Abstract
Purpose
Previous epidemiologic studies have observed positive associations between Trichomonas vaginalis (Tv) serostatus and both prostate cancer (PCa) risk and mortality. However, only a few small older studies have examined Tv antibody persistence over time, all of which were composed mainly of female patients. Therefore, we examined Tv antibody persistence over time, as well as intra-individual variability, among middle- to older-aged men in the Southern Community Cohort Study (SCCS).
Methods
We tested baseline and repeat plasma specimens (collected 1–3 years later) from 248 male participants for Tv antibodies. We used the same enzyme-linked immunosorbent assay as in previous studies of Tv serostatus and PCa.
Results
At baseline, 46 (18.5%) participants were seropositive for Tv infection. Seventy-six percent of these men were still seropositive 1–3 years later. A similar proportion of men “seroconverted” (4.0%) as “seroreverted” (4.4%), all of whom had absorbance values near the cut-off point for seropositivity. Overall, substantial agreement was observed between baseline and repeat serostatus (kappa=0.72, 95% confidence interval: 0.60–0.83).
Conclusion
Tv seropositivity was largely persistent between plasma specimens collected 1–3 years apart from middle- to older-aged men. These high levels of persistence are similar to those observed for other sexually transmitted infections frequently investigated in relation to PCa.
Keywords: Trichomonas vaginalis, antibody, persistence, reproducibility, males
INTRODUCTION
Trichomonas vaginalis (Tv) infection is the most common, curable sexually transmitted infection (STI) in the US and worldwide [1]. This common protozoan infection has been proposed as a possible risk factor for prostate cancer (PCa) for several reasons, including its ability to infect and elicit inflammation within the prostate; its propensity to establish persistent, subclinical infections; its ability to damage prostate epithelium, alter local polyamine levels, inhibit apoptosis, and upregulate proto-oncogenes; and its more common occurrence in African-American men, who are at highest risk of PCa [2, 3]. A recent study also found that Tv macrophage migration inhibitory factor, a human proinflammatory cytokine homolog, increases growth and invasiveness of benign and malignant prostate cells in vitro [4], and two of three recent sero-epidemiologic studies observed positive associations between a history of Tv infection and risk of PCa, particularly high-grade and metastatic/fatal disease [5, 6].
As with most studies of infections and cancer, methodologic challenges exist for the study of Tv infection and PCa. These include the uncertainty of the possible timing of action of Tv infection (i.e., is infection relevant for tumor initiation, promotion, and/or progression?); the potentially long period of time between infection and PCa diagnosis, as Tv may be acquired as early as puberty, whereas PCa is not typically diagnosed until men reach their 60s–70s [7]; and difficulties in accessing non-diseased control prostate tissue. Therefore, most epidemiologic studies to date have examined Tv infection in relation to PCa by using serology as a marker of cumulative Tv exposure in older men. Although these studies observed good agreement between blinded replicate specimens (89–94% agreement [5, 6, 8]), only two small older studies, to our knowledge, have examined antibody persistence or intra-individual variability over time [9, 10], both of which were composed mainly of women who tend to be more likely to seroconvert or be seropositive for STIs than men [11–14]. Therefore, we examined Tv antibody persistence and intra-individual variability over time in male participants in the Southern Community Cohort Study (SCCS).
MATERIALS AND METHODS
Study population and design
Methods for the establishment of the SCCS and baseline data collection have been described in detail elsewhere [15]. Briefly, from 2002–2009, >85,000 participants (41% men) 40–79 years of age who spoke English and who had not undergone cancer treatment in the past year were enrolled in 12 southeastern US states. Most participants were African-American (65%) or non-Hispanic white (30%). Approximately half provided a 20 mL blood sample at their baseline in-person interview. These participants, all of whom enrolled at a community health center (CHC), serve as the study base for this analysis. Blood samples were refrigerated at the CHCs and then shipped the same day in ice packs for overnight delivery to Vanderbilt University where they were separated into components and frozen at −80°C the same day.
Between May-October 2008, we invited all SCCS participants from our study base who enrolled at one of nine active CHCs either one, two, or three years prior to provide a repeat blood sample (N=1,102). Approximately 66% (N=662) of those assumed to have received the invitation provided a sample (N=1,010; 92 invitations were returned undeliverable). Samples were collected and handled as at baseline. Of the 257 male participants who provided a repeat sample, specimens from nine were not sent to the lab for Tv antibody testing, leaving 248 participants in the analysis.
