Abstract
Background
Amygdala volume abnormalities have been reported in relation to craving in substance-dependent adults, but it remains unclear if these effects are seen in adolescent marijuana (MJ) users, particularly following abstinence.
Objectives
The aim of this study was to examine the relationship between amygdala volume and craving during 28 days of abstinence in adolescent MJ users.
Methods
MJ-using adolescents (n = 22) aged 16–19 were recruited as part of a larger study on brain function in teen drug users. Craving measures were collected twice per week throughout a 28-day abstinence period. High-resolution anatomical magnetic resonance imaging data were collected at the end of the 28 days of confirmed abstinence. Left and right amygdala volumes were traced by hand (ICC>0.86). Composite scores for self-reported craving and withdrawal symptoms throughout the 28-day abstinence period were calculated to provide four composite measures of total craving, mood, sleep, and somatic complaints.
Results
Results revealed that greater craving during abstinence was significantly associated with smaller left and right amygdala volumes, after controlling for age and gender. Other measures of withdrawal, including mood, somatic complaints and sleep problems, were not related to amygdala morphometry.
Conclusion
These results are consistent with previous findings in adult alcohol-and cocaine-dependent individuals, who demonstrated a relationship between reduced amygdala volumes and increased craving. Future studies are needed to determine if these brain-behavior relationships are attributable to MJ use or predate the onset of substance use.
Keywords: Adolescence, amygdala, craving, marijuana, withdrawal
Introduction
Marijuana (MJ) is the most widely used illicit substance in the United States (1). Recreational MJ use is common among teenagers (2), and 40% of high school students who have used MJ in the past year met criteria for MJ abuse or dependence (3). This is of particular concern because MJ use during adolescence may interrupt ongoing neurodevelopment.
While it is known that brain size stabilizes around age five (4), crucial neurodevelopments continue throughout adolescence and into young adulthood, including white matter myelination (5), synaptic refinement (6) and reductions of gray matter volumes (7). The impact of MJ use on amygdala volume is important since the amygdala undergoes volumetric increases during adolescence (8–10), at a time when MJ use is increasingly common. It has been suggested that the increased risk-taking behavior in adolescents is related to earlier development of subcortical limbic regions in relation to the prefrontal cortex (PFC; 11–15). In particular, adolescents have demonstrated exaggerated amygdala versus PFC activation compared with children and adults (16), further emphasizing this region's importance in the context of adolescent brain development.
Chronic MJ use during adolescence has been associated with aberrant brain structure and function. Studies have reported decreased white matter integrity (17,18), volumetric differences (19–22), and abnormalities in brain activation during tests of spatial working memory (23–25), executive function (26), memory (25,27), auditory verbal working memory (28,29), verbal learning (30) and inhibitory processing (31). Our group has also reported that amygdala morphometry may interact with gender (22) such that female MJ users exhibited larger right amygdala volumes and increased internalizing symptoms compared with female controls; male users did not differ from male controls. In young adults, MJ craving is also related with amygdala activation as measured through fMRI (32). However, amygdala morphometry and potential relationships with withdrawal and craving symptoms have not yet been examined in adolescent MJ users.
In adults, smaller amygdala volumes have been associated with greater self-reported craving of alcohol (33) and cocaine (34). Similarly, adults with cannabis dependence demonstrated smaller bilateral amygdala volumes compared with controls (35). The current study examined relationships between amygdala volumes and craving and other withdrawal symptoms in abstinent, MJ-using adolescents. We hypothesized that greater withdrawal symptoms, especially craving, over a one-month period of abstinence would predict smaller amygdala volumes.
Methods
Participants
MJ-using adolescents were recruited from local schools and provided consent in accordance with the UCSD Human Research Protections Program. As part of a larger study (31), adolescents aged 16–19 meeting eligibility requirements (right handedness, parent/guardian consent, and 200 or more lifetime MJ use episodes) were recruited. Exclusionary criteria included history of psychotropic medication use, DSM-IV Axis I disorder other than cannabis abuse or dependence, neurologic problems, learning disabilities, prenatal substance exposure and MRI contraindications.
