Abstract
Objective
Recent studies point to the clinical and research utility of saliva as a valuable diagnostic aid for monitoring periodontal health. The objectives of this study were to detect novel biomarkers attributed to chronic inflammation in saliva and to determine if the levels of these markers correlate with severity of periodontitis and with standard obesity measures in participants in a periodontal maintenance program.
Design
In this cross-sectional assessment of 63 participants, unstimulated whole saliva was collected after recording anthropometric and clinical parameters of obesity and periodontitis, respectively. The levels of interleukin-1 receptor antagonist (IL-1ra), sCD40L, granzyme B and alpha-fetoprotein (AFP) in saliva were determined using multiplex proteomic immunoassays. The correlation between the four tested biomarker concentrations and obesity/periodontal measures was determined.
Results
Positive correlation between fat% and granzyme B levels (r=0.292; p=0.020) and negative correlation between BMI and sCD40L (r=0.256;p=0.043) was observed. In addition, positive correlation between severity of periodontal disease and levels of IL1-ra (r=0.253; p=0.046) and negative correlation between periodontitis severity and sCD40L salivary levels (r=0.272; p=0.031) was noted. None of the above correlations remained statistically significant after multiple comparisons adjustment. After adjustment for clinical covariates, the relationship between sCD40L and periodontal severity remained suggestive (p=0.081).
Conclusions
Levels of four novel biomarkers of periodontitis were detectable in saliva of subjects enrolled in a periodontal maintenance program. Prospective studies with larger sample sizes and other populations are warranted to explore the diagnostic applicability of these markers.
Keywords: Saliva, diagnostics, periodontal disease, obesity
Introduction
Obesity and chronic periodontitis are two highly prevalent conditions of inflammatory nature. The number of people in developed nations who are obese is at rise. In the United States, it was estimated that, in 2009–2010, 35.5% of adult men and 35.8% of adult women were obese.1 Periodontal disease is also highly prevalent, affecting approximately 47% of the United States population over the age of 30, according to 2009–2010 NHANES data.2 A recent update by the same group examined data from 2011–2012, including 7,066 adults over the age of 30.3 This update showed findings statistically similar to the data published in 2012. This study stated that 44.7% of adults in the United States had periodontitis. For the combined period of 2009–2012, 45.9% of adults over the age of 30 in the United States have periodontitis, with 8.9% having disease classified as severe.3 There is growing evidence that obesity may play an important role in the pathogenesis of periodontal disease. Recently published longitudinal studies and systematic reviews/meta-analyses clearly underscored this association.4,5 In addition, our group has reported a positive correlation between select obesity measures and the expression of pro-inflammatory mediators in peri-implant sulcular fluid.6
Predicting the onset and/or progression of chronic periodontitis solely based on clinical and radiographic assessments has inherent limitations, given the site-specific and non-linear nature of this local inflammatory condition. Furthermore, the influence of concomitant systemic inflammatory conditions, such as obesity, on the pathogenesis of chronic periodontitis cannot be fully understood on the basis of conventional diagnostic methods. The analysis of biologic fluids to identify markers that may enable clinicians to accurately predict the onset and progression of chronic periodontitis has been proposed as a complementary diagnostic method to overcome some of the aforementioned limitations.7 These advanced diagnostic approaches have the potential to allow clinicians to design a more effective, personalized treatment plan to either prevent or treat distinct forms of chronic periodontitis that have been traditionally considered to be the same, given its phenotypical similarities.8 Salivary diagnostics, one of these emerging diagnostic tools, has been well received because of the ease of collection, established laboratory protocols for analysis and the availability of extensive data from past studies using saliva that can be used for comparisons.9, 10
The diagnostic or prognostic value of multiple salivary markers has been studied for chronic periodontitis.11 In a separate case-control study, 98 inflammatory biomarkers were assessed in gingival crevicular fluid (GCF), of which only four novel biomarkers were significantly elevated in the chronic periodontitis group.12 These four biomarkers are interleukin-1 receptor antagonist (IL-1ra), sCD40L, granzyme B and alphafetoprotein (AFP). All of them were implicated to play a pivotal role in chronic inflammatory conditions.13,14,15 For example, high serum levels of IL-1ra, which inhibits the pro-inflammatory effect of IL-1, were found in patients with metabolic syndrome.16 Specific biological functions of the selected biomarkers are listed in Table 1. The primary objective of this cross-sectional assessment was to detect the baseline levels of these four novel biomarkers in saliva obtained from periodontal maintenance patients and to correlate these values with severity of periodontitis. Secondarily, the correlation between the levels of these novel markers and body fat indices was also assessed.
