Abstract
A consequence of normal aging is a greater susceptibility to memory impairments following an immune challenge such as infection, surgery, or traumatic brain injury. The neuroinflammatory response, produced by these challenges results in increased and prolonged production of pro-inflammatory cytokines in the otherwise healthy aged brain. Here we discuss the mechanisms by which long-lasting elevations in pro-inflammatory cytokines in the hippocampus produce memory impairments. Sensitized microglia are a primary source of this exaggerated neuroinflammatory response and appear to be a hallmark of the normal aging brain. We review the current understanding of the causes and effects of normal aging-induced microglial sensitization, including dysregulations of the neuroendocrine system, potentiation of neuroinflammatory responses following an immune challenge, and the impairment of memories. We end with a discussion of therapeutic approaches to prevent these deleterious effects.
Keywords: normal aging, neuroinflammation, memory impairments, microglial priming, danger signals, neuroendocrine dysregulation
INTRODUCTION
It has long been noted in the clinical literature that otherwise healthy aging individuals often suffer precipitous declines in cognitive abilities, including long-term memory function, following an inflammatory challenge such as an infection (Anttila, 1992; Craft et al., 2012), a surgery (Bedford, 1955; Ramaiah and Lam, 2009), or a head injury (McAllister, 1992; Tokutomi et al., 2008; Senathi-Raja et al., 2010). Importantly, these mild cognitive impairments have been shown to increase susceptibility to the development of dementia later in life (Alzheimer's Association, 2014). Notably, advanced age is the highest risk factor for suffering these mild cognitive impairments, as well as for developing dementia (Moller et al., 1998; Alzheimer's Association, 2014). With the first of the baby boomer generation now turning 65, by the year 2030, approximately 23% of the U.S. population will be over 65 (Wimo et al., 2013; Alzheimer's Association, 2014), a demographic phenomenon that has earned the term “silver tsunami” to characterize its magnitude. Because our unique memories are what make us individuals and give our lives meaning, insults that threaten to destroy already stored memories or disrupt our ability to form new memories can have devastating consequences. Caring for people with dementia in the U.S. has been projected to cost $20 trillion over the next 40 years (Alzheimer's Association, 2014). Thus, in addition to the tremendous human suffering, the economic strain on the health care system and the federal budget is enormous. Therefore, scientific advances in the area of aging-related cognitive declines are of great and immediate importance. In this article, we will review the current literature related to hippocampal-dependent memory in normal aging and how it is negatively impacted by an inflammatory challenge. An important issue that merits attention here is the distinction between “normal” brain aging and “pathological” brain aging. Our work, as well as the preponderance of studies reviewed here, focuses on studying normal aging in which obvious neurodegeneration and senescence is not a prominent feature. Instead, older animals exhibit primed neuroinflammatory responses that require a secondary challenge for overt neuroinflammation or memory impairments to occur. A considerable amount of literature has also studied senescent animals, which exhibit basal behavioral and brain cytokine profiles dramatically different from those of younger animals, and whose brains are generally classified under the heading of “neurode-generation” (Cacabelos et al., 1994; Luterman et al., 2000; Remarque et al., 2001). Neurodegeneration and the memory changes that depend on neurodegeneration are outside the scope of this review.
COMMUNICATION BETWEEN THE PERIPHERAL IMMUNE SYSTEM AND THE BRAIN
Before considering the literature concerning immune challenge-induced hippocampal memory impairments, it is critical to understand that there is extensive bi-directional communication between the immune system and the central nervous system. Infection or injury initiates a peripheral acute phase response that involves a cascade of local and systemic events. Interleukin-1 beta (IL-1β), a pro-inflammatory cytokine, is a principal player in this cascade. As part of its role in this response, IL-1β enhances T and B lymphocyte proliferation and stimulates natural killer cell cytocidal activity to eliminate the injured cells or invading pathogen. IL-1β also induces the production of other cytokines from many cell types such as tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), which in turn have secondary effects on other cells. Blood-borne and neural routes of communication between the peripheral and central nervous systems have been well defined over the last two decades (Dinarello et al., 1988; Ericsson et al., 1994; Goehler et al., 1997; Gaykema et al., 1998; Banks et al., 1999; Maier, 2003; Bachstetter et al., 2011). As a result of this communication, neural activity is altered quite dramatically during and following a peripheral immune challenge (Maier and Watkins, 1998; Maier, 2003), leading to de novo cytokine production within the brain parenchyma, with this response being driven primarily by activated microglial cells (Van Dam et al., 1995; Laye et al., 1996; Nguyen et al., 1998; Turrin et al., 2001). Conversely, acute and chronic brain injury induces significant cytokine and chemokine expression in the liver causing leukocyte recruitment, liver damage, as well as sickness and depressive-like behaviors (Campbell et al., 2003, 2005, 2007a), and blocking cytokine production in the periphery, modulates the central inflammatory response, and prevents some of the behavioral alterations (Campbell et al., 2007b; Jiang et al., 2008). As we will discuss in more detail in the sections that follow, microglial cells in the normal aging hippocampus become sensitized, or primed, such that in response to a peripheral challenge or insult, the inflammatory response in the hippocampus of aged rats is exaggerated and prolonged compared to that in younger adult rats (Godbout et al., 2005; Barrientos et al., 2009a; Frank et al., 2010b).
MICROGLIAL PHENOTYPE IN NORMAL AGING: A SHIFT TOWARD AN IMMUNOLOGICALLY PRIMED STATE
Microglia, as part of the myelomonocytic lineage, constitute the predominant innate immune cell in the brain and serve many functions including immunosurveillance of the brain microenvironment for pathogen invasion, danger signals, cellular debris, apoptotic cells, and alterations in neuronal phenotype (Kreutzberg, 1996). In the young adult brain, normally quiescent microglia become activated in response to a threat (Colton, 2009). Activated microglia undergo morphological changes, proliferate, and produce pro-inflammatory cytokines (Kreutzberg, 1996). Once the threat is resolved, alternatively activated microglia then produce anti-inflammatory cytokines in addition to other signaling molecules that facilitate a return to homeostasis (Colton, 2009). For example, alternatively activated micro-glial cells secrete growth factors such as brain-derived neurotrophic factor (BDNF), which promotes the growth, survival, and differentiation of neurons (Nakajima et al., 2002; Wine et al., 2009). In contrast, with normal aging, the immunophenotype of microglia is characterized by up-regulation of glial activation markers including major histocompatibility complex II (MHC II) and complement receptor 3 (CD11b), a finding that has been reported in several species including human post-mortem tissue, rodent, canine, and non-human primates (Rogers et al., 1988; Perry et al., 1993; Tafti et al., 1996; Rozovsky et al., 1998; Sheffield and Berman, 1998). This up-regulation of MHCII occurs also at the mRNA level (Frank et al., 2006). Importantly, MHCII is expressed at very low levels on microglia of younger animals under basal conditions (Perry, 1998), providing a clear baseline to detect aging-related changes in microglia immunophenotype.
