of ion/ion proton-transfer reactions in electrospray based approaches. Anal Chem. 2007;79:1073–1081. doi: 10.1021/ac061798t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Nat’l Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao G, Yang J, Cancilla MT, Meng F, McLuckey SA. Top-down interrogation of chemically modified oligonucleotides by negative electron transfer and collision induced dissociation. Anal Chem. 2013;85:4713–4720. doi: 10.1021/ac400448t. [DOI] [PubMed] [Google Scholar]
- 12.Newton KA, McLuckey SA. Gas-phase peptide/protein cationizing agent switching via ion/ion reactions. J Am Chem Soc. 2003;125:12404–12405. doi: 10.1021/ja036924e. [DOI] [PubMed] [Google Scholar]
- 13.Mentinova M, Barefoot NZ, McLuckey SA. Solution versus gas phase modification of peptide cations with NHS-ester reagents. J Am Soc Mass Spectrom. 2011;23:282–289. doi: 10.1007/s13361-011-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee WM, Mentinova M, McLuckey SA. Gas-phase conjugation to arginine residues in polypeptide ions via N-hydroxysuccinimide ester-based reagent ions. J Am Chem Soc. 2012;134:11412–11414. doi: 10.1021/ja304778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee WM, McLuckey SA. Efficient and directed peptide bond formation in the gas phase via ion/ion reactions. Proc Nat Acad Sci USA. 2014;111:1288–1292. doi: 10.1073/pnas.1317914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassell KM, Stutzman JR, McLuckey SA. Gas-phase bioconjugation of peptides via ion/ion charge inversion: Schiff base formation on the conversion of cations to anions. Anal Chem. 2010;82:1594–1597. doi: 10.1021/ac902732v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stutzman JR, Hassell KM, McLuckey SA. Dissociation behavior of tryptic and intramolecular disulfide-linked peptide ions modified in the gas phase via ion/ion reactions. Int J Mass Spectrom. 2012;312:195–200. doi: 10.1016/j.ijms.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilo AL, McLuckey SA. Oxidation of Methionine residues in polypeptide ions via gas-phase ion/ion chemistry. J Am Soc Mass Spectrom. 2014;25:1049–1057. doi: 10.1007/s13361-014-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Z, McGee WM, McLuckey SA. Gas phase reactivity of carboxylates with N-hydroxysuccinimide esters. J Am Soc Mass Spectrom. 2015;26:174–180. doi: 10.1007/s13361-014-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mentinova M, McLuckey SA. Covalent modification of gaseous peptide ions with N-Hydroxysuccinimide ester reagent ions. J Am Chem Soc. 2010;132:18248–18257. doi: 10.1021/ja107286p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mentinova M, McLuckey SA. Intra- and inter-molecular cross-linking of peptide ions in the gas phase: reagents and conditions. J Am Soc Mass Spectrom. 2011;22:912–921. doi: 10.1007/s13361-011-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb IK, Mentinova M, McGee WM, McLuckey SA. Gas-phase intramolecular protein crosslinking via ion/ion reactions: Ubiquitin and a homobifunctionalsulfo-NHS Ester. J Am Soc Mass Spectrom. 2013;24:733–743. doi: 10.1007/s13361-013-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stutzman JR, McLuckey SA. Ion/ion reactions of MALDI-derived peptide ions: increased sequence coverage via covalent and electrostatic modification upon charge inversion. Anal Chem. 2012;84:10679–10685. doi: 10.1021/ac302374p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice BM, Gilbert JD, Stutzman JR, Forrest WP, McLuckey SA. Gas-phase reactivity of carboxylic acid functional groups with carbodiimides. J Am Soc Mass Spectrom. 2013;24:30–37. doi: 10.1007/s13361-012-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han H, Xia Y, McLuckey SA. Ion trap collisional activation of c and z ions formed via gas-phase ion/ion electron transfer dissociation. J Proteome Res. 2007;6:3062–3069. doi: 10.1021/pr070177t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hager JW. A new linear ion trap mass spectrometer. Rapid Commun Mass Spectrom. 2002;16:512–526. doi: 10.1002/rcm.1020. [DOI] [PubMed] [Google Scholar]
- 27.Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. Mutual storage mode ion/ion reactions in hybrid linear ion trap. J Am Soc Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Londry FA, Hager JW. Mass selective axial ion ejection from a linear quadrupole ion trap. J Am Soc Mass Spectrom. 2003;14:1130–1147. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 29.Woodward RB, Olafson RA, Mayer H. A new synthesis of peptide. J Am Chem Soc. 1961;83:1010–1012. [Google Scholar]
- 30.Woodward RB, Olafson RA. A useful synthesis of peptides. Tetrahedron. 1966;(Suppl 8):321–346. [Google Scholar]
- 31.Bodlaender P, Feinstein G, Shaw E. The use of isoxazolium salts for carboxyl group modification in proteins. Trypsin Biochemistry. 1969;8:4941–4948. doi: 10.1021/bi00840a043. [DOI] [PubMed] [Google Scholar]
- 32.Bodlaender P, Feinstein G, Shaw E. The modification of essential carboxylic acid side chains of trypsin. Biochemistry. 1969;8:4949–4955. doi: 10.1021/bi00840a044. [DOI] [PubMed] [Google Scholar]
- 33.Komissarov Z, Romanova DV, Debabov VG. Complete inactivation of Escherichia coli uridine phosphorylase by modification of Asp5 with Woodward’s reagent K. J Biol Chem. 1995;270:10050–10055. doi: 10.1074/jbc.270.17.10050. [DOI] [PubMed] [Google Scholar]
- 34.Kosters HA, de Jongh HJH. Spectrophotometric tool for the determination of the total carboxylate content in proteins; Molar extinction coefficient of the enol ester from Woodward’s reagent K reacted with protein carboxylates. Anal Chem. 2003;75:2512–2516. doi: 10.1021/ac026279e. [DOI] [PubMed] [Google Scholar]
- 35.Bensadek D, Monigatti F, Steen JAJ, Steen H. Why by’s? Sodiation-induced tryptic peptide-like fragmentation of non-tryptic peptides. Int J Mass Spectrom. 2007;268:181–189. [Google Scholar]
- 36.Paizs B, Suhai A. Fragmentation pathways of protonated peptides. Mass Spectrom Rev. 2005;24:508–548. doi: 10.1002/mas.20024. [DOI] [PubMed] [Google Scholar]
- 37.Bustos P, Gajardo MI, Gómez C, Glodie H, Cardemil E, Jabalquinto AM. Woodward’s reagent K reacts with histidine and cysteine residues in Escherichia coli and Saccharomyces cerevisiae phosphoenolpyruvate carboxykinases. J Protein Chem. 1996;15:467–472. doi: 10.1007/BF01886854. [DOI] [PubMed] [Google Scholar]
- 38.Johnson AR, Dekker EE. Woodward’sEscherichia coli L-threonine dehydrogenase: Increased absorbance at 340–350 nm is due to modification of cysteine and histidine residues not aspartate or glutamate carboxyl groups. Protein Sci. 1996;5:382–390. doi: 10.1002/pro.5560050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.