Procedure
A detailed description of procedures has been described previously (31). Participants were asked to abstain from all illicit drugs and alcohol during a 28-day monitoring period. Monitored urine toxicology screens were performed every 3–4 days (36) utilizing cloned enzyme donor immunoassay kits (CEDIA DAU, Microgenics, Fremont, CA, USA) detecting recent use of MJ (indicated by THCCOOH), amphetamines, methamphetamines, benzodiazepines, cocaine, barbiturates, codeine, morphine, phencyclidine or ethanol. Quantitative indices were tracked to determine if THC metabolite levels, after controlling for creatinine, decreased during the 28-day period (37). Participants who initially screened positive for THC were accepted and retained, as long as THCCOOH/creatinine ratios decreased continually over the 28 days. Self-report of withdrawal and craving symptoms were collected at each drug toxicology session. Following confirmed abstinence for 28 days, all adolescents completed a MRI examination.
Measures
Demographics and verbal intelligence estimate
A structured clinical interview assessed demographics and psychosocial functioning. The Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary subtest provided an estimate of verbal intellectual functioning (38).
Drug use, craving and withdrawal
The Customary Drinking and Drug Use Record (39) assessed lifetime and past three-month alcohol, nicotine and other drug use, lifetime and current DSM-IV abuse and dependence criteria, withdrawal symptoms and other consequences of substance use. The Timeline Follow-Back (40) determined substance use patterns for the 30-days prior to enrolling in the study. The Cannabis Withdrawal Symptom Checklist (41) assessed MJ-related craving and withdrawal symptoms, including mood, somatic complaints, and sleep problems, on a 10-point Likert scale (0 = no intensity; 10 = most intense). Composite scores were calculated by averaging across all time-points.
Psychiatric history
The computerized NIMH Diagnostic Interview Schedule for Children was administered to youth and parents to detect major psychiatric disorders in adolescents (42). Similar modules of the Computerized Diagnostic Interview Schedule (43) were used for 18 and 19-year-old participants living independently (44).
Current mood
The Beck Depression Inventory (BDI) (45) total score assessed current mood and has previously been used with adolescents (46). The Hamilton Anxiety Rating Scale (HARS) (47) was used to determine anxiety levels and has also been used with adolescents (48).
MRI image acquisition and processing
Anatomical images were collected on a 3T GE scanner using a sagittally-acquired structural image collected with an inversion recovery prepared 3D T1-weighted SPGR sequence (TR = 8 ms, TE = 3 ms, flip angle = 12°, field of view = 240 mm, 176 continuous slices, 1 mm3 voxels, acquisition time = 7:19). This acquisition provides high tissue contrast, permitting volumetric analyses.
Intracranial volumes were obtained to separate brain from non-brain material from each anatomical dataset. Semi-automated skull stripping (49) was used, followed by manual editing in Analysis of Functional NeuroImages (AFNI) (50). Manual tracing of the amygdala region of interest occurred on high-resolution grayscale images in standard AC-PC space. Amygdala regions were reliably (ICC>0.86) traced by trained research assistants (TM, CP, and JP) blind to participant characteristics, on contiguous slices in the coronal plane using a protocol based on Richardson and colleagues (51) and Sheline and colleagues (52). Boundaries were as follows: (i) Anterior boundary: first coronal slice in which the temporal stalk merges with the white matter of the insula; (ii) Dorsal boundary: entorhinal sulcus separating the basal forebrain and temporal lobe; (iii) Posterior boundary: head of hippocampus as evident in sagittal view; (iv) Ventral boundary: horizontal boundary extending from the anterior and ventral hippocampal edge; (v) Medial boundary: presence of the subarachnoid space; and (vi) Lateral boundary: surrounding the white matter. Ratios were created from amygdala divided by intracranial volumes to control for individual variability in intracranial space in all analyses (8).
Data analyses
Descriptive statistics were performed to describe the sample and determine if substance use characteristics, age, gender, and family history of SUD were potential covariates. Descriptive characteristics correlated with the outcome measure were included in all subsequent analyses. Primary analyses included a series of linear regressions to determine whether craving or other withdrawal symptoms (mood, somatic complaints, appetite or sleep problems) reported during abstinence predicted amygdala volumes after controlling for covariates. Exploratory analyses were performed to examine factors that may be related to significant results from the primary analysis. Statistical decisions were made if p<0.05.
Results
Sample characteristics
MJ users [lifetime use episodes M (SD) = 436.9 (361.0)] reported low to no affective symptoms [BDI M (SD) = 3.1 (2.6) and HARS M (SD) = 3.7 (5.5)], and were generally from high socioeconomic backgrounds [parental salary M (SD) = 109.3 (93.8)]. MJ users endorsed numerous lifetime alcohol use episodes [M (SD) = 220.5 (178.6)] and performed within the average to high-average ranges in verbal intellectual ability (Vocabulary T score), [M (SD) = 56.1 (8.0)] (see Table 1). None of the potential covariates tested were significantly correlated with amygdala volumes and were therefore not included in the regression model.
