Abstract
In India, the Chhattisgarh State screening programme for sickle haemoglobin focuses on children aged 3–15 years and has screened over 1,050,440 subjects over the last 6 years. Commencing in the District around the capital Raipur, this programme has now completed screening in 7 of the 27 Districts of Chhattisgarh State. Screening is initially performed by solubility tests on fingerprick samples in the field and those with positive tests have venipunctures for haemoglobin electrophoresis. The frequency of the sickle cell trait was 9.64 % and of the SS phenotype 0.29 % with only two Districts in Hardy-Weinberg equilibrium, most Districts showing an excess of the SS ‘phenotype’ most readily explained by symptomatic selection. The estimated costs were US$0.28 (solubility tests alone) and US$0.60 (haemoglobin electrophoresis). Of the social groupings commonly used in India, the OBC’s (other backward classes) had the highest frequencies of the sickle cell gene mutations, followed by the Scheduled Tribes and the Scheduled Castes. The objectives of the programme were the detection of sickle cell disease for prospective clinical management and of the sickle cell trait for purposes of genetic counselling. The former objective is being met for diagnosis although the success of referral to clinic services requires audit. The objective of genetic counselling is compromised by the failure of the screening test to detect other genes of potential clinical significance such as HbD Punjab and the beta thalassaemia trait. Despite these exceptions, the detection of HbS appears relatively robust and could be another condition factored into the traditions of partner selection amongst the underprivileged communities of this state. Overall, the Chhattisgarh programme seeks to address the daunting challenges of large populations carrying the sickle cell gene and maybe a useful model for elsewhere.
Keywords: Chhattisgarh, Haemoglobin S, Sickle cell disease, Solubility test
Introduction
Long known to be common amongst African populations, the sickle cell gene was first described in India in 1952 (Lehmann and Cutbush 1952). This initial report amongst tribal peoples in the Nilgiri Hills of Tamil Nadu was considered of anthropological interest, and it was some years before the extent of sickle cell disease amongst Indian people was revealed. Extensive studies by the Anthropological Survey of India (Rao 1988) showed the sickle cell trait to be widespread with frequencies as high as 35 % across eastern Gujarat, western Odisha, Maharastra, Madhya Pradesh and Chhattisgarh and a small focus in the north of Kerala and Tamil Nadu. The resulting sickle cell disease is now known to be a major health problem especially common amongst the scheduled tribes and schedules castes of central India but spreads throughout Indian society. The sickle cell gene in India is of the Asian haplotype (Kulozik et al. 1986) representing a fourth and independent occurrence of the mutation against a different genetic background from that in African people. Indian sickle cell disease tends to be milder than that observed in Africa because it is associated with high frequencies of alpha thalassaemia and markedly increased foetal haemoglobin (HbF), both factors likely to reduce sickling. Despite this, bone pain crises and other serious problems continue to be common causes of distress and hospital admissions. With the widespread recognition of the disease in India, several States have enacted Sickle Cell Control legislation and population screening has become a priority in Gujarat, Maharastra and Chhattisgarh. The Chhattisgarh programme started in October 2007, and the results from subjects in 2087 villages of Raipur District have already been presented (Patra et al. 2011). This programme has now screened over 1 million subjects in Raipur and six surrounding districts and has two objectives, the detection of patients with an SS phenotype who are referred for clinical care, and the education and counselling of subjects with the sickle cell trait in order to reduce the number of births affected by sickle cell disease. The organization and some results of this programme are presented as a model which others may wish to use.
Material and methods
The target population was aged 3–15 years accessed primarily through the schools. The site of screening was usually the village school and the principal local contacts were the headmaster or the village headman. The programme started in the district around the capital Raipur but has now completed six surrounding districts. Up to four visiting teams are in the field each day and each team comprises a supervisor, counsellor, medical technologist, attendant, and driver.
