Abstract
MMP-10 is expressed by macrophages and epithelium in response to injury, but its functions in wound repair are unknown. We observed increased collagen deposition and skin stiffness in Mmp10−/− wounds with no difference in collagen expression or re-epithelialization. Increased collagen deposition in Mmp10−/− wounds was accompanied by less collagenolytic activity and reduced expression of specific metallocollagenases, particularly MMP-8 and MMP-13, where MMP-13 was the key collagenase. Ablation and adoptive transfer approaches and cell-based models demonstrated that the MMP-10-dependent collagenolytic activity was a product of alternatively activated (M2) resident macrophages. These data demonstrate a critical role for macrophage MMP-10 in controlling the tissue remodeling activity of macrophages and moderating scar formation during wound repair.
Introduction
Scar formation, the deposition of fibrous connective tissue within a wound bed, is a normal consequence of injury and provides temporary strength to damaged tissue. However, excessive, persistent scarring can lead to a variety of clinical problems, such as keloids, hypertrophic scars, and severe life-threatening fibrosis of lung, kidney, and liver. In these conditions, excess scarring is seemingly due to both an over-abundance of collagen production and inadequate resolution (McKleroy et al., 2013).
Although far from being fully understood, resolution of scarring and fibrosis appears to be the responsibility of macrophages and, in particular, alternatively activated (M2) macrophages (Atabai et al., 2009; Madsen et al., 2013). Current models indicate that matrix turnover involves two sequential steps: limited extracellular proteolysis followed by uptake and lysosomal degradation (Madsen et al., 2011). For the first step, some matrix metalloproteinases (MMPs) cleave the large collagen fibrils into fragments that can be endocytosed and degraded intracellularly (Lee et al., 2006; Madsen et al., 2007; Wagenaar-Miller et al., 2007). MMPs can also contribute to resolution of scarring and fibrosis indirectly by shaping the proteolytic phenotype of cells (Giannandrea and Parks, 2014).
In response to injury, stromelysin-2 (MMP-10) is induced by epithelial cells and macrophages in many organs, including skin (Kassim et al., 2007; Koller et al., 2012; Saarialho-Kere et al., 1994). In collaboration with others, we reported that over-expression of active MMP-10 in basal keratinocytes did not affect re-epithelialization and led to only a minor phenotype in basement membrane deposition (Krampert et al., 2004). The function of MMP-10 during wound repair remains to be elucidated.
Like most tissues, skin contains a population of resident macrophages, and following injury, additional macrophages influx into the wound bed (Jameson et al., 2005). Macrophages have distinct roles during different phases of wound repair, but it is not clear how these roles are split between resident and recruited cells. Macrophages contribute to scar formation by producing profibrotic cytokines that activate resident fibroblasts and pericytes into ECM-producing myofibroblasts (Duffield, 2003; Wynn and Barron, 2010). Depletion of macrophages soon after injury impairs formation of vascularized granulation tissue and delays repair (Goren et al., 2009; Lucas et al., 2010). On the other hand, as demonstrated in liver injury, depletion of macrophages during later phases leads to an inability to resolve fibrotic matrix (Duffield et al., 2005; Ramachandran et al., 2012). Early phase macrophages are predominately classically-activated proinflammatory cells (M1-biased), whereas the resolution-phase cells tend to be alternatively activated, remodeling-competent macrophages (M2-biased) (Lichtnekert et al., 2013; Mosser and Edwards, 2008).
Here we report that scarring was increased in wounded skin of Mmp10−/− mice. We found that excess ECM was not associated with increased expression of collagen but rather with reduced metallo-collagenolytic activity of resident macrophages. As MMP-10 lacks collagenolytic activity, these findings indicate that this proteinase regulates the ECM degradative potential of macrophages.
Results
MMP-10 does not Affect Re-epithelialization
Using RNA from uninjured wildtype mouse skin, we detected a Ct range for Mmp10 mRNA between 36–37 (Fig. S1), indicating essentially no or very low levels of expression. Similar to that reported earlier (Krampert et al., 2004), Mmp10 was induced by day 3 post wounding, remained elevated at day 8, and began to decline by day 12 (Fig. S1). To examine the role of MMP-10 in re-epithelialization, we compared the rate of wound closure between wildtype and Mmp10−/− mice (Fig. S2). However, wound closure rates (i.e., slope of the lines) did not differ between wildtype and Mmp10−/− wounds and both visual (Fig. S2) and histologic (not shown) analyses showed complete reepithelialization in both genotypes by day 8 post-injury.
