Abstract
Purpose
The metabolic syndrome (MetS, clustering of elevated blood pressure, triglycerides and glucose, reduced high-density lipoprotein cholesterol (HDL-C), abdominal obesity) has been associated with increased breast cancer risk, but less is known about its association with mammographic breast density, a strong risk factor for breast cancer.
Methods
We collected data on risk factors, body size and blood pressure via in-person interviews and examinations, and measured glucose, triglycerides, and HDL-C from dried blood spots from women recruited through a mammography screening clinic (n=373; 68% Hispanic, 17% African American; 63% foreign born). We performed linear regression models to examine the associations of each MetS component and the MetS cluster (≥ 3 components) with percent density and dense breast area, measured using a computer-assisted technique and Cumulus software.
Results
About 45% of women had the MetS, with the prevalence of the individual components ranging from 68% for abdominal obesity to 33% for elevated triglycerides. The prevalence of the MetS increased with higher body mass index (BMI) and postmenopausal status, but did not vary substantially by ethnicity, immigrant generational status, parity, age at menarche or alcohol consumption. Low HDL-C (< 50 mg/dL), but not the MetS cluster or the other MetS components, was associated with larger dense breast area after adjusting for age, BMI, fasting time, and educational attainment (β=8.77, 95% CI=2.39, 15.14). The MetS and its individual components were not associated with BMI-adjusted percent density.
Conclusions
HDL-C alone may have an influence on dense breast tissue that is independent of BMI, and may be in the same direction as its association with breast cancer risk.
Keywords: mammographic breast density, breast cancer, metabolic syndrome, HDL-Cholesterol, waist circumference, hypertension, triglycerides, glucose, Hispanic, immigrants
Introduction
The metabolic syndrome (MetS) refers to the presence of multiple biochemical abnormalities and related clinical conditions, including elevated blood pressure, triglyceride and glucose levels, low high-density lipoprotein cholesterol (HDL-C) levels, and excess body weight or abdominal obesity [1, 2]. An established risk factor for type II diabetes and cardiovascular disease, the MetS has more recently been associated with the risk of many common cancer sites, with some associations being stronger in women and for female-specific cancers [3]. A meta-analysis of nine studies reported an overall 52% increased risk of postmenopausal breast cancer in women with MetS, which exceeded the range of 8% to 39% increased risk associated with any individual component of the MetS [4]. Additionally, the MetS and/or its individual components may be more prevalent in breast tumors with more aggressive and poorer prognostic characteristics (e.g., later stage, larger tumors, triple negative tumors), and may lower survival following breast cancer diagnosis [5–9].
Mammographic breast density reflects the amount of epithelial and stromal (dense) breast tissue, and may represent one intermediate marker for breast cancer risk [10]. In addition to strong associations with future breast cancer risk [11–13], the associations of mammographic density with risk factors that reflect estrogen-related exposures (e.g., hormone replacement use, parity) are in the same direction as their associations with breast cancer risk [14]. However, the evidence on the associations between mammographic density and other risk factors relevant to metabolic abnormalities are more mixed with physical activity showing mostly null associations [15], diabetes showing null to inverse associations [16, 17], and larger body size showing consistently strong and inverse associations [18–21]. The few studies that have examined the MetS or its components in relation to mammographic density have produced mixed results of null to modest positive as well as inverse associations [22–28]. These associations have been similar for both absolute and relative measures of mammographic density, which respectively capture the amount of dense breast tissue only and incorporate the amounts of both dense and fat breast tissues [22–24, 26–28].
Given that mammographic density can be monitored over time through routine screening mammography, quantifying its associations with potentially modifiable and easily assessed risk factors such as the MetS components may help in breast cancer risk reduction and prevention efforts. Research in this area can also benefit from studying diverse populations as the risk of the MetS components, breast cancer and mammographic density patterns show variation by race/ethnicity and immigration [29–36]. Here, we examined the associations between the MetS as a cluster and its individual components with mammographic density in a socially diverse urban midlife sample of women.
Methods
Study Population
Between November 2012 and May 2014, we recruited 400 women as they presented for routine screening mammography appointments at Columbia University Medical Center (age range 40–64 years; mean±standard deviation [SD]: 50.5±5.8). The study sample represents the catchment community served by Columbia University Medical Center in terms of race/ethnicity and immigrant background, but the study sample has higher educational profile than the community [37]. The rate of recent mammography among women aged 40 and older in this community is about 80%, and thus, the study population also represents the majority of community women in this age group [37, 38]. Trained research personnel interviewed women in English (56%) or Spanish (44%) on sociodemographic and risk factors data, obtained anthropometric and blood pressure measurements, and collected blood samples. We collected copies of digital mammograms, performed on the same day as the enrollment and data collection, for 395 participants. We excluded participants with breast implants (n=9), and missing data on blood biomarkers of the MetS (n=13), leaving a final sample of 373 (93% of total sample) for the current analysis.
Measures
Body Size and Metabolic Syndrome
We measured women’s height using a stadiometer, weight using a digital scale and waist circumference using a measuring tape; all measurements were done in light clothing and without shoes. After at least 5 minutes of rest time in sitting position, we obtained three consecutive blood pressure measures using an automated blood pressure machine, and used the average of the last two measures in data analysis.
