Abstract
Apolipoprotein B (apoB) and nonHDL-cholesterol (nonHDL-C) are cardiovascular disease (CVD) risk markers, although data in adults with type 1 diabetes mellitus (DM) are limited. We hypothesized that elevated apoB and nonHDL-C would be associated with greater odds of coronary artery calcification progression (CACp), a measure of coronary atherosclerosis, than either category alone in adults with type 1 DM. We grouped subjects with type 1 DM (n=652) into four groups; elevated apoB (≥90mg/dL) and elevated nonHDL-C (≥130mg/dL), elevated nonHDL-C alone, elevated apoB alone, and normal apoB and nonHDL-C. We employed logistic regression to examine the associations between the groups and CACp over 6-years. We performed sensitivity analyses with elevated apoB and nonHDL-C re-defined as ≥ cohort means (91.4, 119.0 mg/dL respectively). Subjects with elevated apoB and nonHDL-C had greater odds of CACp compared to subjects with normal apoB and nonHDL-C (OR: 1.90, 95% CI 1.15-3.15), and compared to subjects with elevated apoB alone (OR: 2.86, 95% CI 1.43-5.74) adjusting for age, sex, duration, HbA1c and statins. Similar results were obtained with elevated apoB and nonHDL-C defined as ≥ the cohort means. In conclusion, elevated apoB and nonHDL-C carry a greater risk of atherosclerosis than elevated apoB in the absence of elevated nonHDL-C in adults with type 1 DM. These data suggest that apoB and nonHDL-C should be viewed as complementary rather than competitive indices of CVD risk in type 1 DM.
Keywords: apoB, nonHDL-C, coronary artery calcification, type 1 diabetes mellitus (type 1 DM)
Introduction
Apolipoprotein B (apoB) and non-high density lipoprotein-cholesterol (nonHDL-C) have been proposed to be superior indicators of cardiovascular (CV) risk than total cholesterol and/or low density lipoprotein-cholesterol (LDL-C) [1-5]. Some authors argue that while non-HDL-C and apoB correlate, they are not interchangeable, and may provide unique information about CV risk [6]. There are insufficient data on the concordance between apoB and nonHDL-C in adults with type 1 diabetes mellitus (DM) across a wide range of risk factors for atherosclerosis including triglycerides (TG) [7] and body mass indices (BMI) [8]. Moreover, it remains unclear whether elevated apoB (≥90mg/dL) or elevated nonHDL-C (≥130mg/dL) independently carries the same risk for atherosclerosis as elevation of both lipid indices in type 1 DM. When comparing two tests, the clinical consequences of the tests are best understood through their agreement and disagreement [9]. In cases where elevated apoB or elevated nonHDL-C are individually associated with the same risk for atherosclerosis as elevation of both lipid indices, risk may be equally served by either test. This is clinically important as measurement of apoB incurs an additional cost. Accordingly in this study, we sought to examine the correlation of nonHDL-C and apoB across a wide range of lipid, metabolic profiles and cardiovascular profiles in adults with type 1 DM in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Second, we sought to examine whether adults with elevated measures of apoB (≥90mg/dL) and nonHDL-C (≥130mg/dL) had greater odds of CAC progression (CACp) compared to adults with elevated apoB alone or elevated nonHDL-C alone, and compared to adults with normal measures of both apoB and nonHDL-C.
Methods
The CACTI Study enrolled 1416 subjects 19-56 years old, 652 with type 1 DM and 764 without diabetes, who were asymptomatic for cardiovascular disease (CVD) at the baseline visit in 2000-02 and then were re-examined 6 years later. The study was approved by the Colorado Multiple Institutional Review Board and all participants provided informed consent.
We measured height and weight, and calculated BMI in kg/m2. Resting systolic (SBP) and fifth-phase diastolic blood pressure (DBP) were measured three times while the patient was seated, and the second and third measurements were averaged. After an overnight fast, blood was collected, centrifuged, and separated. Plasma was stored at 4°C until assayed. High performance liquid chromatography was used to measure HbA1c (HPLC, BioRad variant). Total plasma cholesterol and triglyceride (TG) levels were measured using standard enzymatic methods, HDL-C was separated using dextran sulfate and LDL-C was calculated using the Friedewald formula. NonHDL-C was calculated by subtracting HDL-C from total cholesterol, and the ratio of TG to HDL-C was calculated by dividing TG by HDL-C. ApoB was measured by Beckman Array Nephelometer (Beckman Coulter Inc., Brea, CA). Elevated apoB was defined as ≥90mg/dL and elevated nonHDL-C as ≥130mg/dL per consensus report from the American Diabetes Association (ADA) and the American College of Cardiology Foundation (ACC) [10].