MMP-10 Moderates Collagen Deposition
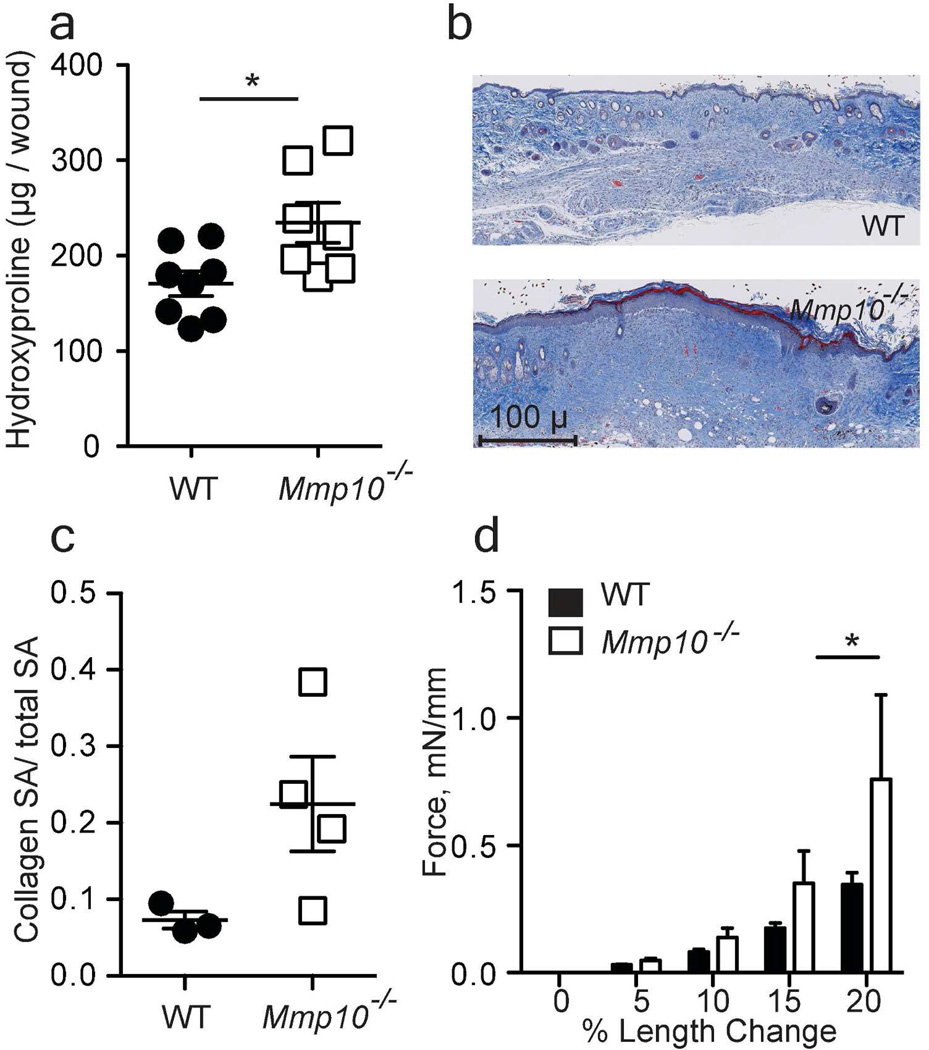
During wound healing, granulation tissue is replaced with newly deposited type I collagen forming a scar. Net collagen deposition is the sum of syntheses minus turnover. In skin wounds, collagen production begins 3–5 days after wounding and peaks around day 10 (Wu et al., 2003). Between days 3–8, we observed a trend for more deposition of collagen in Mmp10−/− wounds compared to wildtypes (not shown), which led to significantly elevated levels of accumulated collagen deposition by day 12 in null mice (Fig. 1a–c). Consistent with more collagen deposition, we found, using an established method that measures the dynamic mechanical strength of skin wounds (Jørgensen et al., 1993), that day-12 Mmp10−/− wounds were significantly stiffer than wildtype wounds (Fig. 1d). These data indicate that MMP-10 moderates scar formation. In intact skin, collagen levels did not differ significantly between wildtype (155 ± 26.8 µg/biopsy) and Mmp10−/− (165.7 ± 26.0) mice.
Figure 1. MMP-10 moderates collagen accumulation at the site of injury.
(a) Uniform 8-mm wound samples were collected on day 12 post-injury, and hydroxyproline was quantified and normalized per wound sample (n = 8 mice/ genotype); WT = wildtype; * p <0.05. (b) Representative images of day-12 wounds stained with Masson’s trichrome. Scale bar = 100 µm. (c) Quantification of collagen density in Masson’s trichrome stained samples. Data are the ratio of collagen-positive (blue) area to total surface area (SA). Each point represents samples from an individual mouse. (d) Tissue stiffness of wounds was measured using 12-day wound samples. Data are presented as the amount of force change per percent of length change. (n = 4 WT, 3 Mmp10−/−), * p <0.05.
MMP-10 does not Affect Collagen mRNA Levels
We assessed if increased collagen deposition in Mmp10−/− wounds was due to increased synthesis. Basal levels of Col1a1 mRNA and its increase and decline in response to injury did not differ between genotypes (Fig. S3a). In addition, we found no significant difference in α-SMA protein levels, a marker of myofibroblasts, the major cell source of collagen synthesis in scars, between wildtype and Mmp10−/− wounds at days 3 (not shown), 8, or 12 (Fig. S3b). Furthermore, we found no difference in the proliferation rate, the basal or TGFβ1-stimulated expression of α-SMA, or collagen uptake between primary dermal fibroblasts from wildtype or Mmp10−/− mice (Fig. S4).
