Abstract
Objective
We sought to determine if the atrial natriuretic peptide (ANP) precursor proANP is biologically active compared to ANP and BNP.
Background
ProANP is produced in the atria and processed to ANP and activates the guanylyl cyclase receptor-A (GC-A) and its second messenger cGMP. ProANP is found in the human circulation but its bioavailability is undefined.
Methods
We investigated the in vivo actions of proANP compared to ANP, BNP, or placebo in normal canines (667 pmol/kg, n=5/group). We also determined cGMP activation in GC-A or GC-B expressing HEK293 cells. ProANP processing and degradation were observed in serum from normal subjects (n=13) and patients with heart failure (HF) (n=14) ex vivo.
Results
ProANP had greater diuretic and natriuretic properties with more sustained renal tubular actions compared to ANP or BNP in vivo in normal canines including marked renal vasodilation not observed with ANP or BNP. ProANP also resulted in greater and more prolonged cardiac unloading than ANP, but much less hypotensive effects than BNP. We observed that proANP stimulated cGMP generation by GC-A, as much as ANP. ProANP was processed to ANP in serum from normals and HF patients ex vivo and the processed peptide activated cGMP in GC-A cells.
Conclusions
ProANP represents a novel activator of GC-A with enhanced renal diuretic, natriuretic and renal vasodilating properties which may represent a key circulating natriuretic peptide in cardiorenal and blood pressure homeostasis as well as a potential innovative therapeutic beyond ANP or BNP for cardiorenal diseases including HF.
Keywords: Atrial natriuretic peptides, cGMP, kidney
INTRODUCTION
The prevalence of acute decompensated heart failure (HF) continues to grow and the prognosis remains poor despite many drugs to treat HF (1). Conventional diuretics and vasodilators remain mainstays of therapy (1) but currently no new drugs have improved prognosis of for acute decompensated HF (2-5). In addition, myocardial remodeling is driven by decreased cGMP activity in cardiac myocytes in both HF with preserved ejection fraction (HFpEF) and advanced HF with reduced EF (HFrEF) (6), suggesting cGMP replacement therapy may be useful in HF.
The cardiac the guanylyl cyclase receptor (GC) –A activators, atrial natriuretic peptide (ANP) and B-type NP (BNP) play roles in circulating volume and blood pressure homeostasis (7,8). Carperitide, recombinant human ANP and nesiritide, recombinant human BNP have been used as therapeutic agents for HF. The use of carperitide continues in Japan with favorable results from clinical trials (9,10), however, the use of nesiritide in HF has decreased in the US since the ASCEND-HF trial as there was no evidence of improved prognosis beyond conventional therapies (4).
The molecular precursor of ANP, proANP is formed following removal of the signal peptide from preproANP which is produced from the NPPA gene in the heart (11,12). ProANP is then processed into amino-terminal (NT)-proANP and the biologically active ANP by corin (13,14) (Supplemental Fig. 1). ProANP is stored in secretory granules in atrial cardiomyocytes and cleaved to ANP upon secretion, as in response to stretch (15). ANP is then degraded into smaller molecular fragments by neprilysin and insulin degrading enzyme (16-18). Hunter and colleagues reported that proANP circulates in canines and humans (19). While initially circulating proNPs were not thought to be biologically active, we and others have recently established proB-type NP (proBNP) is active, has a longer half-life than BNP in rats (20) and can be processed to BNP in human serum (21), however, it is unclear if this is true for proANP.
As with proBNP (20), we hypothesized that proANP would be biologically active in vivo with a longer half-life and longer lasting cardiorenal actions than ANP or BNP. Further, we hypothesized that proANP can stimulate cGMP production via GC-A receptors and be processed to ANP in the circulation. These studies advance our understanding of proANP/ANP/GC-A/cGMP signaling in the circulation with potential physiologic and therapeutic implications.
