Abstract
The aim of this study is to determine the diagnostic value of cardiac magnetic resonance imaging (CMR) with late gadolinium enhancement (LGE), cine imaging, and resting first-pass perfusion (FPP) in the evaluation for ischemic (IC) versus non-ischemic (NIC) cardiomyopathy in new onset heart failure with reduced (≤40%) left ventricular ejection fraction (HFrEF). A retrospective chart review analysis identified 83 patients between January 2009 and June 2012 referred for cardiac magnetic resonance imaging (CMR) evaluation for new onset HFrEF with coronary angiography performed within 6 months of CMR. The diagnosis of IC was established using Felker’s criteria on coronary angiography. CMR sequences were evaluated for the presence of patterns suggestive of severe underlying coronary artery disease as the cause of HFrEF (subendocardial and/or transmural LGE, regional wall motion abnormality on cine, regional hypoperfusion defect on resting FPP). Discriminative power was assessed using receiver operator characteristics curve analysis. Coronary angiography identified 36 patients (43%) with IC. Presence of subendocardial and/or transmural LGE alone demonstrated good discriminative power (c-statistic 0.85, 95% confidence interval 0.76–0.94) for the diagnosis of IC. The presence of an ischemic pattern on both LGE and cine sequences resulted in a specificity of 87% for the diagnosis of IC, while the absence of an ischemic pattern on both LGE and cine sequences resulted in a specificity of 94% for the diagnosis of NIC. Addition of resting FPP on a subset of patients did not improve diagnostic values. In conclusion, CMR has potential value in the diagnostic evaluation of IC versus NIC.
Keywords: Cardiac magnetic resonance imaging, late gadolinium enhancement, heart failure reduced ejection fraction
Heart failure affects 5.1 million people in the United States, and the prevalence is expected to rise as the population ages and the prognosis after acute coronary events improves [1]. Over 650,000 new diagnoses of heart failure are made yearly, more than half of which are associated with a reduced ejection fraction [2]. Heart failure with reduced ejection fraction (HFrEF) may be secondary to severe coronary artery disease (CAD) in up to two thirds of cases, leading to the categorization of ischemic (IC) versus non-ischemic (NIC) cardiomyopathy. Early diagnosis and identification of etiology is crucial because the prognosis of patients with IC may improve following revascularization. Current guidelines recommend invasive coronary angiography in all patients presenting with new onset heart failure [3]. However, as invasive angiography presents a small risk of serious morbidity, alternative non-invasive strategies to diagnose IC versus NIC should be explored [4]. Cardiac MRI (CMR) has superior safety profile and the ability to identify myocardial fibrosis or scar. In this study, we aimed to evaluate the utility of CMR in the diagnosis of IC versus NIC in patients presenting with newly diagnosed HFrEF.
Methods
This was a retrospective study of patients undergoing CMR within 2 months of a new diagnosis of heart failure with left ventricular ejection fraction ≤40% and a coronary angiogram within 6 months of the CMR scan. Consecutive patients were included from January 2009 to June 2012 at 2 tertiary care sites, New York University (NYU) Langone Medical Center and Bellevue Hospital Center (BHC). The former is a private academic tertiary referral center, while the latter serves as the cardiac referral center for the underserved population within the New York City Health and Hospital Corporation system. Both hospitals are affiliated with the NYU School of Medicine. CMR images and coronary angiograms were interpreted by the same group of cardiac radiologists and interventional cardiologists at both sites. Patients were excluded if they met one of the following criteria: (1) known history of severe CAD, prior myocardial infarction, or prior coronary revascularization; (2) known history of structural heart disease such as hypertrophic cardiomyopathy or congenital heart disease; (3) evidence of severe left-sided valvular disease; or (4) diagnosis of ST-segment elevation myocardial infarction on admission. The study was approved by the NYU School of Medicine and BHC Institutional Review Boards.
Baseline demographic, clinical, and CMR variables were recorded from review of the electronic medical record (EMR). Obesity was defined as a body mass index ≥30 kg/m2. History of hypertension was defined per prior documentation in the EMR. Dyslipidemia was defined as a low-density lipoprotein-cholesterol >130 mg/dL or prior documentation in the EMR. Diabetes mellitus was defined HbA1c ≥6.5% or prior documentation in the EMR.