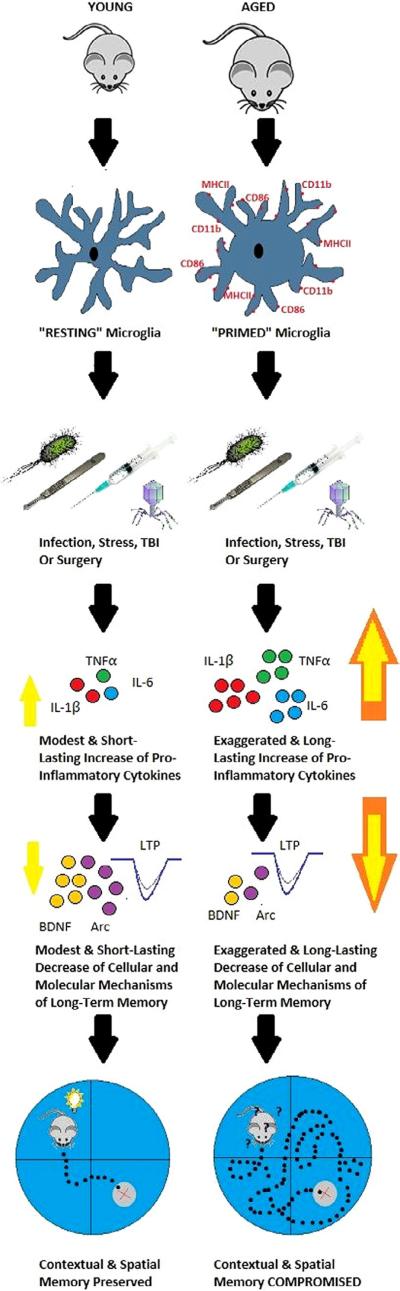
Increased MHCII could result from aging-induced increases in microglia number, or from increases in per microglial cell expression. Although there are not a large number of studies, they favor the idea that there is increased per microglial cell expression, and therefore sensitization. A stereological assessment of microglia numbers in hippocampal sub-regions indicated that microglia numbers appear to remain stable across the life span (Long et al., 1998). Moreover, flow cytometric analysis of microglia isolated from young and aged mice conclusively showed that microglia MHCII expression increased on a per cell basis in aged animals (Henry et al., 2009). Further, we have rapidly isolated microglia from the hippocampus in young and aged rats, and MHCII, CD11b, and Iba-1 gene expression were all strongly up-regulated in aged animals, while controlling for microglia cell number (Frank et al., 2006). This pheno-type represents a progressive shift in the state of micro-glia from quiescent to primed. As we will discuss in more detail in later sections, in the primed state, microglia are skewed toward exaggerated pro-inflammatory immune responses to subsequent challenges, but typically do not exhibit exaggerated responses in the absence of such challenges (Barrientos et al., 2009a, 2010, 2012, 2015b; Frank et al., 2010b), and these exaggerated proinflammatory responses correlate with long-lasting memory deficits (Barrientos et al., 2006, 2009a, 2011, 2012; Abraham et al., 2008; Chen et al., 2008; Abraham and Johnson, 2009b) (See Fig. 1).
Fig. 1.
Schematic depiction of the current state of the literature. Upon being challenged with a bacterial or viral infection, a surgery, or a head injury, once quiescent microglia of the young adult animal are rapidly and transiently activated. Pro-inflammatory cytokines are released resulting in only modest elevations above basal levels, and lasting no longer than 24 h. Synaptic facilitation (LTP) and molecular mediators of long-term memory such as BDNF and Arc are also modestly decreased resulting in mild to negligible memory impairments. In contrast, aged microglia exhibit immunological and morphological markers of activation (i.e., MHCII, CD11b, CD86), rendering them primed for a subsequent challenge. Upon a challenge, these microglia produce a potentiated neuroinflammatory response. Pro-inflammatory cytokine release is exaggerated and prolonged, lasting at least 8 days. Synaptic facilitation (LTP) and molecular mediators of long-term memory are profoundly reduced, and long-term contextual, and spatial memory is significantly impaired.
AGING-RELATED POTENTIATION OF THE NEUROINFLAMMATORY RESPONSE TO CHALLENGE
Significantly elevated levels of pro-inflammatory cytokines such as IL-1β, in key brain regions responsible for mediating memory such as the hippocampus, have been shown to impair memory in young adult rats (Gibertini et al., 1995; Hauss-Wegrzyniak et al., 1998,1999, 2002; Pugh et al., 1998, 1999; Akana et al., 1999; Barrientos et al., 2002a, 2003, 2004; Hein et al., 2010; Jurgens et al., 2012). Thus, an obvious question is whether the neuroinflammatory response to an immune challenge is sensitized in normal aging. Though healthy aged animals exhibit a marked shift in microglia phenotype, it cannot be assumed that this shift results in a potentiated neuroinflammatory response to an immune challenge. To explore this issue, our laboratory, along with several others, have compared the neuroinflammatory response of aged and young animals following a variety of insults and immune challenges. Peripheral infection with bacteria or viruses (Godbout et al., 2005, 2008; Sparkman et al., 2005; Barrientos et al., 2006, 2009a, 2011, 2015a; Abraham et al., 2008), a stressor (Buchanan et al., 2008; Sparkman and Johnson, 2008), surgical intervention (Rosczyk et al., 2008; Cao et al., 2010; Barrientos et al., 2012), or a traumatic brain injury (Sandhir et al., 2008; Kumar et al., 2013) all produced a clear and exaggerated neuroinflammatory response in aged rats compared to their young adult counterparts. Our laboratory, for example, showed that adult and aged F344xBN rats displayed dramatically different cytokine responses to a live, replicating peripheral Escherichia coli (E. coli) infection. IL-1β protein was measured 2, 4, 24 h, 4, 8, and 14 days following infection. Both adult and aged rats had elevated levels of IL-1β in the hippocampus at 2 h post-E. coli, with IL-1β in adult rats returning to vehicle control levels by 24 h. However, aged rats showed sustained elevations through day 8 post-infection, with a return to vehicle control levels at day 14 (Barrientos et al., 2009a). Godbout and colleagues found similar results in the BALB/c mouse. Here, a peripheral injection of lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, resulted in both exaggerated and prolonged elevations in IL-1β and IL-6 (both protein and mRNA) in aged mice (Godbout et al., 2005). Importantly, in these and other studies, the exaggerated cytokine response was restricted to the brain and did not occur in the periphery, suggesting that a central mechanism was the source of this response (Godbout et al., 2005; Barrientos et al., 2009a, 2015a). Moreover, our laboratory found this amplified response to be particular to the hippocampus. We did not observe exaggerated or protracted responses in the hypothalamus, parietal cortex, pre-frontal cortex, spleen or serum (Barrientos et al., 2009a). Similarly, Buchanan and colleagues reported that 30 min of restraint stress repeated over 4 days resulted in IL-1β mRNA expression that was exaggerated in the hippocampus of aged mice compared to non-stressed age-matched controls (Buchanan et al., 2008). This augmented response was not observed in the hypothalamus or in peripheral plasma samples, implicating a hippocampus-selective response. In addition, various surgical procedures have been reported to produce an IL-1β increase in the hippocampus of aged animals for up to several days compared to age-matched sham controls (Rosczyk et al., 2008; Cao et al., 2010; Barrientos et al., 2012). However, young adult animals that underwent the same surgical procedure showed no differences in IL-1β expression compared to their age-matched sham controls. Similarly, traumatic brain injury produced exacerbated inflammation and neurological damage in aged mice compared to younger control mice (Kumar et al., 2013). Controlled cortical impact (CCI) injury caused larger lesions associated with a shift in M1/M2 balance resulting in a pro-inflammatory profile in hippocampal microglia of aged mice compared to that of young adult mice. Sandhir and colleagues showed that up to 28 days after CCI aged mice had higher mRNA levels of CD11b and Iba-1, markers for microglia activation, and these correlated with a longer-lasting neuroinflammatory response (Sandhir et al., 2008).
Taken together, these studies provide an abundance of evidence indicating that either peripheral or central challenges with an inflammatory component indeed result in a potentiated neuroinflammatory response in the aged animal, and in many cases, this response is specific to the hippocampal formation. There are at least two possible reasons for these hippocampus selective effects. First, there is high constitutive expression of IL-1 receptors on neurons and glia in the granule cells of the dentate gyrus and the pyramidal cell layer of the hippocampus (Takao et al., 1990; Ban et al., 1991, 1993; Rubio, 1994; Cullinan et al., 1998). Second, the hippocampus has a particularly dense microglial population (Lawson et al., 1990; Yang et al., 2013b). The hippocampus has been shown to exhibit higher and faster expression of pro-inflammatory cytokines following a peripheral immune challenge compared to other brain regions (Pitossi et al., 1997).Whether this is due to its close proximity to the lateral ventricles and circumventricular organs, which have extensive vasculature and lack a normal blood brain barrier (Katsuura et al., 1990), remains controversial and requires further investigation.