METHODS
All human and animal experimental protocols used in the current study were approved by the Institutional Review Board and Animal Care and Use Committee at Mayo Clinic.
Detailed methods are provided in the Supplemental Materials.
ProANP, ANP, BNP, and CNP synthesis and reagents
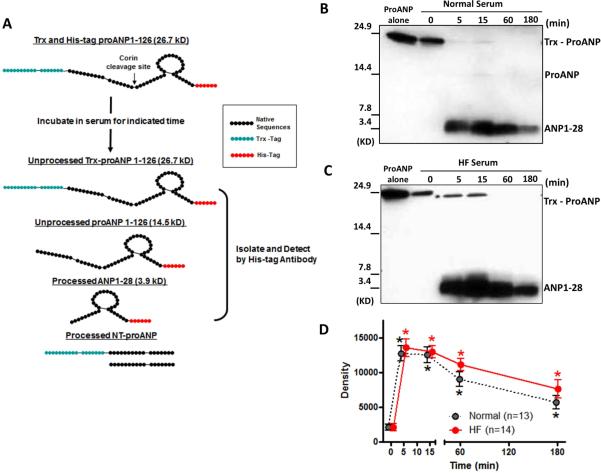
Recombinant human and canine proANP were synthesized by ProMab Biotechnologies, Inc (Richmond, CA). ANP, canine BNP, and CNP were from Phoenix Laboratories, Inc (Burlingame, CA). Human proANP was tagged with Trx on the N-terminus and with 6-Histidine (His) on the C-terminus for peptide isolation (Fig. 5A for human proANP1-126), but canine proANP was untagged.
Figure 5. Ex vivo analysis of proANP processing.
A; Exogenous proANP was incubated in fresh human serum for indicated times at 37°C. Unprocessed or processed proANP were isolated by immunoprecipitation, and detected by Western blot. B and C: Representative Western blot for normal serum (B) and HF serum (C). D: Densitometric analysis of ANP. *p<0.05 vs 0 min.
In vivo studies in normal canines
We performed acute procedures (22-24) to assess the actions by intravenous bolus injection of equimolar doses (667 pmol/kg) (25) of canine proANP, ANP, canine BNP and placebo (0.9% saline) in normal canines (21-30 kg, n=5 of each group). Acute procedures permitted characterization of pharmacokinetics and cardiorenal function up to 3 hours after peptide or placebo injection. In brief, lithium carbonate tablets were given the night before the acute study to assess renal tubular function. Hemodynamic parameters were collected via arterial line and Swan-Ganz catheter. Blood and urine were collected from an arterial line and ureter catheter, respectively. Renal blood flow (RBF) was monitored via electromagnetic flow probe placed on the renal artery. Inulin was continuously infused for measurement of glomerular filtration rate (GFR) which was calculated by inulin clearance. Plasma and urinary sodium (Na), potassium (K), and lithium (Li) levels were determined. Urinary excretion of each parameter (X) such as cGMP, Na or K were determined as X x urine volume (UV) (ml/min). Employing the lithium clearance (CLLi) technique, we calculated both renal tubular function, proximal fractional reabsorption of sodium (PFRNa) and distal fractional reabsorption of sodium (DFRNa) (23,24). Cyclic GMP levels were assayed using a RIA cGMP kit (Perkin-Elmer, Waltham, MA) (22-24). Pharmacokinetic profiles were calculated by the non-compartmental analysis using Phoenix WinNonline 6.3 Software (Pharsight, USA) (26).
In vitro study in HEK 293 cells
Human embryonic kidney (HEK) 293 cells were stably transfected with either human GC-A or GC-B using Lipofectamine (Invitrogen, Grand Island, NY) (27). Cells were treated with or without 10−10 to 10−8 M of proANP, ANP, BNP or CNP for 10 min, and the intracellular cGMP levels were measured.