CAC measurements were obtained in duplicate using an ultrafast Imatron C-150XLP electron beam computed tomography scanner (Imatron, San Francisco, CA) and the two scores were averaged. The average of the two scores was used as the CAC score for that visit. Scans were repeated on follow-up, an average of 6.2±0.6 years after the baseline exam. Presence of CAC was defined as a CAC score > 0. Progression of CAC was defined as an increase in volume of CAC of ≥2.5 square root transformed units.
Differences between men and women were assessed using Chi-Square for categorical variables and t-test for continuous variables. To examine the relationships between apoB and nonHDL-C we employed Pearson's correlation and scatter plots. We explored the relationship in entire cohort, and also stratified the analyses by tertiles of TG (low: <67, mid: 67-95 and high: ≥ 95 mg/dL), BMI (low: <24.1, mid: 24.1-27.3 and high: ≥ 27.3 kg/m2), statin use and presence / absence of CACp respectively. The agreement between elevated apoB (≥90mg/dL) and elevated nonHDL-C (≥130mg/dL) were tested with Chi-squared and Kappa-test with Kappa coefficient < 0 indicating no agreement, <0.40 as poor, 0.40-0.60 as moderate, over 0.61-0.80 as good and above 0.81 as excellent. We stratified subjects into four groups; those with elevated apoB and elevated nonHDL-C (n=196), those with elevated apoB and normal nonHDL-C (n=116), those with normal apoB and elevated nonHDL-C (n=10) and those with normal apoB and normal nonHDL-C (n=330). Due to the limited number of subjects with normal apoB and elevated nonHDL-C (n=10), we also grouped subjects by mean apoB (91.4mg/dL) and mean nonHDL-C (119.0mg/dL); those with apoB and nonHDL-C ≥ to cohort means (n=247), those with only nonHDL-C ≥ cohort mean (n=41), those with only apoB ≥ cohort mean (n=39) and those with apoB and nonHDL-C < cohort means (n=325). Multivariable logistic regression models were applied to examine the odds of CAC progression among the groups; unadjusted and adjusted for sex, age, HbA1c, SBP and statin use. Gender was evaluated for effect modification of the 4-level categorical apoB/nonHDL-C variables by adding the gender by apoB/nonHDL-C variable interaction term in the logistic regression models. The interaction terms were not significant and gender was considered a confounder. We also performed C-statistics and examined the area under the receiver operating characteristics (ROC) curves for CACp by elevated apoB and elevated nonHDL-C, elevated apoB and normal nonHDL-C and normal apoB and elevated nonHDL-C. The C-statistic has been criticized for insensitivities to changes in clinical decisions yielded for information gained [11-14]. Therefore, we also integrated discrimination index (IDI), which uses probability differences to examine prediction performance [11-14]. All analyses were performed in in SAS (version 9.3 for Windows; SAS Institute, Cary, NC). A value of P < 0.05 was considered statistically significant.
