Abstract
The mechanisms by which intracellular pathogens trigger immunosuppressive pathways are critical for understanding the pathogenesis of microbial infection. One pathway that inhibits host defense responses involves the induction of type I interferons and subsequently IL-10, yet the mechanism by which type I IFN induces IL-10 remains unclear. Our studies of gene expression profiles derived from leprosy skin lesions suggested a link between IL-27 and the IFN-β induced IL-10 pathway. Here, we demonstrate that the IL-27p28 subunit is upregulated following treatment of monocytes with IFN-β and Mycobacterium leprae, the intracellular bacterium that causes leprosy. The ability of IFN-β and M. leprae to induce IL-10 was diminished by IL-27 knockdown. Additionally, treatment of monocytes with recombinant IL-27 was sufficient to induce the production of IL-10. Functionally, IL-27 inhibited the ability of IFN-γ to trigger antimicrobial activity against M. leprae in infected monocytes. At the site of disease, IL-27 was more strongly expressed in skin lesions of patients with progressive lepromatous leprosy, correlating and colocalizing with IFN-β and IL-10 in macrophages. Together, these data provide evidence that in the human cutaneous immune responses to microbial infection, IL-27 contributes to the suppression of host antimicrobial responses.
Introduction
The ability of microbial pathogens to trigger immune responses which counteract host defense is critical for the pathogenesis of infectious disease. Among such pathways, the induction of IL-10 represents a key mechanism by which microbes suppress host antimicrobial responses. In some circumstances, the induction of type I interferons (IFNs) by the pathogen represents one trigger for IL-10 production, resulting in a profound inhibitory effect on innate and adaptive immunity. As such, the induction of the type I IFN to IL-10 pathway contributes to chronic progressive disease in bacterial (Teles et al., 2013) and viral (Teijaro et al., 2013; Wilson et al., 2013) infections. Therefore, elucidation of the mechanism(s) by which IL-10 is induced in human immune responses is central for understanding how microbial pathogens suppress antimicrobial defense strategies.
Leprosy, a human infectious disease of skin caused by the intracellular bacterium Mycobacterium leprae, presents as a spectrum in which the clinical manifestations correlate with the immune response to the pathogen. As such, leprosy is a valuable model for investigating human immune responses to infection. In the skin lesions of leprosy patients, an inverse correlation exists between the expression of IFN-β and IFN-γ, as well as their respective downstream gene programs (Teles et al., 2013). The skin lesions from patients with the progressive, L-lep form of leprosy display an IFN-β gene signature, whereas lesions from patients with the localized, T-lep form of leprosy are characterized by a low IFN-β and high IFN-γ gene signature. Similarly, IFN-β and IL-10 expression in lesions were significantly correlated, which led to the observation that M. leprae induction of IL-10 was type I IFN dependent. Both IFN-β and IL-10 inhibit the antimicrobial effect of IFN-γ on intracellular M. leprae, identifying the IFN-β and IL-10 axis as a key immunosuppressive pathway in human mycobacterial infection.
The ability of IFN-β to induce IL-10 is relevant to the pathogenesis of other microbial infections. For example, overlap of the IFN-β gene signature in leprosy lesions with the IFN-β signatures from peripheral blood cells in tuberculosis patients (Berry et al., 2010; Maertzdorf et al., 2012), identified a common signature of 16 IFN-β–induced genes (Teles et al., 2013). One of these IFN-β downstream genes, IL-27, has been shown to induce IL-10, leading to the suppression of lymphocyte responses (Liu et al., 2013; Murugaiyan et al., 2009). IL-27 functions as a heterodimer composed of two subunits: p28 and EBV-induced gene 3 (EBI3), of which the transcriptional regulation of IL-27p28 has been studied in cells of the monocyte/macrophage lineage (Liu et al., 2007). The IL-27 heterodimer activates via an IL-27R complex consisting of the unique subunit IL-27R (also referred to a TCCR and WSX-1) and the gp130 chain of IL-6R. Here, we investigated the role of IL-27 in suppressing human host defense responses against a cutaneous pathogen.