Ex vivo human studies
Fresh serum was obtained from healthy volunteers (normals) and patients with HF, NYHA Class II-IV with informed consent. Fresh serum (100 ul) with or without 500 ng of Trx- and His-tagged proANP (1.9 × 10−7M) added were incubated for varying times from 5 to 180 min at 37°C, then immunoprecipitated and Western blotted using His-tag antibody (21,28).
Statistical analysis
Data are reported as mean ± SEM. Unpaired t-test was performed for comparison between groups. Comparisons within a group in time course ex vivo and in vivo studies were made by 1-way ANOVA followed by Bonferroni multiple comparison posttest analyses when the global test was significant. Two-way ANOVA was used to compare between groups in time course in vivo studies, followed by Bonferroni posttests. GraphPad Prism 5 (GraphPad Software, La Jolla, CA) and JMP 10 were used for the above calculations. Statistical significance was accepted as p<0.05.
RESULTS
In vivo renal actions
Supplemental Table 1 illustrates baseline characteristics in each group. There was no statistical difference among the groups. We calculated the value of the difference from baseline (=0 min) before drug injection for each parameter to compare among the groups.
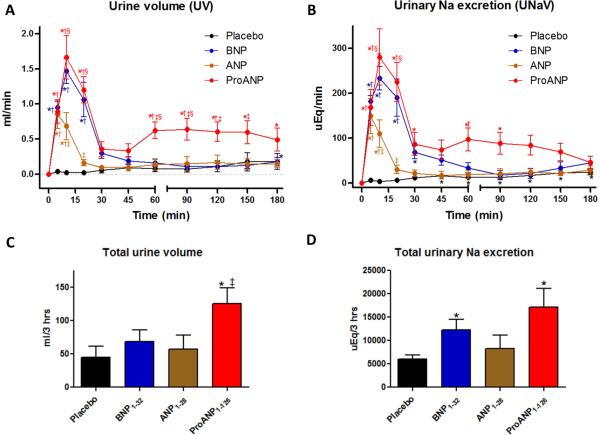
ProANP resulted in a biphasic increase in urine volume (UV) and urinary sodium excretion (UNaV) with 1st peak at 10 min and 2nd peak between 60 and 90 min whereas ANP and BNP showed a mono-phasic increase peak at 10 min (Figs. 1A and 1B). ANP also had a lower peak than proANP and BNP. Urinary K excretion (UKV) followed the same pattern with proANP, whereas ANP and BNP decreased sharply in the first 45 min, with BNP remaining low while ANP slowly increased over the next 150 min (Supplemental Table 2). ProANP had significantly greater total UV for 3 hours but neither ANP nor BNP significantly changed from placebo (Fig. 1C). ProANP and BNP had significantly greater total sodium excretion than placebo but ANP did not (Fig. 1D). All 3 peptides have no significant differences compared to placebo in total potassium excretion (data not shown).
Figure 1. In vivo urinary changes.
A and B: Time course of urine volume (UV; A) and urinary sodium excretion (UNaV; B) following peptide injections, data are calculated from the difference from baseline. *p<0.05 vs baseline (= 0 min), †p<0.05 vs placebo, ‡p<0.05 vs ANP, and §p<0.05 vs BNP,. C and D: Total urine volume (C) and sodium (Na) excretion (B) in kidney for 180 min after drug injection.
ProANP significantly increased RBF from 45 min to 150 min compared to ANP and BNP (Fig. 2A), and proANP had a greater increase in GFR at 10 min compared to ANP or BNP (Supplemental Table 2). ProANP showed the greatest increase in hematocrit (Hct) from 30 min to 180 min, possibly due to volume loss caused by diuresis (Fig. 2B). To determine which nephron segment was involved in the greater natriuretic response to proANP, we analyzed PFRNa and DFRNa as shown in Supplemental Table 2. ProANP significantly decreased PFRNa at 10 min similar to ANP and BNP but showed a bi-phasic effect like UV (Fig. 1A) and UNaV (Fig. 1B) with a significantly prolonged decrease of PFRNa over 120 min compared to the other 3 groups. ProANP also significantly decreased DFRNa with a peak at 10 min and showed a significantly prolonged decrease of DFRNa for 60 min compared to its baseline as well as placebo. ANP and BNP lacked the prolonged effects.
