Abstract
Chronotropic incompetence (CI) is common in heart failure with preserved ejection fraction (HFpEF), and may be a key reason underlying exercise intolerance in these patients. However, the determinants of CI in HFpEF are unknown. We prospectively studied 157 consecutive HFpEF patients undergoing cardiopulmonary exercise testing (CPET), and defined CI according to specific thresholds of the percent heart rate reserve (%HRR). CI was diagnosed as present if %HRR < 80 if not taking a β-blocker and < 62 if taking β-blockers. Participants who achieved inadequate exercise effort (respiratory exchange ratio ≤ 1.05) on CPET were excluded. Multivariable-adjusted logistic regression was used to determine the factors associated with CI. Of the 157 participants, 108 (69%) achieved a respiratory exchange ratio > 1.05 and were included in the final analysis. Of these 108 participants, 70% were women, 62% were taking β-blockers, and 38% had chronic kidney disease (CKD). The majority of HFpEF patients met criteria for CI (81/108; 75%). Lower estimated glomerular filtration rate (GFR), higher B-type natriuretic peptide, and higher pulmonary artery systolic pressure were each associated with CI. A 1-standard deviation decrease in GFR was independently associated with CI after multivariable adjustment (adjusted odds ratio 2.2, 95% confidence interval 1.1-4.4; P = 0.02). The association between reduced GFR and CI persisted when considering a variety of measures of chronotropic response. In conclusion, reduced GFR is the major clinical correlate of CI in patients with HFpEF, and further study of the relationship between CKD and CI may provide insight into the pathophysiology of CI in HFpEF.
Keywords: diastolic heart failure, glomerular filtration rate, chronotropic incompetence, cardiopulmonary exercise testing, chronic kidney disease
Despite a growing appreciation of the presence of chronotropic incompetence (CI) as a pathophysiologic abnormality in heart failure with preserved ejection fraction (HFpEF),1 the overall prevalence and clinical factors associated with CI in HFpEF remain unclear. An understanding of factors associated with CI in HFpEF may enhance our understanding of the pathophysiology of CI in HFpEF, and may allow clinicians to identify at-risk patients who may benefit from exercise testing to evaluate for CI and who may potentially benefit from rate adaptive pacing to alleviate CI.2 We therefore evaluated the prevalence and clinical correlates of CI in a well-characterized HFpEF cohort.
METHODS
Consecutive patients willing to undergo cardiopulmonary exercise testing (CPET) were prospectively recruited from the outpatient clinic of the Northwestern University HFpEF Program between March 2008 and January 2011 as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier #NCT01030991). Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital using the following search criteria: (1) diagnosis of HF or the words “heart failure” in the hospital notes; or (2) BNP >100 pg/ml; or (3) administration of 2 or more doses of intravenous diuretics. The list of patients generated was screened daily, and only those patients who had LV ejection fraction (EF) > 50% and who met Framingham criteria for HF were offered post-discharge follow-up in a specialized HFpEF outpatient program. Once evaluated as an outpatient in the HFpEF clinic, a cardiologist who specializes in HF confirmed the diagnosis of HF. The diagnosis of HFpEF was based on previously published criteria3 which requires an LV ejection fraction > 50%. In line with previous studies in HFpEF, patients with hemodynamically significant valvular disease (defined as greater than moderate in severity), prior cardiac transplantation, prior history of overt LV systolic dysfunction (i.e., prior LV ejection fraction < 40%), or a diagnosis of constrictive pericarditis were not recruited into the study.
In the Northwestern HFpEF Program, all study procedures (including laboratory testing, electrocardiography (ECG), echocardiography, invasive hemodynamic testing, and CPET) are performed in the outpatient setting. All study participants gave written, informed consent, and the institutional review board at Northwestern University approved the study.
We collected the following data on all study participants: demographics, New York Heart Association (NYHA) functional class, co-morbidities, medications, vital signs, and body mass index, as well as information from laboratory blood work, echocardiography, cardiopulmonary exercise test (CPET), and invasive hemodynamics (when available). Estimated glomerular filtration rate (GFR) was calculated using the Modified Diet in Renal Disease equation. Chronic kidney disease (CKD) was defined as GFR < 60 mL/min/1.73 m2. All patients underwent comprehensive echocardiography, as described previously.4
Symptom-limited CPET using a 10-watt bicycle protocol was performed in all patients. CPET was performed in the outpatient setting after the patient was confirmed to be stabilized with no significant cardiac medication changes within the prior 4 weeks. Respiratory gases were analyzed using a calibrated metabolic cart (Sensormedics Vmax, San Diego, CA, USA). Breath-by-breath respiratory gas analysis for measurement of inspired oxygen and expired carbon dioxide was obtained on-line and averaged every 20 seconds at rest, throughout exercise and during the recovery period. The peak VO2 value was defined as the highest VO2 value achieved at end exercise after reaching anaerobic threshold. The VO2 at anaerobic threshold was determined as the point at which carbon dioxide increases in a nonlinear fashion relative to the rate of oxygen consumption by the V-slope method and confirmed from the nadir of the ventilatory equivalent for VO2. The VO2 at anaerobic threshold for each patient was visually noted and agreed upon by 2 independent cardiopulmonary specialists. Standard 12-lead ECGs were obtained at rest, each minute during exercise and for at least 5 minutes during the recovery phase; blood pressure was measured at rest and at each stage of exercise. CPET variables were measured and calculated based on current guidelines.5
CPET results were used to establish the presence of CI, our main outcome variable. We defined CI based on thresholds of %HRR, which was in turn calculated as %HRR = peak observed HR – resting HR)/(age-predicted maximal HR – resting HR). Age-predicted maximal HR was estimated using the Astrand formula, 220–age.6 Several studies utilize the Astrand formula in developing CI definitions that account for β-blocker use, which may lead to pharmacologically-induced CI irrespective of underlying physiology.7 We adopted the %HRR cutoffs of those studies in defining CI: %HRR < 80 for those not taking β-blockers, and %HRR < 62 for those taking β-blockers.