Results
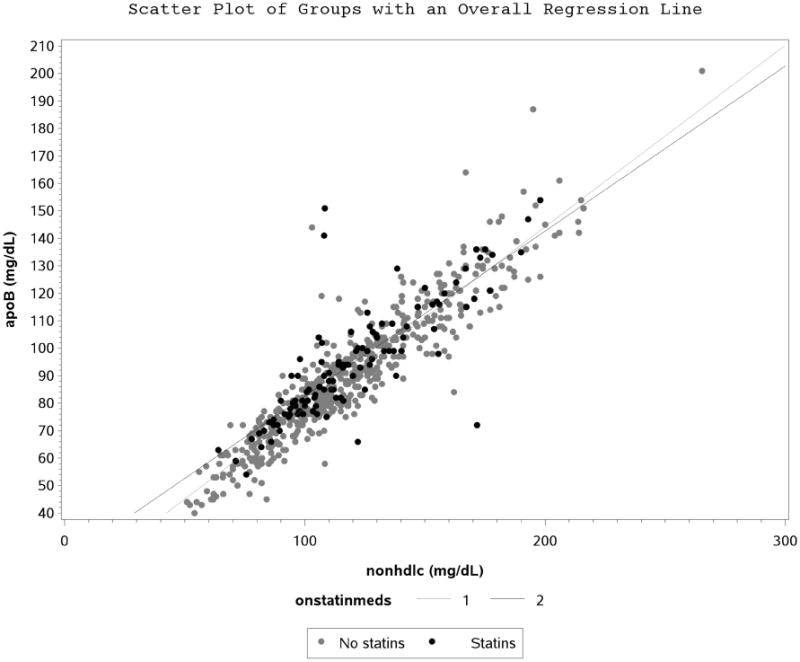
Participant characteristics are shown in Table 1. ApoB and nonHDL-C correlated well at baseline (r=0.91, p<0.0001) in the entire type 1 DM cohort. There were no apparent differences observed when correlation was stratified by CACp status (Figure 1A), tertiles of TG (Figure 1B), statin use (Figure 1C), tertiles of BMI (Supplemental figure 1) and tertiles of LDL-C (Supplemental figure 2).
Table 1. Coronary Artery Calcification in Type 1 Diabetes Study (CACTI) Participants with Type 1 DM at Baseline.
| Men (n=298) | Women (n=354) | p-value | |
|---|---|---|---|
|
| |||
| Age (years) | 37 ± 9 | 36 ± 9 | 0.07 |
|
| |||
| Diabetes duration (years) | 24 ± 9 | 23 ± 9 | 0.29 |
|
| |||
| Hemoglobin A1c [HbA1c] (%) | 8.0 ± 1.2 | 8.0 ± 1.3 | 0.90 |
|
| |||
| Low-density lipoprotein-cholesterol [LDL-C] (mg/dL) | 104 ± 30 | 98 ± 28 | 0.007 |
|
| |||
| High-density lipoprotein-cholesterol [HDL-C] (mg/dL) | 51 ± 14 | 60 ± 17 | <0.0001 |
|
| |||
| Estimated insulin sensitivity [eIS] (mg * kg -1 * min -1) | 3.8 ± 1.4 | 4.8 ± 1.6 | <0.0001 |
|
| |||
| Body mass index [BMI] (kg/m2) | 26.5 ± 3.9 | 26.0 ± 4.7 | 0.09 |
|
| |||
| Triglycerides [TG] (mg/dL) | 80 (61-113) | 77 (61-104) | 0.23 |
|
| |||
| Non-high-density lipoprotein-cholesterol [nonHDL-C] at baseline (mg/dL) | 123 ± 33 | 116 ± 32 | 0.007 |
|
| |||
| Non-high-density lipoprotein-cholesterol [nonHDL-C] at 3-years (mg/dL) | 117 ± 30 | 114 ± 31 | 0.33 |
|
| |||
| Apolipoprotein B at baseline [apoB] (mg/dL) | 94 ± 25 | 89 ± 23 | 0.003 |
|
| |||
| Apolipoprotein B at 3-years [apoB] (mg/dL) | 87 ± 20 | 85 ± 22 | 0.30 |
|
| |||
| Non-high-density lipoprotein-cholesterol [nonHDL-C] ≥130mg/dL | 38% | 27% | 0.004 |
|
| |||
| Apolipoprotein B [apoB] ≥90mg/dL | 50% | 48% | 0.53 |
|
| |||
| On antihypertensive medications | 41% | 35% | 0.09 |
|
| |||
| On statins | 21% | 13% | 0.004 |
|
| |||
| Ever smoker (% yes) | 18% | 22% | 0.21 |
|
| |||
| Any coronary artery calcification [CAC] at baseline | 49% | 28% | <0.0001 |
|
| |||
| Coronary artery calcification progression [CACp] over 6-years | 53% | 34% | <0.0001 |
Data are means ± standard deviation, % or median (25th – 75th %)
Figure 1A. Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Coronary Artery Calcification Progression.