Results
IFN-β induces IL-27 in human monocytes
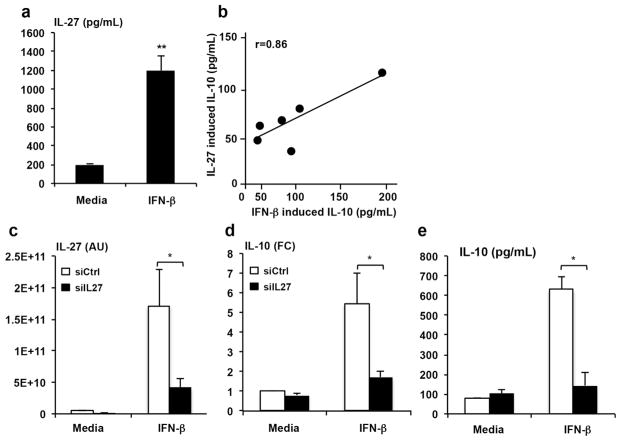
To begin evaluating the role of IL-27 in the IFN-β downstream network, we first examined whether IFN-β induces IL-27 expression in adherent monocytes using an ELISA that measures the levels of the IL-27 heterodimer. Indeed, IFN-β significantly induced IL-27 in human monocytes (Fig. 1a). Both IFN-β and IL-27 induced IL-10, with the induction of IL-10 by IFN-β and IL-27 highly correlated in monocytes from the same donors (Fig. 1b). These data suggested the possibility that IFN-β induction of IL-10 in human monocytes was IL-27 dependent.
Figure 1. Correlation of IL-10 production by IFN-β and IL-27.
Monocytes stimulated for 3 or 24h with IFN-β (273U/mL) and IL-27 (40ng/mL). (a) Production of IL-27 in response to stimulation with IFN-β (n=6). (b). Correlation of IL-10 produced in response to IFN-β (x-axis) and IL-27 (y-axis). (c). Knockdown of IL-27 (ON-TARGET plus; siIL27-black bars) or non-targeting siRNA (siCtrl-white bars). qPCR results displaying arbitrary units (AU) 3hrs following stimulation. IL-27p28 is significantly reduced in siIL27 samples compared to siCtrl (n=11, p=0.03). (d) qPCR showing relative fold change of IL-10 production using ΔΔCT method 3hrs after stimulation with IFN-β (n=11, p=0.02). Levels were normalized to GAPDH and compared to unstimulated cells (media) from siCtrl (n=3, p=0.006). (e) IL- 10 protein 24hrs following stimulation with IFN-β (n=3). Statistical significance was calculated by Student’s t-test; *, p ≤ 0.05 comparing siCtrl to siIL27.
IL-27 participates in IFN-β induced IL-10 production
In order for us to determine whether IFN-β induction of IL-10 was IL-27 dependent, we knocked down IL-27 expression using siRNAs (to our knowledge there is no blocking monoclonal antibody to IL-27). Human peripheral blood mononuclear cells (PBMC) were transfected with a pool of siRNAs specific for the p28 subunit of IL-27 (siIL27) or a pool of non-specific siRNAs (siCtrl), for 24 hrs, then stimulated with IFN-β for 3 hrs or 24 hrs. Transfection of siIL27 inhibited IL-27p28 mRNA expression at 3 hrs by >70% as compared to siCtrl, in both the media and IFN-β stimulated cultures (Fig. 1c). Both IL-10 mRNA (p=0.02, Fig. 1d) measured at 3 hrs and protein (p=0.006, Fig. 1e) measured by ELISA at 24 hrs were significantly inhibited by the knockdown of IL-27 p28 compared to siCtrl cultures.
Live M. leprae induces the production of IFN-β, IL-27, and IL-10 in human monocytes
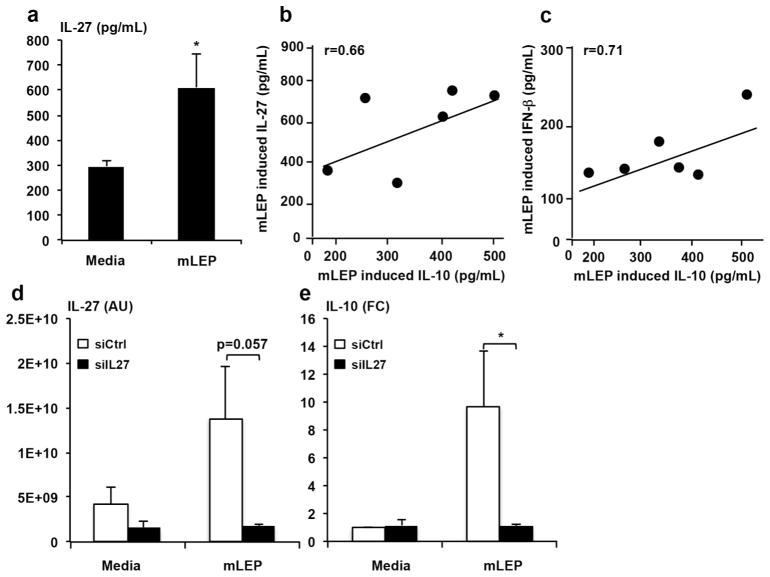
Previously, we found that M. leprae induces IFN-β expression by monocytes (Teles et al., 2013). Here, we investigated a dose titration for the induction of IFN-β, IL-27 and IL-10 by M. leprae (Fig. 2a). Increasing the M. leprae MOI resulted in enhanced IL-27 production in monocytes which plateaued at an MOI of 10. Similarly, IFN-β and IL-10 release plateaued at an MOI of 10, such that this was the dose used for further experiments. We further demonstrate that M. leprae-induced IFN-β and IL-10 production in the same donors correlated significantly (Fig. 2b). In addition, M. leprae induced IL-27 heterodimer and IL-10 produced in the same donors significantly correlated (Fig. 2c). Knockdown of IL-27p28 mRNA significantly inhibited M. leprae induced IL-10 mRNA expression by >80% compared to siCtrl (p=0.03, Fig. 2d and e). Together, these experiments demonstrate a role for IL-27 in both IFN-β and M. leprae induction of IL-10.
