Abstract
Ocean warming and acidification are threatening marine ecosystems. In marine animals, acidification is thought to enhance ion regulatory costs and thereby baseline energy demand, while elevated temperature also increases baseline metabolic rate. Here we investigated standard metabolic rates (SMR) and plasma parameters of Atlantic cod (Gadus morhua) after 3–4 weeks of exposure to ambient and future PCO2 levels (550, 1200 and 2200 µatm) and at two temperatures (10, 18 °C). In vivo branchial ion regulatory costs were studied in isolated, perfused gill preparations. Animals reared at 18 °C responded to increasing CO2 by elevating SMR, in contrast to specimens at 10 °C. Isolated gills at 10 °C and elevated PCO2 (≥1200 µatm) displayed increased soft tissue mass, in parallel to increased gill oxygen demand, indicating an increased fraction of gill in whole animal energy budget. Altered gill size was not found at 18 °C, where a shift in the use of ion regulation mechanisms occurred towards enhanced Na+/H+-exchange and HCO3− transport at high PCO2 (2200 µatm), paralleled by higher Na+/K+-ATPase activities. This shift did not affect total gill energy consumption leaving whole animal energy budget unaffected. Higher Na+/K+-ATPase activities in the warmth might have compensated for enhanced branchial permeability and led to reduced plasma Na+ and/or Cl− concentrations and slightly lowered osmolalities seen at 18 °C and 550 or 2200 µatm PCO2 in vivo. Overall, the gill as a key ion regulation organ seems to be highly effective in supporting the resilience of cod to effects of ocean warming and acidification.
Keywords: Na+/K+-ATPase, H+-ATPase, HCO3− transporter, Na+/H+-exchanger, Standard metabolic rate, Osmolality
Introduction
Anthropogenic climate change has profound impacts on marine ecosystems as the oceans become warmer and are acidified by the uptake of atmospheric carbon dioxide (Pörtner et al. 2014). Depending on emission scenario atmospheric PCO2 levels are projected to reach between 420 and 940 µatm by the year 2100, consistent with an average decrease in surface ocean pH by 0.13–0.42 units. Most of the ocean will continue to warm although rates vary regionally and differ highly between emission scenarios (Collins et al. 2013). Initial studies projected that highly mobile organisms such as marine fishes are excellent osmotic and acid–base regulators and therefore better able to cope with acidification than e.g. more inactive invertebrates with lower ion regulation capacity (Melzner et al. 2009; Wittmann and Pörtner 2013). More recent behavioural studies, mainly of coral reef fish larvae have suggested that some fishes may still be very sensitive when ambient CO2 increases (e.g. Munday et al. 2012; Nilsson et al. 2012; Forsgren et al. 2013). Behavioural effects on sensitive fish species set in at rather low concentrations (<850 µatm CO2); however, the long-term persistence of these phenomena remains to be explored (Wittmann and Pörtner 2013). In fact, Atlantic cod appears resilient to behavioural disturbances at CO2 levels around 1000 µatm (Jutfelt and Hedgärde 2013) and up to 4200 µatm (Maneja et al. 2013). In juvenile cod, aerobic swimming performance was unaffected by exposure to high PCO2 (3000 and 6000 µatm) at close to optimum temperatures (Melzner et al. 2009). However, larval stages may be more sensitive. Severe tissue damage was reported for a fraction of the larval offspring of Norwegian coastal cod (Frommel et al. 2011). In contrast, the tissues of early larval stages of Baltic cod remained unaffected up to 3200 µatm PCO2 (Frommel et al. 2013). Nonetheless, Atlantic cod avoid hypercapnic water, indicating that elevated PCO2 may have unfavourable effects (Jutfelt and Hedgärde 2013).
More is known about the sensitivity of fish including Atlantic cod to temperature changes (for review see Pörtner et al. 2008; Pörtner and Peck 2010). In cod, the thermal range and associated growth performance characterise populations in a latitudinal cline. The thermal window of performance is wide in juvenile fishes and shrinks with increasing body size, especially due to a weight dependent shift in thermal optima and upper thermal limits to cooler temperatures (Pörtner et al. 2008). The warm-induced decline in performance indicates thermal limitation and has been attributed to the onset of a mismatch between oxygen supply capacity and costs on the one hand and oxygen demand on the other hand, at the so-called pejus temperatures. In common eelpout (Zoarces viviparus) pejus limits were identified to parallel the onset of ecological effect, a loss in field abundance due to warming extremes (Pörtner and Knust 2007). Progressive warming beyond a putative critical limit of 16 °C caused a sharp drop in venous PO2 (PVO2), in parallel to the onset of cardiac arrhythmia in cannulated cod (Lannig et al. 2004; it should be noted that cannulation may enhance circulatory cost and cause a downward shift of these limits). As cardiac activity did not compensate for reduced PVO2 in the warmth, reduced oxygen supply to tissues and thus decreased aerobic scope of the whole organism above pejus limits was proposed as the first level of thermal limitation (Lannig et al. 2004; Pörtner et al. 2004). Thermal constraints may affect the scope of energy dependent mechanisms sustaining homeostasis, such as ion and acid–base regulation, and their share in energy budget, especially if challenged by ocean acidification. These changes may feedback on thermal tolerance.
Water breathers exposed to elevated PCO2 in seawater need to re-establish acid–base equilibria through proton equivalent ion exchange. In marine fish, ion regulation mainly takes place in the gills causing a net uptake of bicarbonate within 20–30 min, varying with the extent of acid–base disturbance (Evans et al. 2005). Membrane proteins, including Na+/H+-exchangers, V-type H+-ATPase, HCO3−-transporters and Na+/HCO3 co-transporters contribute to these processes and have associated energetic costs (e.g. Claiborne et al. 2002; Deigweiher et al. 2008; Heuer and Grosell, 2014). Many studies have focused on the identification and localization of the proteins involved in acid–base regulation applying immunological and molecular techniques, while knowledge of in vivo usage of specific transporter and associated metabolic costs is scarce. In branchial tissue, Na+/K+-ATPase is generally thought to be the key transporter driving most energy dependent ion transport processes including those related to acid–base regulation (Deigweiher et al. 2008). An upregulation of this important transport protein, determined via analyses of activity in isolated gills or of enzyme capacity in crude gill homogenates in cod, notothenioids, and eelpout gills was observed under CO2 concentrations of >6000 µatm; ranging from acute exposure to up to 1 year of acclimation (Deigweiher et al. 2008; Melzner et al. 2009; Deigweiher et al. 2010). In contrast, cod acclimated long term to moderately elevated PCO2 (2500 µatm) revealed reduced branchial energy turnover in vivo at unchanged in vitro Na+/K+-ATPase capacity (Kreiss et al. 2015).
Here we conducted a follow-up experiment with transporter specific inhibitors in order to identify the mechanisms contributing to acid–base regulation. We exposed Atlantic cod from the Kattegat/Skagerrak to different levels of PCO2 and temperature for 4 weeks. Atlantic cod is expected to experience strong anthropogenic climate change (Drinkwater 2005), as its habitat on the European Shelf is estimated to undergo one of the highest rates of change in seasonal temperature maxima and minima (Taboada and Anadón 2012). The North Sea region might also exceed the globally predicted average of acidification and reach 1000 µatm CO2 already around the year 2060 (Blackford and Gilbert 2007). In our experiments, we used 10 °C as the optimum temperature, which is close to average habitat temperature and represents the maximum growth temperature of young adults of this species (Pörtner et al. 2008). For the high temperature treatment, we chose 18 °C as this is well in the range of maximum summer values experienced by Atlantic cod (Neat and Righton 2007; Righton et al. 2010). PCO2 levels were selected to cover present and future natural variability at the study site: 550 µatm was chosen as a low value, 1200 µatm as a medium and 2200 µatm as a high CO2 partial pressure. Seawater PCO2 in the Atlantic in general is characterised by a seasonal cycle with surface waters being oversaturated during winter, summer and autumn, while being undersaturated during spring (Takahashi et al. 1993; Gypens et al. 2011). PCO2 also increases in parallel to seasonal temperature. We hypothesise that the combination of elevated temperature and PCO2 will affect the energy demand at whole animal level, as a consequence of shifts in ion regulation and associated energy (re)allocation. We also hypothesise that changes in branchial Na+/K+-ATPase usage under high PCO2 and/or temperature will reflect altered activities of other ion transporters and lead to altered plasma ionic status.
Materials and methods
Experiments using live animals were approved according to ethical commission Dnr.: 23-2012, Sweden’s Ethical Committee on Animal Experiments.
Experimental animals
Atlantic cod Gadus morhua of mixed gender (200.52 ± 95.4 g FW) were caught in fish traps in the Gullmarfjord around the Sven Lovén Centre for Marine Sciences—Kristineberg (Fiskebäckskil, Sweden) in February/March 2012 (permission: Dnr.: 3157-11). At the Marine Station, the fish were held for 2–3 weeks in 1200 l tanks with natural flowing seawater at 10 °C. For the experiments, fish were tagged individually (Visible Implant Elastomer, Northwest Marine Technology Inc. Washington, USA) while anaesthetized with MS-222 (3-aminobenzoic ethyl ester, Applichem, Darmstadt; Germany) at a concentration of 0.2 g/l. Afterwards animals were exposed for 4 weeks to 10.3 ± 0.2 °C and 18.1 ± 0.2 °C at 553 ± 78 µatm CO2, 1470 ± 460 µatm CO2 and 2228 ± 312 µatm CO2 (Table 1). These values encompassed the range of interannual PCO2 fluctuations reported for the Gullmarsfjord from ~500 to 1000 µatm, paralleled by pH oscillations of about 0.15 between 10 °C (more alkaline) and 18 °C (more acidified, Swedish Oceanographic Data Centre (SODC), pH data recorded in the Gullmarsfjord from 1921 to 1989, recalculated by S. Dupont). 8–10 fish were incubated per 1200 l tank, with two replicate tanks per treatment. Fish in the high temperature treatment were directly exposed to 18 °C without gradual warming. CO2-partial pressures of 1200 and 2200 µatm equivalent to pH 7.7 and 7.5 were maintained by a computerised feedback system (Aqua Medic GmbH, Bissendorf, Germany), which regulates pH (NBS scale) by addition of gaseous CO2 to the seawater tank (±0.02 pH units). Fish reared at low PCO2 were maintained in aerated natural seawater. To ensure a consistent mixture of the water, mass pumps with a power of 40 l/min (Eheim GmbH, Deizisau, Germany) were inserted in the tanks. The cod were fed three times a week until satiation with frozen shrimp and blue mussels and were maintained under a 12:12 day: night cycle. Animals were starved for 48 h prior to preparation and experiments. Water chemistry was controlled twice a week by measuring pH and temperature (portable pH metre Profi line pH 3310, WTW GmbH, Weilheim, Germany, NIST Scale corrected to total scale via Dickson standards). Total alkalinity was measured photometrically with an accuracy of 10 µmol/kg−1 seawater according to Sarazin et al. (1999). For total dissolved inorganic carbon (DIC), an autoanalyser (SFA QuAAtro 800 TM, SEAL, Wisconsin, USA) was used; salinity was measured with a conductivity metre (Cond 1970i, WTW GmbH, Weilheim, Germany). PCO2 values were calculated using the CO2sys programme [constants of Mehrbach et al. (1973); refitted by Dickson and Millero (1987) developed by Lewis and Wallace (1998) (Table 1)].