We measured non-fasting glucose, triglycerides and HDL-C in dried blood spots (DBS), and converted the levels for each biomarker to plasma-equivalent levels as described below. We collected capillary whole blood samples from women’s middle or ring finger, which were placed on DBS filter paper (903 protein saver card, Whatman, Piscataway, NJ), dried at room temperature and subsequently frozen in plastic bags with desiccant packs before being shipped to the University of Washington, Department of Laboratory Medicine (UWLM) where the samples were stored with desiccant at −80°c. For each assay, a single 3.2 mm diameter disc was punched from a DBS sample (BSD700 Semi-Automated Dried Sample Puncher; BSD Robotics, Brisbane, QLD, Australia) into a microtiter plate assay well (Greiner Bio-One, Monroe, NC) alongside UWLM assay calibrators and quality control DBS samples. UWLM biomarker-specific elution buffer was added to each microtiter plate well and the microplate was shaken for 1 hour at room temperature. An aliquot from each well was transferred to a microtiter assay plate (Greiner Bio-One), to which UWLM biomarker-specific reagents including a fluorophore were added. The plate was then shaken for 30 seconds, and incubated at 37°c for 30 minutes. The fluorescence intensity (RFU) of each well was read at 530/25 nm excitation and 590/35 nm emission (Synergy HT Microtiter Plate Reader, BioTek, Winooski, VT). A linear regression calibration curve, constructed by plotting the concentrations of the calibrators (UniCel DxC 800 Synchron Clinical System, Beckman Coulter, Miami, FL) against their measured fluorescence values, was used to convert the blank-subtracted RFU value of each DBS sample into a biomarker concentration (Gen5, BioTek). Each DBS biomarker concentration was transformed into a plasma-equivalent biomarker concentration (i.e ., the biomarker concentration that would be expected to be obtained had the DBS sample been a conventional plasma sample) via a linear regression equation derived from analyses of DBS vs. DBS-matched plasma samples from venous blood (UniCel DxC 800 Synchron Clinical System). Assay performance characteristics for the biomarkers of HDL-C, triglycerides and glucose from DBS samples and venous blood plasma samples respectively had Pearson correlation coefficients of 0.86, 0.98, and 0.96 (n=86–132); intra-assay CVs of 5.2%, 3.7% and 5.1%; and inter-assay CVs of 8.0%, 4.4% and 6.5%.
As per modified National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines, we defined the MetS by the presence of at least three of the following components: measured systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or taking anti-hypertensive medication, abdominal obesity or waist circumference of > 88 cm, triglycerides ≥ 150 mg/dL, glucose ≥ 100 mg/dL, HDL-C < 50 mg/dL [39].
Mammographic density
We calculated the total dense area (in squared centimeters) and percent density (dense area/total breast area in percentage) using a computer assisted method, in which a trained reader, blinded to all study data, used a computer thresholding software (Cumulus) to outline the areas corresponding to the total breast and dense breast tissue on digital mammograms [40]. Additionally, we calculated the non-dense area by taking the difference between total breast area and dense area. We evaluated the cranio-caudal images of the left breast for all participants in randomly sorted batches of approximately 50 mammograms, and duplicated the assessment for 10% of mammograms. The intraclass correlation coefficient was 0.95 for percent density, 0.78 for dense area and 0.94 for nondense area. The Pearson correlation coefficients for repeated measures were 0.94 for percent density, 0.92 for dense area, and 0.99 for nondense area.
Covariates
Breast cancer risk factors considered included menopausal status (premenopausal or perimenopausal status defined as menstruating in the last 12 months with no bilateral oophorectomy or hysterectomy and no current hormone replacement therapy (HRT) use, post-menopausal status defined as no menstruation in the last 12 months or history of bilateral oophorectomy or hysterectomy within 18 months of last menstrual period and no HRT use ), parity (nulliparous, 1–2, ≥3 births), age at first live birth (< 25, 25–29, ≥30 years), age at menarche (in years), family history of breast cancer in first degree relatives, alcohol consumption status (never, former, current defined as drinking in the past 12 months), smoking status (never, former, current), fasting time to blood sample taken (< 3, 3–6, ≥ 6 hours), BMI (in kg/m2; continuous and categorized into <25, 25–29, ≥30), self-reported type II diabetes, immigrant generational status (first generation or foreign-born, second generation or U.S.-born to at least one foreign-born parent, third or higher generation or U.S.-born with U.S.-born parents), educational attainment (less than high-school, high school graduate, some college, bachelor’s or higher degree), and race/ethnicity (Hispanic, Non-Hispanic White, Non-Hispanic African American, Non-Hispanic Asian).
Statistical Analysis
We examined descriptive statistics for breast cancer risk factors and sociodemographic variables by the MetS status. We selected age, BMI, and fasting time as a priori covariates in multivariable analysis, and further identified possible confounders as variables that changed the age-adjusted estimates of the association between the MetS and any individual MetS component and either measures of mammographic density by more than 10%. We used linear regression models to examine the associations of the MetS and its components with continuous measures of percent density and dense area. We repeated the final multivariable models without adjustment for BMI given the possible causal relationship of BMI with the MetS. We further tested the interaction of the MetS and its components with: 1) BMI, 2) menopausal status, 3) race/ethnicity and 4) generational status, by introducing cross-product terms between these variables in the linear regression models of percent density and dense area. We repeated our multivariable analysis excluding the participants with type II diabetes and obtained similar results. Finally, we examined the associations between the MetS and its components with non-dense area as the outcome. All tests were 2-sided and performed using SAS 9.3 (SAS Institute, Gary, NC).