Figure 2. In vivo renal blood flow and Hct.

A: Renal blood flow (RBF) and B: Hematocrit (Hct). Data are calculated from the difference from baseline. *p<0.05 vs baseline (= 0 min), †p<0.05 vs placebo, ‡p<0.05 vs ANP, and §p<0.05 vs BNP.
In vivo hemodynamic actions
Fig. 3 and Supplemental Table 2 report hemodynamic responses before and after NP injection compared to placebo. All 3 NPs showed significant decreases in mean arterial pressure (MAP), however only proANP and BNP decreased pulmonary capillary wedge pressure (PCWP) and systemic vascular resistance (SVR) compared to placebo (Figs. 3A, 3B, and 3C). BNP had the most potent sustained decrease of MAP, PCWP and SVR. ProANP decreased MAP similar to ANP, but had a prolonged decrease in PCWP for 2 hours while ANP did not. BNP increased heart rate (HR) for the first 20 min (Fig. 3D), but after 30 min, proANP resulted in the greatest HR increase together with increased Hct (Fig. 2B).
Figure 3. In vivo hemodynamic changes.
A: Mean arterial pressure (MAP), B: Pulmonary capirally wedge pressure (PCWP), C: Systemic vascular resistance (SVR) and D: Heart rate (HR). Data are calculated from the difference from baseline. *p<0.05 vs baseline (= 0 min), †p<0.05 vs placebo, ‡p<0.05 vs ANP, and §p<0.05 vs BNP.
In vivo plasma and urinary cGMP immunoreactivity
Supplemental Figs. 2A and 2B illustrate plasma and urinary cGMP levels. BNP had the greatest and most persistent increase of plasma and urinary cGMP compared to proANP or ANP, while proANP had longer effects on both plasma and urinary cGMP levels than ANP. In pharmacokinetic analyses (Table 1), BNP showed the highest plasma and urinary cGMP Cmax and area under the curve. ProANP had significant increases on both parameters with longer half-life compared to either ANP or BNP.
Table 1.
Cyclic GMP Pharmacokinetics.
| PcGMP Cmax (pmol/ml) | PcGMP Tmax (min) | PcGMP AUClast (pmol*min/ml) | PcGMP T1/2 (min) | UcGMPV Cmax (pmol/ml) | UcGMPV Tmax (min) | UcGMPV AUClast (pmol*min/ml) | UcGMPV T1/2 (min) | |
|---|---|---|---|---|---|---|---|---|
| ANP | 57.7 ± 11.6 | 6.0 ± 0.6 | 1205.6 ± 176.9 | 13.3 ± 1.8 | 5255.4 ± 816.7 | 14.0 ± 2.4 | 191179.3 ± 38770.7 | 25.9 ± 3.6 |
| BNP | 108.3 ± 14.2* | 9.0 ± 1.8 | 3498.6 ± 488.1* | 17.7 ± 1.6 | 10415.2 ± 1190.6* | 18.0 ± 3.7 | 498780.3 ± 51977.7* | 23.6 ± 2.2 |
| ProANP | 51.5 ± 4.7† | 7.6 ± 0.7 | 2720.0 ± 342.9* | 40.3 ± 6.7*† | 5984.9 ± 417.9† | 20.0 ± 3.2 | 383668.1 ± 28010.2* | 44.8 ± 3.2*† |
PcGMP, plasma cGMP levels; UcGMPV, urinary cGMP excretion; Cmax, maximum concentration; Tmax, time to maximum concentration; AUClast, area under the concentration-time curve from time 0 to the last measurable concentration (Tlast=180 min); and T1/2, half-life.
p<0.05 vs ANP
p<0.05 vs BNP.