MMP-10 Promotes Collagen Degradation
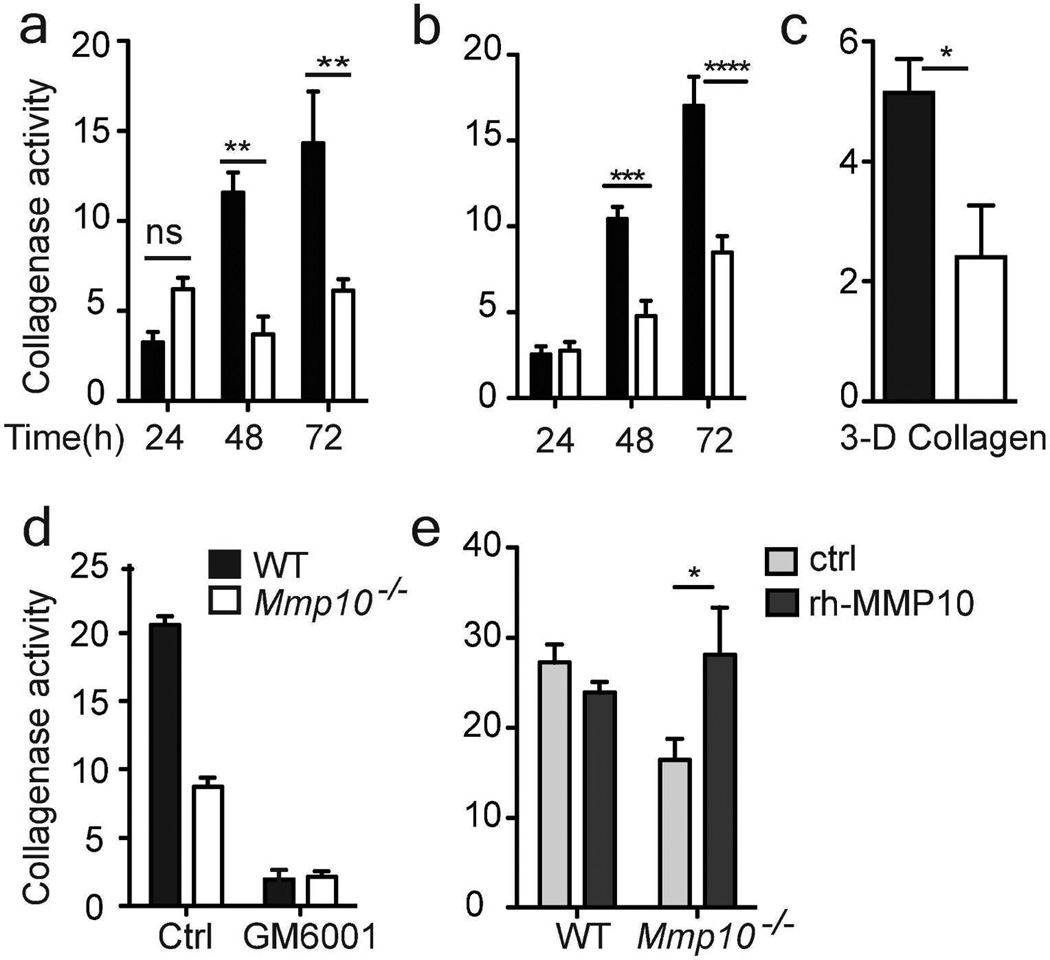
The above data indicate that increased deposition in Mmp10−/− wounds was due to impaired turnover. In defined, in vitro degradation assays MMP-10 does not cleave native type I collagen (Parks et al., 2004; Sternlicht and Werb, 2001). Thus, we assessed if MMP-10 influences the overall collagenolytic activity in wounds. For this, wound samples were incubated with quenched-fluorescent type I collagen (DQ collagen) and release of fluorescence was measured as an indicator of total collagenase activity. Indeed, significantly less collagenase activity was released from Mmp10−/− wounds compared to wildtype samples (Fig. 2a).
Figure 2. Reduced collagenase activity in Mmp10−/− wounds.
(a) Day-8 wounds were incubated with DQ-collagen for 72 h. Collagen degradation was measured every 24 h (n = 3/genotype). (b) Collagenase activity from explants of normal skin (n = 7 WT, 6 Mmp10−/−). (c) Skin explants were seeded on FITC-conjugated type I collagen gels. Collagenase activity was measured in conditioned medium at 72 h (n = 5/genotype). (d) Explants were incubated with 25 µM GM6001, and collagenase activity was assessed 72 h later (n = 4 WT, 3 Mmp10−/−). (e) Explants were treated with rh-MMP10 (100 ng/ml) or vehicle (ctrl), and collagenase activity was assessed 72 h later (n=5/genotype). *p <0.05, ** p <0.01, *** p <0.001, **** p <0.0001.
We used biopsies of intact skin as an ex vivo injury model (Dumin et al., 2001). In wildtype explants, induction of Mmp10 mRNA was detected at 72 h post-biopsy (Fig. S5a), similar to the in vivo pattern (Fig. S1). Using both DQ and native type I collagens, we found significantly less collagenase activity was released from Mmp10−/− explants compared to wildtype samples (Fig. 2b,c). The levels of collagenase activity released from wildtype and Mmp10−/− tissues and the differences between genotypes were comparable for both wound samples and explants (Fig. 2a,b). Because recruited inflammatory cells are not present in explants, the close similarity in the data between these models indicates that the MMP-10-dependent collagenase activity was a product of cells residing in intact skin.