Results
About 45% of participants met the definition of the MetS. As compared with participants without the MetS, those with MetS were on average 3.2 years older, had lower educational attainment (e.g., 30% vs. 14% with less than high school education), were more likely to have BMI ≥30 kg/m2 (63% vs. 28%), be postmenopausal (61% vs. 39%), and have Type II diabetes (18% vs. 3%) (Table 1). Participants with MetS were also slightly more likely to have fasting time of less than 3 hours (48% vs. 39%), but the average fasting time was not significantly different by MetS status, with the average fasting time of 4.7 and 4.5 hours respectively in those with and without the MetS (p<0.67). There were no significant differences in the MetS status by race/ethnicity, generational status, age at menarche, parity, age at first live birth, and alcohol consumption and smoking status (Table 1).
Table 1.
Sample characteristics by the metabolic syndrome status (n=373)
| Characteristics | Total Cohort | With MetS (n=166) | Without MetS (n=207) |
|---|---|---|---|
| Age in years, Mean (SD) | 50.5 (5.9) | 52.3 (5.6) | 49.1 (5.7) |
|
| |||
| Race/Ethnicity, n (%) | |||
| Hispanic | 252 | 116 (69.9) | 136 (65.7) |
| Non-Hispanic White | 45 | 16 (9.6) | 29 (14.0) |
| Non-Hispanic Black | 63 | 30 (18.1) | 33 (15.9) |
| Non-Hispanic Asian | 13 | 4 (2.4) | 9 (4.4) |
| Education, n (%) | |||
| Less Than High School | 79 | 50 (30.1) | 29 (14.0) |
| High School Graduate | 85 | 40 (24.1) | 45 (21.7) |
| Some College | 86 | 36 (21.7) | 50 (24.2) |
| Bachelor’s or higher degree | 123 | 40 (24.1) | 83 (40.1) |
| Immigrant generational status, n (%) | |||
| 1st generation | 236 | 110 (66.3) | 126 (60.9) |
| 2nd generation | 52 | 18 (10.8) | 34 (16.4) |
| 3rd generation | 84 | 38 (22.9) | 46 (22.2) |
| Body Mass Index, n (%) | |||
| <25.0 kg/m2 | 70 | 5 (3.0) | 65 (31.4) |
| 25.0–29.9 kg/m2 | 141 | 56 (33.7) | 85 (41.1) |
| ≥30 kg/m2 | 162 | 105 (63.3) | 57 (27.5) |
| Positive Family history of breast cancer | 45 | 22 (13.3) | 23 (11.1) |
| Menarche, mean (SD) | 12.7 (1.9) | 12.7 (1.9) | 12.7 (1.9) |
| Number of Live Births, n (%) | |||
| 0 | 46 | 17 (10.2) | 29 (14.0) |
| 1–2 | 205 | 87 (52.4) | 118 (57.0) |
| ≥3 | 122 | 62 (37.4) | 60 (29.0) |
| Age at First Birth, n (%) | |||
| <25 Years Old | 176 | 84 (50.9) | 92 (44.9) |
| 25–29 Years Old | 69 | 30 (18.2) | 39 (19.0) |
| 30+ Years Old | 79 | 34 (20.6) | 45 (22.0) |
| No Live Births | 46 | 17 (10.3) | 29 (14.2) |
| Menopausal Status, n (%) | |||
| Pre/Peri-menopausal | 187 | 63 (38.9) | 124 (60.8) |
| Post-menopausal | 179 | 99 (61.1) | 80 (39.2) |
| Alcohol consumption status, n (%) | |||
| Never | 170 | 79 (47.9) | 91 (44.0) |
| Former | 35 | 19 (11.5) | 16 (7.7) |
| Current | 167 | 67 (40.6) | 100 (48.3) |
| Smoking status, n (%) | |||
| Never | 253 | 102 (62.2) | 151 (73.0) |
| Former | 76 | 40 (24.4) | 36 (17.4) |
| Current | 42 | 22 (13.4) | 20 (9.7) |
| Fasting Time, n (%) | |||
| < 3 Hours | 159 | 79 (47.9) | 80 (38.8) |
| 3 ≤ Hours <6 | 131 | 48 (29.1) | 83 (40.3) |
| ≥ 6 Hours | 81 | 38 (23.0) | 43 (20.9) |
| Positive type II diabetes status | 36 | 29 (17.5) | 7 (3.4) |
BMI was not correlated with HDL-C, but was strongly positively correlated with waist circumference (Pearson correlation coefficient [r] =0.84), and modestly positively correlated with other MetS components (range from r=0.12 for triglycerides to r=0.30 for diastolic blood pressure). BMI was also inversely linearly correlated with percent density (r=−0.36), but had minimal correlation with dense area (r=0.10).