In vitro cyclic GMP activation in HEK293 cells
We investigated the ability of proANP to activate cGMP generation compared to ANP and BNP in GC-A and GC-B expressing HEK293 cells (Figs.4A and 4B). CNP was used as a positive GC-B control. BNP, ANP, and proANP all significantly induced GC-A stimulated cGMP production in a dose-dependent manner (Fig. 4A). ProANP had significantly more effects than BNP and a similar effect to ANP at the highest dose (10−8 M). BNP, ANP and proANP showed little cGMP stimulation in GC-B cells (Fig. 4B).
Figure 4. In vitro cyclic GMP response of synthetic peptides.
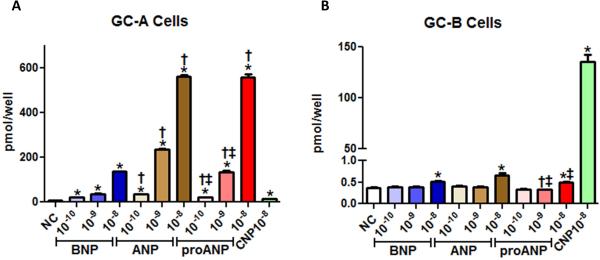
GC-A (A) or GC-B (B) expressing HEK293 cells were treated with or without10−8 to 10−6 M of proANP, ANP, BNP or CNP for 10 min. *p<0.05 vs no treatment, †p<0.05 vs equimolar dose of BNP, and ‡p<0.05 vs equimolar dose of ANP.
Ex vivo proANP processing in human serum
We assessed whether proANP is processed in the normal or HF human circulation ex vivo. Characteristics of normal and HF patients and individual characteristics of HF patients are shown in Supplemental Table 3 and 4. All patients were in-hospital and had moderate to severe HF.
As shown in Fig. 5A, proANP was produced with an N-terminal Trx-tag to aid in peptide purification and a C-terminal His-tag to aid in isolation of ANP forms following processing. His-tag antibodies will detect unprocessed proANP, proANP without the Trx-tag and ANP, but not processed NT-proANP (Fig. 5A). ProANP (approx. 23 KD) was processed into a smaller molecular weight form (approx. 4 KD) (Figs. 5B and 5C) in serum from normals and HF patients which was confirmed to be ANP by sequencing. In some subjects, a faint band around 13 KD was determined to be proANP without the Trx-tag (Fig. 5B).
To more precisely assess proANP processing in the circulation, we examined proANP processing in serum from normals vs patients with HF at 5 different time points; 0, 5, 15, 60 and 180 min. As a control, we pretreated samples for 0 min with EDTA which inhibits proANP processing. In both normals and HF, ANP appeared after 5 min incubation and persisted for 15 min, then began to decrease at 60 min through 180 min (Figs. 5B and 5C). The density of the ANP band from Western blots in each group was significantly higher at each time point compared to 0 min, and there was no significant difference between normals and HF (Fig. 5D).
DISCUSSION
This is the first study to investigate proANP as a GC-A activator, with more sustained natriuretic, diuretic and renal vasodilating actions than ANP or BNP and with greater and more sustained cardiac unloading properties than ANP, suggesting proANP is a biologically active NP with both physiologic and therapeutic implications (Supplemental Fig. 3).
To date, the in vivo physiological actions of proANP have not been defined, although de Bold and colleagues previously reported that extracts from the atrial myocardium, which contained atrial natriuretic factor, but most likely proANP as well, had a very potent diuretic, natriuretic and blood pressure lowering properties (29). Here we observed that proANP had greater and more prolonged cGMP activation than ANP while the AUC of BNP for both plasma cGMP and UcGMPV (Supplemental Fig. 2) was the highest of all three peptides.