Table 1.
Water parameters of different treatments during animal incubation with two replicates each (1 and 2)
| Parameter | Low tank 1 | Low tank 2 | Medium tank 1 | Medium tank 2 | High tank 1 | High tank 2 |
|---|---|---|---|---|---|---|
| Temperature | 10.6 ± 0.2 | 10.4 ± 0.2 | 10.3 ± 0.1 | 10.1 ± 0.1 | 10.3 ± 0.2 | 10.1 ± 0.1 |
| PCO2 [µatm] | 504.36 ± 54.52 | 488.51 ± 52.42 | 1151.00 ± 120.78 | 1111.84 ± 75.67 | 2184.54 ± 338.4 | 2131.62 ± 317.7 |
| pH (NBS scale) | 8.13 ± 0.03 | 8.13 ± 0.03 | 7.76 ± 0.02 | 7.74 ± 0.03 | 7.50 ± 0.06 | 7.50 ± 0.03 |
| pH (total scale) | 7.97 ± 0.04 | 7.98 ± 0.04 | 7.65 ± 0.02 | 7.63 ± 0.04 | 7.37 ± 0.06 | 7.38 ± 0.04 |
| Salinity [PSU] | 32.6 ± 0.3 | 32.6 ± 0.2 | 32.6 ± 0.5 | 32.5 ± 0.5 | 32.6 ± 0.2 | 32.6 ± 0.2 |
| DIC [µmol/L] | 2325.5 ± 65.6 | 2297.1 ± 42.9 | 2363.9 ± 31.4 | 2373.6 ± 66.2 | 2495.0 ± 90.0 | 2325.5 ± 65.6 |
| Alkalinity | 2326.4 ± 68.9 | 2337.0 ± 44.9 | 2330.2 ± 34.5 | 2327.8 ± 24.2 | 2329.6 ± 28.8 | 2327.4 ± 29.8 |
| Temperature | 17.9 ± 0.2 | 18.0 ± 0.1 | 18.2 ± 0.1 | 18.1 ± 0.1 | 18.2 ± 0.2 | 18.2 ± 0.1 |
| PCO2 [µatm] | 595.94 ± 59.75 | 623.08 ± 50.30 | 1278.01 ± 80.16 | 1305.32 ± 82.96 | 2366.74 ± 340.3 | 2214.85 ± 229.4 |
| pH (NBS scale) | 8.04 ± 0.05 | 8.02 ± 0.01 | 7.77 ± 0.02 | 7.76 ± 0.02 | 7.50 ± 0.06 | 7.53 ± 0.05 |
| pH (total scale) | 7.91 ± 0.03 | 7.89 ± 0.03 | 7.61 ± 0.02 | 7.60 ± 0.02 | 7.36 ± 0.06 | 7.39 ± 0.04 |
| Salinity [PSU] | 32.0 ± 1.2 | 32.0 ± 1.0 | 32.6 ± 0.3 | 32.6 ± 0.3 | 32.0 ± 1.1 | 32.1 ± 1.0 |
| DIC [µmol/l] | 2296.5 ± 58.5 | 2279.4 ± 44.9 | 2338.7 ± 4.1 | 2364.5 ± 66.2 | 2370.7 ± 62.4 | 2390.2 ± 81.2 |
| Alkalinity | 2326.0 ± 41.4 | 2315.0 ± 32.7 | 2326.0 ± 28.0 | 2334.0 ± 30.0 | 2324.7 ± 38.8 | 2326.6 ± 38.9 |
Oxygen consumption of whole fish
Standard metabolic rate (MO2min) of Atlantic cod was measured by intermittent-flow respirometry of individual fish (six measured in parallel) starting after 3 weeks of experimental exposure of 12 fish per treatment (including six fish from each replicate tank). The fish were starved for 48 h in their exposure tanks before placing them into the respirometer, a custom-built sealed 3 l horizontal cylindrical acrylic respirometer with a circulation pump (5 l/min) (Eheim GmbH & Co., Deizisau Germany), equipped with an in-line robust 3 mm oxygen optode (FireSting, Pyroscience, Aachen, Germany). The optode was connected to the optical oxygen meter (FireStingO2) which in turn was connected to a PC running the Pyro Oxygen Logger (FireSting Pyroscience, Aachen, Germany). The fish were kept undisturbed in the respirometer for 48 h with continuous 20 min measurements followed by 10 min of flushing, controlled by a timer. The O2 saturation in the respirometers was always above 80 %. The mean of the lowest 10 % of recorded MO2 values is presented as standard metabolic rate, calculated as M(O2) = [µmol (O2) × gFW−1 × h−1]. During respirometry of fish at 18 °C and low PCO2, two fish died from asphyxiation due to water pump failure interrupting the flow. Whole animal as well as gill oxygen consumption rates of the remaining fish from the same tank were significantly reduced compared to the replicate tank. As we cannot preclude that the fish released chemical alarm signals in response to hypoxia (Lebedeva et al. 1994) or excreted catecholamines (Butler et al. 1989), which might induce decreased metabolism (Wahlquivst and Nilsson 1977), we excluded the respective data from further analyses. In later studies, all fish subjected to respiration protocols displayed parameters such as blood ion concentration and osmolality or those investigated in isolated gill experiments identical to those not exposed to respirometry.
Animals and isolated gill preparation
Fish were anaesthetised with 0.2 g/l MS222 and identified by the tag. Blood samples were taken from the ventral vein for later analysis of osmolality and ionic composition. Subsequent to killing the animals by cutting their spine, gill arches were quickly dissected. The first two arches from each side of five fish (10 per treatment and replicate) were used for experiments and immediately placed in ice-cold saline. They were cleared from blood by use of a syringe flushing the efferent blood vessel with saline containing heparin (5000 U/l). The preparation of isolated gill arches was conducted according to Kreiss et al. 2015. Gill arches were suspended by their perfusion tubing attached to the chamber lid, while a magnetic stir bar within the chamber ensured constant mixing of the respiratory medium. The perfusion saline was prepared according to Holmgren and Nilsson (1974) with the exception of HCO3, which was calculated after Heisler (1984, 1986) according to Kreiss et al. 2015 (10 °C, 550 µatm PCO2: 11.50 mM; 10 °C, 1200 µatm PCO2: 14.00 mM; 10 °C, 2200 µatm PCO2: 16.18 mM; 18 °C, 550 µatm PCO2: 7.11 mM; 18 °C, 1200 µatm PCO2: 8.63 mM; 18 °C 2200 µatm PCO2: 10.00 mM).
The pH of the Ringer solution was adjusted by a computerized feedback system using gaseous CO2 (Aqua medic GmbH Bissendorf, Germany) to 7.95 ± 0.05 for treatments at 10 °C and to 7.88 ± 0.07 for treatments at 18 °C. Seawater for respiration measurements was equilibrated with a membrane pump (Schemel & Goetz & Co KG, Offenbach, Germany) and reached pH = 8.09 ± 0.06 at 10 °C and low PCO2 and pH = 8.09 ± 0.06 at 18 °C and low PCO2. Equilibration with defined gas mixtures (AGA gas AB, Sweden) led to pH values matching the desired levels of PCO2 (10 °C, medium (1200 µatm): 7.74 ± 0.01; 10 °C, high (2200 µatm): 7.52 ± 0.02; 18 °C, medium: 7.75 ± 0.01; 18 °C, high: 7.55 ± 0.03).
Energy budget of branchial ion transporters
Gill respiration was analysed according to Kreiss et al. (2015). In brief, the oxygen uptake of isolated perfused gill arches from the respiratory medium (seawater with incubation equivalent CO2 levels) was measured in thermostated (10 or 18 °C) chambers, by use of oxygen micro-optodes (needle type, 140 µm, PreSens, Regensburg, Germany). Fractional respiratory costs of branchial ion transporters were investigated via inhibitors for Na+/K+-ATPase (ouabain), H+-ATPase (bafilomycin A1), Na+/H+-exchanger {[5-(N-ethyl-N-isopropyl) amiloride] (EIPA)} and HCO3−-transporter [4,4′-Diisothiocyano-2,2′stilbenedisulfonic acid (DIDS)] applied to the respiratory medium following earlier methodological improvements (Kreiss et al. 2015). Transport inhibitors (obtained from Applichem, Darmstadt, Germany or Sigma-Aldrich, Taufkirchen, Germany) were dissolved in DMSO at a final DMSO concentration of less than 1 %, which had no effect on respiration in control experiments (data not shown). Final concentrations of inhibitors were in the range of literature values: ouabain 5 mM (Krumschnabel and Wieser 1994; Mark et al. 2005) bafilomycin A1 0.1 µM (Morgan and Iwama 1999; Pörtner et al. 2000), EIPA 80 µM (Wu et al. 2010 (100 µM)) and DIDS 1 mM (Duraton et al. 1997; Parks et al. 2007). Each gill arch was used as its own control prior to application of one inhibitor. Control and inhibitor experiments were conducted for a period of 45 min each while recording oxygen consumption. After experimentation, the gill arches were separated from tubing and blotted dry for determining their total weight. Soft tissue was isolated and weighed after cutting as close as possible to the arch. For each of the six treatments and for each inhibitor, a set of ten gill arches from ten individual fish (five per replicate tank) were measured. Oxygen consumption rates were normalised to soft tissue weight (without the cartilaginous arch) as M(O2) = [µmol (O2) × gFW−1 × h−1].