The studies above did not isolate the cellular source of this age-related potentiated neuroinflammatory response. An early study using mixed glial cultures demonstrated that these cells taken from aged mice exhibited greater spontaneous IL-6 mRNA expression compared to those taken from younger animals (Ye and Johnson, 1999). Flow cytometric analysis strongly suggested that micro-glia were the primary cellular source of this age-related response. In 2005, Cunningham and colleagues first demonstrated that a compromised brain with a phenotype of chronic microglial activation and minimal pro-inflammatory cytokine expression (a phenotype shared by normal aged animals) was primed to produce exaggerated inflammatory cytokines in response to subsequent challenges (Cunningham et al., 2005). More specifically, they showed that an intracerebral administration of LPS in prion-diseased mice indeed resulted in a potentiated pro-inflammatory response compared to the response induced by the same challenge in healthy mice. By double labeling activated microglia with altered morphology and pro-inflammatory markers, their work suggested that microglia were the cells that produced the potentiated inflammatory response. Whether the potentiated neuroinflammatory response was mediated by sensitized micro-glia per se was later addressed by Godbout and colleagues who demonstrated that neuroinflammatory mediators, measured from enriched microglia isolated following an in vivo peripheral LPS challenge, were potentiated in microglia taken from aged mice compared to those taken from adult mice (Henry et al., 2009). These findings strongly pointed to microglia as the sensitized cell producing the exaggerated response. However, it could be argued that the immune challenge, administered in vivo, acted at some other cell type, whether in a central or peripheral compartment, to deliver an exaggerated signal to the microglia in the aging subjects. Therefore, to further address whether a potentiated neuroinflammatory response in aged animals is mediated specifically by sensitized microglia, our laboratory isolated microglia from unchallenged adult and aged animals and stimulated them with LPS ex vivo (Frank et al., 2010b). Our findings supported what others had concluded. That is, we found that hippocampal microglia isolated from normal aged (but not adult) animals were sensitized, as they produced a potentiated pro-inflammatory cytokine response (i.e., IL-1β, TNF-α) when subsequently challenged with LPS (Frank et al., 2010b).
EFFECTS OF POTENTIATED NEUROINFLAMMATORY RESPONSES ON HIPPOCAMPAL MEMORY
The age-related exaggerated neuroinflammatory response to peripheral challenges such as a bacterial infection, surgery, or head trauma does not occur without significant costs to cognitive functions. For the purpose of this special issue review, which focuses on hippocampal vulnerability, we will limit the scope of this section to hippocampal memory. The hippocampus is critical for contextual and spatial learning and awareness, navigation, and episodic memories (Morris et al., 1982; Rudy et al., 2002; Barrientos et al., 2002b; Matus-Amat et al., 2004; Moser et al., 2008). We found that healthy aged rats performed as well as did younger rats in both a short-term and long-term test of reference spatial memory using the Morris water maze. A peripheral bacterial infection with live replicating E. coli did not produce a short-term memory deficit in either young or aged rats, indicating that these animals were capable of learning well. In contrast, the long-term memory test revealed a large deficit in aged infected rats compared to their younger counterparts (Barrientos et al., 2006). Importantly, these long-term memory deficits were detected well beyond the expression of overt sickness behaviors (fever, lethargy, anorexia) had subsided (Barrientos et al., 2009b). Similar findings were reported in a working memory version of the water maze in aged mice injected with LPS (Abraham and Johnson, 2009b). We found this pattern to hold true for hippocampal-dependent contextual memories as well, using the contextual fear-conditioning paradigm (Barrientos et al., 2006, 2009a, 2011; Frank et al., 2010a). In addition, we demonstrated that these memory deficits were restricted to memory processes uniquely dependent on the hippocampus, as hippocampal-independent memories were spared (Barrientos et al., 2006). Buchanan and colleagues reached similar conclusions in their work with repeated mild stress. Hippocampal IL-1β mRNA was elevated in an exaggerated manner in aged mice, and there were spatial memory impairments in the water maze in aged, but not young mice (Buchanan et al., 2008).
Surgery is another peripheral insult often resulting in a constellation of cognitive changes termed post-operative cognitive decline (POCD) experienced by patients shortly following a surgical procedure. These cognitive effects range from feeling disoriented to difficulty remembering events, planning, or sustaining attention, to dementia. Various animal models of POCD have demonstrated elevations in pro-inflammatory cytokines and other proinflammatory mediators in the hippocampus (Cibelli et al., 2010; Barrientos et al., 2012; Terrando et al., 2013; Hovens et al., 2014a,b; Zhang et al., 2014). We, and others, have shown that hippocampal memory function was significantly more impaired by surgery in aged animals compared to younger control animals (Barrientos et al., 2012; Hovens et al., 2013, 2014b; Li et al., 2014; Sun et al., 2014; Yu et al., 2014). This pattern has been found following a variety of surgical procedures including exploratory abdominal surgery (Barrientos et al., 2012; Hovens et al., 2014b; Li et al., 2014), stabilized tibial fracture surgery (Sun et al., 2014), and partial hepatectomy (Li et al., 2013). Importantly, hippocampal memory function was demonstrated to be specifically affected by the surgical procedure and accompanying neuroinflammatory responses and not simply to the anesthesia used during the surgery (Barrientos et al., 2012; Hovens et al., 2013; Li et al., 2013, 2014). Traumatic brain injury (TBI) is yet another insult that has been established to induce a potent pro-inflammatory response in the hippocampus (DeKosky et al., 1994; Knoblach and Faden, 1998; Kinoshita et al., 2002; Lu et al., 2007; Lloyd et al., 2008; Chao et al., 2012; Tomura et al., 2012), and produce impairments in spatial memory of experimental animals (Scheff et al., 1997; Tajiri et al., 2013). Importantly, these inflammatory responses and functional consequences were even greater in aged animals (Shah et al., 2006; Kumar et al., 2013; Monti et al., 2013).
MECHANISMS OF PRO-INFLAMMATORY CYTOKINE-INDUCED HIPPOCAMPAL MEMORY IMPAIRMENTS
As discussed in previous sections, pro-inflammatory cytokines such as IL-1β, when elevated above basal levels in the hippocampus, impair hippocampal-dependent memory. How? Many mechanisms have been implicated in the actions of IL-1β on learning and memory processes. Below, we review several of these.
Elevations in hippocampal IL-1β have been shown to completely block long-term potentiation (LTP) in the hippocampus in vitro and in vivo (Katsuki et al., 1990; Bellinger et al., 1993; Cunningham et al., 1996; Coogan and O'Connor, 1997; Murray and Lynch, 1998a,b; Coogan et al., 1999; Vereker et al., 2000a,b; Kelly et al., 2003; Chapman et al., 2010). LTP is the persistent strengthening of synapses based on recent patterns of activity, and is widely considered to be one of the major cellular mechanisms underlying learning and memory (Bliss and Collingridge, 1993). In addition, blocking IL-1β action with centrally administered IL-1 receptor antagonist (IL-1RA) prevented the LTP deficit induced in aged rats by peripheral infection, suggesting a causal relationship between IL-1β increases and impairments in synaptic plasticity (Chapman et al., 2010). The cellular mechanisms by which IL-1β interferes with LTP are not fully understood. Elevations in IL-1β have been demonstrated to increase reactive oxygen species (ROS) formation in the hippocampus (Vereker et al., 2000b), and in turn, these activated members of the mitogen-activated protein (MAP) kinase family, such as c-jun N-terminal kinase (JNK) (Vereker et al., 2000b; Minogue et al., 2003) and p38 (Vereker et al., 2000b; Kelly et al., 2003). While activation of other members of the MAP kinase family, such as extracellular signal-regulated protein kinase (ERK) has been proven to result in neurite outgrowth, cell proliferation, or differentiation (Seger and Krebs, 1995), activation of JNK and p38 has been proven to induce cell damage or cell death (Maroney et al., 1998). Inhibition of JNK in vivo with D-JNKI1 (Minogue et al., 2003), or in vitro with SP600125 (Curran et al., 2003) blocked IL-1β-induced LTP inhibition in the hippocampus. Moreover, in vivo treatment with the p38 inhibitor, SB203580, attenuated proinflammatory-induced inhibition of hippocampal LTP (Kelly et al., 2003). Furthermore, the inhibition of ROS formation, through an antioxidant diet, reversed IL-1β-induced LTP inhibition as well as IL-1β-induced JNK and p38 activation (Vereker et al., 2000b). Together, these findings strongly suggested that elevated IL-1β plays a prominent role in inhibiting important cellular processes associated with memory formation such as LTP, through activation of these stress-activated protein kinases.