Table 1.
Biomarkers assessed in this study and their biological functions
| Biomarkers | Functional Relevance (References: 10, 11, 12 and 13) |
|---|---|
| Alpha-fetoprotein |
|
| Granzyme B |
|
| IL-1 Receptor Antagonist |
|
| sCD40L |
|
Materials and Methods
Participant Identification and Recruitment
This project stems from a parent study that evaluated the correlation between obesity measures and levels of inflammatory biomarkers in sulcular fluid obtained around dental implants.5 The parent study was conducted after obtaining Institutional Review Board’s approval from the University of Iowa’s Human Subjects Office (IRB # 201109878). Briefly, participants enrolled in the College of Dentistry periodontal recall program and having at least one rough surface implant in function for a minimum of 6 months were identified by searching the electronic health record (EHR). Data and sample collection were done in eligible participants between June 2012 and April 2013. To be eligible for the parent study, participants had to be 18 years of age or older, current non-smokers and had to be enrolled in a collegiate periodontal maintenance program. Participants with aggressive periodontitis and pregnant or nursing women were not eligible. Also, patients who were completely edentulous, had blade-type or smooth surface implants, or who had taken medications such as antibiotics and anti-inflammatory agents for 3 months prior to the study visit were excluded. Participant’s medical history was thoroughly reviewed during the initial telephone calls that we made to assess eligibility and also during the study visit.
Body Composition and Systemic Evaluation
Before clinical examination and sample collection, height (in meters), weight (in kilograms) and waist circumference (in centimeters) were measured and recorded, as described elsewhere.5 Briefly, body mass index (BMI) was calculated using the Quetelet Index [Weight (kg) / Height (meter)2]. Waist circumference (WC) of the subject was then recorded using measuring tape (RJL System, Clinton Township, MI, USA). Body fat content (%) was measured non-invasively using a bioimpedance-based body fat analyzer (RJL System, Clinton Township, USA). At the same visit, blood pressure and fasting blood glucose level (One Touch Ultra 2 Blood Glucose Meter, Milpitas, CA, USA) were also measured.
Saliva Collection and Analysis
Unstimulated whole saliva samples were collected from patients who fasted for at least one hour. Participants were asked to passively drool onto a 50 mL centrifuge tube (placed on ice). The participants were given 15 minutes to get a total volume of 2 mL. If the 2 mL volume was achieved before 15 minutes, the collection was stopped. The samples were aliquoted and stored in −80°C freezer for later use. At the end of the clinical phase, all saliva samples were removed from the −80°C freezer and thawed on ice. Particulates and debris in each sample were pelleted by centrifugation at 16,100 RCF (13,200 RPM, Eppendorf, 5415D centrifuge, Brinkmann Instruments, Inc., Westbury, NY) for 5 minutes at 24°C. The supernatants were removed and held on ice. The pellets were discarded.