Coronary angiograms were evaluated by 2 independent board-certified practicing interventional cardiologists blinded to all clinical, echocardiographic, and CMR data. Significant CAD was defined as a ≥70% diameter stenosis in a coronary artery ≥2 mm in caliber by visual assessment of coronary angiogram or pressure gradient <0.80 if fractional flow reserve measurement was performed. The gold standard etiology of IC was determined using the definition established by Felker et al: presence of ≥70% diameter stenosis in the left main coronary artery, proximal left anterior descending (LAD) artery, or ≥2 epicardial coronary arteries [5]. For those angiograms not categorized to the same IC or NIC classification by the 2 readers (n=5), a 3rd independent blinded interventional cardiologist served as the final reader.
Patients were imaged using a 1.5 or 3.0 Tesla MRI system (Avanto, TimTrio, or Verio; Siemens, Erlangen, Germany). CMR was performed using a standard clinical protocol for evaluation of patients with cardiomyopathy, including use of late gadolinium enhancement (LGE) sequences, cine images, and, when applicable, resting first-pass perfusion (FPP). Imaging was performed in standard 2-chamber, 3-chamber, and 4-chamber long axis views and a short-axis series (base to apex) that was acquired every 10 mm to cover the entire left ventricle.
Per standard clinical protocol, CMR images were analyzed using the American Heart Association 17-segment model, and each segment was evaluated qualitatively on LGE and cine sequences [6]. LGE sequences were used to identify myocardial scar in a pattern suggestive of significant coronary artery disease (ischemic pattern with subendocardial and/or transmural LGE) versus other etiology (non-ischemic pattern with midwall and/or subepicardial LGE or absence of LGE). Since prior reports differ regarding the optimal number of segments used to diagnose IC, separate analyses of LGE were performed using both 1 and 3 segment thresholds. Cine sequences were used to identify regional versus global abnormal myocardial wall motion, with each segment reported as normal, mild/moderate/severe hypokinesia, akinesia, or dyskinesia. In the subset of patients who underwent FPP, resting FPP sequences were used to identify presence versus absence of regional myocardial perfusion defects. All imaging data were obtained from retrospective review of clinical CMR reports, which were generated by the same group of CMR readers at both sites.
CMR sequences were evaluated independently and in combination to diagnose IC versus NIC. A diagnostic algorithm incorporating all 3 sequences, in accordance with current clinical practice, was also used (Figure 1). In this clinically-based CMR algorithm, a third category, termed “mixed” cardiomyopathy, was created for patients with 1 or 2 ischemic LGE segments without perfusion defect on resting FPP and without regional wall motion abnormality on cine [7].
Figure 1.
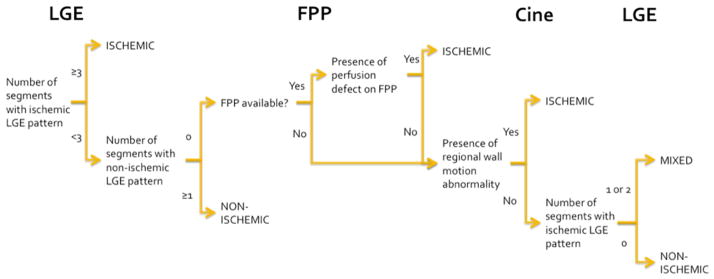
Diagnostic algorithm used to determine ischemic versus non-ischemic cardiomyopathy on cardiac magnetic resonance imaging
FFP = first pass perfusion, LGE = late gadolinium enhancement
All continuous variables were evaluated for normality using the Shapiro-Wilkes test and determined to be normally distributed. Continuous variables, presented as mean ± standard deviation, were compared between IC and NIC groups using independent sample t-test. Categorical variables, presented as proportions, were compared between IC and NIC groups using Fisher’s exact or Chi Square test. To evaluate CMR’s diagnostic utility in differentiating IC versus NIC, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy, and discriminative power were calculated for different CMR sequences using Felker’s criteria on coronary angiography as the gold standard for diagnosis. Diagnostic accuracy was calculated as a percentage of diagnoses that were equivalent between CMR and coronary angiography. Discriminative power was assessed using receiver operator characteristics curve analysis.