Osmolality and ion composition
Blood was centrifuged for 10 min at 1000g and 4 °C. Osmolality was measured in the resultant plasma with a Vapour Pressure Osmometer (Wescor Inc., Utah, USA). Ion composition was determined chromatographically. Na+ concentration was analysed amongst other cations by use of an Ion Chromatography System (DIONEX-ICS 2000, CA, USA) at 40 °C including an IonPac CS 16 column, with methane sulfonic acid (30 mM) as eluent at 0.36 ml min−1 flow rate. Cation concentrations were calculated according to a cation standard (Dionex, Six Cation Standard, CA, USA). Anions were separated on an Ion Pac AS11HC column with potassium hydroxide (30 mM, flow rate 0.30 ml min−1) as an eluent. Dionex Five Anion Standard was used as a reference to calculate anion concentrations. All ion concentrations are presented as mM. Plasma total CO2 (CCO2) of two treatment groups [10 °C, medium PCO2 (n = 4); 18 °C, high PCO2, (n = 46)] was determined with a carbon dioxide analyser (Corning 965, CIBA, Corning diagnostics, UK). Plasma bicarbonate levels were calculated from total CO2 by subtracting physically dissolved CO2 adopting ambient PCO2 levels.
Data analysis and statistics
Statistical analysis was performed using Sigma plot 12.0 and Graphpad Prism 4. Seawater chemistry was tested for differences between PCO2, temperature and replicate tanks performing repeated measures two-way ANOVAs with subsequent Sidak’s multiple comparison tests (Table 2). All data are depicted as mean ± standard error. Outliers at a 99 % confidence level were identified using Nalimov’s test and removed from the plasma data set. Means of plasma ion concentrations, SMR, net O2 demands of isolated perfused gills and of HCO3−-transporter were compared using two-way ANOVAs with subsequent Tukey multiple comparison tests (Table 3). Osmolality, net O2 demand determined in the remaining isolated gill inhibitor experiments (of Na+/K+-ATPase, Na+/H+-exchanger, H+-ATPase) as well as the fractional weight of gill soft tissue were not normally distributed and therefore compared via one-way ANOVAs or Kruskal–Wallis non-parametric ANOVAs per temperature (Table 4). To test for differences between CO2 concentrations within these datasets, subsequent unpaired t tests with Holm-Bonferroni correction was used. Gill total respiration as the fraction of standard metabolic rate of whole fish (%) was compared within treatments at 10 °C performing a one-way ANOVA, while Holm-Bonferroni corrected unpaired t tests were conducted to detect differences between all treatments. Differences between remaining treatments were also tested via unpaired t tests except for the two low PCO2 groups where only means of gill and whole animal respiration could be compared for low PCO2 fish at 18 °C, due to low n numbers at 18 °C. A significance level of p < 0.05 was adopted for all data. Q10 values were calculated for the mean net oxygen demand of the four transporters investigated in the groups exposed to 10 and 18 °C and the respective PCO2 levels. Unfortunately, fish from the two tanks at 18 °C and low PCO2 could not be clearly assigned to their original tank as these were accidently mixed after respiration experiments. For the remaining groups, we performed t tests to detect potential differences between replicates. Except for the standard metabolic rates of the two groups at low PCO2 and 18 °C (see above) no differences in any of the tested parameters were detected between fish groups from replicate tanks.
Table 2.
Results of repeated measures two-way ANOVAs performed to investigate potential differences between acclimation conditions (PCO2; temperature levels) and replicate tanks within and between these treatments (low PCO2 = 550 µatm; medium PCO2 = 1200 µatm and high PCO2 = 2200 µatm at 10 °C; 18 °C)
| Variable | PCO2 effect | Temperature effect | Replicate effect | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | dF | P | F | dF | P | F | dF | P | F | dF | P | |
| PCO2 | 1446 | 2 | <0.0001 | – | – | – | 6.208 | 3 | 0.007 | 2.241 | 6 | 0.045 |
| Temperature | – | – | – | 39.060 | 1 | <0.0001 | 0.4647 | 1 | 0.4970 | 0.0064 | 1 | 0.6951 |
| Low PCO2 | – | – | – | 34.61 | 1 | <0.0001 | 0.0864 | 1 | 0.7709 | 1.251 | 1 | 0.2729 |
| Medium PCO2 | – | – | – | 26.29 | 1 | <0.0001 | 0.0008 | 1 | 0.9765 | 0.7693 | 1 | 0.3879 |
| High PCO2 | – | – | – | 1.593 | 1 | 0.5697 | 1.067 | 1 | 0.3105 | 0.5697 | 1 | 0.3309 |
| 10 °C | 307.8 | 2 | <0.0001 | – | – | – | 0.2700 | 1 | 0.6115 | 0.0385 | 2 | 0.9623 |
| 18 °C | 352.9 | 2 | <0.0001 | – | – | – | 0.462 | 1 | 0.5078 | 1.838 | 2 | 0.1778 |
Significant differences are presented in bold
Table 3.
Results of two-way ANOVAs performed to investigate potential differences of SMR, plasma ion concentrations and gill oxygen consumption between differently exposed fish groups (low PCO2 = 550 µatm; medium PCO2 = 1200 µatm and high PCO2 = 2200 µatm at 10 °C; 18 °C)
| Variable | PCO2 effect | Temperature effect | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | dF | P | F | dF | P | F | dF | P | |
| SMR | 0.0931 | 2 | 0.911 | 16.36 | 1 | 0.0002 | 2.383 | 2 | 0.1025 |
| Plasma Na+ | 0.318 | 2 | 0.729 | 19.298 | 1 | <0.001 | 6.535 | 2 | 0.003 |
| Plasma Cl− | 3.803 | 2 | 0.027 | 3.642 | 1 | 0.060 | 9.024 | 2 | <0.001 |
| Gill MO2 | 0.9367 | 2 | 0.393 | 89.33 | 1 | <0.0001 | 0.1381 | 2 | 0.8711 |
| HCO3 − -transporter | 7.871 | 2 | 0.001 | 4.297 | 1 | 0.043 | 2.287 | 2 | 0.112 |
Significant differences are presented in bold
Table 4.
Results of one-way ANOVAs, respectively, Kruskal–Wallis one-way ANOVAs performed to investigate potential differences of osmolality and ion transporter net O2 demand from isolated gill experiments between differently exposed fish groups (low PCO2 = 550 µatm; medium PCO2 = 1200 µatm and high PCO2 = 2200 µatm) at 10 °C, respectively, 18 °C
| Variable | 10 °C | 18 °C | ||||
|---|---|---|---|---|---|---|
| F | dF | P | F | dF | P | |
| Osmolality | 0.02401 | 2 | 0.9763 | 6.775 | 2 | 0.0032 |
| Na+/K+-ATPase | 0.878 | 2 | 0.428 | H = 1.355 | 2 | 0.508 |
| H+-ATPase | 0.347 | 2 | 0.710 | 0.592 | 2 | 0.560 |
| Na+/H+-exchanger | 4.025 | 2 | 0.030 | 6.064 | 2 | 0.007 |
| % Gill filament weight | H = 14.96 | 2 | <0.001 | H = 1.561 | 2 | 0.458 |
H value instead of F value is given when non-parametric Kruskal–Wallis test was performed
Significant differences are presented in bold
Results
Seawater chemistry and mortality
Seawater chemistry and temperatures of the 12 tanks are summarised in Table 1. PCO2 and temperature differed significantly (p < 0.0001) between tanks according to experimental conditions (Table 2). Statistical interactions found for PCO2 replicates are explained by differences between data measured at 10 and 18 °C. In general, PCO2 values calculated for 18 °C were above those found at 10 °C (Table 1). This was especially pronounced for the low and medium PCO2 groups (Table 2), however, the difference of approximately 100 µatm was considered negligible from a physiological view. Tank replicates did not differ between any treatment neither for temperature nor for PCO2 levels (Table 2). For PCO2 treatments at 10 and 18 °C, replicates were maximally ±0.5° different (p < 0.0001). This difference might be explained by a slight temperature gradient within the rooms and was considered minor.
Total mortality during incubation was 8.2 % (nine fish); however, almost half of the casualties (four fish) were found at 18 °C and low PCO2, while other losses were evenly distributed between exposure groups. Furthermore, two fish died of asphyxiation (see above) and one animal died from the consequences of jumping out of the tank, leaving us with a total of 99 fish.
Oxygen consumption of whole animals
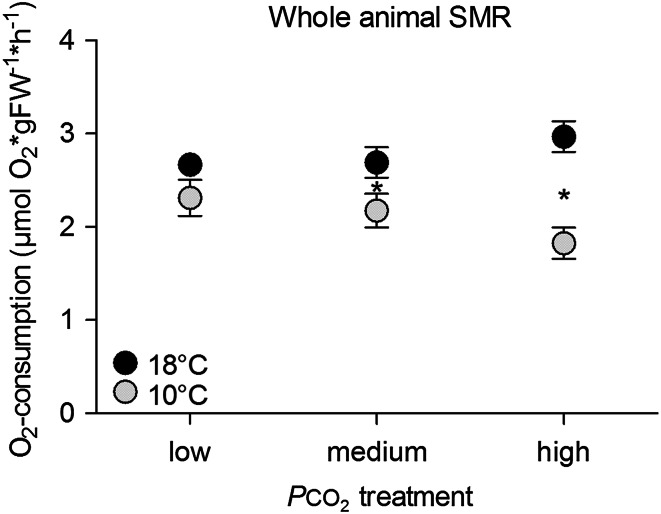
Mean standard metabolic rates related to body mass were in the same range for fish reared at low PCO2 and both incubation temperatures (10 °C: 2.31 ± 0.64 µmol O2 × gFW−1 × h−1; 18 °C: 2.67 ± 0.04 µmol O2 × h−1 × gFW−1) (Fig. 1). Increasing CO2 levels influenced metabolic rate differently depending on incubation temperature (Fig. 1). Significant differences between groups at the two temperatures were observed at either medium or high PCO2 when the fish reared at 18 °C displayed a higher SMR than those acclimated to 10 °C (p = 0.043 at medium PCO2; p < 0.0001 at high PCO2).
