Abstract
We report here the nearly complete 1H, 15N and 13C resonance assignment of the La motif and RNA recognition motif 1 of human LARP6, an RNA binding protein involved in regulating collagen synthesis.
Keywords: LARP6, La motif, RRM1, La related protein, RNA binding protein
Biological context
LARP6 is a member of the La related proteins (LARP) superfamily and it has been implicated in several developmental events including myogenesis, neurogenesis and possibly metastasis (Bayfield et al. 2010; Bousquet-Antonelli and Deragon 2009). In vertebrates, LARP6 regulates collagen synthesis by binding to a conserved stem-loop in the 5′ untranslated region (UTR) of the mRNAs encoding the collagen α1(I) and α2(I) subunits, thereby coordinating their translation into the heterotrimeric collagen type I (Blackstock et al. 2014; Cai et al. 2010a, b). This interaction is mediated by a conserved RNA binding unit present in LARP6, named the La module, which comprises two domains, a La motif (LaM) and an RNA recognition motif (RRM1). The La module was first discovered in the founding member of the LARPs, the La protein, where the LaM and RRM1 were shown to work in synergy to recognise 3′ UUUOH RNA targets (Alfano et al. 2004; Bayfield et al. 2010; Kotik-Kogan et al. 2008). Although the La module is conserved across the LARP superfamily, the recognised RNA targets are not, and this RNA binding versatility is thought, at least in part, to account for the different cellular processes in which LARPs are involved (Bayfield et al. 2010). Contrary to the archetype La protein, for which high resolution structures of several domains in the apo and bound form, as well as biophysical insights into its RNA binding properties, have been reported (Jacks et al. 2003; Alfano et al. 2004; Teplova et al. 2006; Kotik-Kogan et al. 2008; Martino et al. 2012), the LARPs are much less well understood and the mechanism by which La modules of LARPs can recognise a great variety of RNA molecules, with different shapes and sequences, is still elusive.
To understand in detail the RNA recognition mechanism of LARPs we embarked on a structural and biophysical analysis of the LaM and RRM1 from human LARP6 (HsLARP6). Interestingly, this study revealed that the relative orientation of the LaM and RRM1, mainly dictated by the sequence and structure of the interdomain linker, could play a key role in RNA target discrimination by the La module (Martino et al. 2015). These investigations illustrate the complexity of protein-RNA recognition by underscoring the importance of modular types of interaction in achieving binding specificity and affinity.
Since malfunction of collagen production is connected to a number of fibroproliferative disorders the investigation of the RNA binding mechanism of human LARP6 may be exploited in the future for rational drug design. Here we report the backbone and the sidechain NMR assignment of the LaM and RRM1 of human LARP6.
Protein expression and purification
For our NMR studies, two human LARP6 domains, the LaM, encompassing residues 70–183, and RRM1, residues 180–295, were prepared as follows. Both domains were cloned into pET-Duet1 vector (Novagen) with an N-terminal histidine tag followed by a TEV-cleavage site. 15N and 15N/13C labelled recombinant proteins were produced in Escherichia coli Rosetta II, growing transformed bacteria in minimal media enriched with 0.8 g L−115N-ammonium chloride and 2 g L−113C glucose, and induced at 18 °C for 14 h. Cell pellets were resuspended in 50 mM Tris, pH 8.0, 300 mM NaCl, 10 mM imidazole, 5 % glycerol, 2 mM PMSF (phenylmethanesulfonyl fluoride) and lysozyme, and lysed by sonication. Following centrifugation, the proteins were purified by nickel affinity chromatography on a 5 mL HisTrap column (GE Healthcare) following the manufacturer’s protocol. The N-terminal histidine tag was removed by overnight incubation with TEV protease (TEVpro) (at TEVpro:HsLARP6 molar ratio of 1:50) at 4 °C in 50 mM Tris, pH 8.0, 100 mM KCl, 0.2 mM EDTA, 1 mM dithiothreitol (DTT). The reaction mixture was subsequently applied to a Ni–NTA column (Qiagen) to remove the cleaved tags, the His6-tagged TEVpro and any undigested product, and the cleaved LARP6 proteins were dialysed overnight in 50 mM Tris pH 7.25, 100 mM KCl, 0.2 mM EDTA, 1 mM DTT. The proteins were finally purified on a 5 mL Hi-Trap heparin column (GE Healthcare) and eluted with a linear 0–2 M KCl gradient in 25 mM Tris pH 7.25, 10 % glycerol, 1 mM DTT. The LaM and RRM1 were dialysed in 20 mM Tris pH 7.25, 100 mM KCl, 50 mM arginine glutamate salt (Golovanov et al. 2004), 1 mM DTT and 20 mM Tris pH 7.25, 100 mM KCl, 1 mM DTT respectively.