There is some controversy surrounding the appropriate definition of age-predicted maximum HR.8 For instance, Tanaka and colleagues recommend the following formula to estimate: 208 – 0.7×age.9 We therefore re-calculated %HRR and re-established a diagnosis of CI using the Tanaka formula as part of our sensitivity analyses.
For all patients with adequate exercise effort, we obtained the average HR over 20 seconds for up to 140 seconds following peak exercise. HR recovery at 1 and 2 minutes was defined as the difference in peak exercise and HR at 1 and 2 minutes post-peak exercise, respectively. We used the HR difference at 1 minute to calculate a binary indicator of abnormal HR recovery as defined by Watanabe and colleagues, based on their finding that HR recovery ≤ 18 beats is associated with increased mortality.10
Participants were stratified by CI status, and clinical, laboratory, echocardiographic, and CPET parameters were compared between groups using Fisher exact and student t-tests for dichotomous and continuous variables, respectively. False discovery rate methods were used to account for multiple comparisons. Given the number of statistical tests performed in our study, a P-value < 0.003 was considered statistically significant (corresponded to a q-value < 0.05).
A series of multivariable models were generated to examine the association between GFR and CI. Variables associated with CI status on univariate analysis were included as covariates in our multivariable models of chronotropic response. We additionally included smoking status11 and β-blocker usage7,12 as covariates in our multivariable models, because they have been previously shown to impair chronotropic response, as well as LV mass index13 and loop diuretic usage as markers of cardiac and renal disease severity. We conducted multivariable-adjusted logistic regression to examine the association of GFR with CI, and we repeated these analyses using linear regression to examine the association between GFR and %HRR. For the statistical models, we calculated goodness-of-fit measures (Akaike information criteron [AIC] and the adjusted Pearson coefficient [R2]). Models were similarly used to evaluate correlates of HR recovery. The mean HR relative to peak VO2 was estimated from a linear regression model of HR on peak VO2 for those with and without CI. All analyses were performed using Stata v. 12 (StataCorp, College Station, Texas).
RESULTS
A total 157 patients with documented HFpEF enrolled in the study and underwent CPET. Of these, 49 (31.2%) were excluded because of a respiratory exchange ratio < 1.05 at peak exercise, yielding 108 patients for the final analysis. There were no statistically significant demographic or clinical differences between participants included versus excluded based on peak respiratory exchange ratio < 1.05 (Table 1). Although excluded participants were more symptomatic (higher NYHA class) and more likely to have a history of COPD or coronary artery disease, these differences were not significant after correction for multiple comparisons.
Table 1.