To identify the nature of the reduced collagenolytic activity, we treated wound samples with GM6001, a broad-spectrum metalloproteinase inhibitor. In the presence of GM6001, collagenase activity was reduced to the same level in both genotypes (Fig. 2d) indicating that MMP-10 promoted the expression and/or activity of a metallocollagenase(s). Addition of active recombinant MMP-10 (rh-MMP10) increased the level of collagenase activity released from Mmp10−/−explants but had no effect on the activity from wildtype samples (Fig. 2e). Confirming its lack of collagenase activity, rh-MMP10 did not release fluorescence from DQ collagen (not shown).
MMP-10 Controls Expression of Collagenolytic MMPs
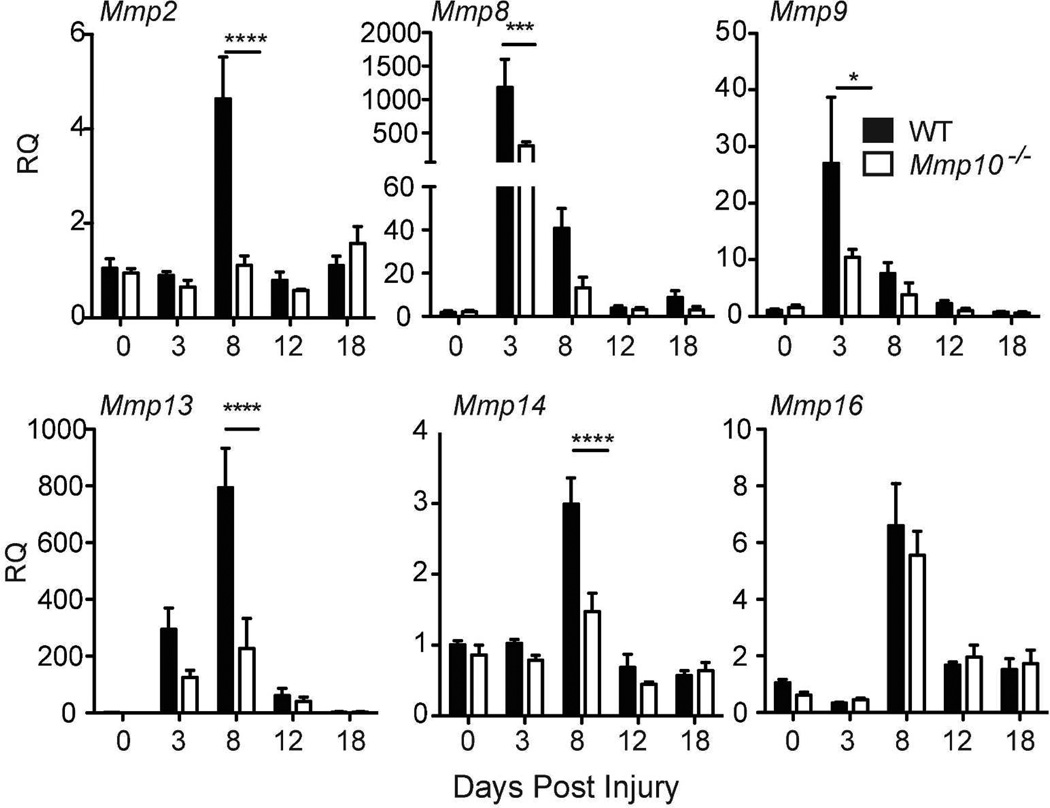
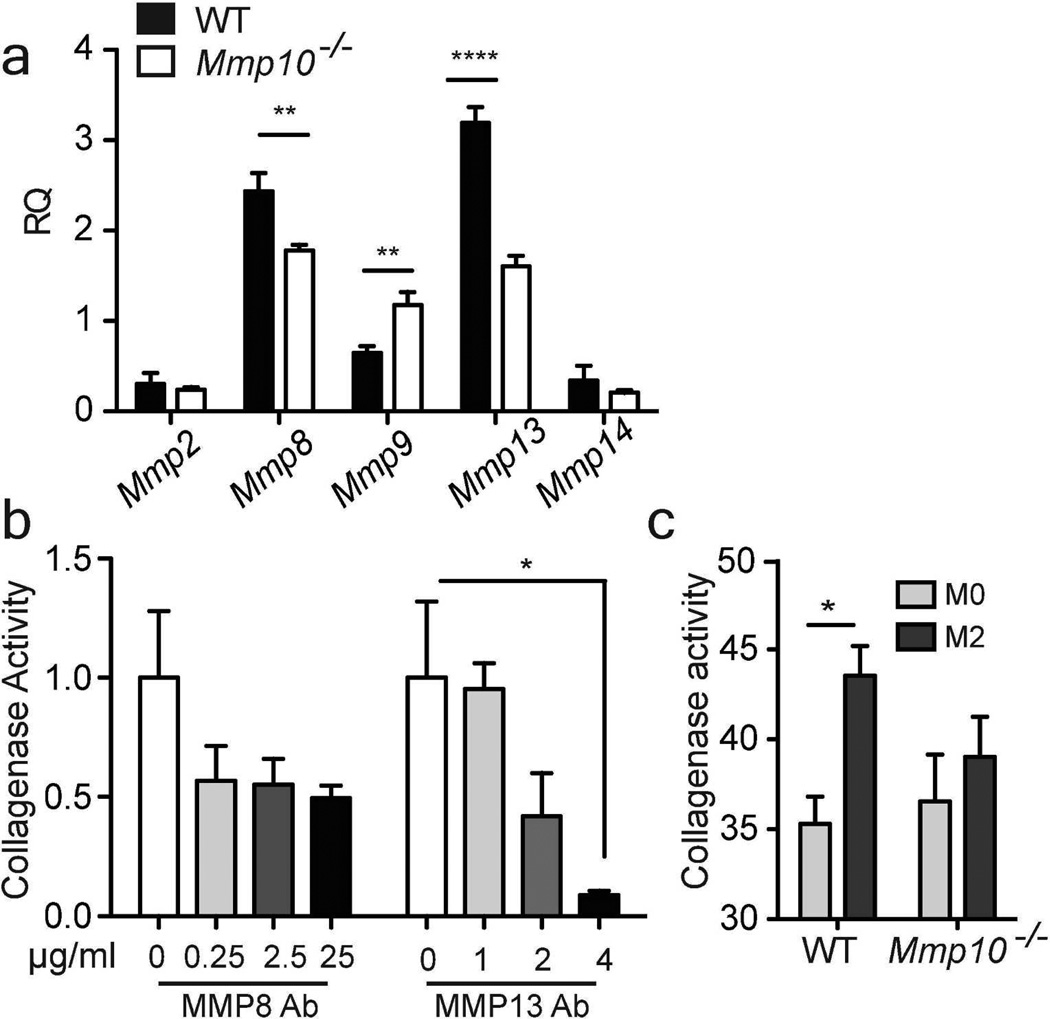
These data indicate that MMP-10 moderates scar formation and promotes resolution by controlling metallocollagenase activity. To identify the specific enzyme(s), we assessed the expression of mRNAs for Mmp2, 8, 9, 13, 14, and 16, which encode known or suspected collagenases (Sabeh et al., 2009; Shi et al., 2008). In day-3 wound samples, we found reduced expression of Mmp8 and Mmp9 mRNAs in Mmp10−/− samples (Fig. 3). In day-8 wound samples, the levels of Mmp2, Mmp13, and Mmp14 mRNAs were significantly lower in Mmp10−/− mice (Fig. 3). Similarly, in explants we found Mmp8, Mmp9, and Mmp13 mRNAs were significantly reduced in Mmp10−/− samples (Fig. S5). These results suggest reduced expression of specific collagenolytic MMPs contributed to increased collagen accumulation in Mmp10−/− wounds.
Figure 3. MMP-10 influences expression of collagenolytic MMPs.
Expression of mRNAs for Mmp 2, 8, 9, 13, 14, and 16 were quantified in wound samples harvested at the indicated days. Data shown are the ratio compared to non-wounded wildtype (WT) samples and normalized to Hprt (n≥ 4/ genotype). *p <0.05, ** p <0.01, *** p <0.001, **** p <0.0001.