Unexpectedly, proANP had significantly greater natriuretic and diuretic actions than ANP or BNP (Figs. 1A and 1B). These greater renal actions were a result of greater tubular actions and greater increases in RBF and GFR. The cardiorenal differences between proANP and mature ANP may be due to: 1) longer half-life of proANP; 2) “dual” activating properties of proANP via proANP and “processed to ANP” forms; and/or 3) conversion of proANP to active ANP forms, such as vessel dilator (ANP31-67) which works in the kidney through a non-cGMP pathway (30). Indeed, we speculate that the vessel dilator form may explain the 2nd peak of proANP urinary actions, which seems to occur via non-cGMP activation since urinary cGMP was not bi-phasic.
Beyond the kidney, we report that proANP reduced cardiac filling pressures including reductions in PAP, PCWP, RAP and SVR with increases in HR and Hct compared to baseline. In contrast, these hemodynamic effects were not observed with ANP. Notably, proANP markedly increased HR over 120 min (Fig.3D). We speculate the increased HR might be in response to volume loss associated with decreased Ht (Fig. 2B), however, this increase in HR may lead to adverse clinical events due to increased myocardial oxygen demand. Further studies are required to observe the HR in a HF model and to investigate the modulating actions of proANP on sympathetic activity.
Both proANP and ANP reduced blood pressure most likely due to actions on both the kidney and the systemic circulation, but with half the hypotensive effects of BNP. Surprisingly, BNP showed stronger and more sustained systemic vasodilatation together with greater cardiac unloading effects, but with much less natriuresis, diuresis and renal vasodilatation as compared to proANP. We cannot explain why BNP plays a different role than ANP or proANP as all are GC-A activators. Recognizing that plasma and urinary cGMP reflects guanylyl cyclase activation, we observed that proANP had greater and more prolonged cGMP activation than ANP, however, the AUC of BNP for both plasma cGMP and UcGMPV was greatest for all three peptides (Supplemental Fig. 2 and Table 1). Possible explanations include BNP signaling through an unknown receptor, differences in processing and degradation, or resistance to degrading enzymes (31).
We demonstrate proANP activates cGMP generation in GC-A but not GC-B overexpressing HEK293 cells. While less than ANP, proANP clearly increased cGMP production consistent with properties of a GC-A activator. This may be a direct action of proANP on GC-A since it has been reported that HEK293 cells lack corin which processes proANP into ANP (13). GC-A activating actions by proANP support our concept that proANP may represent a biologically active peptide itself, complementing mature processed ANP actions.
While our studies support proANP as a direct activator of GC-A, in fact a dual mechanism for GC-A activation may be at play, as we demonstrate that proANP can be processed to biologically active ANP in human serum, similar to our previous studies on proBNP processing in the circulation (21). It has been reported that corin is present in the circulation and kidney (32), supporting potential proANP cleavage to ANP in these locations (13,14). Similar to normal human serum, proANP was rapidly processed to ANP in HF. Thus, our human proANP could activate GC-A either directly or indirectly through processing to ANP in either normals or HF. These results are in contrast to our studies of proBNP processing and degradation, where cGMP activity of proBNP was much less than BNP, proBNP processing to BNP was rapid with degradation complete within 60 min in normal, and proBNP processing was delayed (33). These differences in metabolism and bioactivity between proANP and proBNP may be key factors in cardiovascular homeostasis.
The concept of a cGMP deficient state in HF raises a therapeutic opportunity especially in HFpEF and advanced HFrEF (6,34) despite results from the ASCEND-HF trial, which were negative, and supported by encouraging phase II clinical trials for BNP in HF which were positive. The failure of ASCEND-HF trial may be related to dose, duration, and clinical end-points (4,35). Clinical trials for chronic augmentation of the endogenous NP/cGMP system by angiotensin-neprilysin inhibition (LCZ696) showed positive results compared to angiotensin receptor blockade or ACE inhibitor. Specifically, LCZ696 which inhibits the degradation of endogenous NPs, decreased levels of NT-proBNP in HFpEF patients compared to valsaltan (PARAMOUNT trial) (36), and reduced risk of mortality and re-hospitalization in HFrEF patients compare to enalapril (PARADIGM-HF trial) (37) and increased cGMP (38). These trials suggest that enhancement of the cGMP pathway may be useful treatment for HF.