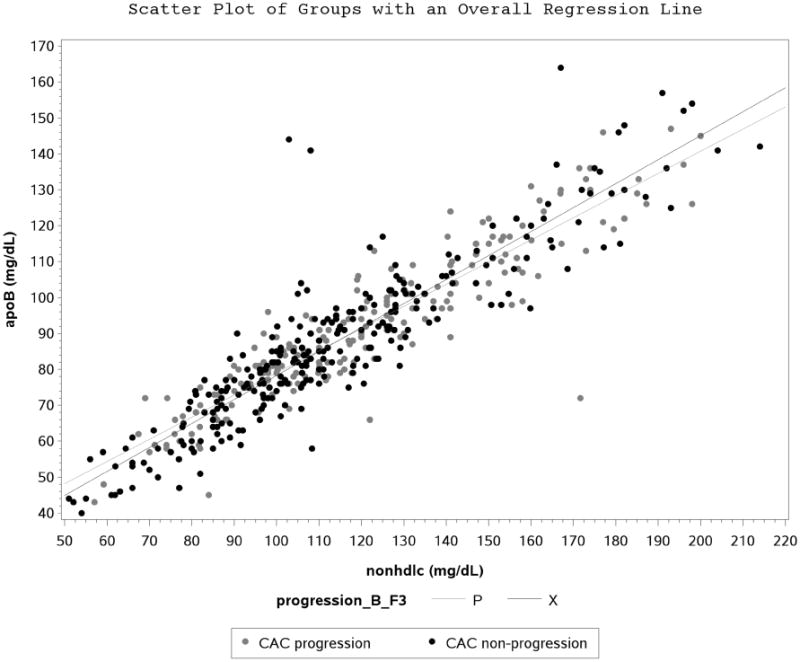
Figure 1B. Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Tertiles of Triglycerides.
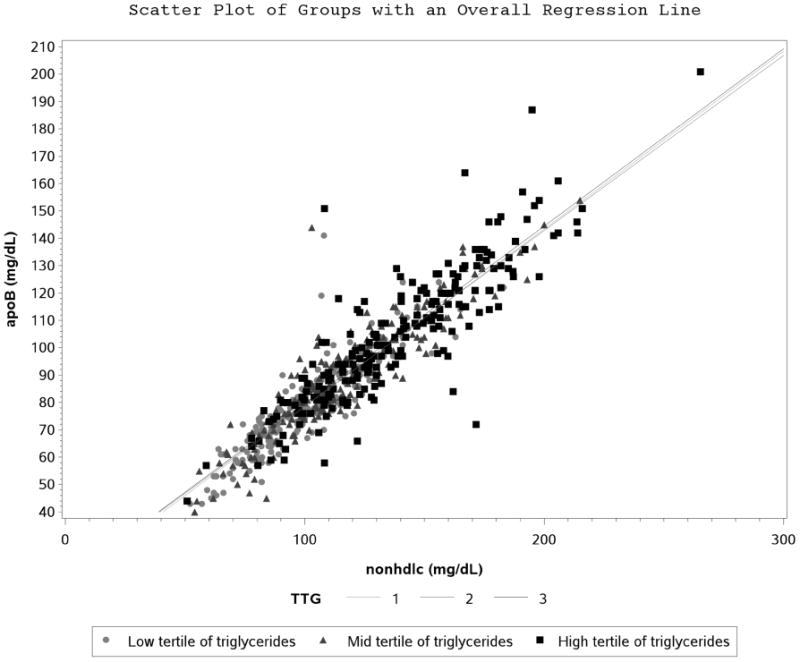
Figure 1C. Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Statin Use.
Elevated apoB and nonHDL-C were concordant, with an overall Kappa coefficient of 0.62 (95% CI 0.56-0.68) indicating good agreement between elevated apoB and nonHDL-C beyond chance. Of the 206 subjects with elevated nonHDL-C, 196 had elevated apoB. In contrast, only 196 of the 312 subjects with elevated apoB had elevated nonHDL-C. Even though apoB and nonHDL-C correlate, and elevated indices of each measure are concordant, it does not necessarily mean that they have the same association with cardiovascular risk. Subjects with elevated apoB and nonHDL-C had significantly greater odds of CACp compared to those with normal apoB and nonHDL-C, but also compared to those with elevated apoB alone after adjusting for sex, age, HbA1c and statin use (Table 2).