Demographic, clinical, and laboratory characteristics of the study participants, stratified by peak respiratory exchange ratio
| Characteristic | Peak Respiratory Exchange Ratio |
P-value* | |
|---|---|---|---|
| < 1.05 (n = 49) | ≥ 1.05 (n = 108) | ||
| Age (years) | 67±12 | 64±12 | 0.24 |
| Women | 37(76%) | 76(70%) | 0.51 |
| Race | 0.27 | ||
| • White | 19(39%) | 56(52%) | |
| • African-American | 26(53%) | 43(40%) | |
| • Other | 4(8%) | 9(9%) | |
| Comorbidities** | |||
| • Coronary artery disease | 22(45%) | 40(37%) | 0.35 |
| • Hypertension | 41(84%) | 84(78%) | 0.40 |
| • Hyperlipidemia | 33(67%) | 65(60%) | 0.39 |
| • Diabetes mellitus | 18(37%) | 35(32%) | 0.60 |
| • Chronic kidney disease | 16(33%) | 34(31%) | 0.88 |
| • Smoker | 20(41%) | 36(33%) | 0.36 |
| • Atrial fibrillation | 13(27%) | 32(30%) | 0.69 |
| • Obesity | 30(61%) | 61(57%) | 0.58 |
| • Chronic obstructive pulmonary disease | 30(61%) | 44(41%) | 0.017 |
| New York Heart Association functional class | 0.053 | ||
| • I | 2(4%) | 13(12%) | |
| • II | 14(29%) | 44(41%) | |
| • III | 33(67%) | 51(47%) | |
| Heart rate (beats/min) | 69±13 | 72±13 | 0.22 |
| Systolic blood pressure (mm Hg) | 128±24 | 125±21 | 0.46 |
| Diastolic blood pressure (mm Hg) | 72±12 | 73±11 | 0.58 |
| Pulse pressure (mm Hg) | 57±15 | 55±16 | 0.40 |
| Body mass index (kg/m2) | 36±11 | 32±9 | 0.033 |
| Serum sodium (mEq/L) | 138±3 | 139±3 | 0.30 |
| Blood urea nitrogen (mg/dl) | 23±13 | 22±15 | 0.73 |
| Serum creatinine (mg/dl) | 1.5±1.6 | 1.4±1.1 | 0.46 |
| Glomerular filtration rate (ml/min/1.73m2) | 60±26 | 61±26 | 0.80 |
| Fasting blood glucose (mg/dl) | 128±74 | 125±64 | 0.78 |
| Hemoglobin (g/dl) | 12.2±1.7 | 12.2±1.6 | 0.85 |
| Log B-type natriuretic peptide (pg/ml) | 5.0±1.4 | 5.0±1.3 | 0.98 |
| Medications | |||
| • Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 33(67%) | 61(56%) | 0.20 |
| • β-blocker | 33(67%) | 63(58%) | 0.28 |
| • Calcium channel blocker | 22(45%) | 36(33%) | 0.16 |
| • Nitrate | 10(20%) | 14(13%) | 0.23 |
| • Loop diuretic | 33(67%) | 56 (52%) | 0.07 |
| • Thiazide diuretic | 13(27%) | 29(27%) | 0.97 |
| • Statin | 27(55%) | 60(56%) | 0.96 |
| • Aspirin | 25(51%) | 42(39%) | 0.15 |
Values expressed as mean ± standard deviation, unless otherwise specified
No comparisons between groups were statistically significant (q < 0.05) after adjustment for false discovery rate
Coronary artery disease was determined using defined protocol that included physician-documented history of coronary disease; known coronary stenosis >50%; history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting; or abnormal stress test results consistent with myocardial ischemia. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, physician-documented history of hypertension, or current use of antihypertensive medications. Hyperlipidemia was defined as physician-documented history of hyperlipidemia or current use of lipid-lowering medications. Obesity was defined as body mass index >30 kg/m2.
The prevalence of CI was 75% (95% confidence interval = 67-83%). Table 2 lists the demographic and clinical characteristics of the study participants, stratified by presence or absence of CI. GFR and log BNP were significantly reduced and elevated, respectively, in participants with CI (P < 0.0001). Participants with CI also had higher prevalence of CKD, higher serum creatinine, increased loop diuretic usage, higher pulse pressure, lower resting HR, and were more likely smokers. An analysis of the subset of study participants who were not using β-blockers showed that those with CI similarly had lower GFR and higher BNP than those without CI (P < 0.05). At the time of CPET, 22 (20%) of the study subjects were in atrial fibrillation. The association between GFR and CI was present in patients with atrial fibrillation (p=0.031) and without atrial fibrillation (p=0.005).
Table 2.
Demographic, clinical, and laboratory characteristics of the study participants who were able to perform maximal exercise, stratified by the presence or absence of chronotropic incompetence