The concentrations (pg/30 second) of IL1RA, sCD40L, GranzymeB, and AFP were determine in each sample using multiplexed fluorescent bead-based immunoassays (Millipore, Billerica, MA) in the Luminex 100 IS Instrument (Luminex, Austin, TX). Briefly, 25.0 µl of saliva supernatant was added to anti-human multi-cytokine magnetic beads (Milliplex immunoassay, Millipore, Billerica, MA) and incubated at 4°C for 18.0 hours. Unbound material was removed by aspiration (ELx405TS magnetic plate washer, BioTek, Winooski, VT USA); anti-human multi-cytokine biotin reporter was added; and the reactions were incubated at room temperature for 1.5 hours in the dark. Streptavidin–phycoerythrin was then added and the plates were incubated at room temperature for an additional 30 minutes. Following which, the plates were washed two times and beads were suspended in sheath fluid prior to reading (Luminex model 100 IS, Austin, TX). The standard curve ranged from 0.64 to 10,000.00 pg/ml for IL1RA and sCD40L; from 0.001 to5.000 ng/ml for GranzymeB, and from 0.14 to 100.00 ng/ml for AFP. Concentrations of IL1RA, sCD40L, GranzymeB, and AFP in each sample were interpolated from these standard curves (xPonent v3.1, Luminex, Austin, TX USA; MILLIPLEX Analyst v5.1, Millipore, Billerica, MA USA).
Periodontal Evaluation
Following sample collection, a comprehensive periodontal examination was conducted by one calibrated examiner (G.A-O.), including plaque levels assessment using the modified Quigley Hein plaque index.17 The following periodontal parameters were assessed and recorded: probing pocket depth, recession, presence or absence of bleeding on probing and suppuration on six points, as well as mobility (Miller scale), furcation involvement18 and width of facial keratinized tissue (if any). Examiner calibration was tested through partial periodontal examination (one quadrant) performed by the examiner on two volunteers on two separate occasions with 3 days apart and Cohen’s Weighted Kappa was used to assess examiner reliability. Participant’s brushing and flossing frequency, time since last prophylaxis, past history of periodontal therapy and familial history of periodontal disease were also recorded. Based on the recently updated case definitions for population-based surveillance of periodontitis, periodontal status of each our participant was categorized by one examiner into one of the following categories: no periodontitis, mild, moderate and severe periodontitis.19