Population sampling
In rural areas, the sampling framework focused on groups of villages (panchayats), then blocks until the district had been covered. For urban areas such as Raipur, different sampling procedures were necessary, offering screening at schools but also providing screening camps in slums, construction sites and community centres. In the State of Chhattisgarh, there are 27 districts of which sampling has been completed in seven. The social categorization was selected by the subject into one of four main groupings according to the Constitution of India into scheduled tribes (ST), scheduled castes (SC), other backward classes (OBC), and general which included all those falling outside these groupings.
Procedure
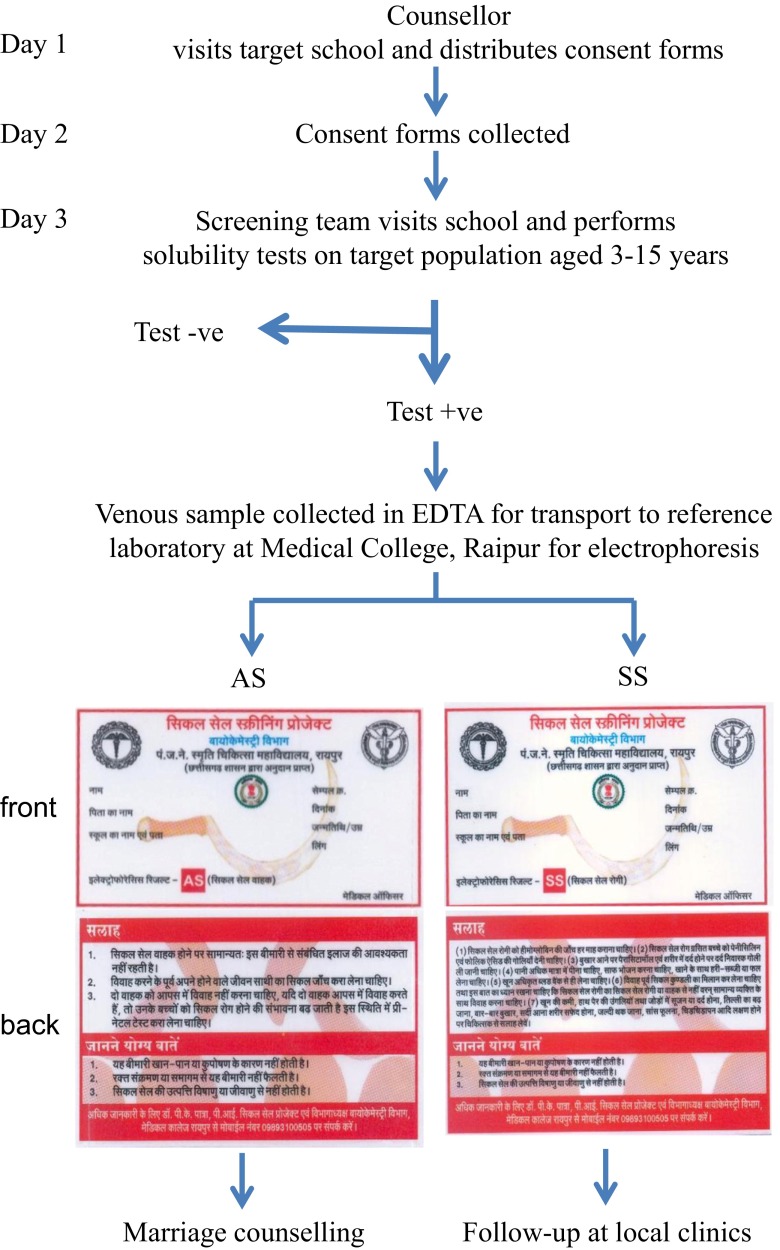
The first village contact was made by the counsellor who visits the village, explains the programme to the head teacher or village headman and provides consent forms for distribution. These forms state the objectives of the programme and the procedures involved. These are collected the next day, and a visit by the screening team is scheduled. At this visit, the technologist collects 10 μl blood by finger prick and performs solubility tests immediately. Subjects with positive solubility tests have a venipuncture of a 3-ml EDTA sample which is carried to the Department of Biochemistry, Pt. JNM Medical College, Raipur for electrophoresis, high pressure liquid chromatography (HPLC) and further tests as necessary. Approximately 5–7 days after the screening visit, a doctor and counsellor visit the site and distributes laminated report cards of abnormal phenotypes along with counselling material. Individuals with an SS phenotype are subjected to health checkup by the doctor and families of subjects with an AS genotype are given genetic and marriage counselling advice. The screening protocol was approved by Institutional Ethical Committee, and the flow sheet is summarized in Fig. 1. The estimated cost of each solubility test, materials and labour is 17 INR (US$0.28), for electrophoresis 37 INR (US$0.60) and for HPLC 308 INR (US$5.00).
