TO THE EDITOR
Recent clinical trials have established a central role for the Th2 cytokines IL-4 and IL-13 in the pathology of atopic dermatitis (AD) (Beck et al., 2014). Although blockade of the IL-4 and IL-13 receptors causes significant clearing of skin lesions, a direct effect of Th2 cytokines on wound healing processes has not yet been demonstrated. Furthermore, AD patients are frequently affected by infection with the pathogen, Staphylococcus aureus (Boguniewicz and Leung, 2011). Elevated levels of staphylococcal products are frequently found on the skin of affected patients (Travers et al., 2010), and these products may affect the wound healing process as well.
A primary event in skin healing is induction of matrix metalloproteinases (MMPs) (Inoue et al., 1995). MMPs-1, 9, and 10 are expressed at the leading edge of the wound (Inoue et al., 1995; Rechardt et al., 2000; Turchi et al., 2003) and are required for keratinocyte migration into the damaged area (Agren, 1999; Pilcher et al., 1997). Inhibition of MMP function effectively blocks keratinocyte migration and wound healing (Mirastschijski et al., 2002b). However, over expression of MMPs has been reported in skin diseases, and can inhibit wound closure as well (Mirastschijski et al., 2002a; Saarialho-Kere et al., 1994). In this study, we determined the effects of staphylococcal products and AD associated Th2 cytokines on MMP expression and on keratinocyte migration.
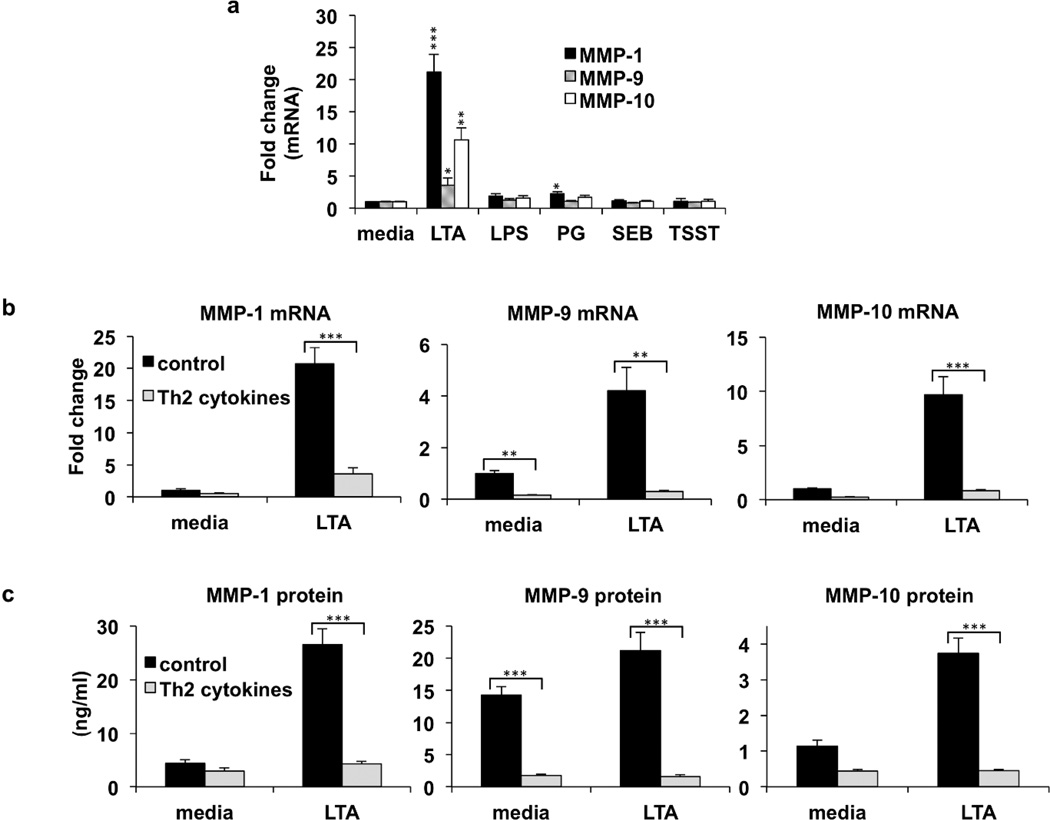
We first examined the effect of bacterial products on MMP levels. Real time PCR and ELISAs were used to quantify mRNA and protein expression of MMP-1, 9, and 10. We found that these MMPs were significantly induced by exposure to staphylococcal LTA (Fig. 1a and supplemental Fig. S1). Peptidoglycan also modestly induced these metalloproteinases. In contrast, neither E. coli derived LPS, nor the staphylococcal toxins, SEB, and TSST, were able to induce expression of MMPs (Fig. 1a). We also found that LTA induced events are dependent upon expression of the TLR signaling adaptor molecule, MyD88, as a MyD88 peptide inhibitor completely blocked gene expression of MMP-1, 9, and 10 (supplemental Fig. S2). We next determined the effect of Th2 cytokines on MMP production. We found that basal level expression of MMP-9 was inhibited by Th2 cytokines (Fig. 1b, c). LTA induced expression of MMP-1, 9, and 10 was also significantly inhibited by Th2 cytokines. (Fig. 1b, c). These results demonstrate