NMR spectroscopy
NMR samples contained ~0.5 mM protein in 95 % H2O/5 % D2O or 99 % D2O at pH 7.25 in 100 mM KCl, 50 mM arginine glutamate salt, 1 mM DTT for the LaM and in 20 mM Tris pH 7.25, 100 mM KCl, 1 mM DTT for RRM1. All NMR spectra for backbone and sidechain resonance assignment were collected at 298 K on a Varian Inova spectrometer operating 18.8 T and on Bruker Avance spectrometers at 14.1 and 16.4 T equipped with triple resonance cryoprobes. Backbone resonances were assigned in a sequential manner using [1H,15N]-HSQC, HNCA, HN(CO)CA, HNCACB, HN(CO)CACB and HNCO experiments (Grzesiek and Bax 1993). Sidechain resonances were obtained using [1H,15N]-HSQC, [1H,13C]-HSQC HCCH-TOCSY15N-edited NOESY-HSQC and 13C-edited NOESY-HSQC spectra (Fesik et al. 1988). NMR data were processed using NMRPipe/NMRDraw (Delaglio et al. 1995) and visualised/assigned using CcpNMR Analysis 2.2 (Vranken et al. 2005) software and/or CARA/Neasy software (Bartels et al. 1995). Chemical shifts were referenced to internal 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS).
Extent of the assignment and data deposition
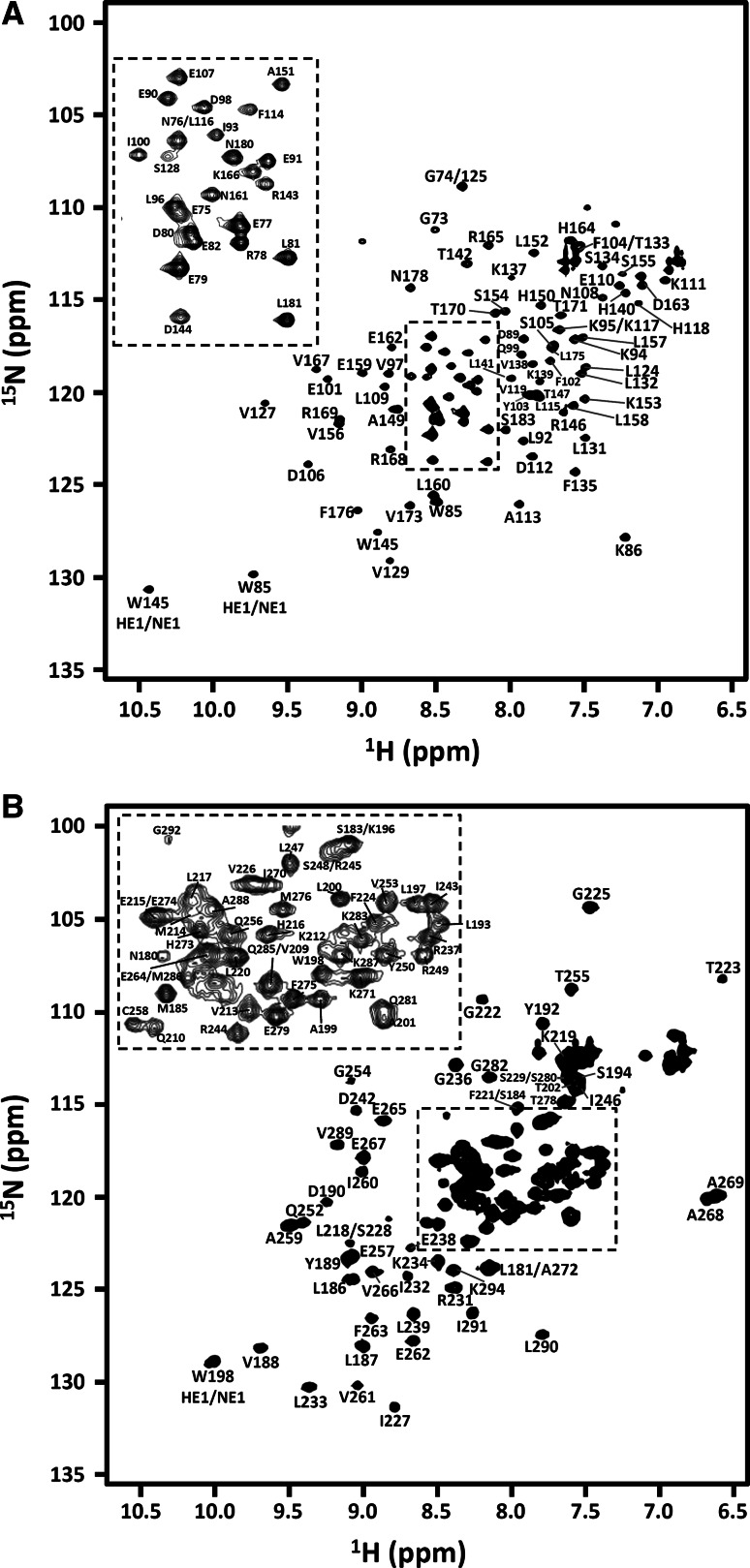
Figure 1 shows the assigned [1H, 15N]-HSQC spectra for the LaM (a) and RRM1 (b) acquired at 298 K. The dispersion of the resonances in both cases suggests that both domains are well folded. The LaM is made of 114 residues of which 6 are prolines and 3 are glycines. For the backbone, 97/107 NH, 102/114 Hα, 84/114 CO, 106/114 Cα and 100/111 Cβ resonances were successfully assigned. This corresponds to 90, 89, 74, 92 and 90 % of the NH, Hα, CO, Cα and Cβ resonances respectively and implies an 87 % complete backbone assignment. Around 78 % of aliphatic sidechain (position γ onwards) and 65 % of the aromatic side chains 1H and 13C assignments have also been made. The RRM1 has 116 residues with 7 prolines and 7 glycines. Here the backbone assignment was 88 % complete: assignments have been obtained for 104/108 NH (95 %), 97/116 Hα (84 %), 93/116 CO (80 %), 111/116 Cα (96 %) and 93/109 Cβ (85 %) resonances. In addition, around 82 % and 72 % of aliphatic and aromatic 13C and 1H sidechain resonances have been assigned respectively.