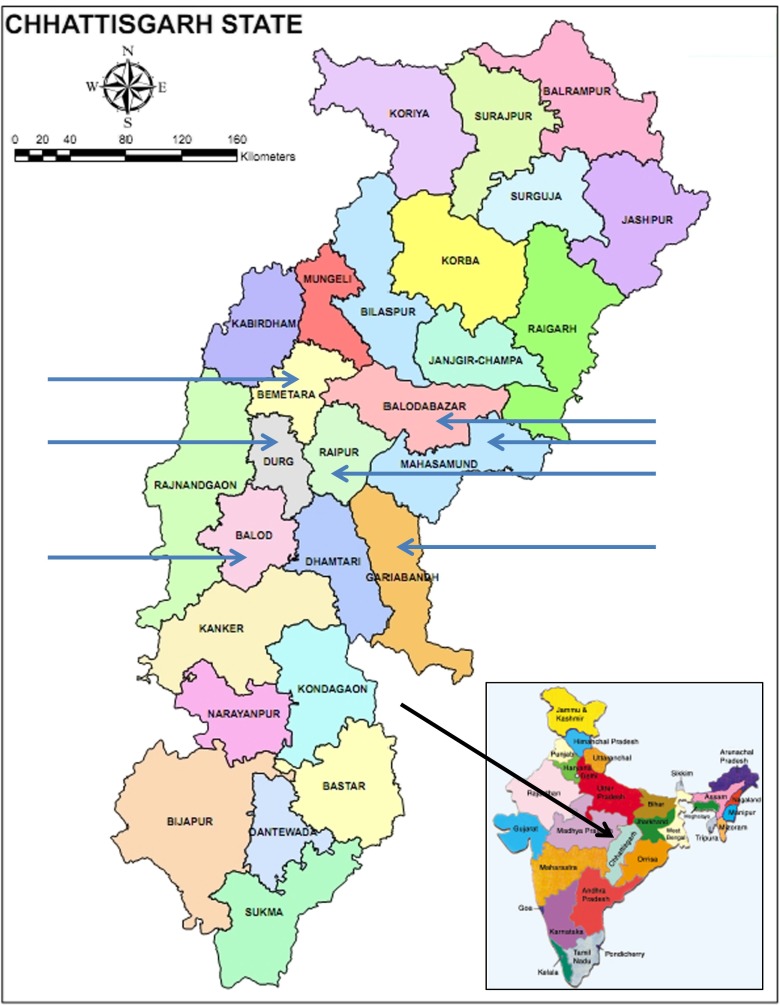
Fig. 1.