The SCCS was approved by Institutional Review Boards at Vanderbilt University and Meharry Medical College. This analysis was also approved by Washington University School of Medicine and the Harvard School of Public Health. All participants provided written informed consent for the main study and separately for repeat blood collection.
Tv serostatus assessment
We assessed Tv serostatus using the same enzyme-linked immunosorbent assay (ELISA) as in previous studies of Tv serostatus and PCa [5, 6, 8]. This assay detects IgG antibodies against recombinant Tv α-actinin protein [5, 16]. We ran baseline and repeat plasma samples for each participant in duplicate in the same batch. Each of these batches had its own control panel, which consisted of a series of specimens of increasing absorbance (absorbance scores 0, 1+, 2+, 3+, 4+, and 5+). We obtained negative control sera (scores ≤2+) from individuals without a history of Tv or other STIs, and positive control sera (scores ≥3+) from patients with Tv infection [17]. Negative control sera had no detectable reactivity to trichomonad proteins blotted onto nitrocellulose after SDS-PAGE of total Tv proteins and immunoblotting, whereas positive control sera readily detected trichomonad proteins by immunoblotting [16, 18]. Sera with scores of 1+ or 2+ gave non-specific reactions above baseline by ELISA.
We included twelve control panels in the testing sequence and used the average of these values in the analysis. We determined Tv score cut-off points by dividing the mean absorbance of each control specimen (1+ through 5+) by the mean absorbance of the 0 score control specimen to generate a positive to negative (P/N) ratio. Cut-off points were then derived by taking the mean of P/N ratios for adjacent categories (e.g., the 3+ score cut-off point was determined by taking the mid-point between the P/N ratios for the 2+ and 3+ control specimens). We calculated Tv scores for each participant by dividing their mean absorbance by the 0 score control absorbance and then by comparing this P/N ratio to the above-mentioned cut-off points. Men with scores ≥3+ were considered seropositive and those with scores ≤2+ were considered seronegative, consistent with the most recent analysis of Tv infection and PCa [6]. Sera with scores ≥3+ are also known to have antibodies to numerous trichomonad proteins by immunoblotting of total Tv proteins [16].
We evaluated assay reproducibility by distributing 18 blinded quality control specimens derived from pooled anonymous plasma randomly across the testing sequence. All of these specimens were seronegative (0 score: N=3; 1+ score: N=10; 2+ score: N=5).
Statistical analysis
We compared Tv seropositive men to seronegative men using t-tests for continuous variables, and chi-square tests for dichotomous and categorical variables. To examine Tv antibody persistence over time, we calculated the proportion of men seropositive at baseline who remained seropositive at their repeat blood draw. Tv antibody agreement over time was investigated by calculating kappa coefficients.
RESULTS
The average age of the 248 participants at baseline was 53 years. The majority of participants were African-American (71.0%), had completed no more than a high school education (71.4%), had a household income <$25,000 (76.9%), had been married (79.8%), were current or former smokers (76.6%), and had an average time between blood draws of 1.4 years (range: 0.9–3.1; Table 1). At baseline, 46 (18.5%) participants were seropositive for Tv infection. These men were non-significantly more likely to be African-American and to be separated, divorced, or widowed than seronegative men. They also had a longer time between blood draws.
Table 1.