STUDY LIMITATIONS
A limitation to our study is the relatively small number of canines in each group, however, large normal canines compared to small animal studies lack hemodynamic variability and are well established in our laboratory, providing statistical significance with smaller numbers of canines. A second limitation is that proANP effects in animals may not translate to humans, therefore clinical studies are needed to confirm these results in humans.
CONCLUSIONS
ProANP represents a novel GC-A activator with a longer half-life and beneficial cardiorenal actions beyond ANP and BNP. The bioactivity of exogenous proANP may be through direct stimulation of GC-A, as well as through processing into ANP, as proANP is processed in the circulation of both normal and HF. Our findings lay the rationale for further investigations of proANP with a focus on not only physiological mechanisms of action but for therapeutic implications in HF and underscore proANP as a novel GC-A receptor activator and cGMP stimulator.
CLINICAL PERSPECTIVES
ProANP may be a unique “dual” activator of GC-A by either direct activation or through processing to ANP, contributing to sodium and volume regulation. ProANP's in vivo cardiorenal actions continued up to 3 hours, and processing of proANP remained intact in patients with HF, therefore HF may be a target disease for proANP treatment. ProANP may serve as a long lasting novel diuretic drug which can be administered by bolus injection and be more useful in the clinical setting compared to carperitide (ANP) or nesiritide (BNP). For widespread clinical application, proANP should be produced as a recombinant peptide based upon its extended amino acid length.
Translational outlook
Our studies advance our understanding of the heart as an endocrine organ and the ProANP/ANP/GC-A/cGMP system with biological, diagnostic and therapeutic implications. As proANP circulates, we must now consider it as a biologically active hormone and it may be useful to develop assays, which explore its biomarker potential. Finally, its unique renal and cardiac unloading actions may also lay the foundation for its development as a new therapy for cardiorenal disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors deeply appreciate the participation of healthy volunteers and patients with HF from our institution. The authors acknowledge the contributions of Dr. Ingrid A Andersen, Gail J Harty, Sharon M Sandberg and Denise M Heublein to the data collection.
Source of Funding: National Institute of Health (R01 HL36634 and P01 HL76611) awarded to Dr. John C. Burnett, Jr., American Heart Association Postdoctoral Fellowship (10POST3600045) and Scientist Development Grant (12SDG11460017) awarded to Dr. Tomoko Ichiki, and the Mayo Foundation.
ABBREVIATIONS
- NP
natriuretic peptide
- GC
guanylyl cyclase receptor
- HEK
human embryonic kidney
- UV
urine volume
- UXV
urinary x excretion
- RBF
renal blood flow
- PFRNa
proximal fractional reabsorption of sodium
- DFRNa
distal fractional reabsorption of sodium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with Industry: None.
