Abstract
Aims
To clarify the role of self-monitoring of blood glucose in the self-management of Type 2 diabetes from the patient’s perspective, using in-depth interviews with non-insulin-treated adults to investigate how they learned to manage their diabetes effectively and whether self-monitoring of blood glucose played a significant role in this.
Methods
Individual interviews were conducted with 14 non-insulin-treated adults with Type 2 diabetes who had significantly improved their glycaemic control [64% women; 50% black; 21% Hispanic; mean age 60 years; mean HbA1c concentration 43 mmol/mol (6.1%)]. Interviews were transcribed and analysed by a coding team, using the concept of illness coherence from the Common Sense Model of Self-Regulation.
Results
The majority of participants relied on self-monitoring of blood glucose to know their self-management efforts were working. Key themes included: adopting an experimental approach; experiencing 'a-ha' moments; provider-assisted problem-solving; using self-monitoring of blood glucose and other feedback to evaluate when their efforts were working; and normalizing diabetes-specific behaviour changes as being healthy for everyone.
Conclusions
Our qualitative data are consistent with the argument that self-monitoring of blood glucose, if implemented appropriately with enough education and provider access, can be a powerful tool for non-insulin-treated adults with Type 2 diabetes to monitor their self-management. Establishing sufficient conditions for illness coherence to develop while individuals are learning to use self-monitoring of blood glucose could increase their sense of personal control in managing a complex and demanding illness.
Introduction
Self-monitoring blood glucose, if used appropriately, provides immediate feedback about behaviours for managing diabetes [1,2]. While it has been proven useful for individuals on insulin [3], results with self-monitoring of blood glucose (SMBG) have been inconsistent for those with non-insulin-treated Type 2 diabetes mellitus; for example, a meta-analysis showed non-clinically meaningful reductions in HbA1c concentration in six randomized trials with non-insulin-treated individuals [4]. With a lack of strong evidence that self-monitoring of blood glucose leads to clinically significant reductions in HbA1c for non-insulin users, some argue that promoting self-monitoring of blood glucose in this population is not a worthwhile use of financial resources [5]. Trials designed to evaluate self-monitoring of blood glucose efficacy as if it were a medication rather than a complex behavioural intervention, however, can be problematic [6]. A review of 11 randomized controlled trials concluded that self-monitoring of blood glucose can result in improved glycaemic control but raised questions about the precise strategies of successful users of self-monitoring of blood glucose [7]. The importance of feedback from clinicians and training in glucose meter use were identified as critical factors for glycaemic improvements in two recent self-monitoring of blood glucose trials with non-insulin-treated adults with Type 2 diabetes [8,9]. One study in non-insulin-treated individuals with poor control reported that adherence to self-monitoring of blood glucose was associated with lowered HbA1c concentration [8]. Intervention participants who received a combination of structured feedback from physicians and training to properly interpret glucose meter readings showed significantly greater improvement in glycaemic control compared with an enhanced usual care comparison group [8]. In a second trial, participants, who performed self-monitoring of blood glucose six times a day on 2 days each week, kept a glucose meter/food log and received structured counselling on diabetes management, showed improved skills in using self-monitoring of blood glucose, a greater sense of self-control, an improved life outlook and significantly lower HbA1c concentrations in comparison with a control group that received a standardized diet and lifestyle counselling [9]. In summary, when treated as a behavioural intervention, trials of self-monitoring of blood glucose have been more successful.
Type 2 diabetes is often an asymptomatic chronic condition with self-management behaviours occurring in the absence of acute symptoms. This dissociation of health behaviours from the results of those behaviours can lead to worse treatment adherence [10]. By narrowing the gap between health behaviours and feedback, ideally self-monitoring of blood glucose can assist people in answering the question, 'Is this treatment working?'. Such questions are important for the development of illness coherence, which is a construct from the Common Sense Model of Self-Regulation [11]; it stems from people's sense that their self-management strategies actually work [11,12]. Experiencing the alignment between particular behaviours and expectations about the influence on levels of blood glucose can help individuals to adopt and maintain self-management strategies that make sense and to develop a stable self-regulatory system [7]. For example, greater frequency of self-monitoring of blood glucose was associated with decreased fat intake in those who reported using self-monitoring of blood glucose feedback to adjust their diet [13]; however, little is known from a patient perspective about how self-monitoring of blood glucose facilitates perceptions of illness coherence, and whether this process can improve self-care practices.