Figure 2. Correlation of IL-10 production by M. leprae and IL-27.
Monocytes were stimulated for 24hrs with mLEP (MOI 1:1, 5:1, 10:1, 20:1) to detect IFN-β, IL-27 and IL-10 levels. (a) Production of IFN- β, IL-27, and IL-10 in response to stimulation with mLEP (n=4, *p<0.05,**p<0.01). (b) Correlation of IL-10 (x-axis) and IL-27 (y-axis) produced in response to mLEP. (c) Correlation of IL-10 (x-axis) and IFN-β (y-axis) produced in response to mLEP. (d) qPCR results displaying arbitrary units (AU) of IL-27p28 transcripts 3hrs following stimulation. IL-27 is reduced in siIL27 (black bars) samples compared to siCtrl (white bars; n=4, p=0.03). (e) qPCR showing relative fold change of IL-10 production using ΔΔCT method 3hrs after stimulation with mLEP. Levels were normalized to GAPDH and compared to unstimulated cells (media) from siCtrl. (n=4, p=0.02). Significance was calculated by Student’s t-test; *, p ≤ 0.05 comparing siCtrl to siIL27.
IL-27 mRNA and protein are differentially expressed in L-Lep lesions and correlate with IFN-β and IL-10 expression in leprosy skin lesions
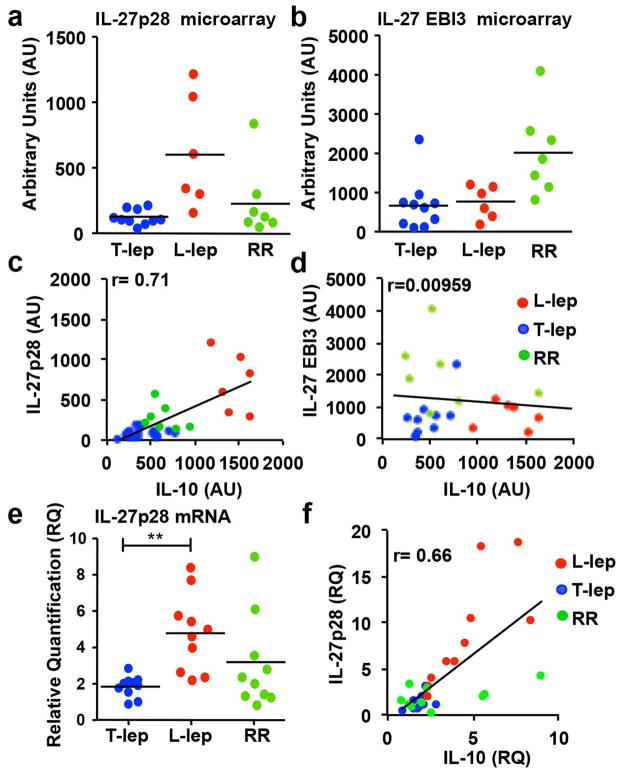
The inverse correlation of IFN-β vs. IFN-γ induced gene expression signatures in leprosy lesions led us to hypothesize that IL-27 contributes to the local immune response in leprosy. In addition to the T-lep and L-lep forms of leprosy, we examined reversal reactions (RR), representing a shift from the L-lep to T-lep part of the disease spectrum, accompanied by a reduction in bacilli in lesions and enhanced Th1 cytokine responses (Yamamura et al., 1992). IL-27p28 but not IL-27 EBI3 mRNAs, was found to be differentially expressed in L-lep compared to T-lep lesions (Fig. 3a and b). RR lesions expressed IL-27p28 mRNA at levels similar to T-lep lesions, and greater than in L-lep lesions. However, IL-27 EBI3 mRNA expression was greater in RR lesions compared to both L-lep and T-lep lesions, though this increase was not significant (Fig. 3b). The expression levels of IL-27p28, but not IL-27 EBI3, directly correlated with IL-10 in the microarray data (r=0.71 and r=0.00959 respectively, Fig. 3c and d). The elevated expression of IL-27p28 transcripts in L-lep vs. T-lep and RR lesions and correlation with IL-10 mRNA expression was corroborated by PCR of an independent set of skin biopsy specimens (Fig. 3e and f).