The location of Chhattisgarh and its Districts. Sampled districts indicated by arrows
Technical methods
The solubility test buffer was made each week in the base laboratory by dissolving 215 g of anhydrous dipotassium hydrogen phosphate (K2HPO4), 169 g of anhydrous potassium dihydrogen phosphate (KH2PO4) and 5 g of saponin in 1 L of distilled water. Adding 0.1 g of sodium dithionite to each 10 ml of buffer shortly before use was found to stabilize the buffer especially under conditions where refrigeration may not be possible. Before release to the field, each buffer was tested with positive and negative controls. Ten microlitre of blood was obtained by finger prick from each subject and was added to glass tubes containing 1 ml of the buffer; if the patient was clinically anaemic, 20 μl of blood was taken by finger prick. After 5 minutes, a cloudy haemolysate denoted a positive test for HbS whereas negative tests remained clear. Venipuncture samples were taken from all subjects with positive solubility tests and were subjected to haemoglobin electrophoresis on cellulose acetate at pH 8.4 stained with Ponceau S which allowed differentiation of the sickle cell trait from SS phenotypes. Subjects with negative solubility tests were assumed to have an AA phenotype since this is the most likely interpretation, but it is recognized that this group could include cases of beta thalassaemia trait and HbD Punjab trait.
Statistical methods
The relative proportions of AA, AS and SS phenotypes were tested for deviation from the Hardy-Weinberg Equilibrium by using the exact test (Wigginton et al. 2005). Variations in phenotype frequency between the seven districts and between the four social groupings were explored by fitting a single multinomial regression model using phenotype as outcome and district and social group as possible determinants of phenotype. The association between each possible predictor and phenotype outcome was tested using a post-regression likelihood ratio. The size of these associations was quantified using relative risk ratios derived from the multinomial regression model, comparing each region to Raipur, and comparing each social class to the General social class.
Results
The seven sampled districts are shown in the map of Chhattisgarh State (Fig. 1) and the flow chart for the sampling procedures and examples of the cards in Fig. 2. The haemoglobin phenotype and social category for 1,050,440 subjects are presented in Table 1 with frequencies of the sickle cell trait of 9.64 % and for the SS phenotype of 0.29 %. The observed and expected frequencies of phenotypes confirmed a significant excess of SS subjects in all regions except for Balod where the frequencies were balanced and for Balodabazar which showed a significant deficiency of the SS phenotype (Table 2). The relative risk ratios for the sickle cell trait amongst the social groups after adjusting for District compared to the General group depicted as unity showed the highest risk amongst the OBC’s (relative risk 1.80, 95 % confidence intervals 1.74–1.86), followed by the Scheduled Castes (1.63, 1.57–1.69), and the Scheduled Tribes (1.35, 1.30–1.40). The relative risk ratios for the SS phenotype were broadly similar. The modelled probability by District of AS subjects is depicted in increasing trait frequency in Fig. 3, and from this, it is clear that there is a deficiency of SS subjects in Baloda. Similar modelling by social group confirms that the OBC’s are most commonly affected.
Fig. 2.
Execution plan of screening programme
Table 1.
Distribution of haemoglobin phenotype results
| Phenotypes | Social categories | ||||