| Characteristic | Chronotropic Incompetence |
P-value* | |
|---|---|---|---|
| Absent (n = 27) | Present (n = 81) | ||
| Age (years) | 65±10 | 64±12 | 0.68 |
| Women | 19(70%) | 57(70%) | 0.99 |
| Race | 0.75 | ||
| • White | 16(5 9%) | 40(49%) | |
| • African-American | 9(33%) | 34(42%) | |
| • Other | 2(7%) | 7(9%) | |
| Comorbidities** | |||
| • Coronary artery disease | 7(26%) | 33(41%) | 0.17 |
| • Hypertension | 20(74%) | 64(79%) | 0.59 |
| • Hyperlipidemia | 15(56%) | 50(62%) | 0.57 |
| • Diabetes mellitus | 6(22%) | 29(36%) | 0.19 |
| • Chronic kidney disease | 3(11%) | 31(38%) | 0.008 |
| • Smoker | 13(48%) | 23(28%) | 0.059 |
| • Atrial fibrillation | 6(22%) | 26(32%) | 0.33 |
| • Obesity | 15(56%) | 46(57%) | 0.91 |
| • Chronic obstructive pulmonary disease | 8(29%) | 36(44%) | 0.18 |
| New York Heart Association functional class | 0.055 | ||
| • I | 7(26%) | 6(7%) | |
| • II | 9(33%) | 35(43%) | |
| • III | 10(41%) | 40(49%) | |
| Heart rate (beats/min) | 77±15 | 70±13 | 0.03 |
| Systolic blood pressure (mm Hg) | 122±21 | 126±21 | 0.39 |
| Diastolic blood pressure (mm Hg) | 74±11 | 72±11 | 0.52 |
| Pulse pressure (mm Hg) | 48±11 | 57±17 | 0.01 |
| Body mass index (kg/m2) | 31±9 | 32±8 | 0.43 |
| Serum sodium (mEq/L) | 139±3 | 139±3 | 0.78 |
| Blood urea nitrogen (mg/dl) | 18±9 | 24±16 | 0.07 |
| Serum creatinine (mg/dl) | 0.9±0.4 | 1.5±1.2 | 0.02 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 79±28 | 56±24 | < 0.001* |
| Fasting blood glucose (mg/dl) | 118±80 | 127±59 | 0.58 |
| Hemoglobin (g/dl) | 12.4±1.4 | 12.2±1.6 | 0.57 |
| Log B-type natriuretic peptide (pg/ml) | 4.1±1.3 | 5.3±1.2 | < 0.001* |
| Medications | |||
| • Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 16(5 9%) | 45(56%) | 0.74 |
| • β-blocker | 13(48%) | 50(62%) | 0.08 |
| • Calcium channel blocker | 6(22%) | 30(37%) | 0.16 |
| • Nitrate | 1(4%) | 13(16%) | 0.18 |
| • Loop diuretic | 9(33%) | 47(58%) | 0.03 |
| • Thiazide diuretic | 11(41%) | 18(22%) | 0.06 |
| • Statin | 14(52%) | 46(57%) | 0.66 |
| • Aspirin | 11(41%) | 31(38%) | 0.82 |
Values expressed as mean ± standard deviation, unless otherwise specified
Statistically significant (q < 0.05) after adjustment for false discovery rate
Coronary artery disease was determined using defined protocol that included physician-documented history of coronary disease; known coronary stenosis >50%; history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting; or abnormal stress test results consistent with myocardial ischemia. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, physician-documented history of hypertension, or current use of antihypertensive medications. Hyperlipidemia was defined as physician-documented history of hyperlipidemia or current use of lipid-lowering medications. Obesity was defined as body mass index >30 kg/m2.
Echocardiographic and CPET parameters are shown in Table 3, stratified by CI status. LV mass index and E/A ratio tended to be higher in HFpEF patients with CI, but did not achieve significance after adjustment for multiple comparisons. There were no significant associations between CI (or %HRR) and RV structure (RV end-diastolic area, RV end-systolic area, RV maximal diameter, RV wall thickness) or function (RV fractional area change, tricuspid annular plane systolic excursion). On echocardiography, pulmonary artery systolic pressure (PASP) was significantly higher in HFpEF patients with CI compared to those without CI. These results were confirmed in a subset of patients (n=60 with CI and n=16 without CI) who underwent invasive hemodynamic testing: invasive PASP 56±19 vs. 39±7 mmHg (p=0.001) in those with vs. without CI, respectively.
Table 3.
Echocardiographic and cardiopulmonary exercise testing parameters by presence or absence of chronotropic incompetence
| Parameter | Chronotropic Incompetence |
P-value | |
|---|---|---|---|
| Absent (n = 27) | Present (n = 81) | ||
| Echocardiography: | |||
| • Left ventricular end-systolic volume (ml/m2) | 30±11 | 31±11 | 0.81 |