Results
Eighty three consecutive patients met inclusion/exclusion criteria. Approximately 61% (n=51) were from the BHC site, and the proportion of patients with IC was similar between both sites (45% at NYU Langone Medical Center and 41% at BHC, p=0.86). The majority of patients had a coronary angiogram performed before CMR in the IC and NIC groups (69% and 55%, p=0.28). Baseline characteristics are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics by study group
| Variable | Total (n=83) | Type of Cardiomyopathy | p-value† | |
|---|---|---|---|---|
| Ischemic* (n=36) | Non-ischemic* (n=47) | |||
| Age (years) | 58.8 ± 12.1 | 61.7 ± 11.1 | 56.5 ± 12.5 | 0.06 |
| Men | 59 (71%) | 29 (81%) | 30 (64%) | 0.14 |
| White, not Hispanic | 28 (34%) | 12 (33%) | 16 (34%) | 0.03 |
| Black, not Hispanic | 24 (29%) | 5 (14%) | 19 (40%) | |
| Hispanic | 20 (24%) | 11 (31%) | 9 (19%) | |
| Asian | 6 (7%) | 5 (14%) | 1 (2%) | |
| Other | 5 (6%) | 3 (8%) | 2 (4%) | |
| Body mass index (kg/m2) | 29.5 ± 7.5 | 28.4 ± 4.6 | 30.4 ± 9.1 | 0.23 |
| Obesity‡ | 32 (39%) | 12 (33%) | 20 (43%) | 0.50 |
| Hypertension | 49 (59%) | 24 (67%) | 25 (53%) | 0.26 |
| Dyslipidemia§ | 40 (48%) | 24 (67%) | 16 (34%) | 0.004 |
| Diabetes mellitus | 29 (35%) | 20 (56%) | 9 (19%) | 0.001 |
| Chest pain | 39 (47%) | 16 (44%) | 23 (49%) | 0.83 |
| Smoker | 45 (56%) | 22 (61%) | 23 (51%) | 0.50 |
| Troponin >99th percentile of the upper reference limit | 54 (70%) | 26 (84%) | 28 (61%) | 0.04 |
Diagnosed using Felker’s criteria on coronary angiography
Ischemic versus non-ischemic cardiomyopathy cohort comparison
Obesity defined as body mass index ≥30 kg/m2
Dyslipidemia defined as low-density lipoprotein-cholesterol >130 mg/dL or prior documentation in the electronic medical record
Continuous variables are presented as mean ± standard deviation and compared using independent sample t-test. Categorical variables are presented as n (proportion) and compared using the Fisher’s exact test.
All 83 patients had results available for LGE and cine sequences, while 68 (82%) had a resting FPP sequence performed as part of their CMR protocol. Left ventricular characteristics by CMR are shown in Table 2. Among the 4 patients in the IC group who did not demonstrate an ischemic pattern on LGE, 3 had nonproximal coronary artery stenoses and 1 had a severe stenosis in the proximal LAD artery.
Table 2.