The present qualitative study reports on in-depth interviews with non-insulin-treated people with Type 2 diabetes who had significantly improved their glycaemic control through self-management efforts; we refer to them as 'exemplary' patients. Through the theoretical lens of the Common Sense Model of Self-Regulation and illness coherence, this study aimed: 1) to understand how people with Type 2 diabetes learned to improve glycaemic control; 2) to understand how they evaluated their efforts; and 3) to explore how self-monitoring of blood glucose assisted them in their efforts.
Patients and methods
Recruitment
We recruited adults (age >18 years) with Type 2 diabetes at two sites: the Montefiore Clinical Diabetes Program and affiliated primary care clinics in Bronx County, NY, and the General Internal Medicine Program at the University of Medicine and Dentistry of New Jersey. The population of Bronx County, NY is 54% Hispanic/Latino; 43% black/African-American; and 10% non-Hispanic white; a third of residents live below the poverty level. Middlesex County in New Jersey is 20% Hispanic/Latino; 11% black/African-American; and 46% non-Hispanic white, with 8% of residents living below the poverty level [14,15]. Recruitment letters were sent to participants identified through clinician referrals and electronic medical search. Letters indicated that individuals could call to opt out of the study; participants were called if they did not opt out via a telephone call. We used critical case sampling [16] with the aim of identifying individuals who had achieved clinically significant reductions in HbA1c concentration through a combination of medication and self-management behaviours. The research team consulted with an endocrinologist and a family medicine physician specializing in diabetes to develop the inclusion criteria. Physicians advised that a decrease in HbA1c concentration of at least 1.5% would demonstrate significant improvement probably achieved by behaviour change in addition to medication; therefore, eligible participants needed three consecutive HbA1c readings of the most recent past < 53 mmol/mol (7%) and at least one previous HbA1c reading > 69 mmol/mol (8.5%) since diagnosis. Establishing criteria of having HbA1c readings < 53 mmol/mol (7%) was based on the recommended glycaemic goal for most non-pregnant adults [17]. Additionally, as we aimed to interview participants who had improved their glycaemic control and maintained it over time through sustained behaviour change, participants had to have been diagnosed with Type 2 diabetes for at least 2 years and had to have been on glucose-lowering oral medication(s) for Type 2 diabetes with a stable or reduced dosage for at least 1 year. Participants with significant cognitive impairment, legal blindness that could interfere with completing self-report measures, or a past diagnosis of gestational diabetes were ineligible; however, no contacted participants were excluded from the study as a result of those criteria. Active self-monitoring of blood glucose use was not an inclusion criterion, as our aim was to learn about how individuals improved their glycaemic control and whether or not their strategies included self-monitoring of blood glucose. The institutional review boards at the Albert Einstein College of Medicine, Rutgers, the State University of New Jersey, and the University of Medicine and Dentistry of New Jersey approved study procedures, and participants provided informed consent.
Study visit
Participants came to the Albert Einstein College of Medicine or the Institute for Health at Rutgers University to take part in a face-to-face, individual, in-depth, semi-structured interview. Interviews were conducted by experienced graduate clinical psychology students trained in qualitative research and clinical interviewing. M.T. conducted 11 interviews, J.S. and J.Y. each conducted an interview and one interview was conducted by a research coordinator with a masters degree in social work. An interview guide was used to promote consistency across interviews and between interviewers. Interviews used a 'bottom-up' approach [18] of starting with a general, open-ended question: 'Tell me about your experience getting diagnosed with diabetes.' This question enabled participants to provide their own narrative about learning that they had Type 2 diabetes, their initial reactions to the diagnosis and the first steps they took. Additional questions provided prompts to guide participants to reflect back and recount how they learned to manage diabetes, what feedback and strategies they found most helpful and how they learned to use self-monitoring of blood glucose. Table 1 shows the overarching interview questions. The interview guide included additional sub-questions and prompts if participants did not spontaneously bring up certain topic areas. Interviews lasted between 1 and 2 h, and were audio-recorded and transcribed. Demographic information was collected via self-report and participants were compensated with $30. Recruitment stopped when no new themes arose and theoretical saturation was reached; this occurred with the 13th and 14th participants.
Table 1.