Table 1.
Characteristics of abstinent adolescent marijuana users (n = 22).
| M (SD) | |
|---|---|
| Age | 17.8 (0.9) |
| % Female | 23% |
| % Caucasian | 55% |
| Pubertal development scale score | 3.5 (0.4) |
| Parent annual salary | 109.3 (93.8) |
| WASI Vocabulary T-score | 56.1 (8.0) |
| Beck Depression Inventory Score | 3.1 (2.6) |
| Hamilton Anxiety Rating Scale | 3.7 (5.5) |
| Lifetime alcohol use episodes | 220.5 (178.6) |
| Years of MJ use | 2.6 (1.6) |
| Age first used MJ regularly (weekly) | 14.5 (3.6) |
| Lifetime MJ use episodes | 436.9 (361.0) |
| MJ craving score (1 question) | 16.3 (23.2) |
| Mood score (5 questions) | 44.4 (45.3) |
| Sleep score (5 questions) | 82.4 (57.6) |
| Appetite score (1 question) | 8.9 (12.9) |
| Somatic score (5 questions) | 21.7 (23.4) |
| Intracranial volume (in cc3) | 1574.6 (129.0) |
| Left amygdala volume (in cc3) | 2.0 (0.3) |
| Right amygdala volume (in cc3) | 2.3 (0.4) |
WASI, Wechsler Abbreviated Scale of Intelligence; MJ, marijuana.
Primary results
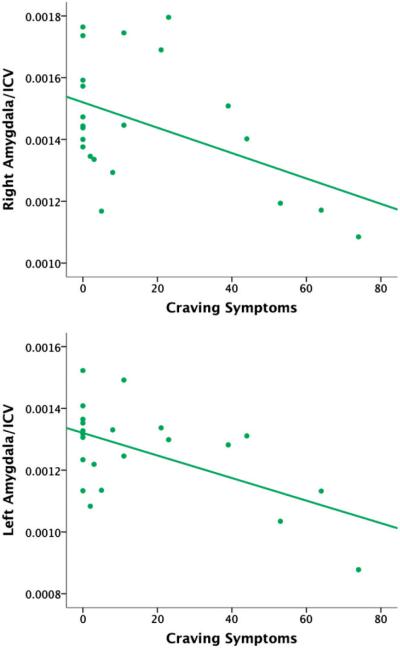
Primary results revealed that following 28 days of abstinence, average craving across the period of abstinence significantly predicted of smaller right (t = −2.32, B = −0.46, p = 0.031, see Figure 1) and left (t = −3.09, B = −0.57, p = 0.006, see Figure 1) amygdala volumes. Follow-up repeated measures general linear model showed that self-reported craving across the nine visits did not significantly change over time (F = 0.49, p = 0.70) and are graphically represented in Figure 2. The relationship between total craving and amygdala volume did not differ for males as compared to females in this sample and remained unchanged when controlling for age and gender for both right (t = −2.46, B = −0.51, p = 0.024) and left (t = −2.87, B = −0.57, p = 0.010) amygdala volumes. Craving was correlated with a number of marijuana dependence symptoms as well as number of hits smoked per month in the three months prior to study enrollment. After adjusting for these variables as covariates, left (t = −2.93, B = −0.77, p = 0.009) and marginally right (t = −1.78, B = −0.53, p = 0.091) amygdala volumes were significantly related to self-reported craving. Other symptoms of MJ withdrawal, including mood, sleep, appetite and somatic complaints, were not related to amygdala morphometry in this sample.
Figure 1.
Scatterplot of craving symptom scores predicting right and left amygdala to intracranial volume ratios in 22 abstinent adolescent marijuana users.
Figure 2.
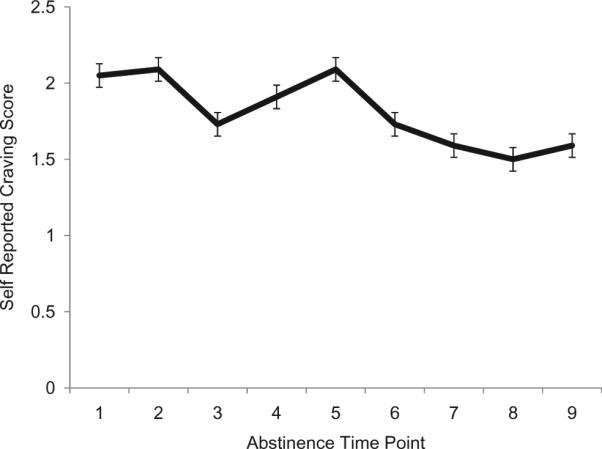
Average craving rating for chronic marijuana users across nine time-points collected over 28 days of abstinence.