that Th2 cytokines interfere with both basal and LTA induced MMP expression. For comparison, TNF-α does not interfere with MMP expression (supplemental Fig. S3), as previously described (Han et al., 2001). Expression of tissue inhibitor of metalloproteinases (TIMPs) was also examined. In contrast to MMPs, TIMP levels were not substantially altered by LTA or Th2 cytokines (supplemental Fig. S4).
Fig. 1. Staphylococcus aureus LTA induced expression of MMPs is inhibited by Th2 cytokines.
(a) Normal human epidermal keratinocytes were cultured in medium or medium additionally containing 5 µg/ml of LTA, 5 µg/ml lipopolysaccharide, (LPS), 5 µg/ml peptidoglycan (PG), 5 µg/ml S. aureus enterotoxin B (SEB), or 5 µg/ml Toxic Shock Syndrome Toxin (TSST) for 24 hours. Real-time PCR was used to quantify mRNA, and levels were normalized to beta actin. Fold change values were calculated relative to media control for MMP-1, MMP-9, and MMP-10. (b) Keratinocytes were pre-treated with medium, or IL-4/IL-13 for 24 hours. Following pre-treatment, cells were then cultured in the presence or absence of LTA for an additional 24 hours. Gene expression was analyzed by real-time PCR, for MMP-1, MMP-9, and MMP-10, and normalized to beta actin. Fold change in MMP expression was measured relative to medium control. (c) Protein levels were measured by ELISA for MMP-1, MMP-9, and MMP-10. Data are mean ± SEM, n = 3. *P<0.05; **P<0.01; ***P<0.001 (as compared to the cells gown in medium alone).
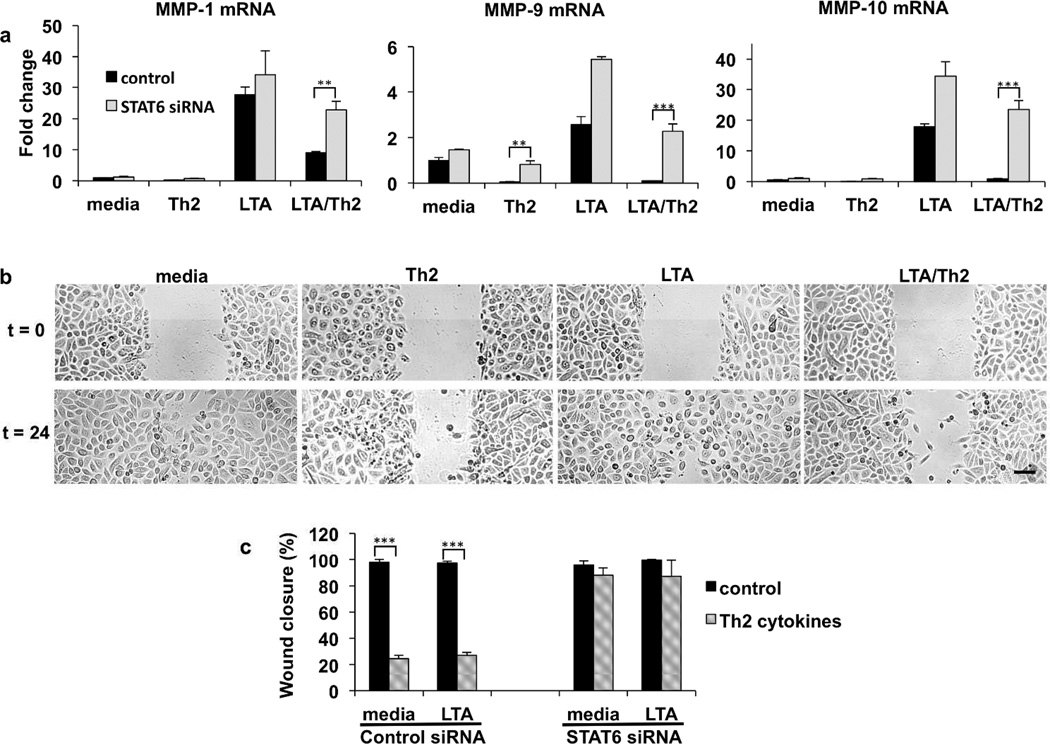
We next focused on determining the molecular events induced by Th2 cytokines that influence MMP gene expression. Signal transducer and activator of transcription 6 (STAT6) is a transcription factor activated by ligation of the IL-4 and IL-13 receptors (Albanesi et al., 2007). We therefore used siRNA directed against STAT6 to determine whether Th2 cytokines signal through STAT6 to modulate MMP levels. Fig. 2a demonstrates that basal MMP-9 expression is inhibited by Th2 cytokines in control, but not in STAT6 siRNA treated cells. Furthermore, the Th2 mediated inhibition of LTA induced MMP expression was no longer observed in STAT6 siRNA treated keratinocytes (Fig. 2a). The increased expression of MMPs in STAT6 knockdown cells was significant. Therefore, we conclude that the inhibition of MMP expression by Th2 cytokines is dependent upon STAT6.
Fig. 2. Th2 cytokine inhibition of MMP expression and keratinocyte migration requires STAT6.