Primary interview questions
|
Data analysis
Qualitative data were analysed according to Auerbach and Silverstein's grounded theory approach [18]. In the first phase, a team of five coders (four clinical psychology graduate students with advanced training in qualitative methods and one clinical psychology professor with expertise in grounded theory) independently read four transcripts and highlighted relevant text. Concepts mentioned by at least two participants were grouped into repeating ideas, and clustered into themes to create an initial list of codes. In the second phase, two researchers (M.T. and J.Y. or J.Y.B.) used this initial list to code each transcript and added new codes as needed. Coders met weekly to review each interview, and to discuss and resolve discrepancies between coders. Earlier transcripts were then reviewed by M.T. to determine if later codes were present in them. Codes were compiled by themes and reviewed by M.T., J.Y., J.Y.B. and J.S. to ensure that quotations belonged under the same code and to ensure accuracy of the descriptions of each code. To support reflexivity [19], the multidisciplinary research team (clinical health psychologists, a social psychologist and a researcher who was also a diabetes nurse specialist and certified diabetes educator) acknowledged its predominately behavioural medicine focus and consulted with endocrinologists when establishing inclusion criteria, collecting data and interpreting results. The present study inherently emphasizes the role of patient behaviour in diabetes management; however, it is important to note that many factors are outside patients' control, including: complexity of medication regimens, provider expertise, limited patient visit times, limited use of self-monitoring of blood glucose data by physicians, and failure to intensify treatment when needed [20]. An audit trail was kept throughout the coding process to document decision-making across different coding stages, and to support dependability of the data [21].
Results
At the time of the interview, all 14 participants (Table 2) were currently using glucose meters, with testing frequencies ranging from once a week to several times a day. Key themes are presented in chronological order: from the experience of diagnosis to the development of a self-regulatory system for managing diabetes.
Table 2.
Participant characteristics (n = 14)
| Characteristic | |
|---|---|
| Sex: female, n (%) | 9 (64.3) |
| Race, n (%) | |
| White | 4 (28.6) |
| Black | 7 (50.0) |
| Ethnicity, n (%) | |
| Hispanic | 3 (21.4) |
| Non-Hispanic | 11 (78.6) |
| Age, years | |
| Mean (SD) | 60.3 (8.9) |
| Range | 44–71 |
| HbA1c | |
| Mean (SD) | |
| mmol/mol | 43 (5.5) |
| % | 6.1 (0.5) |
| Range | |
| mmol/mol | 34–50 |
| % | 5.3–6.7 |
| Years since diagnosis | |
| Mean (SD) | 10.57 (11.9) |
| Range | 2–42 |
| Education level, n (%) | |
| GED/High-School diploma | 8 (57.1) |
| 2-year college | 1 (7.1) |
| University degree | 3 (21.4) |
| MA degree | 2 (14.3) |
| Employment Status, n (%) | |
| Not working/retired | 6 (42.9) |
| Disabled/on disability | 4 (28.6) |
| Working part-time | 1 (7.1) |
| Working full-time | 3 (21.4) |
Committing to change, but not expecting immediate results
Nearly all participants described a range of negative emotions after diagnosis, and many expressed fears about severe complications; however, after this initial adjustment, one strikingly common feature involved descriptions of making a clear decision to prioritize health and initiate behaviour change for diabetes management. As one participant stated, 'You just cannot be a bystander in this disease'. For others, fear of future complications served as a strong motivator for committing to change, and they asked themselves questions such as: Do you want to live or do you want to die? And if you want to live, do you want to live on one leg or one arm? Do you want to live all together like you are now, or do you want to live in pieces?
Despite their recollection of a clear commitment to change, many participants described a gradual approach to behaviour changes. Rather than expecting overnight transformation, they took 'one day at a time' (e.g. allowing themselves time to adjust to new exercise routines; adopting new dietary patterns, little by little). Setting a realistic time frame for observing change was critical for developing and translating response efficacy into self-efficacy.
Experimenting with health behaviour change
Participants experimented with several new health behaviours simultaneously, learning to test their blood glucose, while trying exercises, attending classes or support groups, meeting with a nutritionist, and conducting research on the Internet. All participants but one mentioned receiving diabetes education. Most began exercising (e.g. walking, joining a gym) and others incorporated physical activity into workdays. Participants started improving their diets, eliminating 'sugary stuff', decreasing portion sizes, eating baked instead of fried foods, and making other dietary changes. Participants also began to use self-monitoring of blood glucose to evaluate the efficacy of multiple, specific behavioural changes.
'I tested a lot at first because I was trying to test out my food': an experimental approach to starting out with self-monitoring of blood glucose
When participants initiated self-monitoring of blood glucose, they approached this new task with an attitude of curiosity. Five participants started by checking their blood glucose more often than their doctors recommended, while others followed doctors' instructions to check at certain times of the day in relation to meals. Participants expressed a desire to check frequently in order to understand the numbers and to look for patterns. '[I] started using the [blood glucose meter] a lot…before I ate, after I ate, at night-time, in the morning, just to get familiar with what the numbers for myself look like.'