Fig. 1.
[1H, 15N]-HSQC spectra for human LARP6 LaM (a) and RRM1 (b) recorded at 298 K on a Bruker Avance spectrometer working at 16.4 T. Backbone chemical shifts assignments are labelled for both domains. Residue types and numbers are indicated. Residue numbering corresponds to native sequence. For both spectra, top left panels in dotted squares show an expansion of the central crowded [1H, 15N] HSQC region for clarity. The difference in linewidths between the two domains reflects a different sample behavior in the experimental conditions used, confirmed by relaxation analysis (Martino et al. 2015)
Most of the missing assignments in the two domains correspond to residues T70, A71, S72, Q83, R120, R121, N122, K123, Y126, K130, K136 for the LaM and Q204, K205, N206, G207, S251 for the RRM1, for which peaks in the [1H,15N]-HSQC spectra could not be observed. None of the exchangeable sidechain protons of Arg and Lys residues was identified, nor the sidechain amide groups of most Asn and Gln.
Secondary structures were derived from backbone chemical shifts and estimates for ψ/φ dihedral angles were obtained using TALOS+ (Shen et al. 2009). As expected, the secondary structure elements predicted for HsLARP6 LaM closely resemble what was previously found for human La LaM (Alfano et al. 2003). Interestingly, the topology for the HsLARP6 RRM1 was found to be β1α0′α1β2α1′β3α2β4; this domain therefore contains two non-canonical secondary structure elements located in the loop between β1 and α1 (α0′) and the loop between β2 and β3 (α1′) in addition to the expected four β-strands (β1–β4) and two α-helices (α1 and α2) typical of the RRM fold. These findings were confirmed in the high resolution structure recently obtained (Martino et al. 2015).
The chemical shift data were deposited in the BioMagResBank (http://www.bmrb.wisc.edu/) under the accession numbers 25159 and 25160 for the LaM and RRM1 respectively.
Acknowledgments
This work was supported by the Wellcome Trust through a Capital Award for the Centre for Biomolecular Spectroscopy to MRC (085944/Z/08/Z). LM was recipient of a long-term EMBO Fellowship and NJHS of a MRC studentship funding his MRes in Molecular Biophysics.