Statistical Analysis
Spearman rank correlations were used to assess associations among periodontal status /obesity measures (waist circumference, BMI, and fat %), and the four biomarkers of interest. Bivariate associations were re-evaluated after adjustment for clinical covariates (brushing frequency, flossing, fasting blood glucose, days since professional cleaning, and plaque index) using regression models. Multiple comparisons adjustment was made by the standard Bonferroni method.
Results
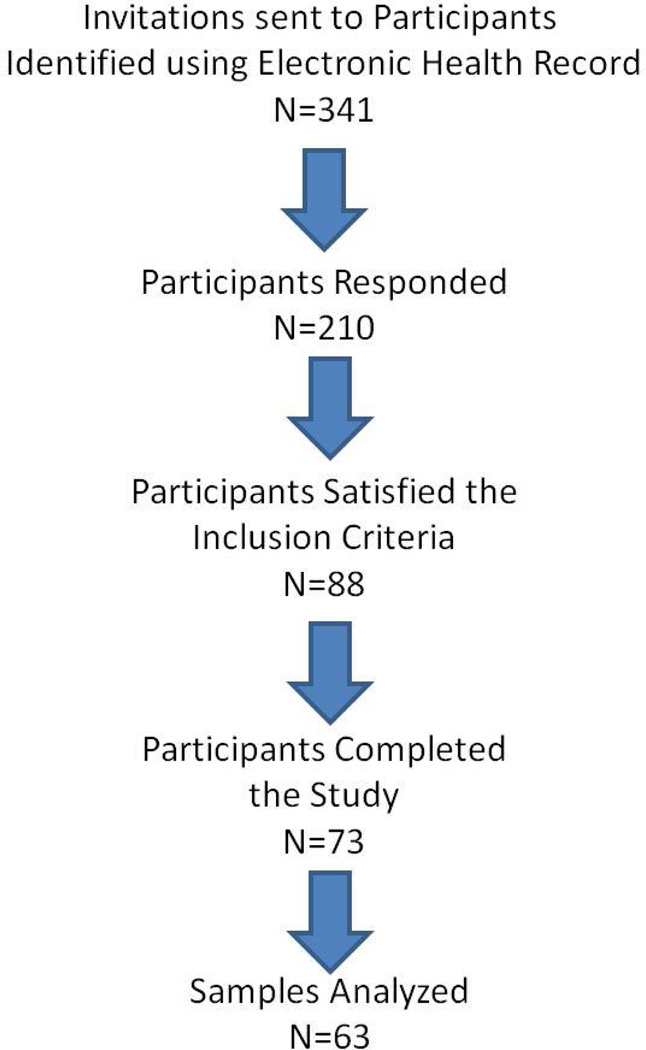
Of the 341 invitations sent to potential participants, 210 responded, 88 of which fulfilled the inclusion criteria. Of the 88 participants scheduled, 73 completed the study but the final analysis included samples from 63 participants as samples from 10 participants were deemed non-usable due to sampling errors or lack of sufficient volume of saliva for multiplex assay (Figure 1). Please see tables 2 and 3 for descriptive statistics for our study population. Though the participants were in active periodontal maintenance and exhibited low plaque index scores, it was clear that, based on the case definitions employed, close to 54% of them had moderate periodontitis at the time of the appointment and a smaller proportion (20.6%) were categorized as severe periodontitis subjects. Close to 60% and 78% of our participants reported that they brushed twice a day and flossed at least once a day, respectively.
Figure 1.
Flowchart depicting the study participant selection process
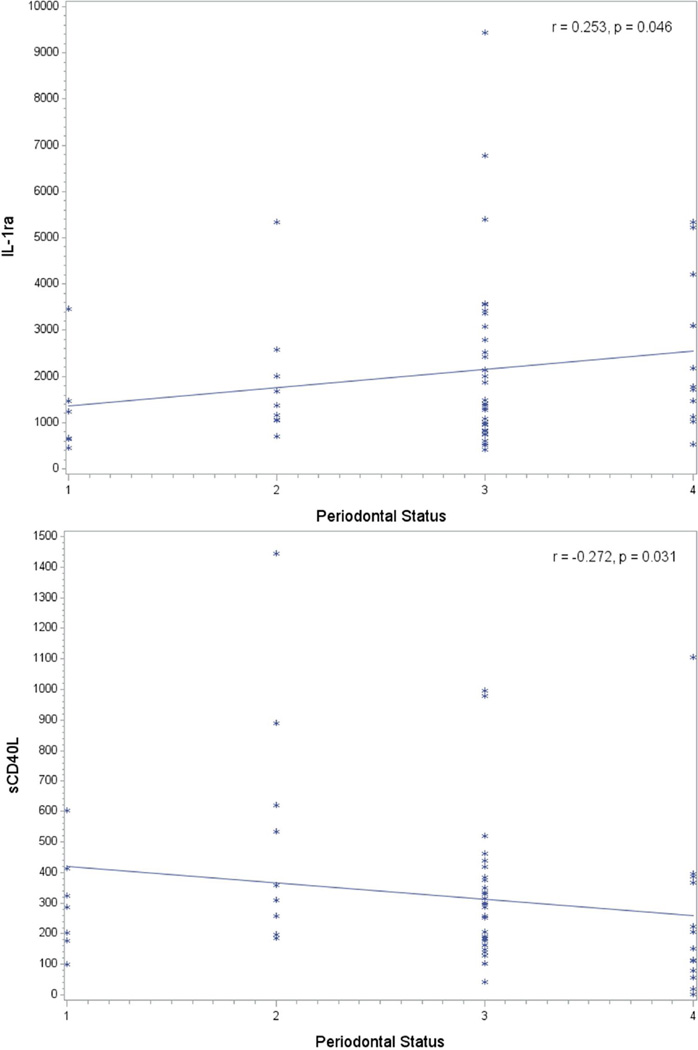
We show for the first time that these four novel biomarkers (IL-1ra, sCD40L and Granzyme and AFP) were detectable in saliva, but AFP levels were towards the lower end of the detection range. The mean levels and range of concentrations of these biomarkers in our subjects are displayed in Table 2. With regard to the correlation between periodontal status and levels of these four biomarkers, we observed a positive correlation between periodontitis severity and levels of IL1-ra (r=0.253; p=0.046) (Figure 2). We also observed a significant negative correlation between periodontitis severity and sCD40L salivary levels (r=0.272; p=0.031) (Figure 2). None of the above correlations remained statistically significant after multiple comparisons adjustment. After adjustment for clinical covariates, the relationship between sCD40L and periodontal severity remained suggestive (p=0.081).