Excitatory amino acid transporters (EAATs), also known as glutamate transporters, serve to regulate concentrations of the excitatory neurotransmitter glutamate at the synapse (Danbolt, 2001). Optimal gluta-mate concentrations are important for proper synaptic transmission and plasticity, and prevent neurotoxicity (Bergles and Jahr, 1998; Tzingounis and Wadiche, 2007). Different mechanisms responsible for the increase in gluta-mate uptake during early- and late-phase LTP (E-LTP and L-LTP) have been described (Pita-Almenar et al., 2006). During E-LTP, new protein synthesis is not necessary, and increases in glutamate uptake are mediated by activation of PKC and excitatory amino acid carrier-1 (EAAC1), a neuronal glutamate transporter. During L-LTP, new protein synthesis is required and is mediated by the cAMP-PKA (protein kinase A) pathway, and it involves a different gluta-mate transporter, glutamate transporter subtype 1 (GLT1) which is expressed on astrocytes (Pita-Almenar et al., 2006). Reductions in the widely distributed GLT1 have been known to result in impairments of LTP (Katagiri et al., 2001), deficits in hippocampal spatial memory (Yang et al., 2013a), and excitotoxicity (Lievens et al., 2000). Relevant to the current review, pro-inflammatory mediators such as IL-1β severely impair glutamate transport, further exacerbating disease states (Takahashi et al., 2003; Zou and Crews, 2005; Tilleux and Hermans, 2007; Brothers et al., 2013; Tan et al., 2014). Furthermore, pharmacological treatment with Riluzole, a drug that enhances glutamate clearance by increasing GLT1 expression in the hippocampus, prevented neuroinflammation-induced memory impairments in aged animals (Brothers et al., 2013).
IL-1β has also been shown to interfere with LTP and memory indirectly by modulating downstream mediators that in turn cause the impairment. BDNF is a plausible candidate capable of affecting memory processes due to its critical role in late phase synaptic plasticity processes (Pang and Lu, 2004) and long-term memory (Ma et al., 1998; Hall et al., 2000; Mizuno et al., 2000). BDNF has been shown to be rapidly and selectively induced in the hippocampus following contextual fear conditioning (Hall et al., 2000). Various peripheral immune challenges such as stress or the administration of either E. coli, IL-1β or LPS (Lapchak et al., 1993; Nguyen et al., 1998; Pugh et al., 1998; Barrientos et al., 2006; Richwine et al., 2008) that have been shown to induce IL-1β levels in the hippocampus have also been shown to robustly decrease BDNF mRNA in the hippocampus (Lapchak et al., 1993; Barrientos et al., 2003, 2004, 2011; Bilbo et al., 2008; Richwine et al., 2008; Cortese et al., 2011; Chapman et al., 2012). Furthermore, this neuro-inflammation-induced reduction in BDNF resulted in hippocampal-dependent memory deficits (Barrientos et al., 2004, 2006; Bilbo et al., 2005; Barrientos, 2011). Moreover, intra-hippocampal administration of IL-1RA prevented both the BDNF mRNA downregulation, and the memory impairments produced by the challenge, again suggesting a causal relationship between IL-1β increases and memory impairments (Barrientos et al., 2003). Moreover, these findings have recently been confirmed at the protein level. Cortese et al. (2011) found that mature-BDNF was markedly reduced in hippocampal synaptoneurosomes prepared from aged animals following infection. It is important to note that the cleaved protein, mature-BDNF binds to tyrosine kinase B receptors, promotes cell survival, and facilitates some forms of LTP. In contrast, the un-cleaved protein, pro-BDNF binds preferentially to the pan-neurotrophin receptor p75NTR, activates apoptosis-related signaling pathways, and may facilitate long-term depression in the hippocampus. Interestingly, pro-BDNF, was not reduced in the aged inflammatory hippocampus samples. Furthermore, a central administration (intra cisterna magna, ICM) of IL-1RA prevented the mature BDNF reduction (Cortese et al., 2011). Similarly, our laboratory reported that physical exercise in aged rats not only prevented E. coli-induced IL-1β elevations in the hippocampus, but also prevented BDNF mRNA reductions in CA1 of the hippocampus and reversed memory deficits caused by the infection (Barrientos et al., 2011). Taken together, these studies provide strong evidence for the idea that pro-inflammatory cytokine-induced memory impairments may involve the downregulation of BDNF.
The immediate early gene activity-dependent cytoskeletal-associated protein (Arc) is another mediator downstream of IL-1β that has been identified as a key modulator of hippocampal memory consolidation (Bramham et al., 2008). Arc mRNA is rapidly and specifically distributed throughout the dendritic arbor of the hippocampus after neuronal activity (Link et al., 1995; Lyford et al., 1995) and localized to regions that receive direct synaptic activation (Steward et al., 1998). The inhibition of Arc protein expression with antisense oligodeoxynucleotides resulted in impaired long-term memory consolidation and L-LTP (Guzowski et al., 2000). Importantly, short-term memory and E-LTP were not impaired by Arc inhibition. This is noted because this is a pattern we have observed in E. coli-challenged aged rats who exhibit long-lasting neuroinflammatory responses (Barrientos et al., 2006; Chapman et al., 2010). In a separate study, our laboratory reported that a peripheral E. coli challenge in young and aged rats resulted in a profound suppression of hippocampal Arc mRNA expression in only aged rats (Frank et al., 2010a). This suppression correlated with long-term memory deficits, and an elevation in hippocampal IL-6 protein. Furthermore, administration of IL-1RA blocked all of these effects, underscoring the potential role of Arc in neuroinflammatory-induced memory impairments. To further support this notion, in a model of chronic neuroinflammation, young adult rats exhibited activity-related alterations in Arc expression in the hippocampus and these alterations correlated with microglial activation (Rosi et al., 2005). Also, IL-1βXAT transgenic mice with sustained hippocampal IL-1β overexpression exhibited significantly suppressed Arc mRNA expression, and this was highly correlated with contextual and spatial memory impairments (Hein et al., 2010). At the protein level, Arc was recently demonstrated to be significantly reduced in aged animals that showed impairments in spatial memory consolidation (Menard and Quirion, 2012). However, neuroinflammatory markers were not measured in this study. Although to the best of our knowledge Arc protein expression has not yet been examined in aged animals following an immune challenge, one could reasonably expect Arc protein to be dramatically blunted following a learning experience in these animals.
CAUSES OF MICROGLIAL PRIMING
Understanding the mechanism(s) that sensitize microglia during normal brain aging is of considerable importance in the context of developing effective treatments to attenuate neuroinflammatory-induced cognitive impairments. Several lines of research have recently emerged to shed some light on this issue. The first of these lines implicates various proteins that activate anti-inflammatory signals following ligand receptor interactions (Griffiths et al., 2009). We will focus on two such proteins here. The ligands, CD200 and fractalkine (CX3CL1), which are expressed preferentially on neurons, function to inhibit microglia through their cognate receptor (CD200R and CX3CR1, respectively) expressed predominately on microglia (Webb and Barclay, 1984). Young adult CD200 knockout mice exhibited a chronically activated microglial phenotype remarkably similar to the phenotype of aged animals, characterized by a less ramified morphology and increased expression of CD11b (Hoek et al., 2000). Interestingly, CD200 protein and gene expression were reported to be significantly reduced in the normal aging hippocampus (Lyons et al., 2007; Frank et al., 2006), and CD200 ligand receptor interactions were shown to modulate microglial activation via up-regulation of the anti-inflammatory cytokine IL-4 (Lyons et al., 2007).
The other of these proteins, fractalkine, is equally important in maintaining the functional plasticity of microglia. Decreases in either the ligand or the receptor can lead to microglial activation. Young mice deficient in the CX3C receptor (CX3CR1−/−) exhibited long-lasting microglial activation in response to an immune challenge (Corona et al., 2010), and also exhibited decreased hippocampal neurogenesis (Vukovic et al., 2012). Interestingly, with advanced age, both the receptor and the ligand exhibited a progressive decrease in the hippocampus (Wynne et al., 2010; Bachstetter et al., 2011), and this was also associated with a significant decline in hippocampal neurogenesis (Vukovic et al., 2012). Importantly, treatment with exogenous CX3CL1 reversed the age-related decrease in hippocampal neurogenesis and reduced the number of MHCII+ cells in the hippocampus (Bachstetter et al., 2011). These studies provide compelling evidence that neuronal inhibitory control over microglial pro-inflammatory processes may be compromised with normal aging, thereby predis-posing the aged brain to exaggerated pro-inflammatory response in the face of immune challenges.