Conflict of interest
None declared.
References
- Alfano C, Babon J, Kelly G, Curry S, Conte MR. Resonance assignment and secondary structure of an N-terminal fragment of the human La protein. J Biomol NMR. 2003;27:93–94. doi: 10.1023/A:1024741228802. [DOI] [PubMed] [Google Scholar]
- Alfano C, Sanfelice D, Babon J, Kelly G, Jacks A, Curry S, Conte MR. Structural analysis of cooperative RNA binding by the La motif and central RRM domain of human La protein. Nat Struct Mol Biol. 2004;11:323–329. doi: 10.1038/nsmb747. [DOI] [PubMed] [Google Scholar]
- Bartels C, Xia TH, Billeter M, Guntert P, Wuthrich K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J Biomol NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- Bayfield MA, Yang R, Maraia RJ. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs) Biochim Biophys Acta. 2010;1799:365–378. doi: 10.1016/j.bbagrm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstock CD, Higashi Y, Sukhanov S, Shai SY, Stefanivic B, Tabony AM, Yoshida T, Delafontaine P. Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5′ stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem. 2014;289:7264–7274. doi: 10.1074/jbc.M113.518951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Antonelli C, Deragon JM. A comprehensive analysis of the La-motif protein superfamily. RNA. 2009;15:750–764. doi: 10.1261/rna.1478709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Fritz D, Stefanovic L, Stefanovic B. Binding of LARP6 to the conserved 5′ stem-loop regulates translation of mRNAs encoding type I collagen. J Mol Biol. 2010;395:309–326. doi: 10.1016/j.jmb.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Fritz D, Stefanovic L, Stefanovic B. Nonmuscle myosin-dependent synthesis of type I collagen. J Mol Biol. 2010;401:564–578. doi: 10.1016/j.jmb.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Fesik SW, Luly JR, Erickson JW, Abad-Zapatero C. Isotope-edited proton NMR study on the structure of a pepsin/inhibitor complex. Biochemistry. 1988;27:8297–8301. doi: 10.1021/bi00422a001. [DOI] [PubMed] [Google Scholar]
- Golovanov AP, Hautbergue GM, Wilson SA, Lian LY. A simple method for improving protein solubility and long-term stability. J Am Chem Soc. 2004;126:8933–8939. doi: 10.1021/ja049297h. [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Bax A. Measurement of amide proton exchange rates and NOEs with water in 13C/15N-enriched calcineurin B. J Biomol NMR. 1993;3:627–638. doi: 10.1007/BF00198368. [DOI] [PubMed] [Google Scholar]
- Jacks A, Babon J, Kelly G, Manolaridis I, Cary PD, Curry S, Conte MR. Structure of the C-terminal domain of human La protein reveals a novel RNA recognition motif coupled to a helical nuclear retention element. Structure. 2003;11:833–843. doi: 10.1016/S0969-2126(03)00121-7. [DOI] [PubMed] [Google Scholar]
- Kotik-Kogan O, Valentine ER, Sanfelice D, Conte MR, Curry S. Structural analysis reveals conformational plasticity in the recognition of RNA 3′ ends by the human La protein. Structure. 2008;16:852–862. doi: 10.1016/j.str.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino L, Pennell S, Kelly G, Bui TT, Kotik-Kogan O, Smerdon SJ, Drake AF, Curry S, Conte MR. Analysis of the interaction with the hepatitis C virus mRNA reveals an alternative mode of RNA recognition by the human La protein. Nucleic Acids Res. 2012;40:1381–1394. doi: 10.1093/nar/gkr890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino L, Pennell S, Kelly G, Busi B, Brown P, Atkinson RA, Salisbury NJH, Ooi ZH, See KW, Smerdon SJ, Alfano C, Bui TT, Conte MR. Synergic interplay of the La motif, RRM1 and the interdomain linker of LARP6 in the recognition of collagen mRNA expands the RNA binding repertoire of the La module. Nucleic Acids Res. 2015;43:645–660. doi: 10.1093/nar/gku1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplova M, Yuan YR, Phan AT, Malinina L, Ilin S, Teplov A, Patel DJ. Structural basis for recognition and sequestration of UUU(OH) 3′ temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol Cell. 2006;21:75–85. doi: 10.1016/j.molcel.2005.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]