Baseline characteristics of men included in a Trichomonas vaginalis (Tv) serostatus reproducibility study, Southern Community Cohort Study 2005–8
| All participants (n=248) |
Baseline Tv serostatus | |||
|---|---|---|---|---|
| Positive (n=46) |
Negative (n=202) |
P-valuea | ||
| Age at baseline blood draw (years, mean (range)) | 53 (40–77) | 55 (40–74) | 53 (40–77) | 0.30 |
| Race (%): | ||||
| African-American | 71.0 | 80.4 | 68.8 | 0.12 |
| White | 29.0 | 19.6 | 31.2 | |
| Education (%): | ||||
| <9 years | 14.5 | 17.4 | 13.9 | 0.66 |
| 9–11 years | 25.4 | 28.3 | 24.8 | |
| 12 years, completed high school, or received a General Education Diploma | 31.5 | 23.9 | 33.2 | |
| Any post-secondary education | 28.6 | 30.4 | 28.2 | |
| Annual household income (dollars, %): | ||||
| <15,000 | 58.3 | 60.0 | 57.9 | 0.97 |
| 15,000–24,000 | 18.6 | 17.8 | 18.8 | |
| ≥25,000 | 23.1 | 22.2 | 23.3 | |
| Marital status (%): | ||||
| Married or living as married with a partner | 45.5 | 45.7 | 45.5 | 0.15 |
| Separated, divorced, or widowed | 34.3 | 43.5 | 32.2 | |
| Never married | 20.2 | 10.9 | 22.3 | |
| Cigarette smoking status (%): | ||||
| Current | 43.6 | 41.3 | 44.1 | 0.82 |
| Former | 33.1 | 37.0 | 32.2 | |
| Never | 23.4 | 21.7 | 23.8 | |
| Years between blood samples (%) | ||||
| 1 | 73.4 | 58.7 | 76.7 | 0.04 |
| 2 | 20.2 | 30.4 | 17.8 | |
| 3 | 6.5 | 10.9 | 5.4 | |
| Time between specimens (years, mean (range)) | 1.37 (0.93–3.12) | 1.54 (0.94–3.08) | 1.33 (0.93–3.12) | 0.03 |
Calculated by t-tests for continuous variables and chi-square tests for dichotomous and categorical variables.
Comparing baseline and repeat Tv serostatus, 77.4% of men were persistently seronegative, 4.0% seroconverted (i.e., seronegative at baseline, seropositive on repeat testing), 4.4% seroreverted (seropositive, seronegative), and 14.1% were persistently seropositive (Table 2). Seventy-six percent of men seropositive at baseline were still seropositive on average 1.4 years later (1 year post-baseline: 20/27=74.1%; 2 years: 11/14=78.6%; 3 years: 4/5=80.0%). The kappa coefficient for serostatus agreement over time was 0.72 (95% confidence interval: 0.60–0.83). Similar results were observed in analyses stratified by race, age (<50, ≥50 years), and follow-up time (data not shown).