Fig. 1.
Standard metabolic rate (µmol O2 × gFW−1 × h−1) of cod 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles). n = 4–12 per treatment. Symbols indicate significant differences between temperature groups (p < 0.05)
Plasma osmolality and ion concentrations
Plasma osmolality (Fig. 2) was independent of CO2 in cod reared long term at 10 °C (optimum temperature, total mean 354.53 ± 31.52 mmol kg−1). It was significantly lower in cod at 18 °C under low and high CO2 compared to fish reared at 18 °C and medium PCO2 (p < 0.05). No statistical temperature effect was detected between fish reared at 10 °C and 18 °C. Plasma ion concentrations (Fig. 3a, b) at low PCO2 and 10 °C were in the range of 188 ± 16 mM for Na+, and 175 ± 21 mM for Cl− (Fig. 3a, b). For fish at 10 °C, both ions were found at ~10 % lower concentrations at medium PCO2 than under low PCO2 (p = 0.057 Na+, p = 0.025 Cl−). At 10 °C and high PCO2, Cl− concentrations fell below values of animals at low PCO2 (15 %; p = 0.004); whereas Na+ plasma levels were only marginally reduced (3 %). Plasma bicarbonate for the medium PCO2 group at 10 °C was in the range of 10.99 ± 0.69 mM.
Fig. 2.
Plasma osmolality (mmol kg−1) of cod 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles). n = 18–20 per treatment. Different letters indicate significant differences between treatment groups (p < 0.05)
Fig. 3.
a, b Blood plasma Na+ and Cl− concentrations (mM) in plasma of cod 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles). n = 18–20 per treatment. Different letters indicate significant differences between treatment groups (p < 0.05)
Warming to 18 °C under low PCO2 led to a ~20 % reduction in the concentrations of both ions (p = 0.012, Fig. 3a, b). At 18 °C and medium PCO2, Na+ and Cl− concentrations were less distinct from the group at 10 °C and low PCO2. This pattern was not observed at high PCO2 and 18 °C such that plasma ion levels at 18 °C were reduced in fish under low and high CO2 levels compared to medium PCO2 (Na+: low PCO2p = 0.002, high PCO2p = 0.004; Cl−: low PCO2p = 0.002, high PCO2p = 0.006) (Fig. 3a, b). For animals from all treatments, mean plasma Na+ concentration exceeded that of Cl− by 14–31 mM positive charges. Plasma bicarbonate for the high PCO2 group at 18 °C was in the range of 9.81 ± 1.68 mM.
Gill fresh weight
Mean gill fresh weight determined per single arch after gill respiration experiments was CO2 dependent at 10 °C. Soft gill tissue and thereby also total gill weight increased in fish at 10 °C and medium or high PCO2 (p < 0.05) compared to the group at 10 °C and low PCO2 (Table 5). This pattern was also mirrored in the fraction of gill soft tissue weight (related to whole animal weight) and thereby independent of an allometric effect (Table 5). At high temperature, no CO2 effect was observed. Weights of total gill and gill soft tissue for the three groups at 18 °C were in the range of those from low PCO2 fish at 10 °C, being significantly lower than the size-increased gills in fish at 10 °C and medium or high PCO2 treatments.
Table 5.
Weight of total gill, respectively, gill soft tissue without cartilaginous arch, as well as fractional soft tissue weight related to whole animal weight of the differently exposed groups (low PCO2 = 550 µatm; medium PCO2 = 1200 µatm and high PCO2 = 2200 µatm at 10 °C; 18 °C) given as mean ± standard deviation, n = 40 per treatment for total gill and soft tissue weight, n = 3–8 for fractional (%) gill weight
| weight (g) | 10 °C low | 10 °C medium | 10 °C high | 18 °C low | 18 °C medium | 18 °C high |
|---|---|---|---|---|---|---|
| Gill arch | 0.52 ± 0.15a | 0.65 ± 0.25bc | 0.69 ± 0.25b | 0.56 ± 0.25ac | 0.57 ± 0.21ac | 0.52 ± 0.20a |
| Gill soft tissue | 0.28 ± 0.08a | 0.36 ± 0.13bc | 0.38 ± 0.15b | 0.26 ± 0.13a | 0.29 ± 0.11ac | 0.26 ± 0.12a |
| % Weight | 0.13 ± 0.07a | 0.27 ± 0.22b | 0.18 ± 0.11bc | 0.17 ± 0.14ab | 0.12 ± 0.04a | 0.16 ± 0.10ab |
Gill respiration and energy budget of ion regulation transporters
Mean oxygen consumption related to gill soft tissue was 10.8 µmol O2 × gFW−1 × h−1 at 10 °C and low PCO2, whereas gills from fish reared at 10 °C and medium PCO2 displayed respiration rates about 15 % lower than those at low PCO2 (p = 0.013) (Fig. 4). Gill oxygen consumption rates at high PCO2 and 10 °C were found between those at the other PCO2 levels, but did not differ significantly from those. Fish acclimated to 18 °C had higher branchial respiration rates than those reared at 10 °C (p < 0.001) (Fig. 4), following a Q10 of 1.8 ± 0.07. Gill oxygen consumption rates at the three PCO2 levels and 18 °C were not statistically different. Again, respiration rates were lowest at medium PCO2 as observed before at 10 °C (p = 0.057, Fig. 4).
Fig. 4.
Gill total oxygen consumption (µmol O2 × gFW−1 × h−1) of cod 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles). n = 40 per treatment. Different letters indicate significant differences between treatment groups (p < 0.05)
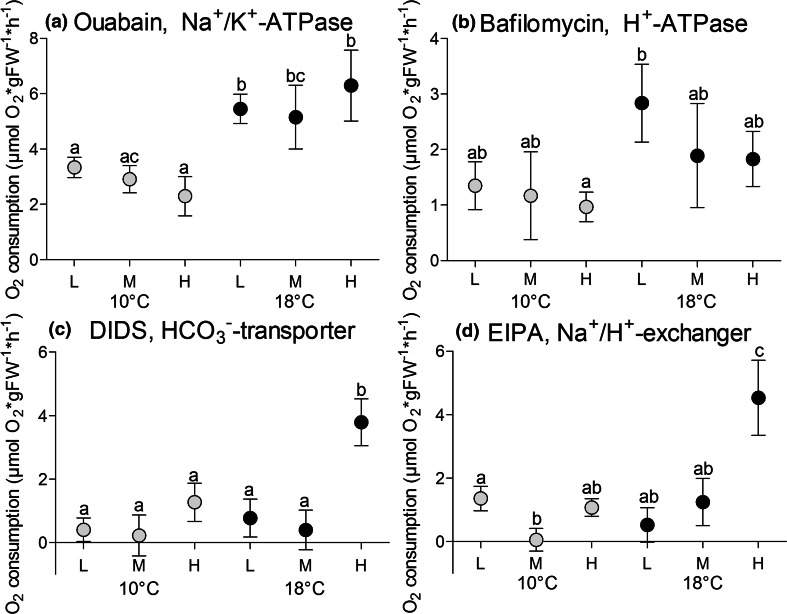
Net O2 demand of ion transporters, calculated as the difference between respiration rates of untreated and inhibitor-exposed gills, is presented in Fig. 6a–d; fractional costs in percent of total gill respiration are listed in Table 6. The inhibition of Na+/H+-exchanger (EIPA) and HCO3−-transporter (DIDS) occasionally led to respiration rates above controls, especially at medium PCO2 and 10 °C, indicating a compensatory increase in the activity of other transporters (Fig. 5c, d). For the transporters investigated, the largest fraction of branchial energy was claimed by Na+/K+-ATPase, at 3.3 ± 1.1 and 5.4 ± 1.7 µmol O2 × gFW−1 × h−1 at 10 and 18 °C, respectively (Fig. 5a), equivalent to 30 % of total gill respiration at low PCO2 conditions. This fraction remained independent of temperature at medium CO2 concentrations, however, a significantly elevated Na+/K+-ATPase fraction (38.80 %) was observed at 18 °C and high PCO2, larger than at high PCO2 and 10 °C (21.5 %) (p = 0.022) (Table 6). Oxygen demand by H+-ATPase (1.35 ± 1.30 µmol O2 × gFW−1 × h−1) and Na+/H+-exchanger (1.36 ± 1.21 µmol O2 × gFW−1 × h−1) at low PCO2 and 10 °C both comprised about 11 % of total gill respiration. HCO3−-transporter consumed about 3.7 ± 12.8 % (0.4 ± 1.2 µmol O2 × gFW−1 × h−1) (Fig. 5b–d; Table 6). At 18 °C, net O2 demand of Na+/K+-ATPase was elevated above rates seen in gills at 10 °C (p < 0.05), except when comparing the two medium PCO2 treatments which differed less (p = 0.098). O2-demand of H+-ATPase was also elevated at high temperature, but this effect became statistically significant only between gills at high PCO2 and 10 °C and those at low PCO2 and 18 °C (p = 0.023). CO2 dependent shifts in the usage of ion transporters were observed for Na+/H+-exchanger at both temperatures and for HCO3− transport at 18 °C. Na+/H+-exchanger was reduced in terms of oxygen demand at medium PCO2 and 10 °C below rates at low PCO2 (p = 0.017) and rose at high PCO2 and 18 °C compared to gills of all other treatments (18 °C low PCO2 7.6-fold, p = 0.017; 18 °C medium PCO2 2.6-fold, p = 0.025; 10 °C low PCO2 2.3-fold, p = 0.016; 10 °C medium PCO2 82.8-fold p = 0.001, 10 °C high PCO2 4.2-fold, p = 0.008). Mean absolute oxygen demand of HCO3− transport was eightfold higher at high PCO2 and 18 °C than at medium PCO2 and 18 °C (p = 0.002) and at low PCO2 conditions and 10 °C (p = 0.0006), whereas oxygen demand of HCO3− transport was increased fourfold at high PCO2 and 18 °C compared to low PCO2 gills at 18 °C (p = 0.007) (Fig. 5c).