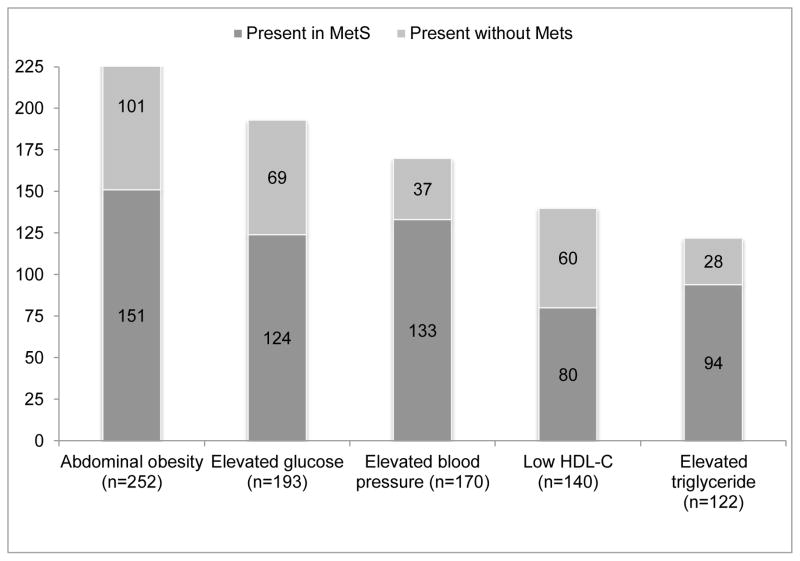
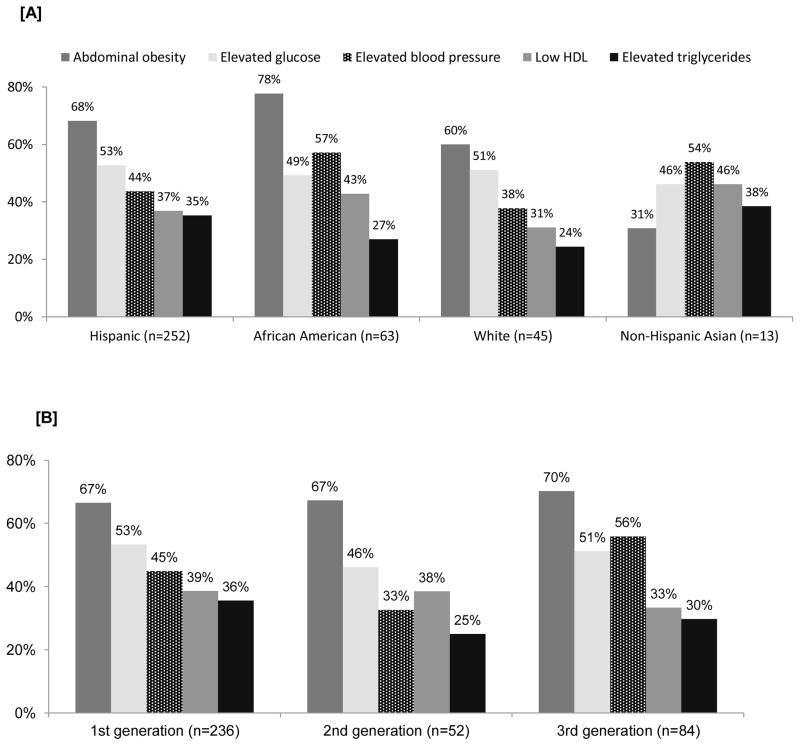
The majority of participants with any individual MetS component had two or more other risk factors, and were therefore classified as having the MetS (Figure 1). Abdominal obesity, the most common individual MetS component, was present in 68% of all participants; about 60% of those with abdominal obesity met the MetS definition. The presence of elevated triglycerides was the least prevalent MetS component in the overall sample at 33%, but 77% of those with elevated triglycerides met the MetS definition (Figure 1). Abdominal obesity was the most prevalent MetS component among all ethnic groups with the exception of the 13 participants of Asian ethnicity, for whom elevated blood pressure or taking hypertensive medications was the most common component (Figure 2, Panel A). About half of
Figure 1.
Number of participants with each metabolic risk factors by metabolic syndrome status
Figure 2.
Percentage of participants with each metabolic risk factor by race/ethnicity [A] and immigrant generational status [B]
Hispanic and white participants had elevated glucose while 57% of African Americans had elevated blood pressure or were taking antihypertensive medications. The relative distribution of MetS components varied slightly across generational status, with abdominal obesity as the most prevalent and elevated triglycerides as the least common MetS component in all immigrant generation levels (Figure 2, Panel B). The largest differences were observed for elevated blood pressure, which had lower prevalence in first-generation (45%) and second-generation women (33%) than in third-generation women (56%); however, this higher prevalence in third generation may have been due to the larger proportion of African Americans in this group (~64% of third-generation women with elevated blood pressure were African Americans, as compared to 8% Hispanics and 28% whites).
Beyond the selected a priori covariates of age, BMI and fasting time, all measured at the time of the mammographic screening, the only factor that altered the associations of the MetS and any individual MetS component with percent density or dense area was educational attainment; this variable was added to the final multivariable models. The MetS was inversely associated with percent density in the multivariable model that adjusted for age, fasting time and educational attainment (, but this association was largely accounted for after further adjustment for BMI (β=−1.05, 95% CI: −4.47, 2.37 in the fully adjusted model) (Table 2, Panel 1). Similarly, the inverse association between abdominal obesity and percent density was reduced and no longer statistically significant with adjustment for BMI. No other associations were observed between any of the MetS components and percent density.
Table 2.