| • Left ventricular end-diastolic volume (ml/m2) | 76±17 | 80± 22 | 0.43 |
| • Left ventricular mass index (g/m2) | 90±37 | 105±33 | 0.05 |
| • Left ventricular ejection fraction (% units) | 61±8 | 62±6 | 0.55 |
| • Left atrial volume index (ml/m2) | 32±14 | 32±9 | 0.90 |
| • Ratio of early to late mitral inflow (E/A ratio) | 1.1±0.4 | 1.4±0.7 | 0.04 |
| • Tissue Doppler e' velocity (cm/s) | 7.4±2.6 | 7.2±2.4 | 0.73 |
| • E/e' ratio | 13.8±6.4 | 15.1±6.8 | 0.39 |
| • Diastolic function grade | 0.09 | ||
| ○ Normal diastolic function | 2(7%) | 8(10%) | |
| ○ Grade I (mild) diastolic dysfunction | 4(15%) | 5(6%) | |
| ○ Grade II (moderate) diastolic dysfunction | 13(48%) | 29(36%) | |
| ○ Grade III (severe) diastolic dysfunction | 4(15%) | 32(40%) | |
| ○ Indeterminate diastolic function | 4(15%) | 7(9%) | |
| • Pulmonary artery systolic pressure (mm Hg) | 36±9 | 47±15 | 0.003* |
| Cardiopulmonary exercise testing: | |||
| • Workload (watts) | 85±53 | 67±30 | 0.060 |
| • Exercise time (seconds) | 8.7±3.2 | 7.0±3.1 | 0.019 |
| • Resting heart rate (beats/min) | 77±15 | 70±13 | 0.031 |
| • Resting respiratory rate (breaths/min) | 19±6 | 18±5 | 0.33 |
| • Peak heart rate (beats/min) | 145±12 | 108±21 | < 0.001* |
| • Peak respiratory rate (breaths/min) | 41±9 | 36±8 | 0.015 |
| • Peak systolic blood pressure (mm Hg) | 184±24 | 177±32 | 0.25 |
| • Peak diastolic blood pressure (mm Hg) | 77±14 | 73±14 | 0.18 |
| • Anaerobic threshold | 12±5 | 9.8±2 | 0.001* |
| • Absolute VO2 (L/kg) | 1.5±0.6 | 1.2±0.4 | 0.005 |
| • Relative VO2 (ml/kg/min) | 18±7 | 13±4 | < 0.001* |
| • Oxygen pulse (mL/beat) | 10±4 | 11±4 | 0.25 |
| • Peak VE (L/min) | 62±25 | 46±14 | < 0.001* |
| • VE/VCO2 ratio at anaerobic threshold | 33±5 | 32±4 | 0.32 |
| • Breathing reserve | 26±14 | 31±19 | 0.15 |
| • Respiratory exchange ratio | 1.2±0.1 | 1.2±0.1 | 0.12 |
| • HR recovery at 1 min (beats/min) | 26±2 | 16±1 | < 0.001* |
| • HR recovery at 2 min (beats/min) | 40±3 | 25±2 | < 0.001* |
VE = ventilatory equivalents; VO2 = maximal oxygen consumption; VCO2 = maximal carbon dioxide output
Values expressed as mean ± standard deviation, unless otherwise specified
Statistically significant (q < 0.05) after adjustment for false discovery rate
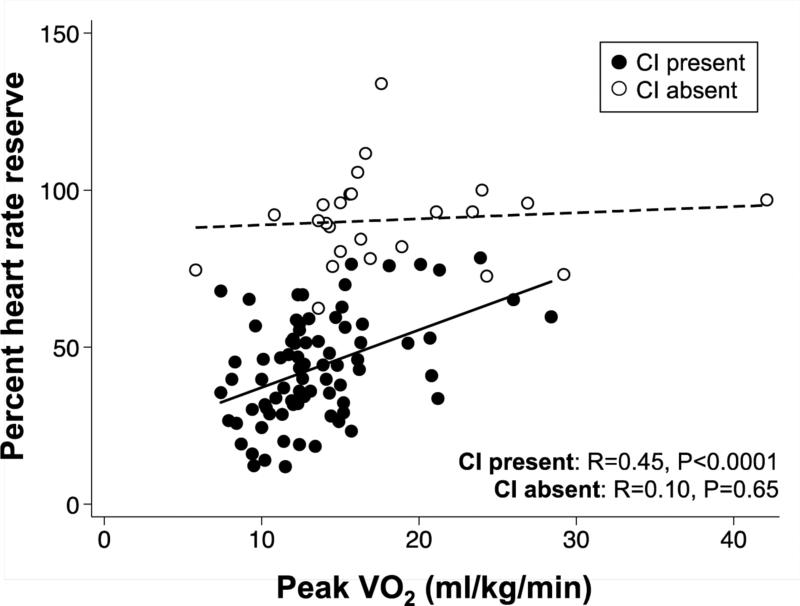
On CPET, the presence of CI was associated with reduced peak HR, anaerobic threshold, relative VO2, peak VE, and HR recovery at 1 and 2 minutes after accounting for multiple comparisons (Table 3). Patients with CI were more likely to have abnormal HR recovery compared to those without CI (65% versus 27%, P = 0.001). Poorer HR reserve was associated with worse exercise capacity (as measured by peak VO2) in patients with CI, but not in those without CI (Figure 1). Though trends were similar during recovery, differences in the mean [SE] HR recovery relative to ΔVO2 at 1 minute (9.0 [4.1] versus 14.7 [3.3] bpm; P = 0.44) and 2 minutes (9.4 [4.1] versus 18.3 [3.3] bpm; P = 0.21) for those with CI versus those without CI, respectively, were not statistically significant.
Figure 1. Scatterplot of Heart Rate Reserve versus Maximal Oxygen Consumption, Stratified by Presence or Absence of Chronotropic Incompetence.
Percent heart rate reserve was associated with peak VO2 in HFpEF patients with CI but not those without CI. In addition, for any given peak VO2, percent heart rate reserve was lower in HFpEF patients with CI versus those without CI. VO2 = oxygen consumption; HFpEF = heart failure with preserved ejection fraction; CI = chronotropic incompetence.
Figure 2 depicts the relationship between renal function (GFR) and CI (%HRR) in the HFpEF study patients, stratified by beta-blocker usage. There was a linear correlation between GFR and %HRR such that reduced GFR was associated with worse HR reserve. The progressive increase in OR for CI with advancing stages of CKD was in line with the linear relationship between GFR and %HRR: CKD Stage 2 OR=8.2, P=0.003; CKD Stage 3 OR=12.1, P=0.001; CKD Stage 4 or 5 OR=22.5, P=0.010 (using CKD Stage 1 as the referent group).