Some conducted specific experiments, testing before and after eating certain foods to get feedback about how those foods affected their blood glucose levels. For example, one participant described how she tested the effects of a particular cereal: 'Take for instance with the Raisin Bran [breakfast cereal], I have a small portion of that and then I wait a couple of hours and check my blood sugar.'
In addition, six participants kept logs of their glucose meter readings throughout this process, either with books provided by their doctors' office, taking their own notes, or using an iPad app. Participants did not mention clinicians using their meters to graph out self-monitoring of blood glucose results nor using their meters' memory to review their self-monitoring of blood glucose history.
'If it was that high, I'd have no qualms about calling the doctor': clinician-assisted problem-solving to respond to out-of-range numbers
Beyond adopting a curious, experimental approach to self-monitoring of blood glucose, participants became active problem-solvers when confronted with out-of-range numbers. One participant stated, 'If I got a high reading…I'd think back and say "Maybe I shouldn't have had that"...A lot of that was trial and error.' Some participants described waiting a short amount of time and re-testing, and some sought expert advice if they could not problem-solve on their own. One participant described an instance of a high reading in which, after waiting 2 h and rechecking, she decided to ask for help. 'I called my coach at the clinic,' she said, 'and we started talking and it clicked…'. She realized the high number was the result of meter error and was able to address it. With pro-active problem-solving and a willingness to consult providers when needed, participants continued learning how foods affected glucose levels and improved their ability to approach confusing or out-of-range readings.
How to tell things are working: sources of feedback
Participants discussed a variety of feedback sources they used to know whether their self-management strategies were working: the body (e.g. physical symptoms, sense of health/vigour/strength), self-monitoring of blood glucose, HbA1c level, and feedback from healthcare providers and other people. Eleven participants said they relied on self-monitoring of blood glucose to know their efforts were working. One participant stated: 'I just use the reading. The reading every day'. The same proportion of participants also used a range of bodily symptoms and feelings to evaluate self-management strategies. For example, one participant said, 'I haven't had not one cold…I'm in good shape…I can walk up steps now just like that'. Nine participants used doctor visits and HbA1c. As one participant noted, 'I know every 3 months I'm going to get the HbA1c reading, so that's how I know it's working'. Six participants also used their mood (e.g. 'I'm less depressed, I'm happier') as an indicator of their overall progress with diabetes management. Finally, 10 participants noted the objective feedback of losing substantial weight as tangible evidence of their efforts paying off. Identifying meaningful signals and minimizing noise strengthened response and self-efficacy and sustained self-management.
From novel to routine: turning self-monitoring of blood glucose into habit
Participants used several strategies for incorporating self-monitoring of blood glucose into their daily lives, included keeping a glucose meter in a specific place (e.g. bedside, kitchen counter); owning several meters in different locations (e.g. car, purse, home, work); pairing self-monitoring of blood glucose with taking medications or blood pressure in the morning; and pairing their self-management routine with their spouse's. Eventually, the act of testing became second nature for several, 'like tying my shoes,' involving little forethought. One participant described her morning routine: 'Wash my face, brush my teeth, test my blood'.
'Ah, so this is the trick': using self-monitoring of blood glucose to facilitate coherence
Eight participants recounted a clear moment when they first realized their actions were working. Here are two examples of moments they described:
I was eating Honey Nut Cheerios [breakfast cereal]…maybe two cups…I thought it was okay…so when I didn’t eat Cheerios a couple of nights after that, my sugar started going down and down and down and I was getting proper readings. And then I would try again and eat another bowl of cereal and it would be real high. And I said, 'Ah, I got it.' And then I bring it to my nutritionist who said, ‘Try half a cup of Cheerios'.
[My number] was up at 395…I said, 'It’s a nice night, let me walk and let’s see what happens.' And I walked…and by the time I got home it was down to 180…I said, 'Ah, so this is the trick'.
Several participants who recalled an initial experience of coherence, or an awareness that their efforts were working, said that those experiences were facilitated by self-monitoring of blood glucose. By seeking well-timed feedback from their glucose meters, participants could then connect the readings to recent behaviours. In these instances, self-monitoring of blood glucose was instrumental in fostering a sense of understanding that efforts (including medications) were working. In other cases, this realization resulted from more distal feedback, including HbA1c results or weight loss (e.g. clothes fitting differently). Behaviours that were appropriately timed met expectations, created coherence and strengthened response- and self-efficacy.