Linear regression coefficients (β) and 95% confidence intervals (CI) for the associations between metabolic risk factors and mammographic density
| Adjusted for age | Adjusted for age and BMI | Adjusted for age, fasting time, education | Adjusted for age, fasting time, education, BMI | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | |
| Percent Density | ||||||||
| Metabolic syndrome (≥3 metabolic components) | −4.71 | (−8.06, −1.35) | −1.05 | (−4.47, 2.37) | −4.47 | (−7.84, −1.10) | −0.70 | (−4.15, 2.74) |
|
Individual components Abdominal Adiposity (WC ≥88cm vs. < 88cm) |
−8.19 | (−11.69, −4.69) | −2.16 | (−6.42, 2.10) | −7.89 | (−11.40, −4.39) | −1.86 | (−6.09, 2.36) |
| Elevated Glucose (>100 mg/dL vs. ≤100 mg/dL) | −1.52 | (−4.79, 1.74) | −0.22 | (−3.34, 2.89) | −1.67 | (−4.91, 1.58) | −0.28 | (−3.38, 2.82) |
| Elevated Blood Pressure (SBP ≥130 or DBP≥85 or hypertensive med vs. SBP<130 & DBP<85 and no hypertensive medication) | −2.41 | (−5.77, 0.96) | 0.86 | (−2.48, 4.20) | −1.99 | (−5.35, 1.37) | 1.44 | (−1.91, 4.79) |
| Low HDL (<50 mg/dL vs. ≥50 mg/dL) | 2.26 | (−1.08, 5.60) | 2.65 | (−0.50, 5.81) | 1.97 | (−1.38, 5.33) | 2.28 | (−0.90, 5.45) |
| Elevated Triglycerides (≥150 mg/dL vs. <150 mg/dL) | −1.40 | (−4.87, 2.07) | −0.44 | (−3.73, 2.86) | −0.96 | (−4.46, 2.53) | 0.16 | (−3.17, 3.48) |
| Dense Area | ||||||||
| Metabolic syndrome (≥3 metabolic components) | 5.65 | (−0.84, 12.13) | 3.10 | (−3.78, 9.98) | 6.23 | (−0.29, 12.75) | 3.87 | (−3.08, 10.82) |
|
Individual components Abdominal Adiposity (WC ≥88cm vs. < 88cm) |
9.36 | (2.58, 16.14) | 5.90 | (−2.57, 14.36) | 9.30 | (2.51, 16.09) | 6.39 | (−2.01, 14.80) |
| Elevated Glucose (>100 mg/dL vs. ≤100 mg/dL) | 3.20 | (−3.06, 9.47) | 2.23 | (−4.04, 8.50) | 3.06 | (−3.19, 9.31) | 2.08 | (−4.18, 8.35) |
| Elevated Blood Pressure (SBP ≥130 or DBP≥85 or hypertensive medication vs. SBP<130 & DBP<85 and no hypertensive medication) | 5.46 | (−1.00, 11.92) | 3.28 | (−3.44, 10.00) | 6.43 | (−0.03, 12.89) | 4.43 | (−2.33, 11.19) |
| Low HDL (<50 mg/dL vs. ≥50 mg/dL) | 9.55 | (3.20, 15.90) | 9.26 | (2.95, 15.57) | 8.91 | (2.50, 15.33) | 8.77 | (2.39, 15.14) |
| Elevated Triglycerides (≥150 mg/dL vs. <150 mg/dL) | −4.67 | (−11.32, 1.98) | −5.46 | (−12.07, 1.15) | −3.73 | (−10.46, 2.99) | −4.56 | (−11.26, 2.15) |
With the exception of elevated triglycerides, the presence of the MetS and the remaining MetS components showed positive associations with dense area in age adjusted models, although only the associations for low HDL-C (β=9.55 95% CI: 3.20, 15.90) and abdominal obesity (β=9.36 95% CI: 2.58, 16.14) reached statistical significance (Table 2, Panel 2). The association between low HDL-C and larger dense area persisted after adjustment for BMI, fasting time, and educational attainment (β=8.77, 95% CI: 2.39, 15.14).
We did not find support for additive statistical interactions between the MetS or its components and BMI, menopausal status, race/ethnicity and immigrant generational status (all p-values for interaction terms > 0.05). We further explored variations in the observed associations through stratified analysis by obesity (obese or BMI≥30 and nonobese or BMI<30) and menopausal status (pre/peri- and postmenopausal) (data not shown). There were some indication of differences in the strength of the association between low HDL-C and larger dense area by menopausal status, with the association being stronger and statistically significant in premenopausal (β=12.48, 95% CI: 3.71, 21.26) as compared with postmenopausal women (β=4.52, 95% CI: −4.54, 13.58). Abdominal obesity also had a stronger association with lower percent density (β=−5.37, 95% CI:−10.10, −0.64) in non-obese womenthan in obese women (β=−0.38, 95% CI:−15.02, 14.26).
The MetS cluster, abdominal obesity, elevated glucose and high blood pressure were positively associated with non-dense area in age adjusted model, but there were no associations between any of these factors and nondensea area after adjustment for BMI (e.g., β=8.54, 95% CI: −4.22, 21.30 for MetS vs. no MetS) (Data not shown).
Given that we measured biomarkers from nonfasting blood, we repeated the multivariable models in the sample restricted to those who had fasted ≥3 hours and again to those who had fasted ≥ 6 hours. The results similarly showed only a positive association between low HDL-C and larger dense area (e.g., low HDL-C measured in blood with ≥ 3 hours and with ≥ 6 hours of fasting was associated respectively with 11.82 cm2 (95% CI: 4.14, 19.50) and 7.19 cm2 larger dense area (95% CI: −2.85, 17.22).
Discussion
In a predominantly racial/ethnic minority and immigrant study population of women in their midlife, we did not observe an association between the MetS as defined by the presence of three or more metabolic risk components and the absolute amount of fibroglandular breast tissues on mammograms as measured by dense breast area. However, we observed an association between low HDL-C and larger dense area, which persisted even after accounting for factors with strong influences on HDL-C and mammographic density including BMI. There were no associations between the MetS cluster or any of its individual components with percent density, capturing the amount of dense area relative to total breast area, after accounting for differences in BMI.