Another line of emerging evidence suggests that significant and prolonged elevations in hippocampal corticosterone (CORT), the principal glucocorticoid (GC) in rodents, leads to microglial priming. Although CORT is well-known for its anti-inflammatory and immunosuppressant activity in both humans and animals (Selye, 1955), new evidence has begun to emerge over the past decade that suggests that CORT can also have immunoenhancing effects. A series of papers (Sapolsky and Pulsinelli, 1985; Sapolsky, 1999; Johnson et al., 2002; Dinkel et al., 2003; MacPherson et al., 2005; de Pablos et al., 2006; Munhoz et al., 2006) demonstrated that chronic exposure to stress levels of CORT exacerbated, rather than inhibited, the neuroinflammatory response to subsequent challenge. Frank and colleagues (Frank et al., 2010c) reported that exogenous CORT administered acutely prior to a bacterial immune challenge facilitated the inflammatory response to the challenge, both in the periphery and in the brain. In contrast, CORT administered after the same immune challenge resulted in suppression of the inflammatory response, suggesting that the temporal relationship between CORT increases and an immune challenge determines whether CORT will facilitate or suppress the inflammatory response. More recent evidence in young adult rats has demonstrated that chronic exposure to exogenous GCs primed hippocampal microglia, resulting in a potentiated pro-inflammatory response to a bacterial challenge (Frank et al., 2014). Together, these findings prompted our own laboratory to examine whether normal aging-associated elevations in basal GCs might be associated with microglial priming. Although circulating levels of GCs were not elevated by aging, CORT levels within the hippocampus were indeed elevated throughout the light phase (Barrientos et al., 2015b). The dissociation between circulating and brain levels of CORT is thought to be driven by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). 11β-HSD1 has reductase activity, converting the inactive derivative of CORT (11-dehydrocorticosterone) to biologically active CORT, thereby amplifying CORT action locally (Seckl, 1997; Wyrwoll et al., 2011). 11β-HSD1 protein and gene expression was significantly elevated in the normal aged hippocampus (Holmes et al., 2010; Barrientos et al., 2015b), resulting in greater CORT and greater glucocorticoid receptor (GR) activity there (Yau et al., 2011, 2015; Barrientos et al., 2015b). Moreover, this increased GR activation primed hippocampal microglial, as blocking GR activation prevented the bacteria-induced potentiated pro-inflammatory response, and associated memory declines (Barrientos et al., 2015b). Similarly, hippocampal learning and memory declines were prevented in aged 11β-HSD1−/− knockout mice (Yau et al., 2011, 2015). Taken together, there is mounting and converging evidence that elevations of CORT in the aged hippocampus prior to an immune challenge plays a critical role in priming microglia, amplifying the inflammatory response to a challenge, and ultimately impairing hippocampal memory.
While the phenomenon of stress and GC-induced neuroinflammatory priming has been well supported in recent years (de Pablos et al., 2006; Munhoz et al., 2006, 2010; Frank et al., 2010c, 2014; Espinosa-Oliva et al., 2011; Kelly et al., 2012), mechanism(s) by which GCs prime microglia remain largely unknown. There is a growing literature suggesting that GCs induce the expression of the multi-protein complex known as the NLRP3 (Nucleotide-binding domain, Leucine-Rich Repeat, Pyrin domain containing protein 3) inflammasome (Busillo et al., 2011; Frank et al., 2014). The NLRP3 inflamma-some, expressed in microglia (Hanamsagar et al., 2012) regulates the activation of caspase-1 and the subsequent maturation and release of IL-1β (Martinon et al., 2002; Bauernfeind et al., 2011). Busillo et al. (2011), found that GCs induce NLRP3 expression in THP-1 cells, bone marrow-derived macrophages, and primary human monocytes in vitro, thereby priming NLRP3 inflammasome formation to a subsequent stimulus and potentiating the pro-inflammatory cytokine response. Frank et al. (2014) found that chronic CORT exposure increased mRNA expression of NLRP3 in the hippocampus in vivo, in a dose-dependent manner. Chronic CORT exposure also produced a primed microglial immunophenotype (elevated Iba-1 and MHCII gene expression), and potentiated the neuroinflammatory response to LPS. Together, these studies suggest that GCs can operate as an endogenous danger signal by increasing expression of the NLRP3 inflammasome. Whether GCs modulate the expression of CD200, fractalkine, or their receptors has yet to be examined.
THERAPEUTIC INTERVENTIONS
The evidence reviewed indicates that normal aging potentiates neuroinflammatory responses to immune challenges. The majority of studies have shown that an immune challenge in aged animals induced exaggerated brain cytokine responses causing impairments in long-term memory. Here we will discuss various studies demonstrating that pharmacological, dietary, and exercise-induced modulation of cytokines, or microglial phenotype, prior to an immune challenge blocks the behavioral effects of such challenges in aged animals.
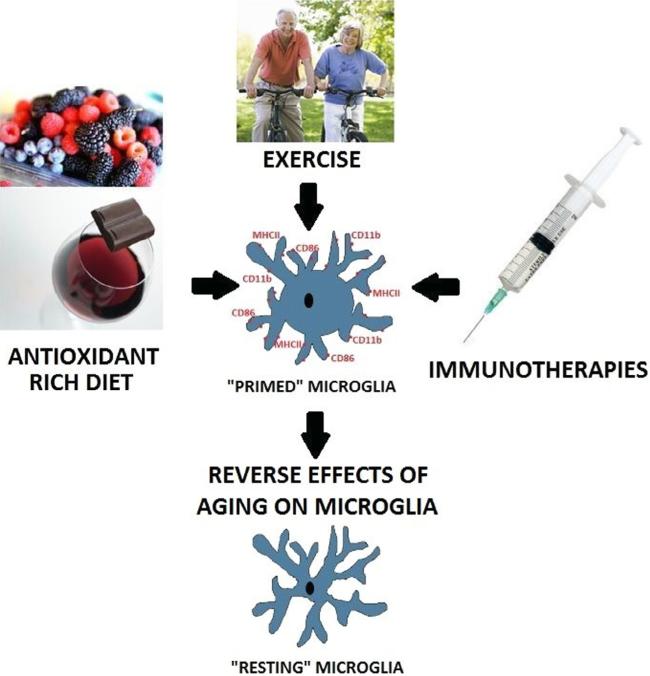
Some of these studies were touched upon in an earlier section, and are expanded upon here (See Fig. 2).
Fig. 2.
Therapeutic interventions. Pharmaceutical (e.g., IL-1RA, minocycline) interventions, exercise, and anti-oxidant rich diets have all been demonstrated to normalize the immunophenotype of the aged hippocampal microglia, thus reducing neuroinflammatory responses, and preventing long-term memory impairments.
Pharmacological treatments
In order to understand whether the neuroinflammatory sequelae of peripheral infection is causal rather than merely correlated with impaired LTM in older animals, the IL-1RA was administered ICM immediately prior to a peripheral E. coli infection (Frank et al., 2010a) or prior to an abdominal surgery (Barrientos et al., 2012). In the first of these studies, prior treatment with IL-1RA prevented the E. coli-induced suppression of Arc, increases in IL-6, and long-lasting long-term memory impairments (Frank et al., 2010a). In the second experiment, similar findings were reported. Central administration of IL-1RA prior to abdominal surgery prevented surgery induced hippocampal IL-1 increases, and long-term memory impairments (Barrientos et al., 2012). These data suggest that preventing the cascade of neuroinflammatory sequelae in the aged brain may protect against the deleterious effects of the otherwise unrestrained inflammatory response following a challenge. Notably, ICM administration of IL-1RA treatment exerted its effects up to 8 days post-treatment (Frank et al., 2010a, 2012; Barrientos et al., 2012), which was remarkable given the reported short half-life (1–2 h) of peripherally administered (i.v. or i.p.) IL-1RA (Granowitz et al., 1992). Thus, an alternative interpretation is that IL-1RA is effective because it persists in the brain for such a long period of time.
As discussed earlier, aged animals showed deficits in LTP, which were accompanied by increases in proinflammatory cytokines (Lynch, 1998; Chapman et al., 2010), and these effects were further amplified by an immune challenge (Chapman et al., 2010). Chapman et al. also examined the effects of IL-1RA and found that a single central administration of the antagonist, at the time of a peripheral E. coli injection, abrogated the aging-associated, infection-induced impairment of theta-burst L-LTP (Chapman et al., 2010). Consistent with these findings, Abraham and Johnson (2009a) reported that central administration of IL-1RA blocks LPS-induced sickness behaviors in aged mice. These studies provide evidence that an exaggerated pro-inflammatory cytokine response in aged animals plays an important role in their cognitive health following an immune challenge. Age-related alterations to hippocampal microglial phenotype also play a critical role in the neuroinflammatory and cognitive responses to immune challenge. Thus, pharmacological interventions that control microglial activation states hold considerable promise with regard to neuroinflammatory-induced memory impairments in the aged population. The microglia inhibitor, minocycline, was found to attenuate the age-related increase in hippocampal IL-1β, while partially restoring deficits in LTP (Griffin et al., 2006). It is important to note this study was conducted in immunologically unchallenged animals. However, a study by Henry et al. showed that pre-treating aged animals with minocycline reduced the hippocampal pro-inflammatory response to LPS (Henry et al., 2008). Furthermore, minocycline was also demonstrated to be effective at improving surgery-induced and isoflurane-induced memory impairments in aged rodents (Kong et al., 2013; Jin et al., 2014). Together these studies suggest that pharmacological treatments that aim to prevent hippocampal microglial priming or reduce the exaggerated neuroinflammatory response to an immune challenge are effective at attenuating memory deficits in the aged population.