|---|---|---|---|---|---|
| ST | SC | OBC | General | Total | |
| Raipur district | |||||
| Solubility -ve | 10,051 | 42,969 | 135,402 | 27,209 | 215,631 |
| AS | 1380 (12.04 %) | 3407 (7.33 %) | 15,168 (10.05 %) | 1595 (5.53 %) | 21,550 |
| SS | 32 (0.25 %) | 102 (0.22 %) | 418 (0.28 %) | 34 (0.12 %) | 586 |
| Total | 11,463 | 46,478 | 150,988 | 28,838 | 237,767 |
| Baloda Bazar District | |||||
| Solubility -ve | 21,009 | 44,761 | 100,828 | 6193 | 172,791 |
| AS | 2286 (9.79 %) | 2690 (5.67 %) | 12,814 (11.25 %) | 519 (7.72 %) | 18,309 |
| SS | 45 (0.19 %) | 33 (0.07 %) | 257 (0.23 %) | 14 (0.21 %) | 349 |
| Total | 23,340 | 47,484 | 113,899 | 6726 | 191,449 |
| Gariyaband District | |||||
| Solubility -ve | 23,261 | 10,146 | 38,659 | 3056 | 75,122 |
| AS | 2608 (10.05 %) | 1560 (13.23 %) | 4723 (10.85 %) | 243 (7.34 %) | 9134 |
| SS | 92 (0.35 %) | 84 (0.71 %) | 144 (0.33 %) | 12 (0.36 %) | 332 |
| Total | 25,961 | 11,790 | 43,526 | 3311 | 84,588 |
| Mahasamund District | |||||
| Solubility -ve | 35,901 | 20,153 | 70,164 | 8977 | 135,195 |
| AS | 3880 (9.72 %) | 3242 (13.78 %) | 10,484 (12.93 %) | 684 (7.06 %) | 18,290 |
| SS | 133 (0.33 %) | 146 (0.62 %) | 412 (0.51 %) | 22 (0.23 %) | 713 |
| Total | 39,914 | 23,541 | 81,060 | 9683 | 154,198 |
| Durg District | |||||
| Solubility -ve | 8885 | 14,009 | 97,388 | 11,861 | 132,143 |
| AS | 996 (10.04 %) | 1121 (7.39 %) | 9391 (8.77 %) | 610 (4.88 %) | 12,118 |
| SS | 32 (0.32 %) | 30 (0.20 %) | 344 (0.32 %) | 18 (0.14 %) | 424 |
| Total | 9913 | 15,160 | 107,123 | 12,489 | 144,685 |
| Balod District | |||||
| Solubility -ve | 23,806 | 8039 | 56,858 | 1928 | 90,631 |
| AS | 3133 (11.58 %) | 1051 (11.53 %) | 6567 (10.32 %) | 135 (6.54 %) | 10,886 |
| SS | 115 (0.42 %) | 29 (0.32 %) | 201 (0.32 %) | 1 (0.05 %) | 346 |
| Total | 27,054 | 9119 | 63,626 | 2064 | 101,863 |
| Bemetara District | |||||
| Solubility -ve | 6089 | 22,902 | 89,673 | 5972 | 124,636 |
| AS | 667 (9.85 %) | 1227 (5.08 %) | 8,664 (8.79 %) | 385 (6.05 %) | 10,943 |
| SS | 18 (0.27 %) | 24 (0.10 %) | 263 (0.27 %) | 6 (0.09 %) | 311 |
| Total | 6774 | 24,153 | 98,600 | 6363 | 135,890 |
| Combined totals | |||||
| Solubility -ve | 129,002 | 162,979 | 588,972 | 65,196 | 946,149 (90.07 %) |
| AS | 14,950 | 14,298 | 67,811 | 4171 | 101,230 (9.64 %) |
| SS | 467 | 448 | 2039 | 107 | 3061 (0.29 %) |
| Grand totals | 144,419 | 177,725 | 658,822 | 69,474 | 1,050,440 |
Solubility -ve refers to the initial screening test performed in the villages
Social categories; ST scheduled tribe, SC scheduled caste, OBC other backward classes, General other social groups
Table 2.
Observed and expected phenotype frequencies by district
| District | Phenotype | Equilibrium p valuea | |||
|---|---|---|---|---|---|
| AA | AS | SS | |||
| Raipur | Observed | 215,631 | 21,550 | 586 | 0.0526 |
| Expected | 215,587 | 21,636 | 542 | ||
| Baloda bazar | Observed | 172,791 | 18,309 | 349 | <0.001 |
| Expected | 172,913 | 18,063 | 471 | ||
| Gariyaband | Observed | 75,122 | 9134 | 332 | 0.003 |
| Expected | 75,073 | 9230 | 283 | ||
| Mahasamund | Observed | 135,195 | 18,290 | 713 | <0.001 |
| Expected | 135,112 | 18,455 | 630 | ||
| Durg | Observed | 132,143 | 12,118 | 424 | <0.001 |
| Expected | 132,009 | 12,385 | 290 | ||
| Balod | Observed | 90,631 | 10,886 | 346 | 0.320 |
| Expected | 90,614 | 10,920 | 329 | ||
| Bemetara | Observed | 124,636 | 10,943 | 311 | <0.001 |
| Expected | 124,571 | 11,072 | 246 | ||
| All | Observed | 946,149 | 101,230 | 3061 | <0.001 |
| Expected | 945,830 | 101,866 | 2742 | ||
a p values of the exact test of Hardy-Weinberg Equilibrium (Wigginton et al. 2005)
Fig. 3.