Fig. 6.
a, b Gill total respiration (a) and weight (b) as the fraction of standard metabolic rate, respectively, weight of whole animal 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles). n = 4–8 per treatment, for 18 °C low PCO2 a mean relation was calculated. Letters indicate significant differences in net O2 demand of processes between treatment groups (p < 0.05) (a); symbols indicate significant differences between treatments (b) as not all groups could be tested against each other (p < 0.05)
Table 6.
Cod gill fractional costs (%) of Na+/K+-ATPase, H+-ATPase, HCO3 − -transporter and Na+/H+-exchanger in the different exposed groups (low PCO2 = 550 µatm; medium PCO2 = 1200 µatm and high PCO2 = 2200 µatm at 10 °C; 18 °C) given as mean ± standard deviation
| % | 10 °C low | 10 °C medium | 10 °C high | 18 °C low | 18 °C medium | 18 °C high |
|---|---|---|---|---|---|---|
| Na+/K+-ATPase | 29.95 ± 5.64 ab | 28.60 ± 10.99ab | 21.53 ± 18.77b | 29.95 ± 8.53ab | 30.65 ± 14.83ab | 38.8 ± 11.04ac |
| H+-ATPase | 10.37 ± 10.45 | 6.23 ± 21.89 | 8.70 ± 7.66 | 14.81 ± 8.84 | 11.25 ± 19.16 | 11.18 ± 9.21 |
| HCO3 −-transporter | 3.68 ± 12.84ab | 1.79 ± 20.81ab | 11.33 ± 17.59ab | 4.93 ± 10.47ac | 3.59 ± 11.25ad | 23.57 ± 12.21b |
| Na+/H+-exchanger | 11.37 ± 9.59ab | -3.48 ± 17.06ab | 10.64 ± 7.80 ab | 3.67 ± 12.43ac | 7.47 ± 14.19ad | 19.28 ± 8.46b |
Different letters indicate significant differences in fractional costs of processes between treatments (p < 0.05), n = 8 per treatment
Fig. 5.
a–d Net O2 demand of cod gill Na+/K+-ATPase (a), H+-ATPase (b), HCO3 −-transporter (c) and Na+/H+-exchanger (d) (µmol O2 × gFW−1 × h−1) of cod 4 weeks exposed to low PCO2 = 550 µatm (L), medium PCO2 = 1200 µatm (M) and high PCO2 = 2200 µatm (H) at 10 °C (grey circles) and at 18 °C (black circles), given as means with standard error of the mean. Letters indicate significant differences in net O2 demand of processes between treatment groups (p < 0.05), n = 10 per treatment
In gills at 18 °C compared to those at 10 °C and the same level of PCO2 warming led to a strong PCO2 effect as indicated by elevated net O2 demand and an increase in the respective Q10 values (Fig. 5a–d; Table 7). Three of the four transporters investigated had a significantly higher net O2-demand at high PCO2: Na+/K+-ATPase, (p = 0.013), Na+/H+-exchanger, (p = 0.008), HCO3−-transporter, (p = 0.016). The related Q10 values for transporter activity between 10 and 18 °C under high PCO2 ranged between 2.6 and 5.8 (Table 7).
Table 7.
Q 10 values calculated for the mean net oxygen demand of the four transporters investigated between 10 and 18 °C groups at the respective PCO2 levels
| Q 10 | Low PCO2 | Medium PCO2 | High PCO2 |
|---|---|---|---|
| Na+/K+-ATPase | 1.78 | 2.05 | 3.78 |
| H+-ATPase | 1.93 | 1.80 | 2.58 |
| HCO3 −-transporter | 0.88 | 0.65 | 3.18 |
| Na+/H+-exchanger | 0.32 | 29.68 | 5.81 |
Discussion
Atlantic cod reared under various levels of PCO2 and temperature responded by adjustments in branchial ion regulation and associated costs with implications for whole animal oxygen demand, osmolality, and Na+ and Cl− concentrations in the plasma.
Whole animal respiration
Standard metabolic rate (SMR) of cod (mean weight 193.34 ± 62.4 g) at 10 °C and low PCO2 (2.3 ± 0.6 µmol O2 × gFW−1 × h−1 or 74.0 ± 20.6 mg O2 × kg−1 × h−1) compare well with values published for 200 g Atlantic cod reared at 10 °C, ranging from 55–121 mg O2 × kg−1 × h−1 (Schurmann and Steffensen 1997). The potential impact of elevated PCO2 on aerobic scope and metabolic rates have been assessed in a number of fish species from the tropics to the Antarctic with the general hypothesis that the cost of coping with elevated PCO2 (acid–base and osmoregulation as well as cardiorespiratory adjustments) would increase SMR and/or cause a shift in energy budget and reduce aerobic scope and finally fitness (cf. Heuer and Grosell 2014). In Atlantic cod at 10 °C, SMR remained unaffected by elevated ambient PCO2. At low PCO2, SMR was compensated for during long-term warm exposure leading to similar rates in 10 and 18 °C animals (2.67 ± 0.04 µmol O2 × h−1 × gFW−1 or 85.38 ± 1.3 mg O2 × kg−1 × h−1), similar to those in Atlantic cod from Øresund, near to our study location, after thermal acclimation for several months to 10 and 15 °C (Schurmann and Steffensen 1997). Such compensation for changing temperatures is in line with earlier findings that cod can undergo thermal acclimation and thereby endure warm periods. Despite the down-regulation of SMR during warm acclimation, branchial respiration rates were enhanced at 18 °C (all treatments) indicating a shift in energy budget (see above) possibly constraining residual aerobic scope (cf. Pörtner et al. 2010). Further careful analysis of various components of aerobic energy budget and their trade-offs under elevated CO2 levels is thus warranted. In line with a high capacity of warm acclimatisation, wild southern North Sea cod remained for several weeks in their natural habitat at temperatures above 16 °C (Righton et al. 2010). In contrast, Atlantic cod from Scotlands’ west coast exposed to the same acclimation conditions as in the present study (10 and 18 °C for 4 weeks) displayed a 70 % increase in routine metabolic rates during long-term warm exposure (Soofiani and Hawkins 1982), indicating that thermal acclimatisation capacity differs between cod populations (cf. Pörtner et al. 2008).
The response of SMR to increasing PCO2 levels was different at the two acclimation temperatures. While SMR at low PCO2 was similar due to effective compensation, SMR at 18 °C under medium and high PCO2 was higher than in fish reared under the same PCO2 levels at 10 °C (Fig. 1). Elevated PCO2 may thus offset the compensation under warming and cause an increase in metabolic cost and/or capacity. Both warming and acidification occur regularly in summer in the cod’s natural habitat with as yet unclear functional consequences.
Gill maintenance costs
Oxygen consumption rates of gills isolated from Kattegat/Skagerrak cod reared at low PCO2 and 10 °C (5.81 ± 1.19 µatm O2 × gFW−1 × h−1) (Fig. 1) were in the range found in previous studies (Lyndon 1994; Deigweiher et al. 2010), but 50 % lower than those reported for Southern North Sea cod (Kreiss et al. 2015). Lower total gill oxygen demand might be attributed to seasonal changes or population differences.
Relating the weight and metabolic costs of soft gill tissue to the weight of the whole fish (Fig. 6a, b) revealed an interesting pattern. The fraction of soft gill tissue was significantly elevated in medium and high PCO2 fish at 10 °C compared to fish reared at low PCO2 and 10 °C (twofold at medium PCO2, 1.4-fold at high PCO2; p < 0.001) (Fig. 6a). Such an increase in gill soft tissue mass in relation to whole animal weight may indicate branchial remodelling, a mechanism contributing to altered gill mass. Gill remodelling has also been reported in freshwater fish in response to various environmental factors (Goss et al. 1998; Nilsson 2007; Mitrovic and Perry 2009), and is supported by results from Atlantic halibut, where elevated PCO2 for 4 months caused an upregulation of proteins related to cellular turnover in the gill soft tissue (de Souza et al. 2014). A potentially higher total oxygen demand of larger gill arches will likely impact whole animal energy budget. Under low and medium PCO2 at 10 °C the fractional oxygen consumption of gills accounted for approx. 5 % of whole animal oxygen demand at rest, while this fraction increased with falling whole animal rates at high PCO2 reaching >7 % (p = 0.056) (Fig. 6b). For fish reared at 18 °C increasing PCO2 caused the fraction to decline with rising whole animal metabolic rates from about 7 % at low PCO2 to ~4 % at high PCO2. Taking into account that gill MO2 per gram soft tissue was maintained at high PCO2 and 10 °C (Fig. 4), we can postulate that branchial oxygen demand was increased due to the increase in tissue weight under this treatment (Fig. 6a) As gill oxygen consumption was stable in all PCO2 groups at 18 °C (Fig. 6a), higher demand for oxygen by other tissues would explain the reduced fractions of gill oxygen demand (Fig. 6b).