Table 2.
Descriptive statistics of clinical and obesity measures and analyzed biomarkers in saliva
| Variables of Interest | N | Mean | Std Dev | Median |
|---|---|---|---|---|
| Age | 63 | 59.905 | 14.081 | 60 |
| Fasting blood glucose (mg/dL) | 59 | 89.915 | 10.70496 | 88 |
| BMI | 63 | 28.114 | 4.646777 | 27.9 |
| WC (mm) | 63 | 91.383 | 15.71448 | 93.25 |
| Fat content (%) | 63 | 31.12381 | 7.348112 | 29.7 |
| Plaque index | 63 | 0.793 | 0.486734 | 0.7 |
| Days since last cleaning | 63 | 230.714 | 296.8732 | 146 |
| IL1RA (pg/ml) | 63 | 2086.4 | 1717.1 | 1478 |
| sCD40L (pg/ml) | 63 | 321.731 | 268.5999 | 257.98 |
| Granzyme B (pg/ml) | 63 | 7.642 | 28.90583 | 0.005 |
| AFP (pg/ml) | 63 | 0.014 | 0.026807 | 0 |
Figure 2.
Relationship between periodontal status (1- no periodontitis, 2-mild periodontitis, 3-moderate periodontitis and 4 –severe periodontitis) and the levels of IL-1ra (top) and sCD40L (below) levels in saliva (in pg/ml)
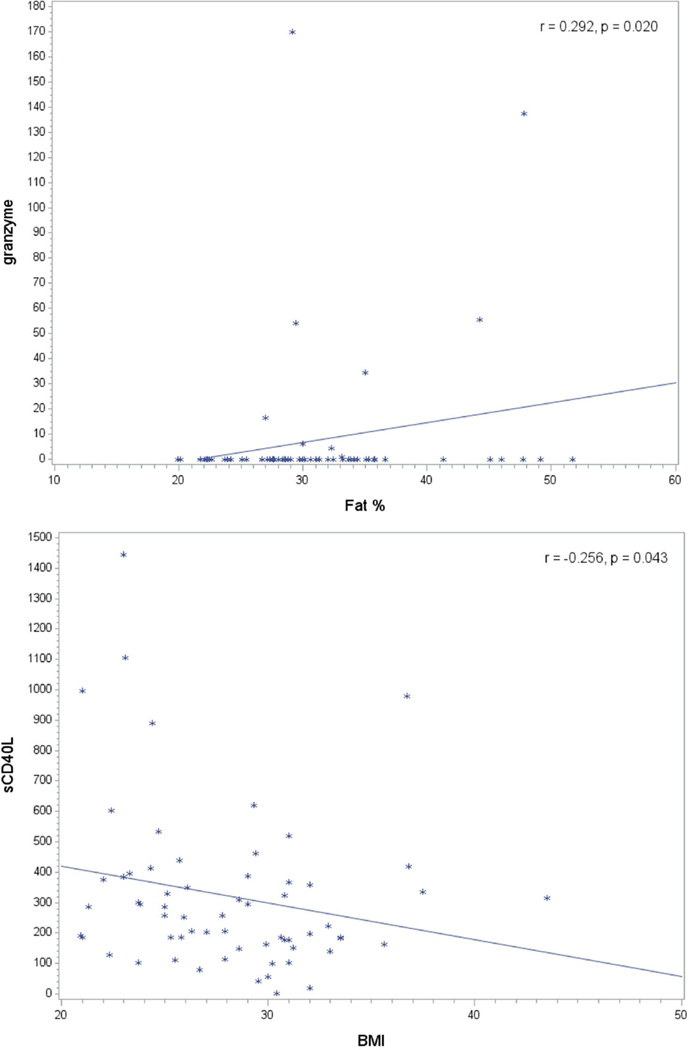
With regard to the correlation between obesity measures and selected biomarker levels in saliva, we noticed a significant positive correlation between fat% and Granzyme B levels (r=0.292; p=0.020). Additionally, a strong negative correlation was observed between BMI and sCD40L levels in saliva (r=0.256;p=0.043) (Figure 3). Nevertheless, none of the above correlations remained statistically significant after multiple comparisons adjustment. Pairwise correlation between biomarkers, explanatory variables and periodontal status revealed other correlations. Importantly, plaque index correlated positively with periodontitis severity (r=0.432; p=0.0004); this correlation remained significant after adjustment for multiple comparisons. Other correlations of interest included correlations of periodontitis severity with IL-1ra levels (r=0.262; p=0.038) and AFP levels (r=0.25; p=0.048) (Table 4). Another significant positive correlation was seen between waist circumference of participants and severity of periodontitis (r=0.262; p=0.038), which is important in the context of adiposity – periodontitis association (Table 4).