Left ventricular characteristics on cardiac magnetic resonance (CMR) imaging by study group
| Variable | Total (n=83) | Type of Cardiomyopathy | p-value† | |
|---|---|---|---|---|
| Ischemic* (n=36) | Non-ischemic* (n=47) | |||
| Ejection fraction (%) | 27.1 ± 8.1 | 26.7 ± 6.9 | 27.4 ± 8.9 | 0.71 |
| End-diastolic volume (mL) | 240.0 ± 80.3 | 226.2 ± 62.3 | 250.3 ± 91.0 | 0.18 |
| End-systolic volume (mL) | 178.2 ± 74.2 | 167.9 ± 57.7 | 186.2 ± 84.4 | 0.27 |
| Late gadolinium enhancement‡ | ||||
| Absent | 19 (23%) | 3 (8%) | 16 (34%) | 0.01 |
| Non-ischemic only pattern | 17 (20%) | 1 (3%) | 16 (34%) | <0.001 |
| Ischemic only pattern | 38 (46%) | 27 (75%) | 11 (23%) | <0.001 |
| Ischemic and non-ischemic patterns | 9 (11%) | 5 (14%) | 4 (9%) | 0.50 |
| Cine (regional wall motion abnormality) | 30 (36%) | 20 (56%) | 10 (21%) | 0.003 |
| FPP (presence of hypoperfusion) (n=68) | 34 (50%) | 20 (69%) | 14 (36%) | 0.01 |
| Clinical algorithm§ | 47 (57%) | 31 (86%) | 16 (34%) | <0.001 |
Diagnosed using Felker’s criteria on coronary angiography
Ischemic versus non-ischemic cardiomyopathy cohort comparison
Non-ischemic pattern is defined as midwall and/or subepicardial late gadolinium enhancement, and ischemic pattern is defined as subendocardial and/or transmural late gadolinium enhancement
Patients categorized as mixed cardiomyopathy by the clinical algorithm (see Figure 1) are categorized as ischemic cardiomyopathy by CMR
Continuous variables are presented as mean ± standard deviation and compared using independent sample t-test. Categorical variables are presented as n (proportion) and compared using the Fisher’s exact test.
The sensitivity, specificity, PPV, NPV, and diagnostic accuracy of the individual CMR sequences and in combination are shown in Tables 3 and 4. LGE-CMR had the highest diagnostic accuracy for the diagnosis of IC, with good discriminative power (c-statistic 0.85, 95% confidence interval 0.76–0.94).
Table 3.
Diagnostic value of an ischemic pattern on different cardiac magnetic resonance imaging sequences in the diagnosis of ischemic cardiomyopathy
| Variable | n | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|
| LGE* | 47/83 | 89 | 68 | 68 | 89 | 77 |
| LGE† | 39/83 | 78 | 77 | 72 | 82 | 77 |
| Cine‡ | 30/83 | 56 | 79 | 67 | 70 | 69 |
| LGE* or cine | 53/83 | 94 | 60 | 64 | 93 | 75 |
| LGE* and cine | 24/83 | 50 | 87 | 75 | 69 | 71 |
| LGE*, cine, or FPP | 46/68 | 93 | 51 | 59 | 91 | 69 |
| LGE*, cine, and FPP | 17/68 | 41 | 87 | 71 | 67 | 67 |
| Clinical algorithm | 47/83 | 86 | 66 | 66 | 86 | 75 |
Presence of subendocardial and/or transmural late gadolinium enhancement (LGE) in ≥1 segment of the American Heart Association 17-segment model
Presence of subendocardial and/or transmural LGE in ≥3 segments
Presence of regional wall motion abnormality in ≥1 segment on cine imaging
Table 4.
Diagnostic value of a non-ischemic pattern on different cardiac magnetic resonance imaging sequences in the diagnosis of non-ischemic cardiomyopathy
| Variable | n | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|
| LGE* | 36/83 | 68 | 89 | 89 | 68 | 77 |
| LGE† | 17/83 | 34 | 97 | 94 | 53 | 61 |
| Cine‡ | 53/83 | 79 | 56 | 70 | 67 | 69 |
| LGE* or cine | 59/83 | 87 | 50 | 69 | 75 | 71 |
| LGE* and cine | 30/83 | 60 | 94 | 93 | 64 | 75 |
| LGE*, cine, or FPP | 51/68 | 87 | 41 | 67 | 71 | 68 |
| LGE*, cine, and FPP | 22/68 | 51 | 93 | 91 | 59 | 69 |
| Clinical algorithm | 36/83 | 66 | 86 | 86 | 66 | 75 |
Absence of subendocardial and/or transmural late gadolinium enhancement (LGE)
Absence of subendocardial and/or transmural LGE, but presence of midwall and/or subepicardial LGE in ≥1 segments of the American Heart Association 17-segment model
Absence of regional wall motion abnormalities on cine imaging
Four patients (4.8%) were categorized as “mixed” cardiomyopathy by the clinically-based CMR algorithm (Figure 1). Two of these patients were categorized as IC by Felker’s criteria. The diagnostic value of the individual and combination CMR sequences did not differ when these 4 “mixed” cardiomyopathy patients were excluded from the analyses.