Exploratory analyses
Bivariate correlations demonstrated that self-reported craving was not associated with THC metabolites (p<0.05) in this sample, even when controlling for creatinine levels. Similarly, THC metabolites were not related to duration of marijuana use or lifetime marijuana use. THC metabolite levels were correlated with a number of marijuana dependence symptoms at the first four toxicology visits (r = 0.55, p = 0.009; r = 0.60, p = 0.003; r = 0.53, p = 0.01; r = 0.48, p = 0.024, respectively) as well as average THC metabolite level across the 28 days (r = 0.45, p = 0.034). However these results were no longer significant after controlling for creatinine levels (p>0.05).
Discussion
The current study revealed that higher levels of self-reported craving during a 28-day monitored abstinence period were significantly associated with smaller left and right amygdala volumes in a sample of chronic MJ-using adolescents. This association was present even after controlling for age and gender. Findings are consistent with previous reports in adult alcohol and cocaine users, who demonstrated relationships between smaller amygdala volumes and greater craving (33,34). However, the results are novel in that this was a community sample of non-treatment seeking heavy MJ users as young as 16 years old.
Despite consistent findings of smaller amygdala volumes with greater drug craving for various substances in adults (33,34) and now adolescents, the underlying mechanism is not fully understood. Candidate theories include focal damage specific to the amygdala, or more widespread alterations to brain networks involved in craving, such as frontolimbic circuits which play a role in reward evaluation, affective processing and emotional regulation (53,54). Specifically, individuals who are more vulnerable to emotion dysregulation, which is known to be associated with amygdala dysfunction (55), may also experience more self-reported craving while undergoing abstinence. Furthermore, amygdala abnormalities may actually predate drug use and serve as an underlying risk factor for the initiation of heavy drug use, but this hypothesis could not be examined using the current cross-sectional data. Filbey and colleagues found greater amygdala activation to cue-induced craving in the amygdala (32). The observed volume reductions may help explain why greater activation is associated with craving (i.e. hyperactivation of regions to compensate for reduced volumes).
Heavy exposure to MJ that is sufficient to cause withdrawal symptoms, especially craving, may cause cellular changes in the CB1-dense amygdala (55–58). It is also possible that heavy MJ use and subsequent craving may disrupt normal amygdala development that is typically characterized by an increase in volume during adolescence (9). However, the current sample did not reveal a relationship between THC metabolites and self-reported craving across the 28 days of abstinence. THC metabolites were related to number of marijuana dependence symptoms in this sample, suggesting a relationship with dependence severity. Future longitudinal studies should examine how MJ use may affect the developmental trajectory of the amygdala as well as moderating factors that may indicate a more severe substance use patterns that elicit craving, such as number of dependence symptoms as observed in the current study. Drug craving may also serve as a marker for MJ addiction severity, emotional reactivity to withdrawal or neurocognitive impact of acute abstinence. Additionally, amygdala volumes may undergo continued normalization with prolonged sobriety; Makris et al. (59) reported increases in limbic region volumes following abstinence among adult alcohol-dependent individuals (59).
Limitations of the present study should be considered. The study was cross-sectional so it is not possible to infer cause-effect relationships. Additionally the sample was relatively small. Adolescent MJ users typically consume alcohol (2) but alcohol was not related to amygdala volumes in this study. Nonetheless, it is difficult to fully disentangle the effects that may be attributable to alcohol use or cessation. Unfortunately due to a shortage in female MJ users, we had insufficient power to properly examine gender differences beyond examining gender as a covariate. This will be crucial in future studies given the known gender differences in amygdala development (14) as well as drug use (60). Lastly, due to the high socioeconomic status and lack of comorbid psychological disorders, these results may not generalize to other samples.
In conclusion, the present study suggested that heavy MJ-using adolescents with their levels of craving during one month of abstinence show smaller bilateral amygdala volumes. It is unknown if this relationship predates or is a consequence of chronic MJ exposure during adolescence. Future studies are needed to examine amygdala morphometry and function longitudinally to test whether abnormal brain-behavior relationships predict relapse.