Table 2.
Agreement between baseline Trichomonas vaginalis (Tv) serostatus and serostatus 1–3 years later among 248 male participants in the Southern Community Cohort Study 2005–8
|
Tv serostatus (baseline/repeat specimen) |
||||
|---|---|---|---|---|
| −/− | −/+ | +/− | +/+ | |
| N | 192 | 10 | 11 | 35 |
| Time between baseline and repeat specimen collection (years, mean) | 1.3 | 1.7 | 1.5 | 1.6 |
| Baseline score (%): | ||||
| 0 | 18.8 | 0.0 | 0.0 | 0.0 |
| 1+ | 45.8 | 0.0 | 0.0 | 0.0 |
| 2+ | 35.4 | 100.0 | 0.0 | 0.0 |
| 3+ | 0.0 | 0.0 | 100.0 | 91.4 |
| 4+ | 0.0 | 0.0 | 0.0 | 8.6 |
| Difference between the baseline P/N ratio and the cut-off point for seropositivity (mean) | −0.81 | −0.21 | 0.30 | 0.57 |
| Repeat score (%): | ||||
| 0 | 20.8 | 0.0 | 9.1 | 0.0 |
| 1+ | 41.1 | 0.0 | 9.1 | 0.0 |
| 2+ | 38.0 | 0.0 | 81.8 | 0.0 |
| 3+ | 0.0 | 90.0 | 0.0 | 97.1 |
| 4+ | 0.0 | 10.0 | 0.0 | 2.9 |
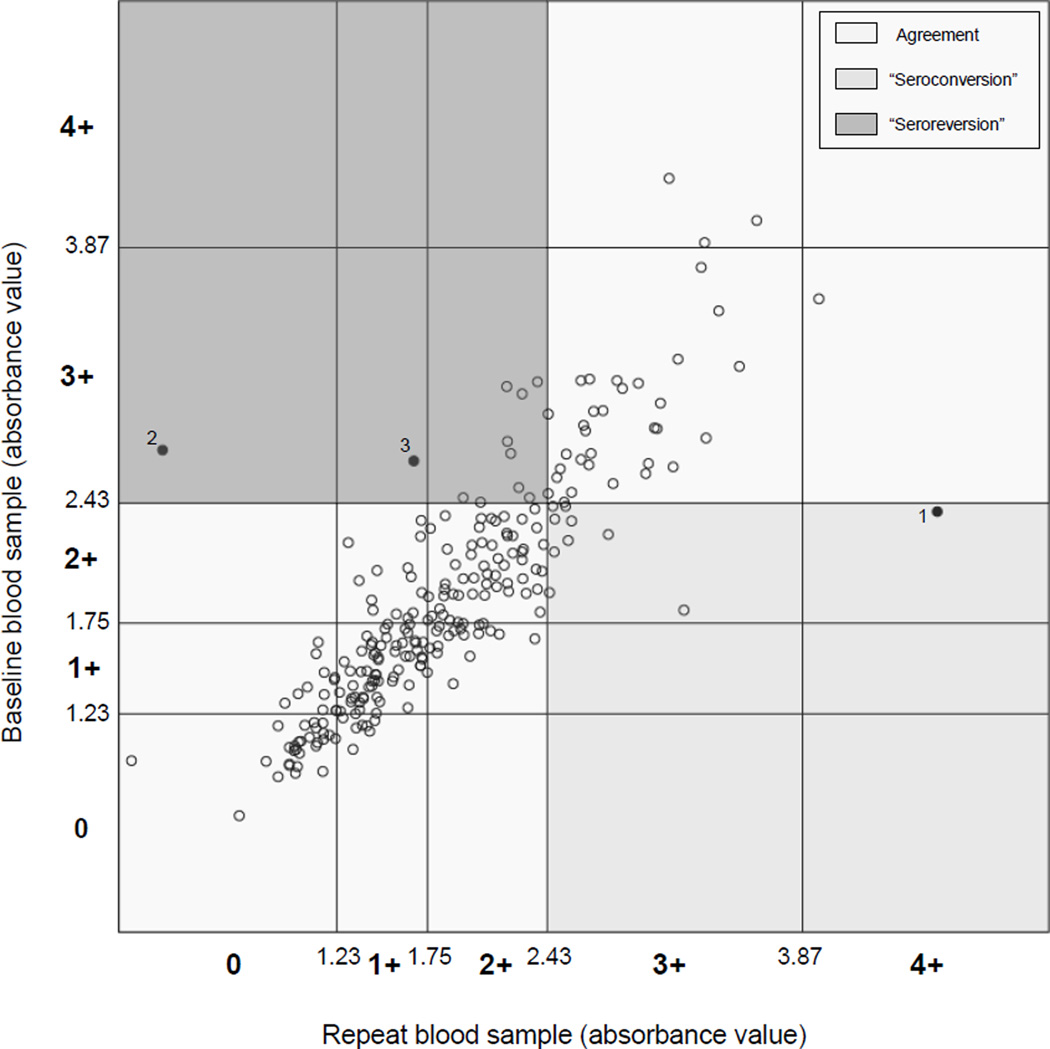
Men who seroconverted were significantly more likely to have baseline 2+ scores (rather than 0 or 1+ scores; 100% of seroconverters vs 35.4% of persistently seronegative men, p<0.0001), as well as P/N ratios closer to the seropositivity cut-off point than persistently seronegative men (mean difference=−0.21 vs −0.81, p<0.0001; Table 2 and Figure 1). Even considering only participants with baseline 2+ scores, those who seroconverted had P/N ratios closer to the seropositivity cut-off point than those who remained seronegative (mean difference=−0.21 vs −0.37, p=0.008). Most men who seroconverted had 3+ scores on their repeat specimen; only one man (datapoint 1; Figure 1) had a 4+ score. Considering seroreversion, all participants who seroreverted had baseline 3+ (as opposed to 4+) scores and P/N ratios marginally closer to the seropositivity cut-off point than those who remained seropositive (mean difference=0.30 vs 0.57, p=0.07). These men also tended to have scores close to the seropositivity cut-off point on their repeat specimen; 9 (81.8%) had 2+ scores and 2 (18.2%) had 1+ or 0 scores (datapoints 3 and 2; Figure 1). None of the seroconversions or reversions was explained by differences in batch for baseline and repeat specimens.
Figure 1.