REFERENCES
- 1.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–96. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 2.Konstam MA, Gheorghiade M, Burnett JC, Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA : the journal of the American Medical Association. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine. 2010;363:1419–28. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 6.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 7.van den Akker F. Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J Mol Biol. 2001;311:923–37. doi: 10.1006/jmbi.2001.4922. [DOI] [PubMed] [Google Scholar]
- 8.Espiner EA, Richards AM. Atrial natriuretic peptide. An important factor in sodium and blood pressure regulation. Lancet. 1989;1:707–10. doi: 10.1016/s0140-6736(89)92217-4. [DOI] [PubMed] [Google Scholar]
- 9.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circulation journal : official journal of the Japanese Circulation Society. 2005;69:283–90. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 10.Hata N, Seino Y, Tsutamoto T, et al. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circulation journal : official journal of the Japanese Circulation Society. 2008;72:1787–93. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 11.Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovascular research. 2005;68:8–17. doi: 10.1016/j.cardiores.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Nemer M, Chamberland M, Sirois D, et al. Gene structure of human cardiac hormone precursor, pronatriodilatin. Nature. 1984;312:654–6. doi: 10.1038/312654a0. [DOI] [PubMed] [Google Scholar]
- 13.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 15.Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. Journal of Clinical Investigation. 1991;87:1402–12. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potter LR. Natriuretic peptide metabolism, clearance and degradation. The FEBS journal. 2011;278:1808–17. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC., Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. European Heart Journal. 2013;34:886–893c. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralat LA, Guo Q, Ren M, et al. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. The Journal of biological chemistry. 2011;286:4670–9. doi: 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter I, Rehfeld JF, Goetze JP. Measurement of the total proANP product in mammals by processing independent analysis. Journal of immunological methods. 2011;370:104–10. doi: 10.1016/j.jim.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Semenov AG, Seferian KR, Tamm NN, et al. Human pro-B-type natriuretic peptide is processed in the circulation in a rat model. Clinical chemistry. 2011;57:883–90. doi: 10.1373/clinchem.2010.161125. [DOI] [PubMed] [Google Scholar]
- 21.Ichiki T, Huntley BK, Heublein DM, et al. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–7. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 22.Ichiki T, Boerrigter G, Huntley BK, et al. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;304:R102–9. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HH, Cataliotti A, Schirger JA, Martin FL, Burnett JC., Jr. Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1093–7. doi: 10.1152/ajpregu.00682.2004. [DOI] [PubMed] [Google Scholar]
- 24.McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC., Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol. 2009;54:1024–32. doi: 10.1016/j.jacc.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida Y, Morita H, Minamino N, Kangawa K, Matsuo H, Hosomi H. Effects of brain natriuretic peptide on hemodynamics and renal function in dogs. The Japanese journal of physiology. 1990;40:531–40. doi: 10.2170/jjphysiol.40.531. [DOI] [PubMed] [Google Scholar]
- 26.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods in molecular biology. 2012;929:377–89. doi: 10.1007/978-1-62703-050-2_16. [DOI] [PubMed] [Google Scholar]
- 27.Martin FL, Sangaralingham SJ, Huntley BK, et al. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLOS ONE. 2012;7:e52422. doi: 10.1371/journal.pone.0052422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Jr., Ichiki T. ProBNP1-108 Processing and Degradation in Human Heart Failure. Circulation. Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bold AJ. Atrial natriuretic factor of the rat heart. Studies on isolation and properties. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1982;170:133–8. doi: 10.3181/00379727-170-41408. [DOI] [PubMed] [Google Scholar]
- 30.Vesely DL. Which of the cardiac natriuretic peptides is most effective for the treatment of congestive heart failure, renal failure and cancer? Clinical and experimental pharmacology & physiology. 2006;33:169–76. doi: 10.1111/j.1440-1681.2006.04344.x. [DOI] [PubMed] [Google Scholar]
- 31.Pankow K, Schwiebs A, Becker M, Siems WE, Krause G, Walther T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic Peptide degradation. Journal of molecular biology. 2009;393:496–503. doi: 10.1016/j.jmb.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Wu S, Wang W, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–72. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huntley BK, Sandberg S, Heublein D, Sangaralingham J, Burnett J, Ichiki T. ProBNP1-108 Processing and Degradation in Human Heart Failure. Circ Heart Fail. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001174. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene SJ, Gheorghiade M, Borlaug BA, et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. Journal of the American Heart Association. 2013;2:e000536. doi: 10.1161/JAHA.113.000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J. The disconnect between phase II and phase III trials of drugs for heart failure. Nature reviews. Cardiology. 2013;10:85–97. doi: 10.1038/nrcardio.2012.181. [DOI] [PubMed] [Google Scholar]
- 36.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 37.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.