MMP-10 Does Not Impact Macrophage Influx into Skin Wounds
To analyze the cellular mechanism by which MMP-10 governs collagen cleavage, we focused on macrophages. Although the similar levels of activity released from wound biopsies and explants (Fig. 2) suggested that MMP-10-dependent collagenolysis was a function of resident cells, impaired influx of macrophage could also have contributed to reduced collagenase activity in Mmp10−/− wounds. However, we found no difference in the number of resident macrophages between wildtype and Mmp10−/−skin (Fig. S6a,b) or in the total number of macrophages (resident plus recruited) in wildtype and Mmp10−/− wounds (Fig. S6c,d). Furthermore, we detected no difference in the ability of wildtype and Mmp10−/− macrophages to migrate toward serum (Fig. S6e) or in macrophage chemotactic activity in wildtype and Mmp10−/− wounds (Fig. S6f). These data indicate that the reduction in collagenase activity in Mmp10−/− wounds was not due to fewer macrophages.
MMP-10 Modulates Collagenolytic Activity of Alternatively Activated Macrophages
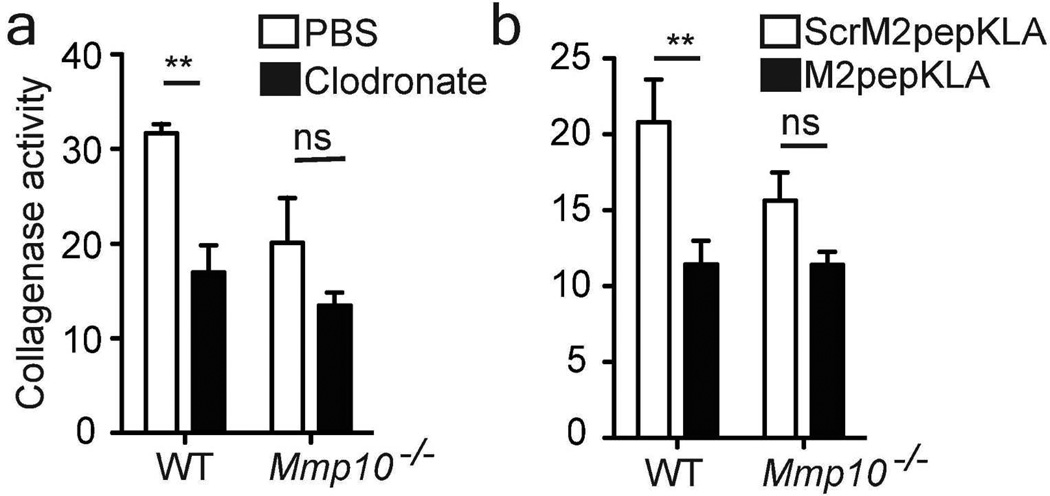
To assess a functional role for macrophages, we depleted these cells in explants using clodronate liposomes (Fig. S7a). Whereas depletion of macrophages in wildtype explants significantly reduced total collagenase activity to the levels seen in Mmp10−/− samples, ablation did not further lower the reduced activity in Mmp10−/− tissue (Fig. 4a). Skin resident macrophages are categorized as alternatively activated (M2) cells (Davies et al., 2013), and M2 macrophages are considered to be the cells responsible for tissue remodeling (Madsen et al., 2013). Hence, we selectively ablated M2-like macrophages using the M2pepKLA fusion peptide (Cieslewicz et al., 2013). The effectiveness of M2pepKLA was confirmed by reduced levels of MRC-1 (Fig. S7b), an M2 macrophage marker. Similar to the clodronate data, ablation of M2 macrophages with M2pepKLA significantly decreased collagenase activity in wildtype explants but not in Mmp10−/− samples (Fig. 4b).
Figure 4. MMP-10 regulates collagenase activity of tissue macrophages.
Wildtype (WT) and Mmp10−/− skin explants were cultured in the upper chambers of a 24-transwell plate containing (a) PBS or clodronate liposomes (n = 3/genotype) or (b) the apoptosis-inducing peptide M2pepKLA or ScrM2peptideKLA (20 µg/ml; n=5/genotype). FITC-conjugated type I collagen was added to the medium in the lower chamber, and collagenase activity was measured 72 h later. ** p <0.01.
The macrophage ablation data indicated that MMP-10 influenced the expression of collagenolytic MMPs in M2 macrophages. To test this idea, we isolated bone marrow-derived macrophages (BMDM) from wildtype and Mmp10−/− mice and assessed the expression of collagenolytic MMPs in naïve (M0) and M1 and M2-differentiated cells. Although the expression levels of metallocollagenases differed between M0 and M1 macrophages, these cell subtype-specific expression patterns did not differ between wildtype and Mmp10−/− cells (Fig. S8). However, we did find reduced expression of Mmp8 and Mmp13 mRNAs and elevated levels of Mmp9 in Mmp10−/− M2 macrophages (Fig. 5a). These data agree with the reduced expression of Mmp8 and Mmp13 in Mmp10−/− wounds (Fig. 3) and explants (Fig. S5b). Antibody-clearance demonstrated that MMP-13 accounted for the bulk of collagenase activity produced by wildtype M2 macrophages (Fig. 5b).
Figure 5. MMP10 influences expression of collagenolytic MMPs and activity in M2 macrophages.
(a) Total RNA was isolated from M2-polarized wildtype and Mmp10−/− BMDMs and analyzed by qRT-PCR. Data are presented as fold change relative to naïve BMDM from wildtype mice. (b) Wildtype skin explants were cultured in the upper chambers of 24-transwell plates. DQ-collagen was added to the lower chamber containing antibody against MMP-8 or, antibody against MMP-13. Collagenase activity was measured 72 h later. Values are presented as the ratio of untreated samples. n = 3, *p <0.05, **p <0.01. (c) Naïve and M2-polarized BMDMs were incubated with DQ-collagen for 6 h. Supernatants were assayed for collagenase activity. (n = 4 wildtype, 5 Mmp10−/−).
Furthermore, polarization to M2-like macrophages increased the collagenase activity released from wildtype macrophages but had no effect on the activity released from Mmp10−/− macrophages (Fig. 5c). Expression of Arg1 and Fizz1 increased markedly in M2-differentiated macrophages (Gordon, 2003), but this well-characterized M2 response was not altered in Mmp10−/− cells (Fig. S9). These data indicate that the reduced levels of collagenase activity released from Mmp10−/− M2 macrophage was independent of the ability of Mmp10−/− M0 macrophages to respond to the M2 agonists.