Figure 3.
Relationship between body fat indices and the levels of granzyme (top) and sCD40L (below) levels in saliva (in pg/ml)
Table 4.
Spearman Rank Correlation Coefficients, p-values and sample sizes for the pairwise correlation between biomarkers and explanatory variables, and periodontal status and subject characteristics (N=63)
| Rank Correlation r (P-value)* |
Periodontal Status |
IL1RA | sCD40L | GranzymeB | AFP |
|---|---|---|---|---|---|
| Plaque index | 0.43 (0.0004) | 0.26 (0.039) | −0.25 (0.0459) | 0.020 (0.88) | 0.25 (0.048) |
| BMI | 0.07 (0.59) | 0.17 (0.18) | −0.26 (0.043) | 0.14 (0.28) | −0.19 (0.14) |
| Waist Circumference | 0.26 (0.038) | 0.13 (0.32) | −0.203 (0.11) | −0.103 (0.42) | −0.06 (0.62) |
| Fat % | −0.02 (0.88) | 0.14 (0.28) | −0.20 (0.11) | 0.293 (0.020) | −0.16 (0.22) |
| Periodontal Status | 1 | 0.25 (0.046) | −0.27 (0.031) | −0.154 (0.26) | 0.08 (0.55) |
Significance probability associated with the test of the null hypothesis of zero correlation based upon the Spearman rank test. The r values in bold reached statistical significance (p<0.05).
Discussion
We believe that this is the first study that reports the baPgseline levels of IL-1ra, sCD40L and Granzyme B and AFP in saliva obtained from a homogenous periodontal maintenance population. This analysis is an extension of another study that evaluated the correlation of fat indices and levels of other biomarkers around dental implants.6 We observed that the levels of some of these biomarkers were significantly correlated with periodontitis severity and select obesity measures. However, none of these correlations remained significant after adjusting for multiple comparisons.
Our result on the positive correlation of periodontitis severity and levels of IL1-ra is in agreement with a recently published case-control study. The authors of this previous study reported significantly higher levels of IL1-ra in the gingival crevicular fluid samples collected from periodontitis subjects, compared to healthy volunteers.20 Another recent investigation using an experimental gingivitis model in 168 participants reported IL-1ra (in addition to IL-6) to be a potential indicator for pocket depth increase in these participants.21 Contrary to our result on the negative correlation between periodontitis severity and sCD40L salivary levels, a recent case-control study reported a positive correlation between this marker in GCF and periodontitis severity.22 To our knowledge, there are no published studies on the correlation of periodontal severity and the expression of the other two biomarkers tested (i.e. Granzyme B and AFP).