Figure 3.
IFN-β induces both IL-27 and IL-10. Total mRNA was isolated from L-lep (n=6), T-lep (n=10) and RR (n=7) skin lesions, and the (a) IL-27p28 and (b) IL-27 EBI3 mRNA levels were analyzed by microarray. (c) Correlation of IL-27p28 and IL-10 detected by microarray (arbitrary units (AU)). (d) No correlation was seen between IL-27 EBI3 and IL-10 by microarray (AU). (e) Total mRNA was isolated from L-lep (n=10), Tlep (n=10) and RR (n=10) skin lesions, IL-27p28 and IL-10 mRNA levels were analyzed by TaqMan qPCR. The levels of IL-27p28 were normalized to GAPDH levels in the same tissue. (f) Correlation between IL- 27p28 and IL-10 measured by qPCR. Statistical significance was calculated by One way ANOVA with Kruskal-Wallis post hoc analysis; **, p ≤ 0.01; *, p ≤ 0.05
The distribution and localization of IL-27 protein in leprosy lesions was examined by immunohistochemistry and confocal laser microscopy using a mAb that recognized the IL-27 heterodimer. Similar to IL-27p28 mRNA, IL-27 protein was also more highly expressed in L-lep lesions compared to T-lep and RR lesions (Fig. 4a). In addition, IL-27 protein colocalized with CD209+ and CD163+ macrophages, of relevance since these markers identify the predominant macrophage phenotype in L-lep lesions and is reproduced in vitro by the differentiation of monocytes by IL-10 (Fig. 4b) (Montoya et al., 2009). In addition, some IL-27 was detected in CD3+ T cells (Fig. 4b). Triple immunofluorescence revealed that IFN-β, IL-27 and IL-10 are co-expressed at the cellular level in L-lep lesions, with some cells also expressing individual cytokines, indicating autocrine and paracrine production of the three cytokines (Fig. 4c). Taken together, the immunohistology data indicate that IL-27, along with IFN-β and IL-10, are most strongly expressed in L-lep lesions, therefore associated with the progressive form of leprosy.
Figure 4.
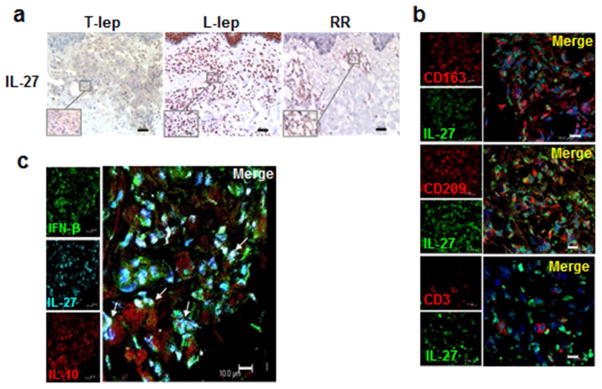
Expression of IFNβ, IL-27 and IL-10 expression in leprosy lesions (a) One representative labeled section displaying IL-27 expression in leprosy lesions, (n=5); scale bar=40 μm. Original magnification: x100. Inserts show higher magnification of inflammatory infiltrate area (x400 original). (b) Co-expression of IL-27 (green) with MΦ markers (CD163 and CD209; red) and T cells marker (CD3; red). Cellular nuclei were visualized using DAPI. Picture represents one individual L-lep sample (n=4); scale bar=10 μm (c) Colocalization of IFN-β (green), IL-27 (cyan) and IL-10 (red), in inflammatory infiltrate of L-lep lesions. Picture represents one individual L-lep sample (n=3); arrows indicate colocalization of the three cytokines; scale bar=10 μm.