Plasma osmolality and ion concentrations
At 10 °C, plasma osmolality was in the range of values reported earlier for cod and independent of ambient CO2 (Larsen et al. 1997; Herbert and Steffensen 2005). However, while osmolality remained constant at 10 °C, the concentration of Cl− was reduced in fish under PCO2 ≥ 1200 µatm at 10 °C. At this temperature, plasma Na+ concentrations were only slightly reduced at medium PCO2 (p = 0.057) but not at high PCO2. This indicates that osmolality was balanced by other anions, and concomitantly raises the question which transport processes are responsible for the unequal reduction in Na+ and Cl− plasma concentrations. Plasma bicarbonate levels, determined in fish at 10 °C and medium PCO2 compensated for the excess of positively charged Na+ over Cl− up to a difference of ~6 mM, supporting earlier findings that HCO3−/Cl− exchange takes place under hypercapnia (Larsen et al. 1997).
Warming close to maximum summer temperatures (18 °C) combined with hypercapnic conditions led to a different response in plasma osmolality and ion concentrations. All parameters showed non-linear “hump-backed-curves”, with reduced ion concentrations and slightly lowered osmolality in animals at 18 °C under low and high PCO2, while plasma parameters of animals at medium PCO2 and 18 °C were significantly higher (Figs. 2, 5a, b). The shape of these curves remains unexplained, but medium PCO2 levels and 18 °C are frequently experienced by cod in warm summers and preadaptation to this conditions may exist.
The reduction of ion concentrations and the slightly lowered osmolality of fish at 18 °C and low and high PCO2 reflects altered gill ion transporter activities indicated by enhanced fractions in oxygen demand. Net O2 demand of Na+/K+-ATPase and less so of H+-ATPase increased in the warmth (Fig. 5a, b). Thermally uncompensated (i.e. stimulated) in vivo activities of Na+/K+-ATPase and increased maximum transporter capacities (Na+/K+-ATPase and H+-ATPase) were also observed in the former study (Kreiss et al. 2015) implying that Na+/K+-ATPase might overcompensate for enhanced branchial permeability in the warmth. Together with the slightly reduced osmolality caused by the loss in ion concentration (Na+ and Cl−) in warm acclimated fish at low and at high PCO2 this indicates a shift in the electrochemical gradient with enhanced NaCl excretion against the inward ion gradient. Enhanced hypo-osmoregulation was reported before for Antarctic fish during warm acclimation (Gonzalez-Cabrera et al. 1995; Brauer et al. 2005). We assume that elevated transport capacities (Q10 effect) cause decreased osmolality despite higher leakage rates according to higher membrane fluidity. Thereby organic osmolytes may play a role in replacing inorganic osmolytes such as Na+ and Cl− for the reasons mentioned above, but this compensation seems less effective for fish incubated at 18 °C under low and high PCO2 than observed in hypercapnic fish at 10 °C.
As the altered plasma ion concentration does not match the observed usage of branchial ion transport for all treatment groups (see below), further ion regulatory organs, such as kidney and the gastrointestinal tract may be involved in NaCl absorption using the same transporter types as found in branchial epithelia (Na+/K+-ATPase, Na+/K+/2Cl− cotransporter). Anion including HCO3− exchange (e.g. Cl− and Na+ dependent HCO3− exchanger) are also known to play an important role in the intestine and might cause altered plasma Na+ and Cl− concentrations not explained by branchial processes (Marshall and Grosell 2005). For European flounder, reduced plasma osmolality was observed in response to experimentally enhanced intestinal bicarbonate excretion demonstrating that such alterations in the intestine can significantly impact whole animal osmolality status (Wilson et al. 2002).
Gill ion regulation mechanisms in vivo
The fractions of Na+/K+-ATPase, H+-ATPase, Na+/H+-exchanger, and HCO3−-transporter in oxygen consumption comprise the activities of all isoforms of the respective transporter families. Na+/K+-ATPase (all isoforms) is located basolaterally (Evans et al. 2005). A basolateral location was also suggested for H+-ATPase in immunolocalization studies on dogfish (Squalus acanthias) and longhorn sculpin Myoxocephalus octodecimspinosus) (Tresguerres et al. 2005; Catches et al. 2006). Several isoforms were described for Na+/H+-exchange (e.g. Edwards et al. 2005; Deigweiher et al. 2008; Rimoldi et al. 2009) and HCO3− transport (e.g. Piermarini et al. 2002; Esbaugh et al. 2012) located either apically or basolaterally.
The isolated tissue model applied here provides first insight into the affected processes, and can be better related to the whole animal level than isolated and reconstituted membrane models. When discussing the results, the apical or basolateral localisation of transporters from the same family should be kept in mind. While the inhibitors applied may have side-effects on other transporters, this did probably not have a large influence on our findings as the observed patterns of the applied inhibitors are in line with a specific mode of action on certain transporter (families). Under all conditions, O2 demand of Na+/K+-ATPase comprised about 30 % of total gill demand and thereby the largest fraction among the transporters investigated, followed by H+-ATPase and Na+/H+-exchanger (approx. 11 % each) and HCO3−-transport (approx. 3 %). This is in line with fractions of Na+/K+-ATPase and H+-ATPase in oxygen demand of cutthroat trout gill, which accounted for 37 % of total gill oxygen consumption (Morgan and Iwama 1999). Further studies examining fractions of oxygen allocation in isolated gills in vivo from marine fish found Na+/K+-ATPase to represent between 11.8 and 23.1 % of total gill respiration in two species of Notothenioidei (Deigweiher et al. 2010). The larger fractions of 43 % in cod (Kreiss et al. 2015) might again be explained by seasonal variation, as observed for other fish species (e.g. in yellow perch Perca flavescens, Packer and Garvin 1998). Differences between populations might involve various ion transporter activities and thereby altered total gill oxygen demand as found between the two cod studies.
Long-term exposure under high PCO2 is known to affect gill ion regulation capacity in teleosts involving increased mRNA levels and protein abundance of basolateral Na+/HCO3− cotransporter and Na+/K+-ATPase, while those of anion exchanger (HCO3−/Cl− transporter) and Na+/H+-exchanger remained at control levels (Zoarces viviparus, Deigweiher et al. 2008). In the present study, in vivo oxygen demand of these transporters at optimum temperature (10 °C) indicates a different pattern: The summed oxygen demand elicited by HCO3−-transporters as well as Na+/H+-exchangers indicates small responses to both hypercapnia levels applied. A slight enhancement of HCO3−-transporter activity occurred at high PCO2 level and the net O2 demand caused by Na+/H+-exchanger seen at the three PCO2 levels indicated minimum activity at medium but elevated activity under high PCO2 conditions. This indicates at least some involvement of both transporter types depending on the hypercapnia levels applied. Thereby, the interdependencies in the involvement of different transporters need to be considered, as in case of DIDS application to gills at 10 °C. DIDS caused a stimulation of oxygen consumption, possibly due to a shift to other, more costly transporters, which compensated for inhibited HCO3−-transport. Such a response has been observed before in isolated tissue of a marine worm (Sipunculus nudus) (Pörtner et al. 2000).
Slightly decreased in vivo Na+/K+-ATPase activities at high PCO2, are in line with earlier observations (Melzner et al. 2009; Esbaugh et al. 2012; Kreiss et al. 2015), where under PCO2 levels ≤3000 µatm, branchial Na+/K+-ATPase capacities remained unchanged or were reduced, contrasting the upregulation observed under high PCO2 ≥ 6000 µatm (Deigweiher et al. 2008, 2010; Melzner et al. 2009). The pathways eliciting these differences remain to be explored. H+-ATPase activity and its net O2 demand remained rather stable across CO2 treatments, indicating that the in vivo costs of proton excretion are not altered in the cod gill at optimum temperature after 4 weeks of exposure. In salmon, reduced levels of V-type H+-ATPase B-subunit mRNA were found under severe hypercapnia (20,000 µatm, Seidelin et al. 2001), again a certain PCO2 threshold for the response may be postulated for the H+-ATPase. Downregulation of this basolateral pump may cause an increase in branchial H+-excretion. Taken together, no clear molecular restructuring in ion transport was observed after 4 weeks of acclimation to prospective PCO2 at optimum temperature.
However, gill remodelling and the increase in gill soft tissue under medium and high PCO2 at 10 °C (Fig. 6a; Table 5; also found in Kreiss et al. 2015) may affect ion transporter usage. Freshwater fish, exposed to acute severe hypercapnia (~2 % CO2), displayed an increased branchial apical surface area of pavement cells, which reduced the surface area of exposed chloride cells and thereby modified the exchange rate of the apical HCO3−/Cl− exchanger (Goss et al. 1998). The authors concluded that this mechanism reduces base-excretion and thus compensated for acidosis. As pavement cells have a lower oxygen demand than chloride cells the relative increment of pavement cells may explain the increase in soft tissue at constant oxygen consumption rate per g gill arch under hypercapnia. Whether these changes occurred and affected the physical exposure of chloride cells and transporters involved, needs to be confirmed by histological and mechanistic analyses. The absence of increased branchial soft tissue mass in the warmth could be prevented by the otherwise counterproductive reduction of the respiratory surface area.
At 18 °C and elevated PCO2, Na+/H+-exchanger and HCO3− transporter both caused elevated O2 and energy demand compared to the respective rates at 10 °C. Na+/H+-exchanger elicited increased net O2 demand at both elevated PCO2 levels, whereas HCO3−-transport only did at high PCO2. Isoforms of Na+/H+-exchanger and HCO3−-transporter are localised either apically or basolaterally (cf. Heuer and Grosell 2014). Three isoforms of Na+/H+-exchangers (NHEs) are involved in fish acid–base regulation, NHE1 on the basolateral and NHE2 and NHE3 on the apical side (Evans et al. 2005; Deigweiher et al. 2008). Various transporters are involved in HCO3−-transport and are blocked by DIDS; the most important in terms of acid–base regulation might be the apical and basolateral HCO3−/Cl− exchangers, called anion exchanger (AEs) and a Na+/HCO3− co-transporter on the basolateral side (NBC1). At 18 °C, NHE costs were elevated in response to both hypercapnia treatments above those at low PCO2, indicating an upregulation of apical NHEs and enhanced acid excretion. In general, acid excretion in marine fish gills is thought to involve the thermodynamically favourable apical Na+/H+-exchanger more than the costly H+-ATPase (Claiborne et al. 1999; Tresguerres et al. 2005). A predominant role for apical Na+/H+-exchange in acid–base regulation was also found in gills of fresh and seawater adapted mummichogs (Fundulus heteroclitus) under acute, more severe hypercapnia (10,000 µatm) (Edwards et al. 2005), as well as during acid infusion (Claiborne et al. 1999). In this line, mRNA of the basolateral isoform of Na+/H+-exchanger was downregulated at least under short-term hypercapnia (1 h–4 days; Deigweiher et al. 2008; Rimoldi et al. 2009). In the present study, in vivo costs of both Na+/H+-exchanger and HCO3−-transport were enhanced under high CO2 levels. This pattern might involve a higher usage and expression of Na+/HCO3−-cotransporter NBC1 as observed before in gill tissues of eelpout (Zoarces viviparus) acclimated for 6 weeks to 10,000 µatm CO2 (Deigweiher et al. 2008).