Though the primary objective of this study was investigate the correlation between periodontal status and the levels of the aforementioned four novel markers in saliva, since we collected anthropometric data from these subjects in our parent study, it provided us the opportunity to explore the correlations between body fat indices and the levels of salivary biomarkers. We know from earlier studies, that WC is a reliable indicator of abdominal obesity, when compared to BMI.23, 24 Therefore, it was interesting to observe a positive correlation with periodontitis severity. Of the body fat indices included in this study, WC showed a strong correlation with the severity of periodontitis. On the contrary, both the fat % and BMI did not correlate with periodontal status, but did correlate with the levels of select biomarkers tested.
Given the cross-sectional nature of the study, causality cannot be established. Though this study has this limitation, its most remarkable strengths include an adequate sample size and the inclusion of novel biomarkers implicated in several chronic diseases for their role in inflammation and immunity. Though we observed significant correlations, except the correlation between plaque index and periodontal status, none of the other correlations with biomarkers remained significant after multiple comparisons, which emphasizes the need for future clinical studies with an even larger sample size and a prospective study design to establish causality. Since this is a branched out study from a parent study that assessed the correlation between obesity indices and peri-implant health, we included subjects with at least one dental implant, resulting in some degree of selection bias. In future studies, it would also be worth analyzing more than one body fluid to increase the odds of identifying appropriate biomarkers with greater sensitivity.25
Conclusions
Four novel biomarkers [interleukin-1 receptor antagonist (IL-1ra), sCD40L, granzyme B and alpha-fetoprotein (AFP) of periodontitis] were detectable in saliva of subjects enrolled in a periodontal maintenance program. Suggestive correlations were identified between the levels of these biomarkers and obesity /periodontal measures, but these did not remain statistically significant after adjusting for multiple comparisons.
Table 3.
Frequencies for Periodontal Status and Brushing habits
| Periodontal Status | N | % |
|---|---|---|
| No Periodontitis | 7 | 11.11 |
| Mild Periodontitis | 9 | 14.29 |
| Moderate Periodontitis | 34 | 53.97 |
| Severe Periodontitis | 13 | 20.63 |
| Brushing Frequency | ||
| Twice a day | 16 | 25.4 |
| Thrice a day | 38 | 60.32 |
| Four times a day | 9 | 14.29 |
| Gender | ||
| Female | 37 | 58.73 |
| Male | 26 | 41.27 |
Highlights.
Levels of novel biomarkers were detectable in saliva periodontal recall patients
Select biomarker levels correlate with periodontal and select obesity measures
Correlations did not remain significant after adjusting for multiple comparisons
Prospective studies with larger sample sizes are warranted
Acknowledgements
This study was supported by a pilot grant from Institute for Clinical and Translational Science (ICTS) Pilot Grant, a part of Clinical and Translational Science Award (CTSA) Program (UL1 TR000422-06), National Institute of Dental and Craniofacial Research (R01DEO14390), Osseointegration Foundation Grant, Iowa Dental Research Grant and University of Iowa College of Dentistry Research Start-Up Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: A portion of this research will be presented at the, 93rd General Session of the International Association for Dental Research, Boston, MA. Charlotte, NC. March 11–14, 2015.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 – 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorman A, Kaye EK, Nunn M, Garcia RI. Changes in body weight and adiposity predict periodontitis progression in men. J Dent Res. 2012;91(10):921–926. doi: 10.1177/0022034512457372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81(12):1708–1724. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elangovan S, Brogden KA, Dawson DV, Blanchette D, Pagan-Rivera K, Stanford CM, Johnson GK, Recker E, Bowers R, Haynes WG, Avila-Ortiz G. Body fat indices and biomarkers of inflammation: a cross-sectional study with implications for obesity and peri-implant oral health. Int J Oral Maxillofac Implants. 2014;29(6):1429–1434. doi: 10.11607/jomi.3758. [DOI] [PubMed] [Google Scholar]