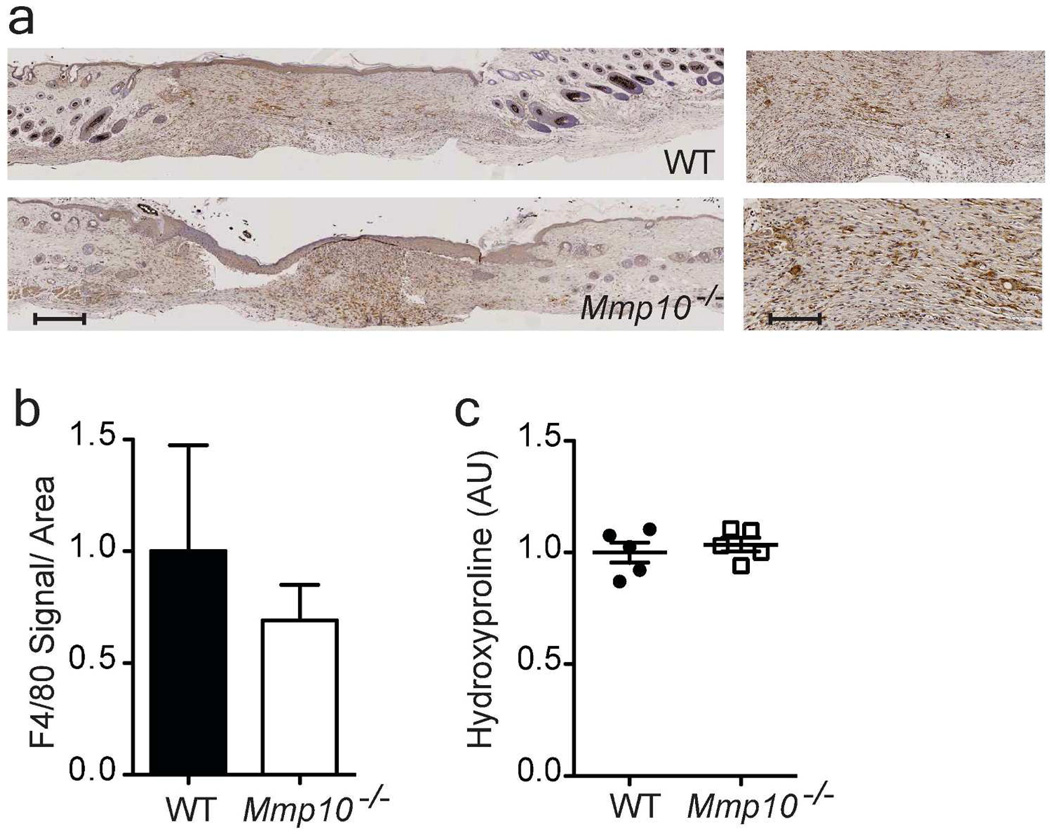
To demonstrate that reduced collagenolytic activity of macrophages contributed to the enhanced scarring in Mmp10−/− wound, we transferred BMDM from wildtype MacGreen mice into Mmp10−/− recipients on day 10 post-injury. Tissues were harvested 2 days later (day 12 after wounding). EGFP+ macrophages were detected in both wildtype and Mmp10−/− wounds (Fig. S10), and similar numbers of F4/80+ macrophages were detected in wounds from both genotypes (Fig. 6a,b). We found that the influx of wildtype macrophages normalized the increased collagen deposition seen in Mmp10−/− wounds (Fig. 6c).
Figure 6. Adoptive transfer of wildtype macrophages normalizes collagen deposition in Mmp10−/− wounds.
Wildtype (WT) and Mmp10−/− mice received 7 × 106 GFP+ BMDM via retro orbital injection at day 10 post-wounding. Wounds were harvested on day 12. (a) Representative images of 12-d wounds sections stained for F4/80. Scale bar = 500 µm (left), 60 µm (right) (b) The total area of F4/80-positive signal was quantified and normalized to total tissue surface area (n = 5/genotype). (c) Hydroxyproline was quantified, normalized per wound sample, and expressed relative to wildtype samples (n=5 mice/genotype).
Discussion
We report that macrophage-derived MMP-10 serves a beneficial role in tissue repair by promoting resolution of scar tissue. Our findings indicate MMP-10 shaped scar deposition indirectly by controlling the collagenolytic activity of M2-biased resident macrophages without impacting collagen production by fibroblasts/myofibroblasts. More specifically, we found that MMP-10, which is not a collagenase, promoted the expression of MMP-8 and MMP-13, two metallocollagenases released by macrophages. However, we found that essentially all collagenolytic activity was due to MMP-13, suggesting that macrophage-derived MMP-8 functions in other processes. Indeed, data supporting that MMP-8 functions as a collagenase are not compelling. Both over-expression and deletion of Mmp8 reduces fibrosis (Craig et al., 2013; Garcia-Prieto et al., 2010; Siller-Lopez et al., 2004).
Studies with Mmp13−/− mice complement our conclusions. The resolution of CCl4-induced liver fibrosis is slowed in Mmp13−/− mice (Fallowfield et al., 2007) and accelerated in mice over-expressing this collagenase (Yang et al., 2014), and both of these studies linked MMP-13 production to scar-associated macrophages. Furthermore, excess type I collagen is deposited in bones of Mmp13−/− mice (Inada et al., 2004; Stickens et al., 2004). On the other hand, liver fibrosis due to bile duct ligation is reduced in Mmp13−/− mice (Uchinami et al., 2006). Regarding skin wounds, disparate findings have been reported for phenotypes in Mmp13−/− mice. Whereas Hattori et al. (2009) concluded that MMP-13 promotes closure of skin wounds, Hartenstein et al. (2006) found no difference in re-epithelialization between wildtype and Mmp13−/− mice. Although neither skin-wound study assessed scarring directly, Hattori et al. reported a reduction in both wound contraction and myofibroblast numbers in Mmp13−/− mice, findings that would suggest that MMP-13 has pro-fibrotic activities, too. As MMP-13 is expressed by many cells types, it is likely that this MMP can affect multiple, distinct processes within and among models.