Figure 2. Relationship between renal function and chronotropic response to exercise in patients with heart failure and preserved ejection fraction.
(A) Probability of chronotropic incompetence across the spectrum of glomerular filtration rate. (B) Percent heart rate reserve versus glomerular filtration rate. CI = chronotropic incompetence; eGFR = estimated glomerular filtration rate; HR = heart rate; LOWESS = locally-weighted smoothed scatterplot
The association between GFR and CI (or %HRR) remained statistically significant after adjustment for potential confounders (Table 4). A 1-standard deviation decrease in GFR was independently associated with CI after multivariable adjustment (adjusted odds ratio [OR] 2.2, 95% confidence interval 1.1-4.4; P = 0.02). We added invasive pulmonary capillary wedge pressure (PCWP) and E/A ratio to the final model to further assess the role of HFpEF severity, but the strength of association with GFR was unchanged. As a sensitivity analysis, we examined the relationships between GFR and chronotropic response after defining %HRR and CI using Tanaka's formula for age-predicted maximum HR.9 Using this formula, the prevalence of CI was 79% (95% confidence interval = 72-87%). The coefficient for GFR is similar to that estimated in models using the Astrand formula, and the association between GFR and chronotropic response remained significant after multivariable adjustment (P<0.05). Next, we examined the linear relationship between GFR and relative peak VO2 (ml/kg/min). GFR was positively associated with peak VO2. Though this relationship was partially explained by %HRR, it remained significant after adjustment for %HRR, age, sex, smoking status, and PASP (P<0.05).
Table 4.
Logistic and linear regression analyses for the association of glomerular filtration rate with chronotropic incompetence
| Logistic regression: predictor = GFR; outcome = chronotropic incompetence |
Linear regression: predictor = GFR; outcome = percent heart rate reserve |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | β (log odds) | SE | P-value | AIC | β | SE | P-value | adjusted R2 |
| Unadjusted model | -0.038 | 0.011 | < 0.001 | 102.9 | 0.439 | 0.087 | < 0.001 | 0.18 |
| Age* | -0.048 | 0.013 | < 0.001 | 100.5 | 0.448 | 0.092 | < 0.001 | 0.19 |
| Sex | -0.038 | 0.011 | < 0.001 | 104.8 | 0.430 | 0.089 | < 0.001 | 0.19 |
| Smoker*† | -0.046 | 0.012 | < 0.001 | 95.8 | 0.462 | 0.086 | < 0.001 | 0.22 |
| Log BNP*† | -0.025 | 0.011 | 0.028 | 97.0 | 0.286 | 0.100 | 0.005 | 0.23 |
| LVMI | -0.035 | 0.011 | 0.001 | 102.8 | 0.394 | 0.090 | < 0.001 | 0.22 |
| β-blocker† | -0.036 | 0.011 | 0.001 | 103.4 | 0.372 | 0.082 | < 0.001 | 0.31 |
| Loop diuretic | -0.034 | 0.011 | 0.002 | 102.9 | 0.413 | 0.090 | < 0.001 | 0.20 |
| PASP*† | -0.046 | 0.017 | 0.007 | 56.6 | 0.317 | 0.115 | 0.008 | 0.20 |
| Smoker* + logBNP*† | -0.034 | 0.013 | 0.007 | 92.9 | 0.322 | 0.102 | 0.002 | 0.24 |
| Smoker* + logBNP + LVMI | -0.033 | 0.013 | 0.009 | 94.5 | 0.320 | 0.104 | 0.003 | 0.24 |
| Smoker* + logBNP + LVMI + β-blocker † | -0.033 | 0.013 | 0.009 | 96.3 | 0.314 | 0.098 | 0.002 | 0.31 |
| Smoker* + logBNP + LVMI + β-blocker† + loop diuretic | -0.030 | 0.013 | 0.020 | 97.7 | 0.300 | 0.100 | 0.004 | 0.31 |
| Smoker + logBNP + LVMI + β-blocker + loop diuretic + PASP* | -0.050 | 0.024 | 0.036 | 60.3 | 0.312 | 0.145 | 0.036 | 0.17 |
GFR = glomerular filtration rate; HR = heart rate; logBNP = log B-type natriuretic peptide; LVMI = left ventricular myocardial infarction; PASP = pulmonary arterial systolic pressure; SE = standard error; AIC = Akaike information criterion
Indicates that covariate was significant at P<0.05 in the logistic regression model
Indicates that covariate was significant at P<0.05 in the linear regression model
Finally, we examined the relationship between GFR and HR recovery. Each standard deviation of GFR below the mean was associated with a 68% increased odds of abnormal HR recovery at 1 minute (P = 0.019). The estimated odds ratio was similar after controlling for smoking, beta-blocker use, and log BNP (OR=0.85, P=0.023). No covariates other than GFR were associated with abnormal HR recovery at 1 minute.