Two prior studies have used similar methodology for assessing the metabolic syndrome and mammographic density [22, 23]. One study, conducted in a cohort of predominantly Caucasian and Asian pre- and early perimenopausal women with a low prevalence of the MetS (15%), reported no associations between the MetS and mammographic density in cross-sectional analysis as well as in analysis involving longitudinal changes in mammographic density [22]. The second study included pre- and postmenopausal women in the Mexican Teacher’s Cohort (ESMaestras) from two states in Mexico with a higher prevalence of the MetS (21–40% in premenopausal women and 39–51% in postmenopausal women) that was similar to the prevalence in our study (32% in premenopausal and 51% in postmenopausal women). The ESMaestras study reported a positive association between the MetS and percent density and dense area (differences of ~5% and 6.3 cm2, respectively) that was limited to premenopausal women in one of the two states. Abdominal obesity was the most common MetS component in our and these two studies, but there were substantial differences in the prevalence and co-occurrence of other components across studies, suggesting different biological profiles of the MetS across these populations. The lack of strong support in these few studies using similar methodology in different study populations suggest that the MetS may not increase breast cancer risk through influencing mammographic density. However, it is also possible that a one-time assessment of the MetS may not sufficiently capture the mechanism linking the MetS to breast cancer risk via mammographic density.
More studies have assessed the associations of individual components of the MetS and mammographic density. Abdominal obesity as measured by waist circumference has been consistently associated with lower percent density and larger dense area, but these associations, as observed in our study, are largely reduced after adjustment for general obesity as measured by BMI [22, 23]. Compatible with our findings, there has been little to no support for associations between elevated glucose [41, 42, 22, 23], triglycerides [23, 22] and blood pressure [23, 22] with either percent density or dense area after accounting for BMI. The results are more mixed for HDL-C. In our study, we observed reduced HDL-C (< 50 mg/dL) to be associated with larger dense area after adjusting for BMI and other covariates, with the association being stronger in premenopausal women. Low HDL-C at the level used in our study was also associated with larger dense area and higher percent density among premenopausal women from one state in the ESTMaestra study [23]. The magnitude of the association between dense area and high HDL-C categories observed in our study is similar to that reported in the ESTMaestra study. HDL-C and percent density were also inversely associated in a small sample of postmenopausal women [26]. In contrast, other studies have reported positive associations between HDL-C levels and mammographic density in premenopausal women [25, 43, 44], as well as null associations in premenopausal [22] and postmenopausal women [24, 27]. Most, but not all [45, 46, 38], studies of HDL-C and breast cancer risk support a protective effect for high HDL-C although results across BMI categories and menopausal status have been inconsistent [47–51, 3]. Taken together, HDL-C may have an influence on breast cancer risk and this may be mediated through mammographic density, but the direction of the association remains unclear and is likely to depend on modification by other factors. The biological mechanisms underlying HDL-C and breast cancer may involve complex processes influencing exposures to proinflammatory factors and endogenous estrogen that stimulate breast tissue proliferation. Additional investigations are clearly needed to better understand these relationships, and are of substantial public health and clinical interest. As HDL-C may be modified through behavioral and pharmaceutical interventions [60], and if related to mammographic density, which is easily monitored through routine mammography, it may offer opportunities for combined efforts to reduce the risks for CVD and breast cancer.
Our results add to the limited empirical literature on the relationship between metabolic risk factors and mammographic density in a diverse study population with sufficient variations in metabolic risk factors and mammographic density. We did not find significant variations in the associations between metabolic risk factors and mammographic density across racial/ethnic groups and between foreign- and U.S.-born women, and only observed differences in the strengths of the association between HDL-C and dense area by menopausal status. However, caution should be exercised when interpreting these results as the small subgroup sizes may have limited the statistical power to detect significant differences. Study participants were not instructed to fast at the time of mammography and enrollment into the study, but we accounted for fasting time in our statistical analysis. Similar to other studies in this area, we used a single measure of metabolic biomarkers collected concurrently with mammographic density data; however, a single measure may not sufficiently capture the long-term risk of metabolic disorders and their associations with mammographic density. We used DBS samples for assessment of blood-based metabolic biomarkers, which while not used in prior investigations of metabolic risk factors with breast cancer risk or mammographic density, have been validated against venous blood samples in prior studies [61–65], and are being increasingly used in large ongoing community surveys including the National Longitudinal Study of Adolescent Health and the National Social Life, Health, and Aging Project [66]. Our prior experience in collecting venous blood from women recruited in community-based mammography clinics similar to the setting in the present study had presented considerable logistical barriers and an overall low response rate for undergoing venipuncture. The DBS collection offers a highly feasible alternative as it is minimally invasive and easily collected by non-phlebotomists, and requires no centrifugation or immediate freezing [66]. However, the laboratory methodologies for DBS samples are less standardized. Given that the laboratory was blinded to mammographic density measures, any measurement error in biomarkers would be nondifferential, and may have contributed to an underestimation of the true association and hence the observed null associations of the MetS and most components with mammographic density in our study. Our study benefited from using digital mammograms that were all obtained at a single institution on the same day as blood sample collection and evaluated by the same reader, and from analyzing all blood samples at a single laboratory, thereby reducing potential errors associated with variations in these factors. Our measures of mammographic density were highly reproducible as determined by a subset of duplicated reads, and any error should be nondifferential with respect to metabolic risk factors as the reader was blinded to this data.
In summary, our results do not support an association between the MetS as a cluster of three or more metabolic risk factors and mammographic density as measured by percent and dense breast area. We found that HDL-C alone may have an influence on dense breast area that is independent of BMI, and may be in the same direction as its association with breast cancer risk. If confirmed in other studies, these results suggest that chronic disease prevention efforts to increase HDL-C levels may potentially have a favorable impact on breast density.