Our findings indicate that MMP-10 controls collagen turnover by macrophages by promoting the expression of specific metallocollagenases, primarily MMP-13. As MMP-13 levels were reduced but not absent in Mmp10−/− macrophages, the residual enzyme likely contributed to the collagenase activity in Mmp10−/− samples and cells and may explain why we did not observe any differences in granulation tissue formation as seen in mice with a complete lack of MMP-13 (Toriseva et al., 2012). Complementary to our conclusion, the transcription factor ANKRD1 influences expression of Mmp13 and Mmp10 (Almodovar-Garcia et al., 2014), suggesting a link in the synthesis of these two MMPs.
In our studies, we used a model of normal skin repair, and we observed no differences in macrophage influx or in the expression of αSMA and type I collagen (or other markers of fibroblast activation) between wildtype and Mmp10−/− mice. In contrast, macrophage influx, αSMA expression, and fibrosis are elevated in Mmp10−/− mice in models of severe liver (Garcia-Irigoyen et al., 2013), colon, (Koller et al., 2012) and lung injury (unpublished findings). Although an explanation for these disparate findings is not yet apparent, we propose that the mechanisms by which MMP-10 affects fibrosis and macrophage influx are context dependent. Thus, the profound pro-inflammatory responses in liver/colon/lung models may lead to MMP-10-dependent roles in macrophage recruitment and activation that, in turn, stimulate myofibroblast differentiation and collagen production. In contrast, because the inflammatory response is less profound in skin wounds, MMP-10-dependent controls on macrophage influx could be modest and, hence, not easily detected in Mmp10−/− tissues.
Compared to the evidence supporting their roles in promoting fibrosis (Pellicoro et al., 2014), there are limited data on how macrophages function during resolution. We found that MMP-10 shapes the tissue-remodeling function of resident skin M2-like macrophages (Davies et al., 2013) by regulating expression of MMP-13. Reduced levels of this metallocollagenase were consistently seen among wound biopsies (Fig. 3), explants (Fig. S5b), and M2-polarized cultured macrophages (Fig. 5a). Reparative or M2-like macrophages are the dominant population of macrophages during the resolution phase (Duffield, 2014; Lichtnekert et al., 2013). M0 macrophages possessed baseline collagenolytic activity (Fig. 5c), and treatment with M2 agonists led to a significant increase in overall collagenase activity released by wildtype macrophages but had no effect on the levels from Mmp10−/− cells. These findings support our central conclusion that MMP-10 controls the collagenolytic activity of M2-biased macrophages. In agreement with our findings, Madsen et al. (2013) demonstrated that M2-like macrophages are the principal cells executing collagen turnover in skin.
Our findings indicated that M2-biased resident macrophages were responsible for scar resolution in excisional skin wounds. Of note, induction of Arg1 and Fizz1, commonly used M2 markers, were not altered in IL-4/IL-13-treated Mmp10−/− macrophages, but a key function - tissue remodeling - attributed to this type of macrophage was markedly impaired. These findings add to the many caveats of the M1/M2 classification scheme and underscore that function is more important in defining macrophage subtype than expression of a few markers (Murray et al., 2014).
Excess scarring (fibrosis) can affect quality of life, with loss of tissue function, restriction of movement, and adverse psychological consequences (Brown et al., 2008). Although fibrotic conditions are oft considered to be irreversible, an understanding how macrophage activation is controlled and how excess ECM is cleared will shed light on means to manipulate these severe diseases. Results from our study demonstrate a role for MMP-10 in controlling the ability of M2-like macrophages to degrade fibrillar collagen during repair of skin wounds. Whether MMP-10 has a similar, beneficial role in mitigating fibrosis in disease settings has yet to be determined.
Materials and Methods
Animals
Mmp10−/− mice (C57BL/6J) (Kassim et al., 2007) and wildtype littermates (male and female) were used for these studies. All procedures were approved by the Office of Animal Welfare, University of Washington, and the IACUC, Cedars-Sinai.
Wounds and Explants
Mice were anesthetized, and their dorsal surface was shaved. Skin was pulled into a fold and laid on a plastic surface. Two full-thickness wounds were created by running a sterile 5-mm punch biopsy (Miltex Inc., New York, NY USA) through the fold. Four wounds were made per mouse and left uncovered. Mice were housed in individual cages. On indicated days post injury, wounds were excised with an 8-mm biopsy punch for further analysis.
RNA
Total RNA was isolated and specific transcripts were quantified by quantitative real-time PCR using TaqMan FAM-labeled probes (Applied Biosystems, Foster City, CA) as described (Rohani et al., 2014).
Hydroxyproline
Excised wounds were weighed and homogenized in deionized water (20 µl/ mg tissue). Equal volumes of homogenates were mixed with 12 N HCl (1:1) and hydrolyzed for 3 h at 120°C. Hydroxyproline was measured using a colorimetric assay (BD BioVision, Milpitas, CA). Data are presented as total micrograms hydroxyproline/biopsies.
Histology
Sections (4 µm) of paraffin-embedded tissues were stained with Masson’s trichrome or processed for immunohistochemistry with anti F4/80 (BM8, eBioscience, San Diego, CA). VisioPharm software (Hørsholm, Denmark) was used to quantify the total area of F4/80 signal or trichrome staining, normalized to total surface area.
Biomechanics
Skin stiffness was measured as described (Rohani et al., 2014). Briefly, 12-d wound samples were dissected into strips and suspended on hooks attached to a force transducer (Aurora Scientific, model 400A) and a length controller (Aurora Scientific, model 308B). Tissue stiffness (dF/dL) was determined by imposing change in length of the skin strip and measuring the resultant change in force normalized per millimeter of sample width. On each strip of tissue, 4 steps of 5% length stretches were taken every 30 sec. Force and length signals were analyzed using custom LabView software.