Primary keratinocytes were transfected with control (non-targeting) or STAT6 siRNA. Transfected cells were then treated with media alone or IL-4/13, LTA, or a combination of LTA and IL-4/13. (a) Gene expression was analyzed by real-time PCR, for MMP-1, MMP-9, and MMP-10, and normalized to beta actin. Fold change in MMP expression was measured relative to medium control. (b) Keratinocytes were cultured with medium alone, or with IL-4/IL-13 for 24 hours. Following pre-treatment, cells were then cultured in the absence or presence of LTA for an additional 24 hours. The cells were then scratched with a pipette tip. The defined area of the wound was photographed under phase-contrast microscopy at time 0 h and at 24 h. Representative fields show the wound gap at the indicated times. Scale bar is 100 µm. (c) Primary keratinocytes were transfected with control (non-targeting) or STAT6 siRNA. Transfected cells were then treated with media alone or IL-4/13, LTA, or a combination of LTA and IL-4/13 as described above. Cells were scratched and the closure of the wounded area at 24 h was quantitated. Data are mean ± SEM, n = 3. ***P<0.001 (as compared to cells grown in medium alone).
As MMPs coordinate epithelial wound healing by enabling cell detachment and migration on collagen (Pilcher et al., 1997), we further investigated whether Th2 cytokines inhibited ‘‘wound’’ closure in a monolayer of human keratinocytes grown on a collagen matrix. Using an in vitro wound scratch assay, we find that cells treated with media alone but disrupted by the scratch, migrated into the depleted area (Fig. 2b). In contrast, pre-treatment with Th2 cytokines inhibited the rate of keratinocyte migration compared with control keratinocytes (Fig. 2b, c). Possibly because of the endogenous activation of MMPs at the leading edge (Pilcher et al., 1997; Turchi et al., 2003), we did not observe an additive effect of LTA on wound closure in a scratch assay (Fig. 2b, c). However, we do observe that Th2 cytokines have a dominant inhibitory effect, blocking migration in all cases. For comparison, the cytokine TNF-α, did not inhibit the closure of keratinocyte monolayers (supplemental Fig. S3), as previously described (Eyerich et al., 2009). Consistent with the observed effects on MMP expression, the inhibition of migration mediated by Th2 cytokines was ablated in Stat6 siRNA treated cells (Fig. 2c). Therefore, Th2 inhibition of migration related to wound closure is also dependent on STAT6.