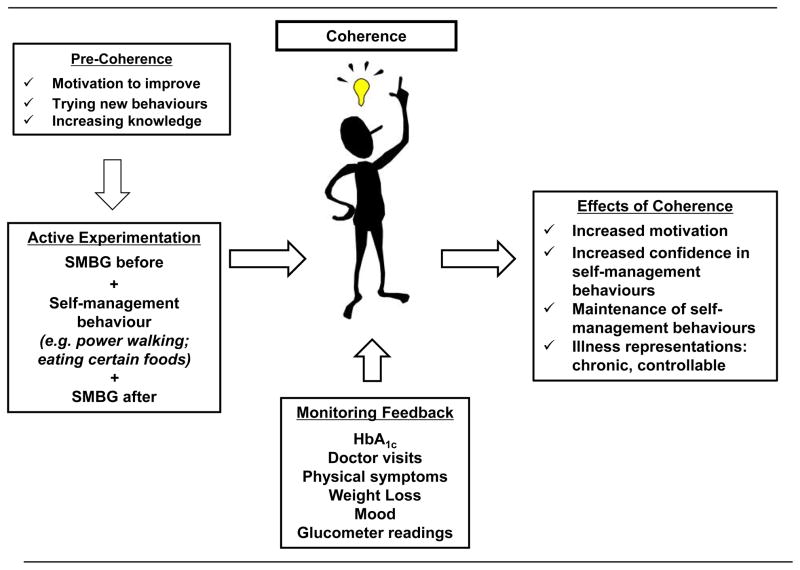
'I feel like Superman': the motivating effect of achieving coherence
Figure 1 shows a hypothesized process by which self-monitoring of blood glucose led to key self-management discoveries, and what resulted from that experience. The motivational result of experiencing confirmatory feedback for their newly adopted behaviours was a key to continuing the effort; effort was minimized when routines became automatic. Participants who saw the fruits of their labour described this experience as providing additional fuel for their focus on diabetes self-management: 'I was pleased once I got the results. I knew I was doing something right…Once I found out what was doing that worked, I stuck with it. You feel like you want to take wings and fly. You feel like, "Now I’ve arrived".'
FIGURE 1.
Development and impact of illness coherence in Type 2 diabetes. SMBG, self-monitoring of blood glucose.
By observing that self-management efforts had an effect, many participants began to view diabetes as controllable, something they could manage through their own actions. This shift in their appraisal of diabetes helped to decrease their fears about future complications of diabetes. One participant described the change in this way: 'You just feel like you just done it all, you got it straight and it’s wonderful, as opposed to the feeling of being out of control and being so terrified that I’m going to wake up and they’re going to be cutting my toes off.'
Several participants also began normalizing the lifestyle changes they had made as healthy for everyone, not just those living with diabetes: 'I’m determined that this is a way of living, eating for the rest of my life. Not just for the diabetic, but a diabetic way of eating is healthy for everybody. I’m not on a diet.'
Some participants described being diagnosed with diabetes as a positive event in their lives in that it made them focus on their health. One participant stated: 'Maybe it’s good that I was diabetic because it makes me go to the gym, it makes me exercise and eat the right things…Just because you’re not diabetic doesn’t mean that you don’t get clogged arteries and so forth. So maybe a diabetic diagnosis is good.'
Finally, participants continued to stress the effort needed to focus on their health. They saw themselves as 'works in progress', and many described new goals, including additional weight loss and medication reduction. Some participants even became promoters of their newly adopted behaviours, sharing knowledge, resources and expertise with others. In summary, self-management was internalized, becoming a facet of self-identity.
Discussion
The majority of participants reported that self-monitoring of blood glucose provided important feedback on their diabetes management progress. It was a key ingredient for reaching coherence after adopting specific health behaviours that met expectations: participants who described achieving coherence using self-monitoring of blood glucose feedback also reported learning about the effects of a particular health behaviour on their blood glucose level. A striking shared feature of many participants' experiences was an 'a-ha' moment—facilitated by self-monitoring of blood glucose—when first realizing a self-management strategy worked. Experiencing coherence between diabetes self-management actions and corresponding results enhanced motivation, a sense of personal control and the development of self-efficacy for maintaining newly adopted health behaviours.