IL-27 prevents IFN-γ dependent antimicrobial activity against M. leprae in human monocytes
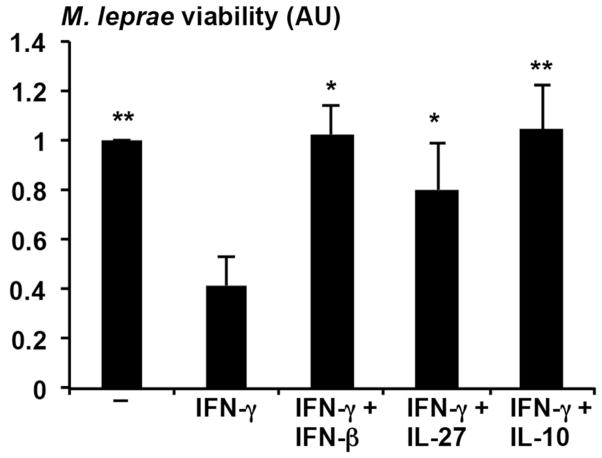
Next, the effect of IL-27 on IFN-γ induced antimicrobial activity against M. leprae was evaluated. Human adherent monocytes were pre-treated with IFN-γ, and then infected with M. leprae at an MOI of 10:1 overnight resulting in infection of approximately 80% of the monocytes infected with about two bacteria per cell. After the overnight infection, monocytes were treated with IFNγ, IFNβ + IFN-γ, IL-27 + IFN-γ or IL-10 + IFN-γ and M. leprae viability measured after five days. IFN-γ induced an antimicrobial activity against M. leprae in infected monocytes, reducing the number of viable bacilli by approximately 60% (Fig. 5). Similar to IFN-β and IL-10, the addition of IL-27 abrogated the IFN-γ induced antimicrobial response (Fig. 5). Because the experiments are performed over a five day period, we could not assess whether IFN-β inhibition of IFN-γ induced antimicrobial activity was IL-27 dependent, as knockdown of IL-27 using siRNA is transient. In summary, these studies suggest that IL-27 inhibits host defense against M. leprae in infected monocytes.
Figure 5.
IL-27 blocks IFNγ induced antimicrobial activity. Human monocytes were pretreated with IFNγ. After infection, cells were treated with IFN-γ alone or in combination with IFN-β, IL-10 or IL-27 for 4 days. Viability of M. leprae was calculated by the ratio of bacterial 16S RNA and DNA (RLEP) detected by qPCR. Data are represented as mean ± SEM, n=7. Statistical significance was calculated by One way ANOVA with Kruskal-Wallis post hoc analysis (two-tailed Student’s t-test).* p 0.05.
Discussion
The ability of intracellular pathogens to inhibit host defense responses contributes to the pathogenesis of human infectious disease. Here, we explored the role of IL-27 in the immune response to a microbial pathogen by studying leprosy as a model. We provide evidence that IL-27 inhibits host defense in human leprosy as: i) the induction of the immunosuppressive cytokine IL-10 by both IFN-β and M. leprae was partially dependent on IL-27, ii) IL-27 colocalized with IFN-β and IL-10 in the skin lesions from the progressive L-lep form vs. the self-limited T-lep of leprosy; and iii) IL-27 suppressed IFN-γ induced antimicrobial activity against M. leprae. Together, these data reveal that the induction of IL-27 provides a mechanism for suppression of host defense responses in the pathogenesis of the cutaneous human infectious disease, leprosy.
Through the analysis of gene expression profiles in leprosy, we identified IL-27, specifically the p28 subunit, as an IFN-β downstream gene associated with the progressive form of the disease (Teles et al., 2013; Waddell et al., 2010). IL-27 was part of a common set of 16 IFN-β downstream genes in the overlap between the gene expression profiles derived from the skin lesions of L-lep patients and the peripheral blood from two cohorts of patients with active tuberculosis (Berry et al., 2010; Maertzdorf et al., 2012). Given that M. leprae induces IFN-β and that IFN-β induces IL-27, M. leprae induced IFN-β is a one key event for the induction of IL-27. Emerging data indicates that mycobacteria induce Type I IFN via cytoplasmic sensors that detect dsDNA and di-cyclic nucleotides that signal through STING, DDX41 and/or cGAS (Dey et al., 2015; Parvatiyar et al., 2012; Watson et al., 2012). It is also possible that M. leprae directly induces IL-27 via a Type I IFN independent pathway.
Here, we provide evidence that IL-27 plays a role in host defense against M. leprae by inhibiting IFN-γ induced antimicrobial activity. In tuberculosis, IL-27 has also been identified at the site of disease (Xia et al., 2014) and inhibits the host defense responses against M. tuberculosis (Robinson and Nau, 2008). IL-27, has been shown to block IFN-γ induced autophagy (Sharma et al., 2014), a key part of the IFN-γ induced antimicrobial pathway against M. tuberculosis (Fabri et al., 2011). Although the present data demonstrate that IL-27 suppresses IFN-γ induced antimicrobial activity against M. leprae, we were unable to experimentally determine whether IFN-β suppression of this antimicrobial activity was dependent on IL-27.
Although IL-27 functions as a heterodimer containing p28 and EBI3, EBI3 has been found to be constitutively expressed, it is suggested that the induction of the p28 subunit is the rate limiting step in the production of bioactive IL-27 (Remoli et al., 2007). This is in agreement with our findings that we see robust induction in the expression of the p28 subunit and very little variability in the expression of EBI3.