Independent of the particular mechanism, our data support that either Na+/HCO3−-cotransporter or HCO3−/Cl−-exchanger, in parallel to Na+/H+-exchanger play a dominant role in cod acid–base regulation under moderate hypercapnia in the warmth. The observed stimulation of ion transporters at elevated PCO2 and 18 °C did not result in alterations of total gill oxygen consumption rates compared to low PCO2 at 18 °C. Stable total gill oxygen consumption despite shifted usage of ion transporter was observed before in Antarctic fish at 10,000 µatm CO2 (Deigweiher et al. 2010). The mechanistic background of this stability remains to be explored.
Conclusions and perspectives
Whole animal standard metabolic rates of Atlantic cod incubated for 3 weeks were independent of temperature (10, 18 °C). These results confirm previous findings that Southern cod populations can acclimate and compensate for temperature, within limits marginally reached at 18 °C. With respect to the different CO2 concentrations applied (550, 1200 and 2200 µatm), these data also provide an evidence that near-term projected future PCO2 levels per se will have a small effect on SMR. Due to the changes in whole animal SMR between medium and high PCO2 groups of the two temperatures studied, we observed increasing fractions of gill in whole organism oxygen demand towards high PCO2 at 10 °C but decreasing fractions under rising PCO2 at 18 °C. This reflects increased overall gill oxygen consumption at 10 °C and high PCO2, whereas gills in fish at 18 °C displayed rather stable branchial oxygen demand that was not involved in fluctuations of cod energy turnover under CO2.
In parallel, our data provide evidence of increased demand for branchial ion regulation through Na+/H+-exchange and HCO3−-transport at 18 °C and elevated PCO2 leading to an associated reduction in inorganic plasma osmolytes via stimulated Na+/K+-ATPase and a shift in plasma ionic balance; the latter effect was also observed at low PCO2 and 18 °C. Reduced net O2 demand of Na+/H+-exchanger under medium PCO2 as well as the slight decrease of net Na+/K+-ATPase O2 demand under high PCO2 at 10 °C is an evidence for a different mechanism at optimum temperature than at 18 °C. To what extent these changes contribute to shifts in somatic energy turnover and budget, remains to be explored. Overall, Atlantic cod appear quite tolerant to elevated CO2 levels and variable temperatures, which may come with its demersal lifestyle and preadaptations to fluctuating CO2 levels and fluctuating temperatures in shallow water habitats.
Acknowledgments
The authors would like to thank Bengt Lundve for his support and assistance in obtaining experimental animals and arranging the experimental facilities as well as Lars Ljungqvist for valuable technical support. Lena Jakob and Caroline Otten are highly acknowledged for their assistance in plasma ion analysis. Furthermore, we would like to thank the constructive criticism by two anonymous reviewers which greatly improved the manuscript. This work contributes to the Bundesministerium für Forschung und Bildung funded project “Biological Impacts of Ocean Acidification” (BIOACID) and is part of the “Polar regions and coasts in a changing earth system” (PACES) research programme of the Alfred-Wegener-Institute for Polar and Marine Research.
References
- Blackford JC, Gilbert FJ. pH variability and CO2 induced acidification in the North Sea. J Marine Syst. 2007;64:229–241. doi: 10.1016/j.jmarsys.2006.03.016. [DOI] [Google Scholar]
- Brauer PR, Sanmann JN, Petzel DH. Effects of warm acclimation on Na+, K+-ATPase α-subunit expression in chloride cells of Antarctic fish. Anat Rec A. 2005;285A:600–609. doi: 10.1002/ar.a.20203. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Axelsson M, Ehrenström F, Metcalfe JD, Nilsson S. Circulating catecholamines and swimming performance in the Atlantic cod, Gadus morhua. J Exp Biol. 1989;141:377–387. [Google Scholar]
- Catches JS, Burns JM, Edwards SL, Claiborne JB. Na+/H+ antiporter, V-H+-ATPase and Na+/K+-ATPase immunolocalization in a marine teleost (Myoxocephalus octodecemspinosus) J Exp Biol. 2006;204:3251–3259. doi: 10.1242/jeb.02384. [DOI] [PubMed] [Google Scholar]
- Claiborne JB, Blackston CR, Choe KP, Dawson DC, Harris SP, Mackenzie LA, Morrison-Shetlar AI. A mechanism for branchial acid excretion in marine fish: identification of multiple Na+/H+ antiporter (NHE) isoforms in gills of two seawater fish. J Exp Biol. 1999;202:315–324. doi: 10.1242/jeb.202.3.315. [DOI] [PubMed] [Google Scholar]
- Claiborne JB, Edwards SL, Morrison-Shetlar AI. Acid-Base Regulation in Fishes: cellular and Molecular Mechanisms. J Exp Biol. 2002;293:302–319. doi: 10.1002/jez.10125. [DOI] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne JL, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner M (2013) The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- De Souza KB, Jutfelt F, Kling P, Förlin L, Sturve J. Effects of increased CO2 on fish gill and plasma proteome. PLoS One. 2014;9(7):e102901. doi: 10.1371/journal.pone.0102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigweiher K, Koschnick N, Pörtner HO, Lucassen M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1660–R1670. doi: 10.1152/ajpregu.90403.2008. [DOI] [PubMed] [Google Scholar]
- Deigweiher K, Hirse T, Bock C, Lucassen M, Pörtner HO. Hypercapnia induced shifts in gill energy budgets of Antarctic notothenioids. J Comp Physiol. 2010;180B:347–359. doi: 10.1007/s00360-009-0413-x. [DOI] [PubMed] [Google Scholar]
- Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. doi: 10.1016/0198-0149(87)90021-5. [DOI] [Google Scholar]
- Drinkwater KF. The response of Atlantic cod (Gadus morhua) to future climate change. ICES J Mar Sci. 2005;62:1327–1337. doi: 10.1016/j.icesjms.2005.05.015. [DOI] [Google Scholar]
- Duraton C, Tauc M, Avella M, Poujeol P. Chloride channels in primary cultures of seawater fish (Dicentrarchus labrax) gill. Cell Physiol. 1997;273:C874–C882. doi: 10.1152/ajpcell.1997.273.3.C874. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Wall BP, Morrison-Shetlar A, Sligh S, Weakley JC, Claiborne JB. The effect of environmental hypercapnia and salinity on the expression of NHE-like isoforms in the gills of a euryhaline fish (Fundulus heteroclitus) J Exp Zool. 2005;303A(6):464–475. doi: 10.1002/jez.a.175. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Heuer R, Grosell M. Impacts of ocean acidification on respiratory gas exchange and acid-base balance in a marine teleost, Opsanus beta. J Comp Physiol. 2012;182B:921–934. doi: 10.1007/s00360-012-0668-5. [DOI] [PubMed] [Google Scholar]
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation and excretion of nitrogenous waste. Physiol Rev. 2005;85:97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- Forsgren E, Dupont S, Jutfelt F, Amundsen T. Elevated CO2 affects embryonic development and larval phototaxis in a temperate marine fish. Ecol Evol. 2013;3(11):3637–3646. doi: 10.1002/ece3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch TBH, Clemmesen C. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Chang. 2011 [Google Scholar]
- Frommel AY, Schubert A, Piatkowski U, Clemmesen C. Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol. 2013;160:1825–1834. doi: 10.1007/s00227-011-1876-3. [DOI] [Google Scholar]
- Gonzalez-Cabrera PJ, Dowd F, Pedibhotla VK, Rosario R, Stanley-Samuelson D, Petzel D. Enhanced hypo-osmoregulation induced by warm-acclimation in Antarctic fish is mediated by increased gill and kidney Na+/K+-ATPase activities. J Exp Biol. 1995;198:2279–2291. doi: 10.1242/jeb.198.11.2279. [DOI] [PubMed] [Google Scholar]
- Goss GG, Perry SF, Fryer JN, Laurent P. Gill morphology and acid-base regulation in freshwater fishes. Comp Biochem Physiol. 1998;119A:107–115. doi: 10.1016/S1095-6433(97)00401-7. [DOI] [PubMed] [Google Scholar]
- Gypens N, Lacroix G, Lancelot C, Borges AV. Seasonal and inter-annual variability of air-sea CO2 fluxes and seawater carbonate chemistry in the Southern North Sea. Prog Oceanogr. 2011;88:59–77. doi: 10.1016/j.pocean.2010.11.004. [DOI] [Google Scholar]
- Heisler N. Acid-base regulation in fishes. In: Hoar WS, Randall DJ, editors. Fish physiology. New York: Academic; 1984. pp. 315–401. [Google Scholar]
- Heisler N. Buffering and transmembrane ion transfer processes. In: Heisler N, editor. Acid-base regulation in animals. Amsterdam: Elsevier Biomedical Press; 1986. pp. 3–47. [Google Scholar]
- Herbert NA, Steffensen JF. The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar Biol. 2005;147:1403–1412. doi: 10.1007/s00227-005-0003-8. [DOI] [Google Scholar]
- Heuer RM, Grosell M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1061–R1084. doi: 10.1152/ajpregu.00064.2014. [DOI] [PubMed] [Google Scholar]