Self-monitoring of blood glucose was neither the only nor the primary source of feedback for participants. HbA1c values, weight loss, provider interactions and the more subjective senses of body and mood were additional key sources of feedback to assist participants in monitoring their progress. Although less useful than self-monitoring of blood glucose for defining the efficacy of specific behaviours in the moment, feedback from experiential sources such as subjective mood helped sustain ongoing motivation for diabetes self-management. The formation of coherence between illness and treatment representations derived from relatively rapid feedback (e.g. self-monitoring of blood glucose and experienced mood), and from temporally remote feedback (e.g. HbA1c value), appears to depend on an array of critical contextual factors, some at an individual level (e.g. adopting a curious, experimental attitude and using a problem-solving approach), and others social in nature (e.g. willingness and availability of a collaborative provider). The benefit of collaboration with providers is consistent with a recent finding that more adherent individuals are more likely to report that providers helped them problem-solve by providing clear instructions and helping set realistic expectations [22].
Given the appropriate personal and social contexts, coherence between actions and feedback leads to the integration of health behaviours such as self-monitoring of blood glucose into automatic routines of everyday life. Our findings related to the importance of habit formation for sustaining self-monitoring of blood glucose mirror results from previous studies in diabetes and asthma [23,24]. Incorporating health behaviours into daily routine (e.g. paired with brushing teeth) was associated with improved metformin adherence for participants in the Diabetes Prevention Program [23]. In addition, a recent study of older adults with asthma found that strategies that promote routine were linked to greater medication adherence [24].
Expertise in diabetes management develops over time as individuals accumulate concrete experiences and form abstract, conceptual templates for approaching future novel situations. Self-monitoring of blood glucose is a tool that provides concrete feedback that serves as a valid alternative to physical symptoms. Using self-monitoring of blood glucose effectively requires being able to bridge the gap between actions in the present and delayed feedback (e.g. linking current behaviours such as diet, exercise and medication-taking to future glucose meter readings or more distal HbA1c results). No single path or action led participants to develop a greater sense of perceived control over their illness nor to an enhanced sense of self-efficacy for being able to problem-solve confusing situations. Rather, participants tested many new behaviours, in conjunction with self-monitoring of blood glucose, and actively sought out connections between action and feedback to bridge that crucial gap.
Our findings are from a deliberately selected set of exemplary self-managers, i.e. expert managers, and do not necessarily reflect the actions of the average individual with diabetes. Our goal was to understand how expertise in diabetes self-management is developed, from the perspective of these exemplary self-managers. Our findings parallel three key requirements that previous research has highlighted as important for developing expertise in any field [25]. Experts 1) engage in 'deliberate practice' to develop and fine-tune a new skill, and intentionally create novel situations in order to learn; 2) rely on immediate feedback, particularly during early training phases; and 3) constantly work toward improvement and building on their progress. Participants deliberately practised self-monitoring of blood glucose alongside other health behaviours to provide immediate feedback to evaluate progress. Despite the progress already made, participants continued to set future health-related goals, including reducing their medication burden and losing additional weight. As a result of this learning process, participants developed a stronger sense of self-efficacy and perceived control of diabetes management, as well as a decrease in emotional distress after the initial negative emotions that accompanied learning of their diagnosis. Self-efficacy has been linked to improved Type 2 diabetes self-management and glycaemic control [26], as has perceived control, or one's perceived ability to achieve a desired outcome [27,28]. Furthermore, perceived control was found to mediate the relationship between diabetes-related emotional distress and medication adherence in Type 2 diabetes [29]. This finding points to the potential importance of developing and sustaining a sense of controllability of one's illness through one's own actions to improve treatment outcomes as was observed in our exemplary participants.
The limitations of the present study should be acknowledged. Interviews were retrospective accounts from participants who were effectively managing their diabetes at the time of the interview, so we cannot assume causality between coherence and improved glycaemic control and could not capture the daily struggles these participants may have experienced while learning to manage diabetes. An interview guide helped promote consistency between interviews and transcripts of all interviews were reviewed by the first author to confirm that protocol was followed; however, the use of multiple interviewers may have led to some differences across interviews. In addition, participants knew they were being interviewed because of their success with self-management, and several knew they had been referred by their provider. The framing of the interview may have motivated participants to focus more on the positive aspects of their experience with diabetes rather than focusing on what did not work, which may have somewhat limited our results. As noted earlier, factors such as disease progression, medication effectiveness, and provider involvement play important roles in 'success' beyond patient behaviour alone, and the term 'expert' in reference to diabetes management places emphasis on behaviour over these other factors. One participant made significant initial gains in managing diabetes, then began to regain weight gain, stopped going to the gym, and began eating foods he had previously eliminated when challenged with the stressor of caring for an ill family member. This example highlights that many patients may have fluctuations in motivation, stress and other psychosocial factors that influence diabetes management and long-term maintenance of behaviour change.