Table 2. Multivariable Logistic Regression Models with Coronary Artery Calcification Progression in Adults with Type 1 DM.
| Baseline apoB (elevated ≥ 90 mg/dL or normal <90 mg/dL) Baseline nonHDL-C (elevated ≥ 130 mg/dL or normal <130 mg/dL) | |||
|---|---|---|---|
| CAC progression over 6-years | OR, 95% CI* | OR, 95% CI* | OR, 95% CI* |
| Elevated apoB and elevated nonHDL-C (n=196) | 190 (1.15-3.15) p=0.01 | 2.86 (1.43-5.74) p=0.003 | 1.11 (0.15-8.45) p=0.92 |
| Normal apoB and elevated nonHDL-C (n=10) | 1.71 (0.23-12.82) p=0.60 | 2.57 (0.33-20.28) p=0.37 | 1.00 (reference) |
| Elevated apoB and normal nonHDL-C (n= 116) | 0.67 (0.35-1.26) p=0.21 | 1.00 (reference) | 0.39 (0.05-3.07) p=0.37 |
| Normal apoB and normal nonHDL-C (n=330) | 1.00 (reference) | 1.50 (0.79-2.85) p=0.21 | 0.59 (0.08-4.38) p=0.60 |
| Baseline apoB (at or above means ≥ 91.4 mg/dL or below means < 91.4 mg/dL) Baseline nonHDL-C (at or above means ≥ 119.0 mg/dL or below means <119.0 mg/dL) | |||
| CAC progression over 6-years | OR, 95% CI* | OR, 95% CI* | OR, 95% CI* |
| Above means for apoB and nonHDL-C (n=247) | 1.67 (1.04-2.67) p=0.03 | 5.24 (1.58-17.43) p=0.007 | 1.57 (0.62-3.97) p=0.34 |
| Above means for nonHDL-C only (n=41) | 1.06 (0.04-2.67) p=0.90 | 3.34 (0.79-14.07) p=0.10 | 1.00 (reference) |
| Above means for apoB only (n=39) | 0.32 (0.10-1.05) p=0.06 | 1.00 (reference) | 0.30 (0.07-1.26) p=0.10 |
| ApoB and nonHDL-C at or below mean (n=325) | 1.00 (reference) | 3.14 (0.95-10.33) p=0.06 | 0.94 (0.38-2.36) p=0.90 |
Each column represents the same logistic regression model, but with a different reference group.
The logistic regression models are adjusted for sex, age, duration, HbA1c and statin use.
The first column reports the associations with CAC progression among the apoB and nonHDL-C groups with normal apoB and normal nonHDL-C as the reference group. The middle column represents the same model but with elevated apoB and normal HDL-C as the reference group. The last column represents the same model but with normal apoB and elevated nonHDL-C as a reference group.
We also examined the area under the curve of ROC for CACp by elevated apoB and elevated nonHDL-C, elevated apoB and normal nonHDL-C and normal apoB and elevated nonHDL-C. The AUC as a relative measure of test efficiency was highest for elevated apoB and elevated nonHDL-C (AUC= 0.57, 95% CI 0.53-0.61) compared to elevated apoB and normal nonHDL-C (AUC = 0.52, 95% CI 0.49-0.56) and normal nonHDL-C and elevated apoB (AUC = 0.51, 95% CI 0.50-0.52). The difference in AUC between participants with elevated apoB and elevated nonHDL-C and those with normal apoB and elevated nonHDL-C was significant (∆AUC 0.06, 95% 0.02-0.11, p=0.006). The difference in AUC between participants with elevated apoB and elevated nonHDL-C and those with elevated apoB and normal nonHDL-C (∆AUC 0.05, 95% 0.004-0.09, p=0.03) was also significant. Furthermore, the addition of apoB to a model with HbA1c, SBP and LDL-C (ABC risk factors) and age, did not improve the IDI for CACp (p=0.32). In contrast, the addition of nonHDL-C to the ABC and age model improved IDI (0.01±0.005, p=0.02) for CACp. Moreover, the addition of nonHDL-C to a model with ABC, age and apoB also improved IDI (0.01±0.005, p=0.02) for CACp. Conversely, the addition of apoB to a model with ABC, age and nonHDL-C did not improve IDI (p=0.70) for CACp.