DISCUSSION
This is the first study to our knowledge that has identified kidney dysfunction as the primary clinical factor associated with CI in HFpEF. Decreased GFR was associated with decreased %HRR, increased CI, and decreased peak VO2. These findings highlight the possibility that CKD is associated with significant autonomic dysfunction in HFpEF. In addition, the presence of CKD may be a valuable index for identifying HFpEF patients that should undergo CPET for evaluation of CI prior to the initiation of beta-blockers or other HR lowering agents that may exacerbate CI. The association of GFR with CI was not explained by different methods of calculating age-predicted maximal HR, nor confounded by β-blocker or loop diuretic usage, smoking status, or severity of HF.
We found a CI prevalence in HFpEF of 75% when defined according to %HRR and the Astrand formula. This is higher than the 57% prevalence estimated by Borlaug et al.14 When employing the Tanaka formula used by Phan and colleagues, we similarly found a higher CI prevalence of 79% compared to their prevalence estimate of 63%.15 The greater prevalence found in our HFpEF cohort likely reflects the more symptomatic and advanced HFpEF in our study of patients with a history of HF-related hospitalization. For example, CI was found in only 6% of participants in the Aldo-DHF trial, though 37% (versus 100% in our study) had a recent history of HF hospitalization only and 14% (versus 45% in our study) were NYHA class III.16
Our results suggest that differences in CI among HFpEF patients with and without CKD are not completely explained by severity of HF. Specifically, we found that adjustment for BNP, LVMI, beta blocker and loop diuretic use, PASP and PCWP, all of which may function as proxies for HF severity, did not attenuate the association between GFR and CI, and fail to explain this relationship in our dataset. HR recovery has been demonstrated to reflect increased vagal activity at end of exercise that is increased in athletes and blunted in patients with chronic HF.17 Our finding that patients with versus without CI were 2.4-times more likely to subsequently demonstrate an abnormal HR recovery is similar to the 2.2-fold difference found by Watanabe and colleagues.10 Combined with our finding that patients with CI had smaller changes in HR at a given peak VO2, we believe these changes likely reflect a cardiac autonomic process.
Indeed, a leading theory for the cause of CI in HFpEF is autonomic dysfunction, including parasympathetic insufficiency as indicated by baroreflex insensitivity18 with reduced parasympathetic drive,19 and sympathetic overdrive.20 Sympathetic overflow can also affect the kidney. For instance, Rundqvist and colleagues21 also reported increased adrenergic tone to the kidneys in severe HF with reduced ejection fraction. Renal sympathetic activity leads to α1-mediated renal arterial vasoconstriction and β1-mediated renin secretion (which also increases sympathetic vascular tone), causing reduced renal plasma flow.22 Sympathetic overdrive is also present in early stages of CKD,23 and reduced HR variability predicts adverse cardiovascular and renal events.24,25 These data indicate that autonomic dysfunction may have contributed to both reduced HR reserve and worse kidney function in our sample.
An alternate possibility is that renal dysfunction contributes directly to CI. There is some evidence that renal transplantation improves autonomic dysfunction as measured by frequency-domain analysis of HR variability.26 Others have shown that renal sympathetic denervation improves diastolic function.27 Current research indicates that these effects may be mediated by renin activation and its downstream effects on myocardial inflammation and fibrosis, as well as cardiac afterload, which can lead to diastolic dysfunction,28 and pulmonary artery smooth muscle proliferation. The latter contributes to pulmonary hypertension, a common finding in HFpEF,29 and may explain the association of PASP and CI demonstrated in our study.
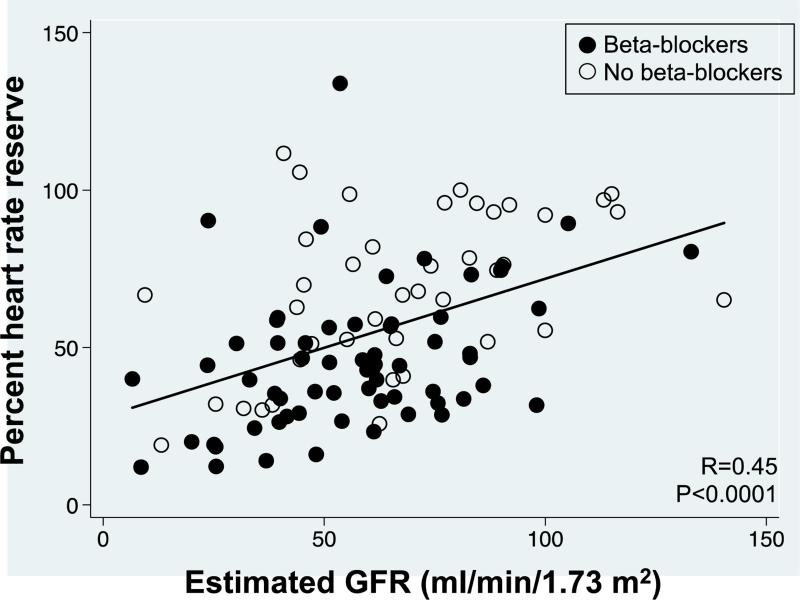
Because of the potential influence of β-blockers on the pathophysiologic mechanisms described above, as well as their influence on chronotropic response, we examined the subset of participants who were not taking β-blockers. In this subgroup of HFpEF patients not taking β-blockers, the prevalence of CI (65%, 95% confidence interval 51-81%) and the mean GFR by CI status (80 versus 61 ml/min/1.73m2) was remarkably similar to that observed in the full sample (79 versus 56 ml/min/1.73m2).