Discussion
This is a real world, all-comers study of a racially and ethnically diverse group of patients with newly diagnosed HFrEF, which demonstrated a combination of CMR sequences to have excellent discriminative power for the diagnosis of IC versus NIC. LGE alone was a powerful tool such that the presence of subendocardial and/or transmural LGE on at least 3 myocardial segments provided 77% specificity in the diagnosis of IC, while the absence of subendocardial and/or transmural LGE provided 89% specificity in the diagnosis of NIC. Diagnostic concordance on cine images provided incremental discriminative value such that the addition of an ischemic pattern on cine images increased specificity for the diagnosis of IC to 87%, while the addition of a non-ischemic pattern on cine images increased specificity for the diagnosis of NIC to 94%. Addition of resting FPP, however, did not further improve diagnostic values. Finally, although only present in one-fifth the study cohort, the presence of only midwall and/or subepicardial LGE provided 97% specificity for the diagnosis of NIC. Thus, CMR is an attractive tool in the evaluation of patients with a new diagnosis of HFrEF.
In the current study, 43% of patients with a new diagnosis of HFrEF had IC, a proportion that is consistent with data from European sites [8]. The lack of underlying severe CAD in more than half of new HFrEF cases highlights the need to investigate non-invasive alternatives to coronary angiography in this setting. The majority of prior studies evaluating the diagnostic utility of CMR in HFrEF had several notable differences from the current study, including: (1) data primarily from European sites, (2) inclusion of patients with longstanding HFrEF, (3) exclusion of patients with any signs or symptoms of CAD, and (4) evaluation of LGE alone [7, 9–11]. Given that the United States has among the highest rates of obesity and diabetes mellitus in the developed nations, the prevalence of these co-morbidities is higher than reports from outside the United States [7, 9–13]. Indeed, diabetes mellitus is associated with cardiomyopathy independent of CAD, possibly through interstitial fibrosis and/or protein glycosylation, though the precise contribution of diabetes mellitus in the absence of CAD to CMR findings remains unclear. Also, given our study did not exclude patients presenting with chest pain or elevated cardiac biomarkers, the current findings are more generalizable than prior reports.
The reported 89% sensitivity of an ischemic pattern on LGE to diagnose IC in the current study is similar to that seen in prior studies (81% to 86%) [9, 11]. However, these prior studies also report a much higher specificity for ischemic patterns on LGE in the diagnosis of IC than the current report. This discrepancy may arise from not only the more selective populations evaluated in prior studies, but also the definition of IC used. The majority of prior reports determined IC by the presence of any angiographically severe CAD, instead of the currently accepted Felker’s criteria [5]. This distinction is important for 2 reasons: (1) survival outcomes in patients with single-vessel disease and HFrEF without proximal LAD involvement are similar to those in patients with no significant CAD and HFrEF, and (2) a significant portion of patients with single-vessel disease without proximal LAD involvement do not have subendocardial/transmural LGE [10]. A more recent study from the United Kingdom demonstrated greater diagnostic accuracy of LGE patterns when compared to a “gold standard” consensus panel, which assembled a diagnosis based on the same CMR findings, along with clinical history and findings on invasive coronary angiography [7]. However, there are currently no standardized diagnostic criteria that integrate clinical and imaging data for the diagnosis of IC versus NIC.
Prior reports suggested different optimal numbers of myocardial segment involvement for the diagnosis of IC [7, 11]. However, regardless of whether 1 or 3 segments were used as the LGE threshold, the diagnostic accuracy of CMR for ICM in our study was similar. Our evaluation also included cine sequences. As both sequences are typically performed as part of routine clinical protocol, it is appropriate to evaluate LGE and cine in combination rather than just LGE alone. A third sequence, FPP, is sometimes performed in CMR protocols to evaluate IC, although perfusion defects have also been reported in NIC [14, 15]. Our study was consistent with prior reports such that resting FPP did not improve diagnostic accuracy of the other 2 CMR sequences. Finally, as a non-invasive modality that can also identify areas of myocardial viability and scar, as well as structural abnormalities, without the use of iodinated contrast media, CMR adds valuable information in the diagnosis of the underlying etiology of HFrEF.