Dietary treatments
ROS are part of the neuroinflammatory cascade (McGahon et al., 1999a,b; Cartford et al., 2002; Gemma et al., 2002; Martin et al., 2002; Godbout et al., 2004; Wenk et al., 2004; Moore et al., 2005) and can play a prominent role in the development of age-related declines in LTP (McGahon et al., 1999a,b; Martin et al., 2002), declines in cognition, sickness behaviors (Perrig et al., 1997; Perkins et al., 1999; Cartford et al., 2002; Berg et al., 2005), and other neuronal functions (Gemma et al., 2002). Several studies have reported age-related decreases in vitamins C and E (alpha-tocopherol), and polyunsaturated fatty acids, such as arachidonic acid in the hippocampus, and increases in hippocampal superoxide dismutase activity, a profile indicative of compromised antioxidative defenses (Murray and Lynch, 1998a; McGahon et al., 1999a,b; Martin et al., 2002; Moore et al., 2005). This profile has also correlated with impaired LTP maintenance and increased hippocampal IL-1β. Thus, a variety of studies have examined the effects of antioxidant treatments on these age-related changes. An 8-week regimen of an alpha-lipoic acid-enriched diet reversed increases in superoxide dismutase activity and decreases in alpha-tocopherol levels in aged rats, as well as restored LTP deficits to levels comparable to those observed in young animals. In addition, this dietary supplement also reduced elevated hippocampal IL-1β levels (McGahon et al., 1999a). In a separate study, supplementing either omega 6 or omega 3 fatty-acids in the diets of aged rats reversed levels of key polyunsaturated fatty acids (arachidonic and docosahexanoic acids) and restored the ability of aged rats to sustain LTP (Martin et al., 2002). Supplementing the diets of aged animals with alpha-tocopherol ameliorated age-related (Murray and Lynch, 1998a; Murray et al., 1999) and LPS-induced sensitized (Berg et al., 2005) responses such as LTP deficits, decreases in membrane arachidonic acid levels, increases in IL-1β, IL-6, and lipid peroxidation, and deficits in social exploration behavior. Vitamin D3, when incorporated into the diets of young and aged rats for 2 weeks, was shown to have significant anti-inflammatory effects. Increased phenotypic (MHCII and CD11b expression) and functional markers of glial activation (IL-1β elevations) found in the hippocampus of aged rats, were abrogated by the vitamin D3-supplemented diet, while causing no change in young rats (Moore et al., 2005).
Strawberries, blueberries, and blackberries have distinguished themselves as being potent antioxidant fruits. As such, several laboratories have incorporated them into the diets of aged animals to examine their ability to reverse age-related changes. Shukitt-Hale et al. found that a 2% blackberry-enriched diet improved short-term memory of aged rats in a spatial memory task, compared to control rats (Shukitt-Hale et al., 2009). Similarly, Joseph et al. found that feeding aged rats for 8 weeks with diets supplemented with spinach, strawberry, or blueberry extracts effectively reversed age-related deficits in neuronal function as well as short-term memory on a spatial memory task (Joseph et al., 1999). Along the same lines, a 4-week diet supplemented with reseveratrol, a polyphenol found in red grapes, reduced the potentiated IL-1β mRNA levels in the hippocampus of aged mice following an LPS challenge (Abraham and Johnson, 2009b). Furthermore, this antioxidant treatment abrogated the LPS-induced deficits in spatial working memory in aged animals (Abraham and Johnson, 2009b). Taken together, these studies suggest that dietary supplements high in antioxidants may be an effective therapy in buffering against age-related neuroinflammatory-induced cognitive impairments, though it should be noted that the effectiveness of these supplements have not been tested with regard to long-term memory paradigms.
Exercise
Many studies have recently reported robust effects of physical exercise in aged animals on hippocampal neurogenesis and hippocampal memory performance (Kim et al., 2010; Barrientos et al., 2011; Kohman et al., 2012; Speisman et al., 2013; Merkley et al., 2014; Snigdha et al., 2014). For example, one study showed that 12 weeks of voluntary wheel running reversed the aging-associated memory deficits in the hippocampal-dependent task of place recognition (Siette et al., 2013). In addition, running restored presynaptic density and neurogenesis in the hippocampus to levels beyond those observed in young adults. Interestingly, they found that exercise might restore memory function by optimizing intrahippocampal neuronal connectivity. Physical exercise has been extensively shown to strongly increase BDNF mRNA and protein levels in the hippocampus (reviewed in Cotman and Berchtold, 2002). Given the prominent role of BDNF in learning and memory processes, the effects of exercise-induced BDNF in aged animals is of great interest. A recent study showed that 28 days of voluntary wheel running in aged mice stimulated hippocampal neurogenesis, increased BDNF, decreased IL-1β production, and improved scores on learning and memory tasks such as Morris water maze and contextual fear conditioning (Gibbons et al., 2014). Our laboratory found that small amounts of voluntary exercise in aged rats were an effective therapeutic intervention to prevent E. coli-induced BDNF blunting, neuroinflammatory responses, and long-term memory impairments. Low-intensity, but consistent exercise, such as daily walking, was recently reported to be associated with greater hippocampal volume in older women (Varma et al., 2014). Similarly, another study found that not only cardiovascular, but also coordinative exercise (focused on the improvement of complex movements for the whole body such as balance, eye–hand coordination, leg–arm coordination as well as spatial orientation and reaction to moving objects/persons) increased hippocampal volume in older men and women (Niemann et al., 2014). With important implications for reducing the neuroinflammatory response, exercise has also been demonstrated to modulate the activation state of microglia and to prevent hippocampal microglial priming (Barrientos et al., 2011), with recent evidence suggesting that this protection may occur through the exercise-induced downregulation of GRs in the hippocampus (Barrientos et al., 2015b). Another study showed that exercise decreased the proportion of CD86+ and MHCII+ microglia in the hippocampus of aged female mice (Kohman et al., 2013). However, the same study showed that while exercise decreased the number of CD86+ microglia, it also increased the number of MHCII+ microglia in the hippocampus of aged male mice, indicating that modulation of microglial activation varies not just by age but sex as well (Kohman et al., 2013).
Exercise has also been shown to ameliorate the deficits shown in rodent models of TBI and Alzheimer's disease. In addition to rescuing impairments in learning and memory tasks, differences in hippocampal formation and volume of lateral ventricles were decreased in animals allowed to run after TBI (Jacotte-Simancas et al., 2014). Another study demonstrated that expression of hippocampal microRNAs were altered to decrease miR-21 after wheel running, something previously shown to have been upregulated in TBI models, and was associated with improved cognitive abilities (Bao et al., 2014). Furthermore, in an AD mouse model male and female mice exhibited increased levels of BDNF and markers for neuroprotection and plasticity after 6 months of voluntary wheel running. This amount of exercise was also associated with activated antioxidant signaling pathways and protected cognitive and dementia-like behaviors (Garcia-Mesa et al., 2011, 2014). Taken together, there is a tremendous amount of evidence to support the beneficial effects of exercise for hippocampal function and thus cognitive health in the aged population, both from a preventative standpoint as well as a restorative one.