Acknowledgments
This work was supported by grants from the Susan G. Komen Foundation [KG110331] and the National Cancer Institute [K07 CA151777].
We thank the study participants for contributing data to this research. We also thank Chidera Agu, Rachel Rhodes, Camille Rodriguez, Meredith Welch and Brenda Umana for data collection and Elizabeth Kerschner for laboratory analysis of blood samples.
List of abbreviations
- MetS
metabolic syndrome
- HDL-C
High density lipoprotein cholesterol
Footnotes
Competing Interests
Authors declare they have no competing interests.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. CIRCULATIONAHA.105.169404 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. CIRCULATIONAHA.109.192644 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes care. 2012;35(11):2402–11. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20(12):1301–9. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 5.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28(7):763–77. doi: 10.1210/er.2006-0019. er.2006-0019 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2009;121(2):479–83. doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 7.Lopez R, Agullo P, Lakshmanaswamy R. Links between obesity, diabetes and ethnic disparities in breast cancer among Hispanic populations. Obes Rev. 2013;14(8):679–91. doi: 10.1111/obr.12030. [DOI] [PubMed] [Google Scholar]
- 8.Berrino F, Villarini A, Traina A, Bonanni B, Panico S, Mano MP, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147(1):159–65. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 9.Bjorge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1737–45. doi: 10.1158/1055-9965.EPI-10-0230. 19/7/1737 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev. 1993;15(1):196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]
- 11.Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to the time since the mammogram. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1110–7. doi: 10.1158/1055-9965.EPI-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. 15/6/1159 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast cancer research : BCR. 13(6):223. doi: 10.1186/bcr2942. bcr2942 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghjyan L, Colditz GA, Wolin K. Physical activity and mammographic breast density: a systematic review. Breast Cancer Res Treat. 2012;135(2):367–80. doi: 10.1007/s10549-012-2152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roubidoux MA, Kaur JS, Griffith KA, Sloan J, Wilson C, Novotny P, et al. Correlates of mammogram density in southwestern Native-American women. Cancer Epidemiol Biomarkers Prev. 2003;12(6):552–8. [PubMed] [Google Scholar]
- 17.Sellers TA, Jensen LE, Vierkant RA, Fredericksen ZS, Brandt KR, Giuliano AR, et al. Association of diabetes with mammographic breast density and breast cancer in the Minnesota breast cancer family study. Cancer Causes Control. 2007;18(5):505–15. doi: 10.1007/s10552-007-0128-9. [DOI] [PubMed] [Google Scholar]
- 18.Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78(9):1233–8. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2086–92. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 20.Reeves KW, Stone RA, Modugno F, Ness RB, Vogel VG, Weissfeld JL, et al. Longitudinal association of anthropometry with mammographic breast density in the Study of Women's Health Across the Nation. Int J Cancer. 2009;124(5):1169–77. doi: 10.1002/ijc.23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Warren RM, Pinney E, Warwick J, Cuzick J. Determinants of percentage and area measures of mammographic density. Am J Epidemiol. 2009;170(12):1571–8. doi: 10.1093/aje/kwp313. kwp313 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Conroy SM, Butler LM, Harvey D, Gold EB, Sternfeld B, Greendale GA, et al. Metabolic syndrome and mammographic density: the Study of Women's Health Across the Nation. Int J Cancer. 2011;129(7):1699–707. doi: 10.1002/ijc.25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice MS, Biessy C, Lajous M, Bertrand KA, Tamimi RM, Torres-Mejia G, et al. Metabolic syndrome and mammographic density in Mexican women. Cancer prevention research. 2013;6(7):701–10. doi: 10.1158/1940-6207.CAPR-12-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamburrini AL, Woolcott CG, Boyd NF, Yaffe MJ, Terry T, Yasui Y, et al. Associations between mammographic density and serum and dietary cholesterol. Breast Cancer Res Treat. 2011;125(1):181–9. doi: 10.1007/s10549-010-0927-7. [DOI] [PubMed] [Google Scholar]
- 25.Boyd NF, Connelly P, Byng J, Yaffe M, Draper H, Little L, et al. Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev. 1995;4(7):727–33. [PubMed] [Google Scholar]
- 26.Maskarinec G, Lyu LC, Meng L, Theriault A, Ursin G. Determinants of mammographic densities among women of Asian, Native Hawaiian, and Caucasian ancestry. Ethnicity & disease. 2001;11(1):44–50. [PubMed] [Google Scholar]
- 27.Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, et al. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1411–7. doi: 10.1158/1055-9965.EPI-04-0920. 14/6/1411 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Tehranifar P, Reynolds D, Fan X, Boden-Albala B, Engmann NJ, Flom JD, et al. Multiple metabolic risk factors and mammographic breast density. Ann Epidemiol. 2014;24(6):479–83. doi: 10.1016/j.annepidem.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keegan TH, John EM, Fish KM, Alfaro-Velcamp T, Clarke CA, Gomez SL. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1208–18. doi: 10.1158/1055-9965.EPI-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borrell LN, Castor D, Conway FP, Terry MB. Influence of nativity status on breast cancer risk among US black women. J Urban Health. 2006;83(2):211–20. doi: 10.1007/s11524-005-9014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habel LA, Capra AM, Oestreicher N, Greendale GA, Cauley JA, Bromberger J, et al. Mammographic density in a multiethnic cohort. Menopause. 2007;14(5):891–9. doi: 10.1097/gme.0b013e318032569c. [DOI] [PubMed] [Google Scholar]
- 33.Tseng M, Byrne C, Evers KA, London WT, Daly MB. Acculturation and breast density in foreign-born, U.S. Chinese women. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1301–5. doi: 10.1158/1055-9965.EPI-06-0159. [DOI] [PubMed] [Google Scholar]
- 34.Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat. 2007;104(1):47–56. doi: 10.1007/s10549-006-9387-5. [DOI] [PubMed] [Google Scholar]
- 35.McCormack VA, Perry N, Vinnicombe SJ, Silva Idos S. Ethnic variations in mammographic density: a British multiethnic longitudinal study. Am J Epidemiol. 2008;168(4):412–21. doi: 10.1093/aje/kwn169. kwn169 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez F, Naderi S, Wang Y, Johnson CE, Foody JM. High prevalence of metabolic syndrome in young Hispanic women: findings from the national Sister to Sister campaign. Metabolic syndrome and related disorders. 2013;11(2):81–6. doi: 10.1089/met.2012.0109. [DOI] [PubMed] [Google Scholar]
- 37.Olson EC, Van Wye G, Kerker B, Thorpe L, Frieden TR. Take care Inwood and Washington Heights. (2) 2006 Retrieved from http://www.nyc.gov/html/doh/downloads/pdf/data/2006chp-301.pdf.