This study has the limitations inherent to a retrospective analysis. We addressed potential reader bias utilizing an expert panel blinded to all clinical and other imaging data to review the coronary angiograms. Second, while this is a small single center study, we enrolled a racially diverse population from a private and public city hospital. Third, the study only included patients who received both an invasive coronary angiogram and a CMR, potentially introducing selection bias. Finally, the criteria established by Felker et al in the diagnosis of IC does not account for whether or not the wall motion abnormalities present are concordant with the coronary anatomy distribution jeopardized, and it does not account for physiologic relevance of an anatomic stenosis. Felker’s criteria also do not account for mixed CM. However, Felker’s criteria are often considered the gold standard criteria for the diagnosis of IC, and sensitivity/specificity of CMR sequences did not differ significantly when mixed CM diagnosed using a clinically-based CMR algorithm were removed. Despite these limitations, this is a real-world study demonstrating the potential utility of 3 CMR sequences in the evaluation of newly diagnosed HFrEF.
This study further demonstrates the utility of CMR in the diagnosis of IC versus NIC. A CMR pattern suggestive of NIC may obviate the need for invasive coronary angiography, or at least allow for consideration of non-invasive coronary angiography. Taken together with prior studies, a consideration should be made to revisit the current guideline driven recommendation for routine invasive coronary angiography in all patients with newly diagnosed HFrEF.
Acknowledgments
We dedicate this article to the memory of Sean O’Rourke, MD, who contributed to the data collection for this study with an aim to enhance the care of patients through evidence-based medicine. This study and Binita Shah were supported in part by the NYU CTSA grant UL1TR000038 from the National Center for Advancing Translational Sciences (NCATS), NIH.
Footnotes
Disclosures
Binita Shah receives research grant support from Siemens. The authors have no conflicts of interest in relation to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 6.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 7.Assomull RG, Shakespeare C, Kalra PR, Lloyd G, Gulati A, Strange J, Bradlow WM, Lyne J, Keegan J, Poole-Wilson P, Cowie MR, Pennell DJ, Prasad SK. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124:1351–1360. doi: 10.1161/CIRCULATIONAHA.110.011346. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 9.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–748. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Soriano CJ, Ridocci F, Estornell J, Pérez-Boscá JL, Pomar F, Trigo A, Planas A, Nadal M, Jacas V, Martinez V, Paya R. Late gadolinium-enhanced cardiovascular magnetic resonance identifies patients with standardized definition of ischemic cardiomyopathy: a single centre experience. Intern J Cardiol. 2007;116:167–173. doi: 10.1016/j.ijcard.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Valle-Munoz A, Estornell-Erill J, Soriano-Navarro CJ, Nadal-Barange M, Martinez-Alzamora N, Pomar-Domingo F, Corbí-Pascual M, Payá-Serrano R, Ridocci-Soriano F. Late gadolinium enhancement-cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure. Eur J Echocardio. 2009;10:968–974. doi: 10.1093/ejechocard/jep115. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Global Status Report on Noncommunicable Diseases 2010. 2011 [Google Scholar]
- 13.Casolo G, Minneci S, Manta R, Sulla A, Del Meglio J, Rega L, Gensini G. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: Diagnostic accuracy of late gadolinium enhancement. Am Heart J. 2006;151:101–188. doi: 10.1016/j.ahj.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 14.Fang W, Zhang J, He ZX. Myocardial ischemia in patients with dilated cardiomyopathy. Nuclear Med Communications. 2010;31:981–984. doi: 10.1097/MNM.0b013e32833f393f. [DOI] [PubMed] [Google Scholar]
- 15.Sobajima M, Nozawa T, Suzuki T, Ohori T, Shida T, Matsuki A, Inoue H. Impact of myocardial perfusion abnormality on prognosis in patients with non-ischemic dilated cardiomyopathy. J Cardiol. 2010;56:280–286. doi: 10.1016/j.jjcc.2010.06.008. [DOI] [PubMed] [Google Scholar]