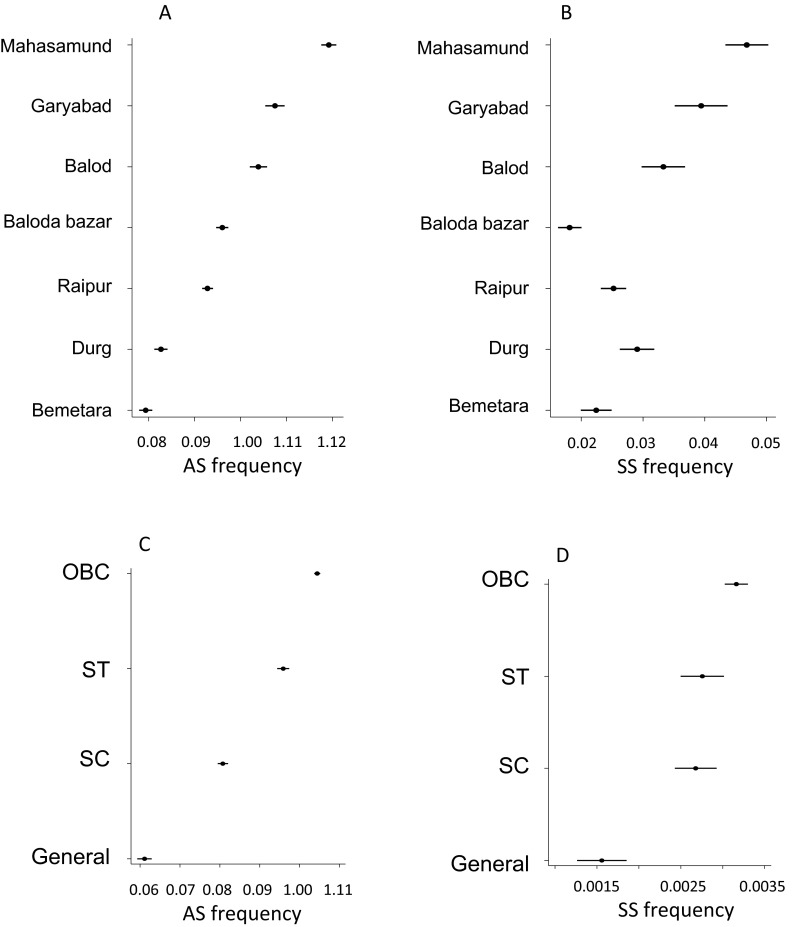
The modelled probability of AS and SS genotypes by region (a, b) and by social group (c, d)
Discussion
The tribal people of India are generally the ancestral inhabitants, usually residing on the Deccan plateau and often in remote rural areas. Tribal people who are especially disadvantaged have been designated Scheduled Tribes by the Constitution of India, and these represent an estimated 8.6 % of the total population of 1.2 billion (Census of India 2011). Similar designation has been applied to the poor lower castes, and the Scheduled Castes represented 16.6 % of the population. The OBC’s or other backward classes are a third group of educationally and socially deprived peoples recognized by the Government of India and amounting to approximately 40 % of the population. The tribal people reach the highest frequency in north-east India but in central India, Chhattisgarh has the highest tribal population.