Discussion
We report strong correlation between apoB and nonHDL-C across a wide range of lipid, metabolic and cardiovascular profiles in adults with type 1 DM. A significant proportion of subjects had elevated apoB in the absence of elevated nonHDL-C, in contrast to vice versa. Elevated apoB and nonHDL-C carried a significantly greater risk of atherosclerosis than elevated apoB in the absence of elevated nonHDL-C. These data suggest that apoB and nonHDL-C may provide unique risk information for atherosclerosis and should be viewed as complementary rather than competitive indices of CVD risk in adults with type 1 DM.
The ADA and the ACC support measuring apoB or LDL particle concentration, in conjunction with using LDL-C and nonHDL-C, in adults at high risk for CVD for assessing risk and guiding therapy [10, 15]. ApoB and nonHDL-C and their association with CVD-risk are well-described in the literature, and most studies, but not all [16, 17], have demonstrated that apoB and nonHDL-C are more closely associated with CVD-risk and all-cause mortality in adults than LDL-C [1-5]. Expert opinion suggests that apoB and nonHDL-C should be considered to be complementary rather than competitive indices to LDL-C in adults with type 1 DM [18], and that it is useful to measure both LDL-C and nonHDL-C or apoB, especially when considering lipid lowering therapy [19]. It remains unclear however whether apoB is superior to nonHDL-C or vice versa for CVD-risk prediction [17, 20, 21], but our data suggest that odds of CACp is highest when apoB and nonHDL-C are concordant.
NonHDL-C was established to improve risk estimation beyond LDL-C from Friedewald's formula in the presence of hypertriglyceridemia, since associated changes in VLDL-TG/VLDL-C ratio may lead to LDL-C underestimation [22]. NonHDL-C represents the cholesterol content of VLDL-C, remnants, and LDL-C particles [23]. ApoB directly measures the aggregate number of all atherogenic lipoproteins since each atherogenic particle contains one apoB molecule [23]. Some authors believe apoB may provide a more complete picture of the lipoprotein profile as it will account for small, dense, more atherogenic particles [24]. Proponents of apoB also argue that the apoB concept is intrinsically easier to understand than nonHDL-C, which represents “a state of otherness” defined by a non-number, instead of a single atherogenic lipid variable [25-27].
The strengths of our paper include longitudinal data over 6-years, a moderately large cohort of adults with type 1 diabetes, and the use of CAC to evaluate coronary atherosclerosis. CAC is accepted as a quantifiable, reliable, noninvasive marker of the extent of coronary atherosclerosis, and CACp predicts both fatal and nonfatal coronary events [28]. Another strength of our study is the use of C-statistics and IDI to evaluate prediction performance of apoB and nonHDL-C for CACp. There are limitations of this study worth mentioning. We employed ≥90mg/dL and ≥130mg/dL to define elevated apoB and nonHDL-C respectively per ADA/ACC consensus statement [10]. Although apoB equates to 90mg/dL for a nonHDL-C of 130mg/dL using published apoB estimating equations, these are not validated for subjects with type 1 DM [26, 27]. In our cohort the mean apoB was > 90mg/dL, in contrast the mean nonHDL-C was lower than 130mg/dL. Due to the discordance between elevated apoB and elevated nonHDL-C by ≥90mg/dL and ≥130mg/dL, which could related to the higher levels of HDL-C in adults with type 1 diabetes, we reran the logistic models with subjects grouped by apoB and nonHDL-C being ≥ cohort means or < means for each lipid index. The 6-year follow-up may be insufficient to fully examine CACp in a cohort of relatively young adults with type 1 DM and fairly favorable lipid profiles. We did not have data on thyroid function which is known to affect apoB and nonHDL-C concentrations and this may have confounded our findings. Furthermore, results from this study may not be generalizable to significantly younger or older subjects with type 1 DM.