The present results suggest that one mechanism of IL-10 production in mycobacterial infection involves an M. leprae→IFN-β→IL-27→IL-10 pathway. We provide evidence that M. leprae induces each of these cytokines, as well as demonstrate that these cytokines in turn induce the downstream pathway, i.e. IFN-β induces IL-27 and IL-10, and IL-27 induces IL-10 directly. Our finding that IL-27 induces IL-10 in human monocytes is consistent with a previous study demonstrating that IL-27 induces IL-10 in mouse bone marrow derived macrophages (Iyer et al., 2010) and human T cells (Batten et al., 2008).
The ability of M. leprae and IFN-β to induce IL-10 was partially dependent on the expression of IL-27. This pathway is consistent with the ability of LPS to induce type I IFN, leading to the IL-27 dependent production of IL-10 (Iyer et al., 2010). IL-10 production in response to Leishmania spp. infection was also found to be IL-27 dependent (Anderson et al., 2009). In addition, IL-27 enhanced leishmania growth in an IL-10 dependent manner (Barreto-de-Souza et al., 2014). Together, these data support the concept that IL-27 directly induces IL-10 expression.
In some experimental settings, IL-27 suppressed IL-10 production, in particular in the presence of other cytokines or stimuli. For example, the addition of IL-27 with M-CSF or monocyte-derived macrophages inhibited TLR-induced IL-10 production (Kalliolias and Ivashkiv, 2008; Zeitvogel et al., 2012). It is noteworthy that our data demonstrates that in the skin lesions of leprosy, IL-27 and IL-10 expression correlates, and the two cytokines co-localize in the progressive form the disease.
Clearly, microbial pathogens can also induce IL-10 in an IL-27 independent manner. In our study, the ability of M. leprae and IFN-β to induce IL-10 was partially blocked by IL-27 knockdown. The induction of IL-10 by the co-addition of live M. tuberculosis and IFN-β to mouse bone marrow derived macrophages was not inhibited by IL-27R deficiency. Live M. tuberculosis is known to induce IL-10 via TLR2 signaling (Jang et al., 2004), a TLR pathway that does not involve induction of type I IFN. Further studies demonstrate that engagement of TLRs can directly induce IL-10 in macrophages (Nair et al., 2009), as can engagement of DC-SIGN through Raf-1 kinase (Gringhuis et al., 2007).
The role of IL-27 in triggering IL-10 production in mycobacterial infection suggests that it might be considered as a therapeutic target. In human cells, type I IFN blocks IL-1 production in response to M. tuberculosis and this blockade was not reversible by addition of type II IFN (Novikov et al., 2011). Therefore, neutralization of IL-27 may favor IFN-γ responses which contribute to host defense against mycobacterial infection. As IFN-β may also activate immune responses such as IL-12 induction (Bohnenkamp et al., 2007), blockade of IL-27 may inhibit the immunosuppressive pathway induced by IFN-β while preserving its immunostimulatory function. In particular, targeting IL-27 might serve as a useful adjuvant to traditional antibiotic therapy, perhaps in the context of multi-drug resistant bacteria.
Materials and Methods
Patients and clinical specimens
Patients with leprosy were classified according to the criteria of Ridley and Jopling (Ridley and Jopling, 1966). The designation of tuberculoid leprosy (T-lep) included patients that were classified as TT/BT and BT and the designation of lepromatous leprosy (L-lep) only included patients that were classified as LL. All T-lep and L-lep skin biopsy specimens were taken at the time of diagnosis, prior to initiating treatment. Reversal reaction (RR) skin biopsy specimens were considered upgrade reactions; consistent with reactivation of cell-mediated immune responses against M. leprae to the tuberculoid pole (usually this reactivation occurs during treatment, but can also occur spontaneously). Specimens were embedded in OCT medium (Ames, Elkhart, IN), snap frozen in liquid nitrogen and stored at −80°C. All leprosy patients were recruited with approval from the Institutional Review Board of University of Southern California School of Medicine and the Institutional Ethics Committee of Oswald Cruz Foundation, as well as the University of California, Los Angeles and gave their written consent. Whole blood was obtained with informed consent from healthy donors (UCLA I.R.B. #11-001274), for all samples not involving leprosy patients. Written consent was received from participants prior to inclusion in the study.
Cytokines
The following human recombinant cytokines were used for in vitro assays, IFN-β (PBL Interferon Source), IFN-γ (BD Biosciences, San Diego, CA), IL-10 and IL-27 (R&D Systems).
Microarray data analysis
Gene expression profiles of mRNAs derived from skin biopsy specimens of 24 leprosy patients (T-lep, n = 10; L-lep, n = 6; RR, n = 7) were determined using Affymetrix Human U133 Plus 2.0 microarrays and analyzed as previously described (Teles et al., 2013). The raw gene expression data analyzed in this study are available online through the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) accession number GSE17763.