Our study possesses a number of strengths. Despite the relatively small study size, ours is one of the largest studies on CPET in HFpEF, and our sample possesses similar demographic and clinical characteristics to that reported in prior HFpEF epidemiology and observational studies, though it should be noted that our sample tended to be younger, more overweight, and less likely to have diabetes mellitus. We also evaluated exercise response by multiple outcomes, including binary and continuous measures of %HRR with two different definitions, as well as peak VO2.
Several limitations bear mentioning. First, our study was largely exploratory using a relatively small number of participants. However, false discovery rate methods provided a stringent threshold for determining statistical significance, and the effect of GFR remained significant despite controlling for multiple potential confounders. Second, the fact that all patients in our study had a history of at least 1 hospitalization for HF resulted in a sicker cohort of HFpEF patients compared to prior studies of CI in HFpEF, which primarily included outpatients presenting with exertional intolerance. Indeed, our study found that roughly 75% of HFpEF patients had CI compared to others’ estimates of approximately 20-30%,8,30 and the estimated CI prevalence of 6% in the Aldo-DHF trial.16 Nonetheless, Phan et al. have found a prevalence of CI as high as 63% in HFpEF. Such difference may be due to variation in CI definitions and in inclusion/exclusion criteria, such as differences in the proportion of patients in an earlier phase of the HF syndrome, or blunted HR response yet not crossing the commonly used thresholds for diagnosing CI.
Evaluation of renal function, including calculation of GFR, was performed only at study enrollment, and not evaluated serially over time. Therefore, we could not examine the relationship between trajectory of renal function and CI. A final limitation is the absence of data on autonomic control of HR, such as measures of HR variability. Direct investigation of the interrelationship between GFR, CI, and indicators of autonomic dysfunction will be critical for improved understanding of the pathophysiology of CKD and CI in HFpEF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kass DA, Kitzman DW, Alvarez GE. The restoration of chronotropic competence in heart failure patients with normal ejection fraction (RESET) study: rationale and design. J Card Fail. 2010;16:17–24. doi: 10.1016/j.cardfail.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman RA, Hopper DL, Mah J, Hummel J, Wilkoff BL. Assessment of pacemaker chronotropic response: implementation of the Wilkoff mathematical model. Pacing Clin Electrophysiol. 2001;24:1748–1754. doi: 10.1046/j.1460-9592.2001.01748.x. [DOI] [PubMed] [Google Scholar]
- 3.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 4.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astrand PO. Physical performance as a function of age. JAMA. 1968;205:729–733. doi: 10.1001/jama.205.11.729. [DOI] [PubMed] [Google Scholar]
- 7.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92:481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 11.Lauer MS, Pashkow FJ, Larson MG, Levy D. Association of cigarette smoking with chronotropic incompetence and prognosis in the Framingham Heart Study. Circulation. 1997;96:897–903. doi: 10.1161/01.cir.96.3.897. [DOI] [PubMed] [Google Scholar]
- 12.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS, Larson MG, Evans JC, Levy D. Association of left ventricular dilatation and hypertrophy with chronotropic incompetence in the Framingham Heart Study. Am Heart J. 1999;137:903–909. doi: 10.1016/s0002-8703(99)70415-1. [DOI] [PubMed] [Google Scholar]
- 14.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann F, Gelbrich G, Duvinage A, Stahrenberg R, Behrens A, Prettin C, Kraigher-Krainer E, Schmidt AG, Dungen HD, Kamke W, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Wachter R, Pieske B. Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction--results of the Aldo-DHF trial. Int J Cardiol. 2013;169:408–417. doi: 10.1016/j.ijcard.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 18.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 19.Poirier P, Bogaty P, Philippon F, Garneau C, Fortin C, Dumesnil JG. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism. 2003;52:1056–1061. doi: 10.1016/s0026-0495(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 20.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 21.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 22.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 24.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, Finkelstein F, Hinderliter A, Pop-Busui R, Rajagopalan S, Saran R. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant. 2012;27:700–709. doi: 10.1093/ndt/gfr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brotman DJ, Bash LD, Qayyum R, Crews D, Whitsel EA, Astor BC, Coresh J. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol. 2010;21:1560–1570. doi: 10.1681/ASN.2009111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang YW, Wu CH, Tsai MK, Kuo TB, Yang CC, Lee PH. Heart rate variability during hemodialysis and following renal transplantation. Transplant Proc. 2010;42:1637–1640. doi: 10.1016/j.transproceed.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 27.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, Volpe M. Role of the reninangiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond) 2009;116:467–477. doi: 10.1042/CS20080390. [DOI] [PubMed] [Google Scholar]
- 29.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]