A critical role for Th2 cytokines in AD skin disease is emerging. Here we directly demonstrate that Th2 cytokines inhibit MMP expression and keratinocyte migration, both essential components of the wound healing process. There is, however, a paradoxical effect of MMPs in wound healing. Although MMPs are required for normal migration leading to wound closure, over-expression is a key feature of chronic wounds and skin disease. It therefore remains possible that over-expression of MMPs induced by LTA may contribute to skin disease as well. Recent studies have evaluated MMP expression in AD skin (Esaki et al., 2015). Using micro-dissection techniques, AD skin was sectioned into dermal and epidermal components. MMP-1, a gene induced by staphylococcal LTA, was identified as the most prominently up-regulated gene in the dermis. Since AD lesions are frequently infected with S. aureus, it seems possible that LTA induced over-expression of MMPs may be a contributing factor in disease. In contrast, increases in MMP-1, 9, or 10 levels in the epidermis of lesional AD skin were not reported (in a list of the top 25 most up-regulated genes). However, this may be a consequence of the inhibitory effects of Th2 cytokines. We propose that Th2 cytokines, as well as staphylococcal LTA, may contribute to delayed wound healing and pathology associated with AD by deregulating MMP production and altering cell migration.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge The Edelstein Family Foundation for their generous support of this work. This research was supported by NIH grants R01 AR41256 and The Atopic Dermatitis Research Network (NIH/NIAID contract NIH/NIAID HHSN272201000020C). This research was also supported in part by Colorado Clinical and Translational Sciences Institute (CCTSI), and in part by Colorado Grant UL1RR025780 from NCRR/NIH and UL1 TR000154 from NIH/NCATS.
Abbreviations
- AD
Atopic Dermatitis
- IL
interleukin
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic Acid
- MMP
Matrix Metalloproteinase
- PG
Peptidoglycan
- RT-PCR
Real Time PCR
- SEB
Staphylococcal Enterotoxin B
- STAT6
Signal Transducer and Activator of Transcription 6
- Th2
T Helper type 2
- TIMP
Tissue Inhibitor of Metalloproteinases
- TLR
Toll Like Receptor
- TSST
Toxic Shock Syndrome Toxin
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Agren MS. Matrix metalloproteinases (MMPs) are required for re-epithelialization of cutaneous wounds. Arch Dermatol Res. 1999;291:583–590. doi: 10.1007/s004030050459. [DOI] [PubMed] [Google Scholar]
- Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki H, Ewald DA, Ungar B, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol. 2015;135:153–163. doi: 10.1016/j.jaci.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YP, Tuan TL, Hughes M, et al. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem. 2001;276:22341–22350. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kratz G, Haegerstrand A, et al. Collagenase expression is rapidly induced in wound-edge keratinocytes after acute injury in human skin, persists during healing, and stops at re-epithelialization. J Invest Dermatol. 1995;104:479–483. doi: 10.1111/1523-1747.ep12605917. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Impola U, Jahkola T, et al. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum Pathol. 2002a;33:355–364. doi: 10.1053/hupa.2002.32221. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Impola U, Karsdal MA, et al. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol. 2002b;118:55–64. doi: 10.1046/j.0022-202x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, et al. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechardt O, Elomaa O, Vaalamo M, et al. Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J Invest Dermatol. 2000;115:778–787. doi: 10.1046/j.1523-1747.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, et al. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Kozman A, Mousdicas N, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol. 2010;125:146–152. e1–e2. doi: 10.1016/j.jaci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi L, Chassot AA, Bourget I, et al. Cross-talk between RhoGTPases and stress activated kinases for matrix metalloproteinase-9 induction in response to keratinocytes injury. J Invest Dermatol. 2003;121:1291–1300. doi: 10.1111/j.1523-1747.2003.12627.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.