Immunoperoxidase labeling and confocal microscopy
Frozen tissue sections were blocked with normal horse serum before incubation with monoclonal antibodies (mAbs) for 60 min, followed by incubation with biotinylated horse anti-mouse IgG for 30 min and were visualized using the ABC Elite system (Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin and mounted in crystal mounting medium (Biomeda, Foster City, CA).
Two- and three-color immunofluorescence with confocal microscopy was used to colocalize cytokines with specific cell markers. Immunofluorescence was performed by serially incubating cryostat tissue sections with mouse anti-human mAbs of different isotypes, anti-CD3 (Clone: UCHT1; IgG1), anti-CD163 (Clone: GH1/61; IgG1), anti-CD209 (Clone: DCN46; IgG2b), anti-IFN-β (Clone: MMHB-3; IgG1 or Clone: MMHB-1; IgG2a), anti-IL-10 (Clone: 127107; IgG1 directly conjugated to PE) and anti-IL-27 (Clone: 307426;IgG2a) followed by incubation with isotype-specific, fluorochrome (A488, A568 or A647)-labeled goat anti-mouse immunoglobulin antibodies (Molecular Probes, Carlsbad, CA). Controls included staining with isotype-matched antibodies as described previously (Ochoa et al., 2008). Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole). Double and triple immunofluorescence of skin sections were examined using a Leica-TCS-SP MP inverted single confocal laser-scanning and a two-photon laser microscope (Leica, Heidelberg, Germany) at the Advanced Microscopy/Spectroscopy Laboratory Macro-Scale Imaging Laboratory, California NanoSystems Institute, University of California at Los Angeles.
Bacterial and human cell culture and treatment
M. leprae was grown in the footpad of nu/nu mice, as described previously (Lahiri et al., 2005) and was provided by Dr. James L. Krahenbuhl of National Hansen’s Disease Programs, Health Resources Service Administration, Baton Rouge, LA. Sonicated M. leprae was provided by Patrick Brennan of the Department of Microbiology, Immunology & Pathology, Colorado State University, Fort Collins, CO.
PBMCs were isolated using Ficoll (GE Healthcare, Piscataway, NJ) gradient centrifugation. Monocytes were purified by plastic adherence for two hours in RPMI 1640 (Invitrogen) supplemented with 1% fetal calf serum (Omega Scientific, Tarzana, CA). Non-adherent cells were removed via vigorous washing and cultured in RPMI supplemented with antibiotics and 10% fetal calf serum. Human adherent monocytes were cultured in RPMI with 10% FCS (Omega Scientific) in the presence or absence of live M. leprae (MOI 10:1). In a separate set of experiments, monocytes were stimulated with IFN-β (200U/ml), IL-27 (40ng/ml) or IL-10 (10ng/ml). After stimulation the cells were cultured for 3, or 24 hours.
Real-time quantitative PCR (qPCR)
Total RNA was isolated from 10 L-lep, 10 T-lep and 10 RR skin lesions, and cDNA was prepared as described previously (Lee et al., 2010). TaqMan gene expression assays were used for detection of IFN-β, IL-10, IL-27 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Applied Biosystems, Foster City, CA). The relative quantities of the gene tested per sample were calculated against the GAPDH mRNA using the CT formula as previously described (Liu et al., 2006).
Antimicrobial assays
To measure Type I IFN induced antimicrobial activity in M. leprae-infected macrophages, we adapted the previously described real time PCR based method for the assessment of bacterial viability, which compares 16S RNA levels to genomic DNA levels (Liu et al., 2012; Martinez et al., 2009). Monocytes were isolated as described above, pre-treated with IFN-γ (273U/ml) for 24 hrs and infected overnight with M. leprae at an MOI of 10:1 followed by stimulation with IFN-β (200U/ml), IFN-γ, IL-10 (10ng/ml), IL-27 (40ng/ml) or medium for three days. Total RNA and DNA were isolated as previously described (Liu et al., 2012). Briefly, cDNA was synthesized from the total RNA as described (Liu et al., 2006) for both human and bacterial mRNAs. The bacterial 16S rRNA and genomic element DNA (RLEP) levels were then assessed using real time PCR. In order to normalize for the total monocytes present in the culture, 36B4 was also evaluated. Comparison of the bacterial DNA to the mammalian 36B4 levels was used to monitor infectivity between all the conditions in the assay as well as PCR quality. The 16S rRNA and genomic DNA values were calculated using the ΔΔCT analysis, with the bacterial DNA value serving as the housekeeping gene. The M. leprae 16S rRNA and M. leprae repetitive genomic element (RLEP) primers used were as previously described (Liu et al., 2012; Martinez et al., 2009).
ELISA
Secreted IFN-β, IL-10 and IL-27 proteins in the supernatant were measured using VeriKine™ Human Interferon-Beta ELISA Kit (PBL Interferon Source), IL-10 (Invitrogen), IL-27 Duoset ELISA (R&D system) following manufacturer protocols.