Acknowledgements
Work for this article was supported, in part, by the Department of Veterans Affairs, Office of Academic Affiliations, VA Advanced Fellowship in Mental Illness Research and Treatment. This research was supported by NIDA grants DA15228 and DA021182. Writing of this manuscript was also supported, in part, by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs, and the Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC).
Footnotes
Because Dr. Padula is an employee of the U.S. Government and contributed to the manuscript as part of her official duties, the work is not subject to US copyright.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- 2.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings, 2008. National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- 3.Chen K, Sheth AJ, Elliot DK, Eagerm A. Prevalence and correlates of past-year substance use, abuse, and dependence in a suburban community sample of high-school students. Addictive Behav. 2004;29:413–423. doi: 10.1016/j.addbeh.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Jernigan TL, Gamst AC. Changes in volume with age – consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Huttenlocher PR, Dabholkar AS. Regional difference in synapto-genesis in human cerebral cortex. J Computat Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent III TF, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giedd JN, Vaituzis AD, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comparat Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AD, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biolog Psychiatry. 1997;21:1185–1205. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 10.Giedd JN. The teen brain: insights from neuroimaging. J Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Casey BJ, Galvan A, Getz S. The adolescent brain. Developmental Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:688–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BJ, Jones RM, Hare TA. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cognit. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biolog Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatric Res. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bava S, Frank L, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang T, Tapert SF. Prefrontal morphometry in abstinent adolescent marijuana users: subtle gender effects. Addiction Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal asymmetry. Exposures during adolescent development: are neurotoxic risks increased? Neurotox Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina KL, Nagel BJ, Tapert SF. Cerebellar vermis abnormality in adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interactions in abstinent adolescent marijuana users. Psychol Addictive Behav. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 26.Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behav. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Millsaps CL, Azrin RL, Mittenberg W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescents. J Child Adolesc Subst Abuse. 1994;3:47–55. [Google Scholar]
- 28.Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- 29.Jager G, Block RI, Luijten M, Ramsey NF. Marijuana use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolescent Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biolog Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berlin) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- 34.Makris N, Gasic GP, Seidmen L, Goldstein J, Gastfriend D, Albaugh DM, Hodge SM, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 36.Fraser AD, Coffin L, Worth D. Drug and chemical metabolites in clinical toxicology investigations: The importance of ethylene glycol, methanol and cannabinoid metabolite analyses. Clinical Biochemistry. 2002;35:501–511. doi: 10.1016/s0009-9120(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 37.Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 39.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 40.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 41.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- 42.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0. (DIS 4.0) Washington University; St Louis, MO: 1996. [Google Scholar]
- 44.Thompson LL, Riggs PD, Mikulich SK, Crowley TJ. Contribution of ADHD symptoms to substance problems and delinquency in conduct disordered adolescents. J Abnormal Child Psychol. 1996;24:325–347. doi: 10.1007/BF01441634. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT. Beck depression inventory (BDI) Psychological Corporation; San Antonio, TX: 1978. [Google Scholar]
- 46.Bennett DS, Ambrosini PJ, Bianchi M, Barnett D, Metz C, Rabinovich H. Relationship of Beck Depression Inventory factors to depression among adolescents. J Affective Disord. 1997;45:127–134. doi: 10.1016/s0165-0327(97)00045-1. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 48.Clark DB, Donovan JE. Reliability and validity of the Hamilton Anxiety Rating Scale in an adolescent sample. J Am Acad Child Adolesc Psychiatry;1994;33:354–360. doi: 10.1097/00004583-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Brady M, Smith S. Segmentation of brain MR images though a hidden random field model and the expectation-maximization algorithm. IEEE Trans Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 50.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 51.Richardson EJ, Griffith HR, Martin RC, Paige AL, Stewart CC, Jones J, Hermann BP, et al. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy Behav. 2007;10:242–249. doi: 10.1016/j.yebeh.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Sheline YL, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 53.LeDoux J. The emotional brain, fear, and the amygdala. Cellular Molec Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pessoa L. On the relationship between emotion and cognition. Nature Rev. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 55.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 56.Marco EM, Rubino T, Adriana W, Viveros MP, Parolaro D, Laviola G. Long term consequences of URB597 administration during adolescence on cannabinoid CB1 receptor binding in brain areas. Brain Res. 2009;1257:25–31. doi: 10.1016/j.brainres.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Molec Cellular Endocrinol. 2008;286:108–113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, et al. Decreased volume of the brain reward system in alcohol. Biolog Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendochrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]