Although previous studies have examined differences in self-management strategies between people with well- and poorly controlled Type 2 diabetes [30], this is the first in-depth qualitative study, to our knowledge, that explores how people with well-controlled Type 2 diabetes successfully learned to manage their illness. Our qualitative data support the argument that, if implemented appropriately with enough education and provider access, self-monitoring of blood glucose can be a powerful tool for providing in-the-moment feedback that can help individuals realize their efforts make tangible differences [6]. Ecological momentary assessment studies following newly diagnosed individuals as they learn to practise self-monitoring of blood glucose could shed light on the learning process as a useful future direction. Furthermore, interviewing people with less success in their metabolic control could provide helpful information as to potential differences in how they incorporate self-monitoring of blood glucose into their overall diabetes self-care. Our findings also have implications for how clinicians work with people to introduce self-monitoring of blood glucose in a way that best facilitates coherence development, e.g. focusing on using self-monitoring of blood glucose feedback to promote coherence for specific health behaviours.
What's new?
This study explored the role of self-monitoring of blood glucose, among other relevant diabetes feedback sources, from a patient perspective, given the lack of consensus on the benefit of self-monitoring of blood glucose for non-insulin-treated adults with Type 2 diabetes mellitus.
We interviewed patients who had successfully improved their glycaemic control through a combination of self-management and oral diabetes medication.
Self-monitoring of blood glucose facilitated 'a-ha' moments at critical points when participants were initiating behaviour change and learning to manage their illness. 'A-ha' moments were motivational turning points for these successful participants.
Acknowledgments
Funding sources: This study was partially supported by a pilot and feasibility grant from the Einstein Diabetes Research Center. J.S.G. and E.A.W. are supported by P60 DK020541; J.S.G.'s efforts were additionally supported by grants and R18 DK098742 from the National Institutes of Health. J.Y.B. is supported by the VA Office of Academic Affiliations and VA Health Services Research and Development Service in conjunction with a VA HSR&D Advanced Fellowship Program.
We are grateful to Louise B. Silverstein, PhD for her valuable qualitative data analysis guidance. We thank L. Alison Phillips, PhD, of Iowa State University, for her feedback on the manuscript and Kimberly A. Convery, MSW, for her assistance with data collection.
Footnotes
Competing interests: None declared.
References
- 1.Hirsch IB, Bode BW, Childs BP, Close KL, Fisher WA, Gavin JR, et al. Self-monitoring of blood glucose (SMBG) in insulin- and non-insulin-using adults with diabetes: consensus recommendations for improving smbg accuracy, utilization, and research. Diabetes Technol Ther. 2008;10:419–439. doi: 10.1089/dia.2008.0104. [DOI] [PubMed] [Google Scholar]
- 2.Sarol JN, Nicodemus NA, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966–2004) Curr Med Res Opin. 2005;21:173–183. doi: 10.1185/030079904X20286. [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, Ackerson LM, Darbinian JA, D’Agostino RB, Jr, Ferrara A, Liu J, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 4.Farmer AJ, Perera R, Ward A, Heneghan C, Oke J, Barnett AH, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ. 2012;344:e486–e486. doi: 10.1136/bmj.e486. [DOI] [PubMed] [Google Scholar]
- 5.Malanda UL, Bot SD, Nijpels G. Self-Monitoring of Blood Glucose in Noninsulin-Using Type 2 Diabetic Patients It is time to face the evidence. Diabetes Care. 2013;36:176–178. doi: 10.2337/dc12-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polonsky WH, Fisher L. Self-Monitoring of Blood Glucose in Noninsulin-Using Type 2 Diabetic Patients Right answer, but wrong question: self-monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care. 2013;36:179–182. doi: 10.2337/dc12-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breland JY, McAndrew LM, Burns E, Leventhal EA, Leventhal H. Using the Common Sense Model of Self-Regulation to Review the Effects of Self-Monitoring of Blood Glucose on Glycemic Control for Non–Insulin-Treated Adults With Type 2 Diabetes. Diabetes Educ. 2013;39:541–559. doi: 10.1177/0145721713490079. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1c levels in poorly controlled, noninsulin-treated type 2 diabetes. Results from the Structured Testing Program study. Diabetes Care. 2011;34:262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siebolds M, Gaedeke O, Schwedes U. Self-monitoring of blood glucose–psychological aspects relevant to changes in HbA1c in type 2 diabetic patients treated with diet or diet plus oral antidiabetic medication. Patient Educ Couns. 2006;62:104–110. doi: 10.1016/j.pec.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz CR, Rein SB, Leventhal H. A story of maladies, misconceptions and mishaps: effective management of heart failure. Soc Sci Med. 2004;58:631–643. doi: 10.1016/s0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res. 1992;16:143–163. [Google Scholar]
- 12.Phillips LA, Leventhal H, Leventhal EA. Assessing theoretical predictors of long-term medication adherence: Patients’ treatment-related beliefs, experiential feedback and habit development. Psychol Health. 2013;28:1–17. doi: 10.1080/08870446.2013.793798. [DOI] [PubMed] [Google Scholar]
- 13.McAndrew LM, Horowitz CR, Lancaster KJ, Quigley KS, Pogach LM, Mora PA, et al. Association between self-monitoring of blood glucose and diet among minority patients with diabetes. J Diabetes. 2011;3:147–152. doi: 10.1111/j.1753-0407.2011.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau. Middlesex County, New Jersey. State Cty. [Last accessed 22 November 2014];QuickFacts. 2014 Available at http://quickfacts.census.gov/qfd/states/34/34023.html.