Agreement between baseline Trichomonas vaginalis (Tv) serostatus and serostatus 1–3 years later among 248 male participants in the Southern Community Cohort Study, 2005–8
Baseline and repeat Tv antibody absorbance values for each participant are plotted. Absorbance cut-off values for Tv scores and the scores themselves (0, 1+, 2+, 3+, and 4+) are indicated for the baseline values on the Y axis and for the repeat values on the X axis. Seropositivity is defined as a score greater or equal to 3+. Baseline and repeat serostatus results that agree are found in the upper right-hand (both seropositive) and lower left-hand (both seronegative) quadrants; baseline seronegative and repeat seropositive results (“seroconversion”) are found in the lower right-hand quadrant; and baseline seropositive and repeat seronegative results (“seroreversion”) are found in the upper left-hand quadrant. All data points are indicated by circles. Unfilled circles represent agreement or disagreement by no more than one score. Filled circles indicate disagreement by more than one score. Datapoint 1 represents a man who had a score of 2+ at baseline and a score of 4+ on his repeat sample. Datapoints 2 and 3 represent men who had scores of 3+ at baseline and 1+ or less on their repeat samples.
DISCUSSION
In this study of middle- to older-aged men, we found that 18.5% of participants were seropositive for Tv infection at baseline and that 76.1% of these men remained seropositive 1–3 years later (kappa=0.72). Our observed seroprevalence is consistent with findings from the most recent study of Tv infection and PCa (21.4%, using the same seropositivity cut-off point [6]), and our high levels of antibody persistence are consistent with findings from two previous small studies of Tv antibody persistence in predominantly female populations (64–71% persistence over “months to years” in 14 patients [9] and 92% persistence over ≥8 months in 25 patients [10]). They are also consistent with findings from previous studies of other sexually transmitted agents that infect the genital mucosal epithelium and cause curable or non-lifelong infections, such as human papillomavirus and Chlamydia trachomatis (persistence range: 30–83% up to seven years [19–21]; kappa range: 0.79–1.00 over one year [22] and 0.52 over 5–7 years [20]).
Although STIs are typically acquired when men are younger (e.g., in their 20s [1]), Tv infection has a wider age range of infection than many other STIs [23, 24]. Therefore, an interesting question in our sample of middle- to older-aged men is whether seroconversions represented first acquisition of Tv infection. Based on the approximately equal numbers of men with P/N ratios just below or above the seropositivity cut-off point who seroconverted or seroreverted, we believe that first acquisition of Tv infection is unlikely. Rather it seems more likely that antibody levels wax and wane over time, leading to apparent “seroconversions” and “reversions” for men near the seropositivity cut-off point. Why antibody levels fluctuate is unknown, but perhaps repeat infections or periodic stimulation of the immune response by a low-grade, persistent Tv infection might contribute to these fluctuations, similar to the argument made for herpesvirus antibody fluctuations (i.e., repeat infections with different strains and periodic reactivation from latency [25]). We did not collect genital specimens in the SCCS to evaluate the influence of repeat or persistent infection on antibody levels, but this possibility could be explored in future studies.
An additional question that cannot be answered with the present data is the proportion of men who seroconvert following infection and the determinants of seroconversion, as not all individuals infected by sexually transmitted agents seroconvert [11, 20, 21]. Therefore, future studies might consider following men from the time of initial Tv infection to determine the percentage of men who seroconvert and the determinants of seroconversion, as well as the percentage who remain seropositive over time and determinants of persistence. One of these determinants might be length of time between infection and blood draw, as men seropositive at times more distant from infection may be more likely to remain seropositive. Therefore, our estimate of Tv antibody persistence among middle- to older-aged men may not generalize to younger men. Other determinants of both seroconversion and antibody persistence might be the number of repeat Tv infections, the duration of infection, and the development of complications, as each of these has been found to influence seroconversion and antibody persistence for other non-lifelong STIs. Importantly, these characteristics are also believed to be relevant for carcinogenesis [2]. Finally, studies with longer time between repeat specimens are necessary to inform the long-term persistence of Tv antibodies, as our study was limited to three years.
In summary, we found high levels of Tv antibody persistence and substantial agreement in serostatus across specimens collected 1–3 years apart from middle- to older-aged men. These high levels of antibody persistence and agreement are consistent with those observed for other STIs frequently investigated in relation to PCa risk.
ACKNOWLEDGMENTS
We thank Ratna Pakpahan for assistance with Figure 1.