SUMMARY
Taken together, the literature suggests that challenges such as a bacterial infection, surgery, or head injury produce neuroinflammatory responses that are exaggerated in the otherwise healthy aged brain. These exaggerated responses appear to be most prominent in the hippocampal formation, the critical brain region mediating contextual and spatial memory, and may be the cause of hippocampal memory impairments in aged individuals. The primary source of this neuroinflammatory response appears to be sensitized microglia. A dysregulated neuroendocrine response in the aged animal is skewed toward higher hippocampal CORT levels, and this may play a critical role in priming microglia to respond in an exaggerated manner following an immune challenge, possibly through the upregulation of the NLRP3 inflammasome. Pro-inflammatory cytokines such as IL-1β may affect cognitive processes by impairing synaptic plasticity through activation of MAP kinases JNK and p38, and/or by inhibiting downstream mediators essential to hippocampal-dependent memory processes such as BDNF and Arc. Preventing microglial priming or blocking the exaggerated brain cytokine response pharmacologically, or through diet and exercise modifications may effectively block the deleterious effects on memory following an immune challenge, not only suggesting that these may be useful therapeutic interventions, but also supporting the view that proinflammatory cytokines have a causal, rather than merely correlational relationship with impaired long-term memory in older individuals.
Acknowledgments
This work was supported by a grant from the National Institute on Aging R01AG028271 to R.M.B., L.R.W., & S.F.M.
Abbreviations
- 11b-HSD1
11b-hydroxysteroid dehydrogenase type 1
- BDNF
brain-derived neurotrophic factor
- CCI
controlled cortical impact
- CD11b
complement receptor 3
- CORT
corticosterone
- ERK
extracellular signal-regulated protein kinase
- GC
glucocorticoid
- GLT1
glutamate transporter subtype 1
- GR
glucocorticoid receptor
- ICM
intra cisterna magna
- IL-1RA
IL-1 receptor antagonist
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- JNK
c-jun N-terminal kinase
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- MAP
mitogen-activated protein
- MHC II
major histocompatibility complex II
- NLRP3
Nucleotide-binding domain, Leucine-Rich Repeat, Pyrin domain containing protein 3
- POCD
post-operative cognitive decline
- ROS
reactive oxygen species
- TBI
traumatic brain injury
- TNF-α
tumor necrosis factor-alpha
REFERENCES
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009a;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009b;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akana SF, Strack AM, Hanson ES, Horsley CJ, Milligan ED, Bhatnagar S, Dallman MF. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–146. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Anttila SK. Diseases and symptoms as predictors of hospital care in an aged population. A prospective register-based study. Scand J Soc Med. 1992;20:79–84. doi: 10.1177/140349489202000203. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (alpha and beta) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- Ban E, Marquette C, Sarrieau A, Fitzpatrick F, Fillion G, Milon G, Rostene W, Haour F. Regulation of interleukin-1 receptor expression in mouse brain and pituitary by lipopolysaccharide and glucocorticoids. Neuroendocrinology. 1993;58:581–587. doi: 10.1159/000126594. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood–brain barrier is enhanced by lipopolysaccharide. Exp Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- Bao TH, Miao W, Han JH, Yin M, Yan Y, Wang WW, Zhu YH. Spontaneous running wheel improves cognitive functions of mouse associated with miRNA expressional alteration in hippocampus following traumatic brain injury. J Mol Neurosci. 2014;54:622–629. doi: 10.1007/s12031-014-0344-1. [DOI] [PubMed] [Google Scholar]
- Barrientos RM. Voluntary exercise as an anti-neuroinflammatory therapeutic. Brain Behav Immun. 2011;25:1061–1062. doi: 10.1016/j.bbi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002a;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002b;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009a;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain Behav Immun. 2009b;23:450–454. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Arnold TH, Frank MG, Watkins LR, Maier SF. The role of hepatic and splenic macrophages in E. coli-induced memory impairments in aged rats. Brain Behav Immun. 2015a;43:60–67. doi: 10.1016/j.bbi.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging. 2015b;36:1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–263. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, Siggins GR. Interleukin-1 Beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Chen J, Kelley KW, Johnson RW. Alpha-tocopherol and selenium facilitate recovery from lipopolysaccharide-induced sickness in aged mice. J Nutr. 2005;135:1157–1163. doi: 10.1093/jn/135.5.1157. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J Neurosci. 1998;18:7709–7716. doi: 10.1523/JNEUROSCI.18-19-07709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers HM, Bardou I, Hopp SC, Kaercher RM, Corona AW, Fenn AM, Godbout JP, Wenk GL. Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory. J Neuroimmune Pharmacol. 2013;8:1098–1105. doi: 10.1007/s11481-013-9476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC. CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J. 2003;17:1168–1170. doi: 10.1096/fj.02-0757fje. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Deacon RM, Jiang Y, Ferrari C, Pitossi FJ, Anthony DC. Overexpression of IL-1beta by adenoviral-mediated gene transfer in the rat brain causes a prolonged hepatic chemokine response, axonal injury and the suppression of spontaneous behaviour. Neurobiol Dis. 2007a;27:151–163. doi: 10.1016/j.nbd.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Jiang Y, Davis AE, Farrands R, Holbrook J, Leppert D, Anthony DC. Immunomodulatory effects of etanercept in a model of brain injury act through attenuation of the acute-phase response. J Neurochem. 2007b;103:2245–2255. doi: 10.1111/j.1471-4159.2007.04928.x. [DOI] [PubMed] [Google Scholar]
- Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, Wang LL, Tan WF. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1426–1432. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Cartford MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha) and TNFbeta in the cerebellum. J Neurosci. 2002;22:5813–5816. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PK, Lu KT, Jhu JY, Wo YY, Huang TC, Ro LS, Yang YL. Indomethacin protects rats from neuronal damage induced by traumatic brain injury and suppresses hippocampal IL-1beta release through the inhibition of Nogo-A expression. J Neuroinflammation. 2012;9:121. doi: 10.1186/1742-2094-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol Aging. 2012;33:832.e1–832.e14. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan A, O'Connor JJ. Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1 beta. NeuroReport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O'Neill LA, O'Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLC{gamma}1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012;67:754–759. doi: 10.1093/gerona/gls112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan EB, Kwee L, Nunes P, Shuster DJ, Ju G, McIntyre KW, Chizzonite RA, Labow MA. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol. 1998;161:5614–5620. [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O'Neil LAJ, Lynch MA, O'Connor JJ. Interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran BP, Murray HJ, O'Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118:347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Goss JR, Miller PD, Styren SD, Kochanek PM, Marion D. Upregulation of nerve growth factor following cortical trauma. Exp Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988;10:168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging. 2011;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. MRNA up-regulation of MHC II and pivotal proinflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010a;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010b;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010c;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. J Neuroimmunol. 2012;252:33–39. doi: 10.1016/j.jneuroim.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, Revilla S, Guerra R, Gruart A, Laferla FM, Cristofol R, Delgado-Garcia JM, Sanfeliu C. Physical exercise protects against Alzheimer's disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24:421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Garcia-Mesa Y, Pareja-Galeano H, Bonet-Costa V, Revilla S, Gomez-Cabrera MC, Gambini J, Gimenez-Llort L, Cristofol R, Vina J, Sanfeliu C. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology. 2014;45:154–166. doi: 10.1016/j.psyneuen.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Tilders FJH, Bol JGJM, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces Fos immunoreactivity in primary afferent neurons of the vagus nerve. NeuroImmunoModulation. 1998;5:234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (–)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav Brain Res. 2014;272:131–140. doi: 10.1016/j.bbr.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Kelley KW, Johnson RW. Alpha-tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, OC J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J Neuropathol Exp Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999;815:36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR, Yau JL. 11beta-hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments. J Neurosci. 2010;30:6916–6920. doi: 10.1523/JNEUROSCI.0731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Nyakas C, van Leeuwen BL. Surgery-induced behavioral changes in aged rats. Exp Gerontol. 2013;48:1204–1211. doi: 10.1016/j.exger.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014a;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA, Schoemaker RG. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol Learn Mem. 2014b;118C:74–79. doi: 10.1016/j.nlm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Jacotte-Simancas A, Costa-Miserachs D, Coll-Andreu M, Torras-Garcia M, Borlongan C, Portell-Cortes I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis and neuroprotection after traumatic brain injury in rats. Journal of neurotrauma. 2014 doi: 10.1089/neu.2014.3502. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Deacon R, Anthony DC, Campbell SJ. Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiol Dis. 2008;32:125–132. doi: 10.1016/j.nbd.