- Holmgren S, Nilsson S. Drug effects on Isolated Artery Strips from two teleosts, Gadus morhua and Salmo gairdneri. Act Physiol Scand. 1974;90:431–437. doi: 10.1111/j.1748-1716.1974.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Jutfelt F, Hedgärde M. Atlantic cod actively avoid CO2 and predator odour, even after long-term CO2 exposure. Front Zool. 2013;10:81. doi: 10.1186/1742-9994-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss CM, Michael K, Bock C, Lucassen M, Pörtner H-O. Impact of long-term hypercapnia on the energy budget of isolated gills of Atlantic cod (Gadus morhua) Comp Biochem Physiol. 2015;182A:102–112. doi: 10.1016/j.cbpa.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Krumschnabel G, Wieser W. Inhibition of the sodium pump does not cause a stoichiometric decrease of ATP-production in energy limited fish hepatocytes. Experentia. 1994;50:483–485. doi: 10.1007/BF01920752. [DOI] [Google Scholar]
- Lannig G, Bock C, Sartoris F, Pörtner HO. Oxygen limitation of thermal tolerance in cod, Gadus morhua L., studied by magnetic resonance imaging and online-venous oxygen monitoring. Am J Physiol Regul Integr Physiol Comp. 2004;287:R902–R910. doi: 10.1152/ajpregu.00700.2003. [DOI] [PubMed] [Google Scholar]
- Larsen BK, Pörtner HO, Jensen FB. Extra- and intracellular acid-base balance and ionic regulation in cod (Gadus morhua) during combined and isolated exposures to hypercapnia and copper. Mar Biol. 1997;128:337–346. doi: 10.1007/s002270050099. [DOI] [Google Scholar]
- Lebedeva NY, Vosilene MZY, Golovkina TV. Aspects of stress in rainbow trout, Salmo gairdneri, release of chemical alarm signals. J Ichthyol. 1994;33:66–74. [Google Scholar]
- Lewis E, Wallace D (1998) Program developed for CO2 system calculations. In: ORNL/CDIAC-105a. Oak Ridge, Tenessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy
- Lyndon AR. A method for measuring oxygen consumption in isolated perfused gills. J Fish Biol. 1994;44(4):707–715. doi: 10.1111/j.1095-8649.1994.tb01246.x. [DOI] [Google Scholar]
- Maneja RH, Frommel AY, Browman HL, Clemmesen C, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB. The swimming kinematics of larval Atlantic cod Gadus morhua L., are resilient to elevated seawater pCO2. Mar Biol. 2013;160:1963–1972. doi: 10.1007/s00227-012-2054-y. [DOI] [Google Scholar]
- Mark FC, Hirse T, Pörtner HO. Thermal sensitivity of cellular energy budgets in some Antarctic fish hepatocytes. Polar Biol. 2005;28:805–814. doi: 10.1007/s00300-005-0018-0. [DOI] [Google Scholar]
- Marshall WS, Grosell M. Ion transport, osmolytes and acid-base balance. In: Evans DH, Claiborne JB, editors. The physiology of fishes. 3. USA: CRC Press Taylor and Francis Group; 2005. pp. 177–229. [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceangr. 1973;18:897–907. doi: 10.4319/lo.1973.18.6.0897. [DOI] [Google Scholar]
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner HO, Lucassen M. Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4-12 months) acclimation to elevated seawater PCO2. Aquat Toxicol. 2009;92:30–37. doi: 10.1016/j.aquatox.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Mitrovic D, Perry SF. The effects of thermally induced gill remodelling on ionocyte distribution and branchial chloride fluxes in goldfish (Carassius auratus) J Exp Biol. 2009;212:843–852. doi: 10.1242/jeb.025999. [DOI] [PubMed] [Google Scholar]
- Morgan JD, Iwama GK. Energy cost of NaCl transport in isolated gills of cutthroat trout. Am J Physiol Regul Integr Comp Physiol. 1999;277:R631–R639. doi: 10.1152/ajpregu.1999.277.3.R631. [DOI] [PubMed] [Google Scholar]
- Munday PL, McCormick MI, Nilsson E. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J Exp Biol. 2012;215:3865–3873. doi: 10.1242/jeb.074765. [DOI] [PubMed] [Google Scholar]
- Neat F, Righton D. Warm water occupancy by North Sea cod. Proc R Soc B. 2007;274:789–798. doi: 10.1098/rspb.2006.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G. Gill remodelling – a new fashion or an ancient secret? J Exp Biol. 2007;210:2403–2409. doi: 10.1242/jeb.000281. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson SW, Munday PL. Near future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang. 2012 [Google Scholar]
- Packer RK, Garvin JL. Seasonal differences in activity of perch (Perca flavenscens) gill Na+/K+-ATPase. Comp Biochem Physiol. 1998;120B:777–783. doi: 10.1016/S0305-0491(98)10078-0. [DOI] [Google Scholar]
- Parks SK, Tresguerres M, Goss GG. Interactions between Na+ channels and Na+-HCO3−–cotransporters in the freshwater fish gill MR cell: a model for transepithelial Na+ uptake. Am J Physiol Cell Physiol. 2007;292:935–944. doi: 10.1152/ajpcell.00604.2005. [DOI] [PubMed] [Google Scholar]
- Piermarini PM, Verlander JW, Royaux IE, Evans DH. Pendrin immunoreactivity in the gill epithelium of a euryhaline elasmobranch. Am J Physiol Integr Comp Physiol. 2002;283:R983–R992. doi: 10.1152/ajpregu.00178.2002. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol. 2010;77:1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Bock C, Reipschläger A. Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J Exp Biol. 2000;203:2417–2428. doi: 10.1242/jeb.203.16.2417. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Mark FC, Bock C. Oxygen limited thermal tolerance in fish? Answers by nuclear magnetic resonance techniques. Respir Physiol Neuro. 2004;141:243–260. doi: 10.1016/j.resp.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Bock C, Knust R, Lannig G, Lucassen M, Mark FC, Sartoris FJ. Cod and climate in a latitudinal cline: physiological analyses of climate effects in marine fishes. Clim Res. 2008;37:253–270. doi: 10.3354/cr00766. [DOI] [Google Scholar]
- Pörtner HO, Schulte PM, Wood CM, Schiemer F. Niche dimensions and limits in fishes: an integrative view. Illustrating the role of physiology in understanding ecological realities. Physiol Biochem Zool. 2010;83:808–826. doi: 10.1086/655977. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Karl DM, Boyd PW, Cheung WL, Lluch-Cota SE, Nojiri Y, Schmidt DN, Zavialov PO (2014) Ocean systems. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- Righton DA, Andersen KH, Neat F, Thorsteinsson V, Steingrund P, Svedäng H, Michalsen K, Hinrichsen HH, Bendall V, Neuenfeldt S, Wright P, Jonsson P, Huse G, van der Kooij J, Mosegaard H, Hüssy K, Metcalfe J. Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Mar Ecol Prog Ser. 2010;420:1–13. doi: 10.3354/meps08889. [DOI] [Google Scholar]
- Rimoldi S, Terova G, Brambilla F, Bernardini G, Gornati R, Saroglia M. Molecular characterization and expression analysis of Na+/H+ exchanger (NHE)-1 and c-Fos genes in sea bass (Dicentrarchus labrax, L) exposed to acute and chronic hypercapnia. J Exp Biol Ecol. 2009;375:32–40. doi: 10.1016/j.jembe.2009.05.002. [DOI] [Google Scholar]
- Sarazin G, Michard G, Prevot F. A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res. 1999;33:290–294. doi: 10.1016/S0043-1354(98)00168-7. [DOI] [Google Scholar]
- Schurmann H, Steffensen JF. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol. 1997;50(6):1166–1180. [Google Scholar]
- Seidelin M, Brauner CJ, Jensen FB, Madsen SS. Vacuolar-type H+-ATPase and Na+, K+-ATPase expression in gills of Atlantic salmon (Salmo salar) during isolated and combined exposure to hyperoxia and hypercapnia in fresh water. Zool Sci. 2001;18:1199–1205. doi: 10.2108/zsj.18.1199. [DOI] [PubMed] [Google Scholar]
- Soofiani NM, Hawkins AD. Energetic costs at different levels of feeding juvenile cod, Gadus morhua L. J Fish Biol. 1982;21(5):577–592. doi: 10.1111/j.1095-8649.1982.tb02861.x. [DOI] [Google Scholar]
- Taboada FG, Anadón R. Patterns of change in sea surface temperature in the North Atlantic during the last three decades: beyond mean trends. Clima Chang. 2012;115:419–431. doi: 10.1007/s10584-012-0485-6. [DOI] [Google Scholar]
- Takahashi T, Olafsson J, Goddard JG, Chipman DW, Sutherland SC. Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: a comparative study. Glob Biogeochem Cyc. 1993;7(4):843–878. doi: 10.1029/93GB02263. [DOI] [Google Scholar]
- Tresguerres M, Katoh F, Jasinska Fenton H, Goss GG. Regulation of branchial V-H+-ATPase, Na+/K+-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias) J Exp Biol. 2005;208:345–354. doi: 10.1242/jeb.01382. [DOI] [PubMed] [Google Scholar]
- Wahlquivst I, Nilsson S. The role of sympathetic fibres and circulating catecholamines in controlling the blood pressure and heart rate in the cod, Gadus morhua. Comp Biochem Physiol. 1977;57C:65–67. doi: 10.1016/0306-4492(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish – why and how? BBA Biomem. 2002;1566(1–2):182–193. doi: 10.1016/S0005-2736(02)00600-4. [DOI] [PubMed] [Google Scholar]
- Wittmann AC, Pörtner HO. Sensitivities of animal taxa to ocean acidification. Nature Clim Chang: 2013 [Google Scholar]
- Wu SC, Horng JL, Liu ST, Hwang PP, Wen ZH, Lin CS, Lin LY. Ammonium-dependent sodium uptake in mitochondrium-rich cells of medaka (Oryzias latipes) larvae. Am J Physiol Cell Physiol. 2010;289(2):237–250. doi: 10.1152/ajpcell.00373.2009. [DOI] [PubMed] [Google Scholar]