Collagenase
Wound and explant samples were placed in the upper chambers of transwells in 50 µl, and 1% (v/v) quenched fluorescein-conjugated DQ™ type I collagen (1 µg/ml; Life Technologies, Grand Island, NY) was added to the bottom chambers in 500 µl of phenol red-free RPMI with 1% FBS and 1% penicillin/streptavidin. Collagenase activity was defined as the fluorescence detected in the bottom chamber (excitation 480 nm, emission 530 nm) minus fluorescence in blank controls (no sample in the upper chamber). For 3D matrix, we mixed native type I collagen (PurCol, Advanced BioMatrix, Carlsbad, CA) with DQ collagen and seeded explants onto the mixed matrix and assessed collagenase activity. For antibody depletion, we cultured samples with antibodies against MMP-8 (R&D Systems, Minneapolis, MN) or MMP-13 (Thermos Fisher Scientific, Waltham, MA). For macrophages, cells were incubated with phenol red-free RPMI containing 1% DQ collagen and 6 h.
Macrophage Depletion
Explants were cultured for 72 h with PBS- or clodronate liposomes (50 mg/ml; http://www.clodronateliposomes.org) added to the upper chamber. To deplete M2 macrophages, explants were cultured with 20 µg/ml M2pepKLA or a control scrambled peptide (scrM2pepKLA) (Cieslewicz et al., 2013).
Macrophage Culture
Isolation and activation of BMDM were done as described (Manicone et al., 2009). Briefly, marrow cells were cultured for 7 days in Mac medium. For M2 activation, BMDM were stimulated with 10 ng/ml IL-4 and IL-13 for 48 h. For M1 activation, cells were cultured with 100 ng/ml E. coli LPS for 6 h, washed, and cultured for another 24 h. For adoptive transfer, macrophages were collected at day 5 after marrow harvest and suspended in PBS. Recipient mice received 7 × 106 BMDM in 50 µl PBS via retro orbital injection.
Statistical Analysis
Statistical analysis was performed using Prism 5 (GraphPad Software, Inc., La Jolla, CA). We used t-test to compare two groups, or two-way ANOVA followed by Bonferroni post-test to test the effect of two factors. Data are presented as mean ± SEM. A p-value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AR060157 (MR), HL098067 (WCP), and HL089455 (WCP). The authors thank Dr. Anne Manicone, Dr. Peter Chen, and Tyler Vandivort for helpful discussions. We also thank Erin McCarty and Cara Leigh Appel of the Histology and Imaging Core in the Center for Lung Biology, University of Washington.
Footnotes
Conflict of Interest. The authors state no conflict of interest.
References
- Almodovar-Garcia K, Kwon M, Samaras SE, et al. ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site. Mol Cell Biol. 2014;34:1500–1511. doi: 10.1128/MCB.01357-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabai K, Jame S, Azhar N, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BC, McKenna SP, Siddhi K, et al. The hidden cost of skin scars: quality of life after skin scarring. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2008;61:1049–1058. doi: 10.1016/j.bjps.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Cieslewicz M, Tang J, Yu JL, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci USA. 2013;110:15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig VJ, Quintero PA, Fyfe SE, et al. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J Immunol. 2013;190:4283–4296. doi: 10.4049/jimmunol.1201043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumin JA, Dickeson SK, Stricker TP, et al. Procollagenase-1 (matrix metalloproteinase-1) binds the integrin α2β1 upon release from keratinocytes migrating on type I collagen. J Biol Chem. 2001;276:29368–29374. doi: 10.1074/jbc.M104179200. [DOI] [PubMed] [Google Scholar]
- Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- Garcia-Irigoyen O, Carotti S, Latasa MU, et al. Matrix metalloproteinase-10 expression is induced during hepatic injury and plays a fundamental role in liver tissue repair. Liver Int. 2013 doi: 10.1111/liv.12337. [DOI] [PubMed] [Google Scholar]
- Garcia-Prieto E, Gonzalez-Lopez A, Cabrera S, et al. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PloS One. 2010;5:e13242. doi: 10.1371/journal.pone.0013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Disease models & mechanisms. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein B, Dittrich BT, Stickens D, et al. Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J Invest Dermatol. 2006;126:486–496. doi: 10.1038/sj.jid.5700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Mochizuki S, Kishi K, et al. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Sharp LL, et al. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen PH, Bang C, Andreassen TT. Mechanical properties of skin graft wounds. Br J Plast Surg. 1993;46:565–569. doi: 10.1016/0007-1226(93)90106-l. [DOI] [PubMed] [Google Scholar]
- Kassim SY, Gharib SA, Mecham BH, et al. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infection and immunity. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller FL, Dozier EA, Nam KT, et al. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest. 2012;92:1749–1759. doi: 10.1038/labinvest.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M, Bloch W, Sasaki T, et al. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell. 2004;15:5242–5254. doi: 10.1091/mbc.E04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Overall CM, McCulloch CA, et al. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17:4812–4826. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtnekert J, Kawakami T, Parks WC, et al. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–564. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Engelholm LH, Ingvarsen S, et al. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282:27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Ingvarsen S, Jurgensen HJ, et al. The non-phagocytic route of collagen uptake: a distinct degradation pathway. J Biol Chem. 2011;286:26996–27010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen DH, Leonard D, Masedunskas A, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202:951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicone AM, Birkland TP, Lin M, et al. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani MG, Chow YH, Razumova MV, et al. uPARAP function in cutaneous wound repair. PLoS One. 2014;9:e92660. doi: 10.1371/journal.pone.0092660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, et al. Distinct populations of keratinocytes express stromelysin-1 and -2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Li X, Saunders TL, et al. Secreted versus membrane-anchored collagenases: Relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Son MY, Yamada S, et al. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-Lopez F, Sandoval A, Salgado S, et al. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122–1133. doi: 10.1053/j.gastro.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriseva M, Laato M, Carpen O, et al. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PloS One. 2012;7:e42596. doi: 10.1371/journal.pone.0042596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchinami H, Seki E, Brenner DA, et al. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- Wagenaar-Miller RA, Engelholm LH, Gavard J, et al. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27:6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–932. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Seminars in liver disease. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339 e1, 50 e1. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.