- 38.http://www.ssa.gov/history/ssn/ssnchron.html.
- 39.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 40.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Physics in medicine and biology. 1994;39(10):1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 41.Borugian MJ, Spinelli JJ, Gordon PB, Abanto Z, Brooks-Wilson A, Pollak MN, et al. Fasting insulin and endogenous hormones in relation to premenopausal breast density (Canada) Cancer Causes Control. 2014;25(3):385–94. doi: 10.1007/s10552-014-0339-9. [DOI] [PubMed] [Google Scholar]
- 42.Woolcott CG, Courneya KS, Boyd NF, Yaffe MJ, McTiernan A, Brant R, et al. Association between sex hormones, glucose homeostasis, adipokines, and inflammatory markers and mammographic density among postmenopausal women. Breast Cancer Res Treat. 2013;139(1):255–65. doi: 10.1007/s10549-013-2534-x. [DOI] [PubMed] [Google Scholar]
- 43.Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol Biomarkers Prev. 2005;14(1):33–40. 14/1/33 [pii] [PubMed] [Google Scholar]
- 44.Flote VG, Frydenberg H, Ursin G, Iversen A, Fagerland MW, Ellison PT, et al. High-density lipoprotein-cholesterol, daily estradiol and progesterone, and mammographic density phenotypes in premenopausal women. Cancer prevention research. 2015;8(6):535–44. doi: 10.1158/1940-6207.CAPR-14-0267. [DOI] [PubMed] [Google Scholar]
- 45.Ferraroni M, Gerber M, Decarli A, Richardson S, Marubini E, Crastes de Paulet P, et al. HDL-cholesterol and breast cancer: a joint study in northern Italy and southern France. Int J Epidemiol. 1993;22(5):772–80. doi: 10.1093/ije/22.5.772. [DOI] [PubMed] [Google Scholar]
- 46.Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: a prospective study of 31,209 Norwegian women. Cancer Causes Control. 1994;5(6):501–9. doi: 10.1007/BF01831377. [DOI] [PubMed] [Google Scholar]
- 47.Llanos AA, Makambi KH, Tucker CA, Wallington SF, Shields PG, Adams-Campbell LL. Cholesterol, lipoproteins, and breast cancer risk in African American women. Ethnicity & disease. 2012;22(3):281–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96(15):1152–60. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y, Park SK, Han W, Kim DH, Hong YC, Ha EH, et al. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol Biomarkers Prev. 2009;18(2):508–15. doi: 10.1158/1055-9965.EPI-08-0133. [DOI] [PubMed] [Google Scholar]
- 50.Moorman PG, Hulka BS, Hiatt RA, Krieger N, Newman B, Vogelman JH, et al. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol Biomarkers Prev. 1998;7(6):483–8. [PubMed] [Google Scholar]
- 51.Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, Folsom AR. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18(9):671–7. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 53.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 54.Beral V Million Women Study C. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 55.Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19(2):492–502. doi: 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 56.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 57.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–15. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 58.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 59.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast cancer research : BCR. 2008;10(1):201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–98. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 61.Lakshmy R, Gupta R, Prabhakaran D, Snehi U, Reddy KS. Utility of dried blood spots for measurement of cholesterol and triglycerides in a surveillance study. J Diabetes Sci Technol. 2010;4(2):258–62. doi: 10.1177/193229681000400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lakshmy R, Mathur P, Gupta R, Shah B, Anand K, Mohan V, et al. Measurement of cholesterol and triglycerides from a dried blood spot in an Indian Council of Medical Research-World Health Organization multicentric survey on risk factors for noncommunicable diseases in India. J Clin Lipidol. 2012;6(1):33–41. doi: 10.1016/j.jacl.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Quraishi R, Lakshmy R, Prabhakaran D, Mukhopadhyay AK, Jailkhani B. Use of filter paper stored dried blood for measurement of triglycerides. Lipids Health Dis. 2006;5:20. doi: 10.1186/1476-511X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abyholm AS. Determination of glucose in dried filter paper blood spots. Scand J Clin Lab Invest. 1981;41(3):269–74. doi: 10.3109/00365518109092044. [DOI] [PubMed] [Google Scholar]
- 65.Winocour PH, McKinnon GA, McMurray JR, Anderson DC. Evaluation of the measurement of blood glucose levels on dried filter paper blood spots. Diabet Med. 1985;2(4):269–71. [PubMed] [Google Scholar]
- 66.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]