The sickle cell gene occurs across central India predominantly on the Deccan plateau and in the Nilgiri Hills of Tamil Nadu and Kerala. For several States, the distribution is heterogeneous occurring in the hilly eastern Gujarat, western Odisha and northern Tamil Nadu and Kerala but it is widespread throughout Maharastra, Madhya Pradesh and Chhattisgarh. Following a separate occurrence of the HbS mutation, it is presumed that the distribution and frequency has been influenced by the widespread falciparum malaria which confers a survival advantage on heterozygotes although the effect of malaria on patients with sickle cell disease remains undocumented in India. Because of the commonly associated genetically determined high levels of foetal haemoglobin and of alpha thalassaemia, both of which inhibit sickling, splenic function tends to persist in Indian patients who may be protected against the serious early complications seen in African patients such as pneumococcal septicaemia and acute splenic sequestration. There are no adequate survival data on Indian sickle cell disease in childhood and this lack of information compromises interpretation of the Hardy-Weinberg results. Furthermore, the marriage practices in Indian society following caste endogamy and clan exogamy may be contributing to its high prevalence although the mathematical computations involved are beyond the expertise of the current authors. Overall, the most likely explanation for the apparent excess of subjects with sickle cell disease is believed to be symptomatic selection within the sampled population.
Although commonly believed to be linked with the tribal peoples, the sickle cell gene occurs throughout Indian society (Balgir and Sharma 1988; Kar 1991). Overall, the association of the HbS gene with social groups is dictated by the coincidence in their distributions and although the State of Chhattisgarh falls firmly in the sickle cell belt of central India and has one of the highest populations of tribal peoples, the OBC’s were the group most commonly affected in the current study. Chhattisgarh and indeed much of India is rural with large populations living in villages. The State population from the 2011 Census was 25 million with 77 % classed as rural. Trying to determine the extent of population coverage for each of the districts was difficult without accurate figures for those aged 3–15 years but as a proportion of the censused total for each district, the surveyed subjects varied between 12.2–17.0 % for five of the districts but in districts with the largest populations fell to 8.4 % of the 1.7 million in Durg and to 5.9 % of the 4.1 million in Raipur. The apparently lower coverage in the more populous states most likely reflects a greater difficulty in accessing the target population. It should also be noted that the sampled total of 237,767 for Raipur District in the current report appears to conflict with 359,823 in a previous report (Patra et al. 2011), but this difference resulted from reorganization of the district boundaries between reports.
The diagnostic procedures used in the present study with solubility tests for the detection of HbS performed in the field and haemoglobin electrophoresis on solubility positive samples appear to be relatively robust for the screening of large populations but there are inevitable limitations. Potentially serious are negative solubility tests for persons with the sickle cell trait in the presence of anaemia, and although false negatives may be corrected by a larger volume of blood, this requires preceding detection of the anaemia. Another limitation is that the solubility test fails to detect other haemoglobin variants such as HbD Punjab, HbE or beta thalassaemia. HbD Punjab reaches frequencies of 1–2 % in northwest India and HbE occurs in northeast India but both are uncommon in central India. The beta thalassaemia trait has been estimated to occur in up to 3 % of some of the scheduled tribal groups in Chhattisgarh (Balgir 2011, 2013), and so, the current paper uses the terminology of AA and SS ‘phenotypes’ in recognition of the fact that these include some cases with beta thalassaemia trait and of sickle cell-beta thalassaemia. Extending the laboratory profile to include estimations of HbA2 levels at a local cost of 308 INR (US$5.00) per sample would be financially unrealistic and failure to detect other haemoglobin variants and beta thalassaemia genes, although at a low prevalence, has to be recognized as a shortcoming of this approach.