- 15.U.S. Census Bureau. Bronx County, New York. State Cty. [Last accessed 22 November 2014];QuickFacts. 2014 Available at: http://quickfacts.census.gov/qfd/states/36/36005.html.
- 16.Patton MQ. Qualitative Evaluation and Research Methods. 2. Newbury Park: Sage; 1990. [Google Scholar]
- 17.American Diabetes Association. Standards of Medical Care in Diabetes–2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach CF, Silverstein LB. Qualitative data: an introduction to coding and analysis. New York, NY: New York University Press; 2003. [Google Scholar]
- 19.Malterud K. Qualitative research: standards, challenges, and guidelines. The Lancet. 2001;358:483–488. doi: 10.1016/S0140-6736(01)05627-6. [DOI] [PubMed] [Google Scholar]
- 20.Vigersky RA. An Overview of Management Issues in Adult Patients with Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2011;5:245–250. doi: 10.1177/193229681100500207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CK, Gregory DM. Evaluation of qualitative research studies. Evid Based Nurs. 2003;6:36–40. doi: 10.1136/ebn.6.2.36. [DOI] [PubMed] [Google Scholar]
- 22.Phillips LA, Leventhal H, Leventhal EA. Physicians’ communication of the common-sense self-regulation model results in greater reported adherence than physicians’ use of interpersonal skills. Br J Health Psychol. 2012;17:244–257. doi: 10.1111/j.2044-8287.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 23.Walker EA, Molitch M, Kramer MK, Kahn S, Ma Y, Edelstein S, et al. Adherence to Preventive Medications Predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29:1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks TL, Leventhal H, Wolf MS, O’Conor R, Morillo J, Martynenko M, et al. Strategies Used by Older Adults with Asthma for Adherence to Inhaled Corticosteroids. J Gen Intern Med. 2014;29:1506–1512. doi: 10.1007/s11606-014-2940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med J Assoc Am Med Coll. 2004;79:S70. doi: 10.1097/00001888-200410001-00022. [DOI] [PubMed] [Google Scholar]
- 26.Sousa VD, Zauszniewski JA, Musil CM, Price Lea PJ, Davis SA. Relationships among self-care agency, self-efficacy, self-care, and glycemic control. Res Theory Nurs Pract. 2005;19:217–230. doi: 10.1891/rtnp.2005.19.3.217. [DOI] [PubMed] [Google Scholar]
- 27.McSharry J, Moss-Morris R, Kendrick T. Illness perceptions and glycaemic control in diabetes: a systematic review with meta-analysis. Diabet Med. 2011;28:1300–1310. doi: 10.1111/j.1464-5491.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- 28.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther. 2008;10:213–219. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez JS, Shreck E, Psaros C, Safren SA. Distress and Diabetes Treatment Adherence: A Mediating Role for Perceived Control. Health Psychol. 2014 doi: 10.1037/hea0000131. http://dx.doi.org/10.1037/hea0000131. [DOI] [PMC free article] [PubMed]
- 30.De Alba Garcia JG, Rocha ALS, Lopez I, Baer RD, Dressler W, Weller SC. ‘Diabetes is my companion’: Lifestyle and self-management among good and poor control Mexican diabetic patients. Soc Sci Med. 2007;64:2223–2235. doi: 10.1016/j.socscimed.2007.02.001. [DOI] [PubMed] [Google Scholar]