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Jin WJ, Feng SW, Feng Z, Lu SM, Qi T, Qian YN. Minocycline improves postoperative cognitive impairment in aged mice by inhibiting astrocytic activation. NeuroReport. 2014;25:1–6. doi: 10.1097/WNR.0000000000000082. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Tanaka K, Manabe T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci. 2001;14:547–553. doi: 10.1046/j.0953-816x.2001.01664.x. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 B inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Arimura A, Koves K, Gottschall PE. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol. 1990;258:E163–E171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Miller DB, Bowyer JF, O'Callaghan JP. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. J Neurochem. 2012;122:995–1009. doi: 10.1111/j.1471-4159.2012.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner Js, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. discussion 203. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2012;26:803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Chen S, Cheng Y, Ma L, Lu H, Zhang H, Hu W. Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One. 2013;8:e61385. doi: 10.1371/journal.pone.0061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Laye S, Goujon E, Combe C, VanHoy R, Kelley KW, Parnet P, Dantzer R. Effects of lipopolysaccharide and glucocorticoids on expression of interleukin-1 beta converting enzyme in the pituitary and brain of mice. J Neuroimmunol. 1996;68:61–66. doi: 10.1016/0165-5728(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Li RL, Zhang ZZ, Peng M, Wu Y, Zhang JJ, Wang CY, Wang YL. Postoperative impairment of cognitive function in old mice: a possible role for neuroinflammation mediated by HMGB1, S100B, and RAGE. J Surg Res. 2013;185:815–824. doi: 10.1016/j.jss.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Li Z, Cao Y, Li L, Liang Y, Tian X, Mo N, Liu Y, Li M, Chui D, Guo X. Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun. 2014;449:74–80. doi: 10.1016/j.bbrc.2014.04.153. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29:222–232. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd E, Somera-Molina K, Van Eldik LJ, Watterson DM, Wainwright MS. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J Neuroinflammation. 2008;5:28. doi: 10.1186/1742-2094-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Lu KT, Wu CY, Yen HH, Peng JH, Wang CL, Yang YL. Bumetanide administration attenuated traumatic brain injury through IL-1 overexpression. Neurol Res. 2007;29:404–409. doi: 10.1179/016164107X204738. [DOI] [PubMed] [Google Scholar]
- Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, Mohs R, Pasinetti GM. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194:376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune–brain communication: implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight E, Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff NT, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DS, Spencer P, Horrobin DF, Lynch MA. Long-term potentiation in aged rats is restored when the age-related decrease in polyunsaturated fatty acid concentration is reversed. Prostaglandins Leukot Essent Fatty Acids. 2002;67:121–130. doi: 10.1054/plef.2002.0408. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999a;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- McGahon BM, Murray CA, Horrobin DF, Lynch Age-related changes in oxidative mechanisms and LTP are reversed by dietary manipulation. Neurobiol Aging. 1999b;20:643–653. doi: 10.1016/s0197-4580(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Menard C, Quirion R. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One. 2012;7:e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley CM, Jian C, Mosa A, Tan YF, Wojtowicz JM. Homeostatic regulation of adult hippocampal neurogenesis in aging rats: long-term effects of early exercise. Front Neurosci. 2014;8:174. doi: 10.3389/fnins.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci. 2013;5:41. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33:573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998a;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998b;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Clements MP, Lynch MA. Interleukin-1 induces lipid peroxidation and membrane changes in rat hippocampus: an age-related study. Gerontology. 1999;45:136–142. doi: 10.1159/000022076. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J Neurochem. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. 2014;6:170. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, Hendrie HC, Callahan CM, Gao S, Unverzagt FW, Xu Y, Hall KS, Hui SL. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Pita-Almenar JD, Collado MS, Colbert CM, Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci. 2006;26:10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Ramaiah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009;27:485–496. doi: 10.1016/j.anclin.2009.07.011. table of contents. [DOI] [PubMed] [Google Scholar]
- Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer's disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36:171–176. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Rubio N. Demonstration of the presence of an interleukin-1 receptor on the surface of murine astrocytes and its regulation by cytokines and Theiler's virus. Immunology. 1994;82:178–183. [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Seckl JR. 11beta-Hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action? Front Neuroendocrinol. 1997;18:49–99. doi: 10.1006/frne.1996.0143. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Selye H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- Senathi-Raja D, Ponsford J, Schonberger M. Impact of age on long-term cognitive function after traumatic brain injury. Neuropsychology. 2010;24:336–344. doi: 10.1037/a0018239. [DOI] [PubMed] [Google Scholar]
- Shah SA, Prough DS, Garcia JM, DeWitt DS, Hellmich HL. Molecular correlates of age-specific responses to traumatic brain injury in mice. Exp Gerontol. 2006;41:1201–1205. doi: 10.1016/j.exger.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Sheffeld LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12:135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- Siette J, Westbrook RF, Cotman C, Sidhu K, Zhu W, Sachdev P, Valenzuela MJ. Age-specific effects of voluntary exercise on memory and the older brain. Biol Psychiatry. 2013;73:435–442. doi: 10.1016/j.biopsych.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, de Rivera C, Milgram NW, Cotman CW. Exercise enhances memory consolidation in the aging brain. Front Aging Neurosci. 2014;6:3. doi: 10.3389/fnagi.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. NeuroImmunoModulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Sun L, Xie K, Zhang C, Song R, Zhang H. Hyperbaric oxygen preconditioning attenuates postoperative cognitive impairment in aged rats. NeuroReport. 2014;25:718–724. doi: 10.1097/WNR.0000000000000181. [DOI] [PubMed] [Google Scholar]
- Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E. Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J Neurosci. 1996;16:4588–4595. doi: 10.1523/JNEUROSCI.16-15-04588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Kellogg SL, Shimizu T, Arendash GW, Borlongan CV. Traumatic brain injury precipitates cognitive impairment and extracellular Abeta aggregation in Alzheimer's disease transgenic mice. PLoS One. 2013;8:e78851. doi: 10.1371/journal.pone.0078851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Takao T, Tracey DE, Mitchell WM, De Souza EB. Interleukin-1 receptors in mouse brain: characterization and neuronal localization. Endocrinology. 1990;127:3070–3078. doi: 10.1210/endo-127-6-3070. [DOI] [PubMed] [Google Scholar]
- Tan H, Cao J, Zhang J, Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. J Neuroinflammation. 2014;11:93. doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, Harding RE, Lindskog M, Eriksson LI. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Tokutomi T, Miyagi T, Ogawa T, Ono J, Kawamata T, Sakamoto T, Shigemori M, Nakamura N. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J Neurotrauma. 2008;25:1407–1414. doi: 10.1089/neu.2008.0577. [DOI] [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab. 2012;32:1939–1947. doi: 10.1038/jcbfm.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- Varma VR, Chuang Y, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2014 doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000a;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000b;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M, Barclay AN. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J Neurochem. 1984;43:1061–1067. doi: 10.1111/j.1471-4159.1984.tb12844.x. [DOI] [PubMed] [Google Scholar]
- Wenk GL, McGann-Gramling K, Hauss-Wegrzyniak B, Ronchetti D, Maucci R, Rosi S, Gasparini L, Ongini E. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89:484–493. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1–11):e13. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Wine RN, McPherson CA, Harry GJ. IGF-1 and pAKT signaling promote hippocampal CA1 neuronal survival following injury to dentate granule cells. Neurotox Res. 2009;16:280–292. doi: 10.1007/s12640-009-9060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwoll CS, Holmes MC, Seckl JR. 11beta-Hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011;32:265–286. doi: 10.1016/j.yfrne.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li MX, Luo Y, Chen T, Liu J, Fang P, Jiang B, Hu ZL, Jin Y, Chen JG, Wang F. Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1. Neuropharmacology. 2013a;75:213–222. doi: 10.1016/j.neuropharm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Yang TT, Lin C, Hsu CT, Wang TF, Ke FY, Kuo YM. Differential distribution and activation of microglia in the brain of male C57BL/6J mice. Brain Struct Funct. 2013b;218:1051–1060. doi: 10.1007/s00429-012-0446-x. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. 11beta-Hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31:4188–4193. doi: 10.1523/JNEUROSCI.6145-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Wheelen N, Noble J, Walker BR, Webster SP, Kenyon CJ, Ludwig M, Seckl JR. Intrahippocampal glucocorticoids generated by 11beta-HSD1 affect memory in aged mice. Neurobiol Aging. 2015;36:334–343. doi: 10.1016/j.neurobiolaging.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Yu L, Sun L, Chen S. Protective effect of senegenin on splenectomy-induced postoperative cognitive dysfunction in elderly rats. Exp Ther Med. 2014;7:821–826. doi: 10.3892/etm.2014.1501. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience. 2014;261:1–10. doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]