Primary cell siRNA transfection
siRNA transfection into primary human PBMCs was accomplished using the Amaxa Nucleofection System and the Human Monocyte Kit according to the manufacturer’s recommendations. ON-TARGET plus SMARTpool; siIL27 siRNA constructs were used at 100 pmol per transfection. Transfection efficiency of siRNA into primary monocytes was assessed using siGLO, a fluorescently labeled control oligo (Dharmacon, Lafayette, CO). Cells were allowed to rest for 18h following transfection before stimulation. At the time of stimulation, non-adherent cells were removed and fresh media was added.
Statistical analysis
Results are reported as pooled data from an entire series of experiments, and described as mean ± the SEM unless otherwise indicated. For data comparison, the Student’s t-test was used with statistical significance at p < 0.05.
Acknowledgments
We thank M. Schibler and the University of California-Los Angeles California NanoSystems Institute, Advanced Light Microscopy Core Facility for assistance with the confocal studies. The live M. leprae was provided by Ramanuj Lahiri from the U.S. National Hansen’s Disease Programs through the generous support of the American Leprosy Missions and Society of St. Lazarus of Jerusalem. This work was supported in parts by NIH grants (P50 AR063020; R01s AI022553, AR040312, and AI047868; T32 training grant AR058921).
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Anderson CF, Stumhofer JS, Hunter CA, et al. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–27. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto-de-Souza V, Ferreira PL, de Carvalho Vivarini A, et al. IL-27 enhances Leishmania amazonensis infection via ds-RNA dependent kinase (PKR) and IL-10 signaling. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Batten M, Kljavin NM, Li J, et al. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–6. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnenkamp HR, Papazisis KT, Burchell JM, et al. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247:72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Dey B, Dey RJ, Cheung LS, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nature medicine. 2015;21:401–6. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Stenger S, Shin D-M, et al. Vitamin D Is Required for IFN-gamma-Mediated Antimicrobial Activity of Human Macrophages. Sci Transl Med. 2011:3. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, den DJ, Litjens M, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–16. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J Immunol. 2010;185:6599–607. doi: 10.4049/jimmunol.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Uematsu S, Akira S, et al. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol. 2004;173:3392–7. doi: 10.4049/jimmunol.173.5.3392. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–33. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- Lahiri R, Randhawa B, Krahenbuhl J. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J Med Microbiol. 2005;54:235–42. doi: 10.1099/jmm.0.45700-0. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Li H, Ochoa MT, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis. 2010;201:558–69. doi: 10.1086/650318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–52. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Wheelwright M, Teles R, et al. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nature medicine. 2012;18:267–73. doi: 10.1038/nm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yu J, Carson WE, 3rd, et al. The role of IL-27 in the induction of anti-tumor cytotoxic T lymphocyte response. American journal of translational research. 2013;5:470–80. [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J, Weiner J, III, Mollenkopf HJ, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A. 2012;109:7853–8. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AN, Lahiri R, Pittman TL, et al. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J Clin Microbiol. 2009;47:2124–30. doi: 10.1128/JCM.00512-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya D, Cruz D, Teles RM, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–53. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Lopez-Diego R, et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–43. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Ramaswamy PA, Ghosh S, et al. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol. 2009;183:6269–81. doi: 10.4049/jimmunol.0901367. [DOI] [PubMed] [Google Scholar]
- Novikov A, Cardone M, Thompson R, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–7. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, et al. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K, Zhang Z, Teles RM, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–61. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoli ME, Gafa V, Giacomini E, et al. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–66. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Dutta RK, Khan MA, et al. IL-27 inhibits IFN-gamma induced autophagy by concomitant induction of JAK/PI3 K/Akt/mTOR cascade and up-regulation of Mcl-1 in Mycobacterium tuberculosis H37Rv infected macrophages. Int J Biochem Cell Biol. 2014;55:335–47. doi: 10.1016/j.biocel.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–11. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles RM, Graeber TG, Krutzik SR, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–53. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell SJ, Popper SJ, Rubins KH, et al. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS ONE. 2010;5:e9753. doi: 10.1371/journal.pone.0009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–15. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–7. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Ye ZJ, Zhou Q, et al. IL-27 and IL-27-producing CD4 T cells in human tuberculous pleural effusion. Tuberculosis (Edinb) 2014;94:579–88. doi: 10.1016/j.tube.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Wang XH, Ohmen JD, et al. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- Zeitvogel J, Werfel T, Wittmann M. IL-27 acts as a priming signal for IL-23 but not IL-12 production on human antigen-presenting cells. Exp Dermatol. 2012;21:426–30. doi: 10.1111/j.1600-0625.2012.01484.x. [DOI] [PubMed] [Google Scholar]