The overall figures of 9.64 % for the sickle cell trait and of 0.29 % for the SS phenotype are consistent with the Hardy-Weinberg equilibrium, but there were variations between districts, an excess of the SS phenotype being observed in four districts and a deficiency in Raipur, Baloda bazar and Bemetara. A deficiency could result from failure of cases with SS disease from entering the sampled population either by early death, ill health on the day of sampling or reluctance for further blood test in cases known to have the disease but an excess of cases most likely reflects symptomatic presentation to the sampling team. The lack of a precise genetic diagnosis is relatively unimportant for the management of patients with sickle cell disease but assumes greater significance for the subsequent genetic counselling of carriers when persons believed to have an AA phenotype may have a child with sickle cell-beta thalassaemia because they were carrying an undiagnosed beta thalassaemia trait. There is no cost-effective solution to this problem although fortunately the areas with the highest frequency of the beta thalassaemia trait do not generally coincide with the high HbS areas.
The distribution of abnormal results by laminated cards which depict additional information appears to be satisfactory although we have been unable to audit the completeness of this distribution or the success of referral of suspected cases of SS disease to the local health care facilities. Within these limitations, the current programme has allowed the screening of over one million subjects, the coverage appears to be greater in rural areas and this has been achieved at a feasible unit cost. The programme is more successful in the detection of sickle cell disease where prophylaxis and follow-up may prevent many complications but less so for the genetic counselling although the lack of coincidence of high frequencies of beta thalassaemia and sickle cell traits may diminish this concern.
Acknowledgments
The programme is funded by the Government of Chhattisgarh, India. We are grateful to Dr. Marianne Jonker of the Department of Epidemiology and Biostatistics, VU University Medical Center, Amsterdam, the Netherlands for performing the Exact Hardy-Weinberg tests and for other advice. We thank Dr. Hrishikesh Mishra and Dr. GK Sahu, and all colleagues at the Pt. J.N.M. Medical College, Raipur, who have participated and contributed to this programme.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The State Government of Chhattisgarh funded this work and believed that the advantages far outweighed the potential disadvantages of blood tests and that participation in the screening programme assumed agreement with its objectives without the collection of written informed consent. This programme is one of several State sickle cell programmes in India in Gujarat, Maharastra, Chhattigarh and Odisha funded either by the individual States or the National Rural Health Mission for the purpose of improving the health status of disadvantaged populations.
Conflict of interest
Pradeep Patra, Prafulla Khodiar, Ian Hambelton and Graham Serjeant declare that they have no conflict of interest.
References
- Balgir RS. Public health challenges of sickle cell disorders, b-thalassaemia syndrome and G6PD deficiency in scheduled caste and scheduled tribe communities of Central India. Int Public Health J. 2011;3:307–318. [Google Scholar]
- Balgir RS. Spectrum of hemoglobinopathies and evaluation of beta-thalassemia in tribal land of Middle India. Int Public Health J. 2013;5:165–177. [Google Scholar]
- Balgir RS, Sharma SK. Distribution of sickle cell hemoglobin in India. Indian J Hematol. 1988;6:1–14. [Google Scholar]
- Census of India (2011) Government of India, Ministry of Home Affairs, Office of the Registrar General and Census Commissioner, India
- Kar BC. Sickle cell disease in India. J Assoc Physicians India. 1991;39:954–960. [PubMed] [Google Scholar]
- Kulozik AE, Wainscoat JS, Serjeant GR, Kar BC, Al-Awamy B, Esan F, Falusi Y, Haque SK, Hilali AM, Kate S, Ranasinghe WAEP, Weatherall DJ. Geographical survey of βs-globin gene haplotypes: evidence for an independent Asian origin of the sickle-cell mutation. Am J Hum Genet. 1986;39:239–244. [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Cutbush M. Sickle-cell trait in Southern India. Br Med J. 1952;1:404–405. doi: 10.1136/bmj.1.4755.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra PK, Chauhan VS, Khodiar PK, Dalla AR, Serjeant GR. Population screening for the sickle cell gene in Chhattisgarh State, India: an approach to a major public health problem. J Community Genet. 2011;2:147–151. doi: 10.1007/s12687-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR. Genetics and epidemiology of sickle cell anemia in India. ICMR Bull. 1988;9:87–90. [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]