- 7.Giannobile WV. Salivary diagnostics for periodontal diseases. J Am Dent Assoc. 2012;143(10 Suppl):6S–11S. doi: 10.14219/jada.archive.2012.0341. [DOI] [PubMed] [Google Scholar]
- 8.Garcia I, Kuska R, Somerman MJ. Expanding the foundation for personalized medicine: implications and challenges for dentistry. J Dent Res. 2013;92(7 Suppl):3–10. doi: 10.1177/0022034513487209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57(5):675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 10.Schulz BL, Cooper-White J, Punyadeera CK. Saliva proteome research: current status and future outlook. Crit Rev Biotechnol. 2013;33(3):246–259. doi: 10.3109/07388551.2012.687361. [DOI] [PubMed] [Google Scholar]
- 11.Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, Shelburne CE, Rayburn LA, Singh AK, Giannobile WV. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90(6):752–758. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr J, Wang X, Martin L, Flaveri M, Cekici A, Teles RP. Journal of Dental Research. A. Vol. 93. AADR; 2014. A Systems Biology Approach to Identify GCF Biomarkers of Periodontitis. Poster #833. [Google Scholar]
- 13.Baena-Fustegueras JA, Pardina E, Balada E, Ferrer R, Catalán R, Rivero J, Casals I, Lecube A, Fort JM, Vargas V, Peinado-Onsurbe J. Soluble CD40 Ligand in Morbidly Obese Patients: Effect of Body Mass Index on Recovery to Normal Levels After Gastric Bypass Surgery. JAMA Surg. 2013;148(2):151–156. doi: 10.1001/jamasurgery.2013.419. [DOI] [PubMed] [Google Scholar]
- 14.El Mesallamy HO, Hamdy NM, Mostafa DM, Amin AI. The serine protease granzyme B as an inflammatory marker, in relation to the insulin receptor cleavage in human obesity and type 2 diabetes mellitus. J Interferon Cytokine Res. 2014;34(3):179–186. doi: 10.1089/jir.2013.0059. [DOI] [PubMed] [Google Scholar]
- 15.Potapovich AI, Pastore S, Kostyuk VA, Lulli D, Mariani V, De Luca C, Dudich EI, Korkina LG. alpha-Fetoprotein as a modulator of the pro-inflammatory response of human keratinocytes. Br J Pharmacol. 2009;158(5):1236–1247. doi: 10.1111/j.1476-5381.2009.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110(25):3842–3848. doi: 10.1161/01.CIR.0000150391.38660.9B. [DOI] [PubMed] [Google Scholar]
- 17.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 18.Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 19.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilowski L, Wiench R, Płocica I, Krzemiński TF. Amount of interleukin-1β and interleukin-1 receptor antagonist in periodontitis and healthy patients. Arch Oral Biol. 2014;59(7):729–734. doi: 10.1016/j.archoralbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Morelli T, Stella M, Barros SP, Marchesan JT, Moss KL, Kim SJ, Yu N, Aspiras MB, Ward M, Offenbacher S. Salivary biomarkers in a biofilm overgrowth model. J Periodontol. 2014;85(12):1770–1778. doi: 10.1902/jop.2014.140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi R, Gupta M, Jain A, Das T, Prashar S. Soluble CD40 ligand: a novel biomarker in the pathogenesis of periodontal disease. Clin Oral Investig. 2015;19(1):45–52. doi: 10.1007/s00784-014-1216-3. [DOI] [PubMed] [Google Scholar]
- 23.Leitzmann MF, Moore SC, Koster A, Harris TB, Park Y, Hollenbeck A, Schatzkin A. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS One. 2011;6:e18582. doi: 10.1371/journal.pone.0018582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia WP, Lu JX, Xiang KS, Bao YQ, Lu HJ, Chen L. Prediction of abdominal visceral obesity from body mass index, waist circumference and waist-hip ratio in Chinese adults: receiver operating characteristic curves analysis. Biomed Environ Sci. 2003;16:206–211. [PubMed] [Google Scholar]
- 25.Khan A. Detection and quantitation of forty eight cytokines, chemokines, growth factors and nine acute phase proteins in healthy human plasma, saliva and urine. J Proteomics. 2012;75(15):4802–4819. doi: 10.1016/j.jprot.2012.05.018. [DOI] [PubMed] [Google Scholar]