Supplementary Material
Supplemental Figure 1 Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Tertiles of Body Mass Index
Supplemental Figure 2 Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Tertiles of Low Density Lipoprotein Cholesterol
Acknowledgments
Support: Support for this study was provided by NHLBI grant R01 HL61753, HL79611, and HL113029, JDRF grant 17-2013-313, and DERC Clinical Investigation Core P30 DK57516. The study was performed at the Adult CTRC at UCD support by NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver, CO. Dr. Snell-Bergeon by an American Diabetes Association Junior Faculty Award (1-10-JF-50).
Drs. Petter Bjornstad, Janet K. Snell-Bergeon and Laura Pyle are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Disclosure statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: do the math. J Am Coll Cardiol. 2011;58:457–463. doi: 10.1016/j.jacc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 3.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 4.Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Jr, Xu P, Marcovina SM. Relationship of Apolipoproteins A-1 and B, and Lipoprotein(a) to Cardiovascular Outcomes: The AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juonala M, Viikari JS, Kahonen M, Solakivi T, Helenius H, Jula A, Marniemi J, Taittonen L, Laitinen T, Nikkari T, Raitakari OT. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2008;52:293–299. doi: 10.1016/j.jacc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Sniderman A, Williams K, de Graaf J. Non-HDL C equals apolipoprotein B: except when it does not! Curr Opin Lipidol. 2010;21:518–524. doi: 10.1097/MOL.0b013e32833ee80c. [DOI] [PubMed] [Google Scholar]
- 7.Bjornstad P, Maahs DM, Wadwa RP, Pyle L, Rewers M, Eckel RH, Snell-Bergeon JK. Plasma triglycerides predict incident albuminuria and progression of coronary artery calcification in adults with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes Study. J Clin Lipidol. 2014;8:576–583. doi: 10.1016/j.jacl.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diab etes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasziou P, Irwig L, Deeks JJ. When should a new test become the current reference standard? Ann Intern Med. 2008;149:816–822. doi: 10.7326/0003-4819-149-11-200812020-00009. [DOI] [PubMed] [Google Scholar]
- 10.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 15.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 17.Bao W, Srinivasan SR, Berenson GS. Tracking of serum apolipoproteins A-I and B in children and young adults: the Bogalusa Heart Study. J Clin Epidemiol. 1993;46:609–616. doi: 10.1016/0895-4356(93)90033-w. [DOI] [PubMed] [Google Scholar]
- 18.Mazanderani AB, Wise SJ, Tildesley HD. Apolipoprotein B levels in adults with type 1 diabetes not receiving lipid-lowering therapy. Clin Biochem. 2009;42:1218–1221. doi: 10.1016/j.clinbiochem.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Sniderman AD, St-Pierre AC, Cantin B, Dagenais GR, Despres JP, Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91:1173–1177. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 20.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora S, Buring JE, Ridker PM. Discordance of Low-Density Lipoprotein (LDL) Cholesterol With Alternative LDL-Related Measures and Future Coronary Events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 23.Martin SS, Michos ED. Are we moving towards concordance on the principle that lipid discordance matters? Circulation. 2014;129:539–541. doi: 10.1161/CIRCULATIONAHA.113.007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Reddy KS, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KM. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson TA. Opening a new lipid “apo-thecary”: incorporating apolipoproteins as potential risk factors and treatment targets to reduce cardiovascular risk. Mayo Clin Proc. 2011;86:762–780. doi: 10.4065/mcp.2011.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermans MP, Ahn SA, Rousseau MF. Discriminant ratio and biometrical equivalence of measured vs. calculated apolipoprotein B100 in patients with T2DM. Cardiovasc Diabetol. 2013;12:39. doi: 10.1186/1475-2840-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermans MP, Sacks FM, Ahn SA, Rousseau MF. Non-HDL-cholesterol as valid surrogate to apolipoprotein B100 measurement in diabetes: Discriminant Ratio and unbiased equivalence. Cardiovasc Diabetol. 2011;10:20. doi: 10.1186/1475-2840-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Tertiles of Body Mass Index
Supplemental Figure 2 Scatter Plot and Regression Line between Apolipoprotein B and Non-High Density Lipoprotein Cholesterol Stratified by Tertiles of Low Density Lipoprotein Cholesterol