Financial support: This study was funded by grant R01 CA092447 from the National Cancer Institute. SS was funded by the Barnes-Jewish Hospital Foundation.
Footnotes
Potential conflicts of interest: none
REFERENCES
- 1.Holmes KK, et al. Sexually transmitted diseases. 4th edition. New York: McGraw-Hill; 2008. [Google Scholar]
- 2.Sutcliffe S. Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses. Future Oncol. 2010;6(8):1289–1311. doi: 10.2217/fon.10.95. [DOI] [PubMed] [Google Scholar]
- 3.Sutcliffe S, et al. Trichomonosis, a common curable STI, prostate carcinogenesis--a proposed molecular mechanism. PLoS Pathog. 2012;8(8):e1002801. doi: 10.1371/journal.ppat.1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twu O, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A. 2014;111(22):8179–8184. doi: 10.1073/pnas.1321884111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutcliffe S, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(5):939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- 6.Stark JR, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J Natl Cancer Inst. 2009;101(20):1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlader N, et al. SEER Cancer Statistics Review, 1975 – 2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. Apr, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 8.Sutcliffe S, et al. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int J Cancer. 2009;124(9):2082–2087. doi: 10.1002/ijc.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogne M, Brasseur P, Ballet JJ. Detection and characterization of serum antitrichomonal antibodies in urogenital trichomoniasis. J Clin Microbiol. 1985;21(4):588–592. doi: 10.1128/jcm.21.4.588-592.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibau L, et al. Enzyme-linked immunosorbent assay for the diagnosis of trichomoniasis in women. Sex Transm Dis. 1987;14(4):216–220. doi: 10.1097/00007435-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Narvanen A, et al. Detection of Antibodies to Chlamydia trachomatis With Peptide-Based Species-Specific Enzyme Immunoassay. Infect Dis Obstet Gynecol. 1997;5(5):349–354. doi: 10.1155/S1064744997000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne EF, et al. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 13.Fleming DT, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337(16):1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 14.van Aar F, et al. Chlamydia trachomatis IgG seroprevalence in the general population of the Netherlands in 1996 and in 2007: differential changes by gender and age. Sex Transm Infect. 2014;90(5):434–440. doi: 10.1136/sextrans-2013-051074. [DOI] [PubMed] [Google Scholar]
- 15.Signorello LB, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972–979. [PMC free article] [PubMed] [Google Scholar]
- 16.Neace CJ, Alderete JF. Epitopes of the highly immunogenic Trichomonas vaginalis alpha-actinin are serodiagnostic targets for both women and men. J Clin Microbiol. 2013;51(8):2483–2490. doi: 10.1128/JCM.00582-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderete JF. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alderete JF, Neace CJ. Identification, characterization, and synthesis of peptide epitopes and a recombinant six-epitope protein for Trichomonas vaginalis serodiagnosis. ImmunoTargets & Therapy. 2013;2:91–103. doi: 10.2147/ITT.S46694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horner PJ, et al. Effect of time since exposure to Chlamydia trachomatis on chlamydia antibody detection in women: a cross-sectional study. Sex Transm Infect. 2013;89(5):398–403. doi: 10.1136/sextrans-2011-050386. [DOI] [PubMed] [Google Scholar]
- 20.Wang SS, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2004;91(7):1269–1274. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clad A, et al. Detection of seroconversion and persistence of Chlamydia trachomatis antibodies in five different serological tests. Eur J Clin Microbiol Infect Dis. 2000;19(12):932–937. doi: 10.1007/s100960000397. [DOI] [PubMed] [Google Scholar]
- 22.Huang WY, et al. Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2374–2381. doi: 10.1158/1055-9965.EPI-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton M, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001 – 2004. Clin Infect Dis. 2007;45(10):1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell HD, et al. Distribution and risk factors of Trichomonas vaginalis infection in England: an epidemiological study using electronic health records from sexually transmitted infection clinics, 2009 – 2011. Epidemiol Infect. 2014;142(8):1678–1687. doi: 10.1017/S0950268813002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waner JL, Weller TH, Kevy SV. Patterns of cytomegaloviral complement-fixing antibody activity: a longitudinal study of blood donors. J Infect Dis. 1973;127(5):538–543. doi: 10.1093/infdis/127.5.538. [DOI] [PubMed] [Google Scholar]