Abstract
Dengue is the most important reemerging mosquito-borne viral disease worldwide. It is caused by any of four Dengue virus types or serotypes (DENV-1 to DENV-4) and is transmitted by mosquitoes from the genus Aedes. Ecological changes have favored the geographic expansion of the vector and, since the dengue pandemic in the Asian and Pacific regions, the infection became widely distributed worldwide, reaching Brazil in 1845. The incidence of dengue in Brazil has been frequently high, and the number of cases in the country has at some point in time represented up to 60% of the dengue reported cases worldwide. This review addresses vector distribution, dengue outbreaks, circulating serotypes and genotypes, and prevention approaches being utilized in Brazil.
1. Introduction
Dengue is an arthropod-borne disease caused by the Dengue virus (DENV) [1]. DENV is an enveloped, single-stranded, positive-strand RNA virus, member of the genus Flavivirus in the Flaviviridae family. It is transmitted from human to human through the bite of mosquitoes of the genus Aedes [2]. DENV transmission by transfused blood is rare; however it indeed occurs and has been documented in Brazil [3].
There are four distinct but antigenically related types or serotypes of DENV (DENV-1 to DENV-4). The outcomes of infection by any of the DENV types range from asymptomatic, subclinical to symptomatic infections [4–7]. Symptomatic infections vary from a mild, flu-like illness known as dengue fever (DF) to a life-threatening form called severe dengue (SD) [8]. Severe dengue was previously known as dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) [8].
The onset of DF is sudden, characterized by high fever accompanied by intense frontal headache, fatigue, retroorbital pain, myalgia, arthralgia, and rash. SD is defined by an increase in vascular permeability (“plasma leakage”), hemorrhagic manifestations, and decreased platelet counts near the time of defervescence, which may progress to clinical hypotension and shock [6, 9–11]. In the absence of supportive care, SD fatalities may occur in approximately 4% of cases [1, 10]. Thrombocytopenia is commonly observed in both DF and SD as a result of platelet activation and apoptosis, triggering platelet clearance [5, 12].
To date, there is no effective vaccine or specific antiviral therapy for dengue. Early diagnosis and appropriate management of SD, including hospitalization, prevent fatalities [13]. Laboratory diagnosis of dengue is based on the detection of the virus and antibodies to the virus or combined detection of antigens and antibodies by serology (detection of specific IgM and IgG anti-DENV) [14], detection of the NS1 antigen of the virus, virus isolation in susceptible mosquitoes or in mosquito or mammalian cell lines, and nucleic acid detection tests (end-point PCR, TaqMan, and other nucleic acid detection assays) [15–18]. The detection of NS1 using ELISA is a sensitive method (>90%) for identification of primary DENV infections. In secondary infections, the sensitivity ranges between 60 and 80% [19, 20]. However, the test is not sensitive enough for blood donor screening. Some NS1 ELISA has low sensitivity for DENV-4 and it has contributed to the failure of reporting epidemiological data concerning DENV-4 cases in Brazil [21, 22].
While there has been no definitive association of the distinct DENV types with clinical course of disease, there are reports suggesting that DENV-2 and DENV-3 cause severe disease more frequently than the other serotypes and that DENV-4 causes a milder illness [23, 24]. DENV-2 was the most prevalent serotype in several outbreaks in the Americas [6] and was epidemiologically classified as the most relevant serotype worldwide due to its association with the highest number of intense outbreaks, followed in sequence by DENV-3, DENV-1, and DENV-4 [25].
Knowledge about the pathogenesis of dengue infection is limited, but both viral and immune host factors appear to be associated with severe illness [26]. Nevertheless, the involved DENV type and the host genetic makeup may explain some differences in clinical manifestations observed, since genetic polymorphisms appear to provide protection or predispose to more severe forms of dengue [27].
Studies carried out in epidemic regions led to the generation of hypotheses about the development of SD [28]. They include antibody-mediated pathogenesis [4], host cytokine storms [29], host genetic factors [24], characteristics of virus isolates [30–32], viral load in the acute phase [33, 34], and the host nutritional status [35]. DENV can interact with dendritic cells, monocytes/macrophages, hepatocytes, and endothelial cells leading to the release of immune mediators during SD [31, 36, 37]. However, how the production of these cytokines is induced and their role in dengue pathogenesis are still not clear.
The global dengue pandemic appears to have begun in the Asian and Pacific regions where the first epidemic of dengue was reported, in 1779-1780 [38]. Ecological changes occurring since that time probably favored the geographic expansion of the vector and its increase in density. The high number of susceptible individuals (local populations, soldiers) and their widespread movement probably created conditions that facilitated the dispersion of the viruses [39, 40]. A number of complex factors contributed to the emergence and reemergence of dengue, as, for instance, population growth and unplanned urbanization associated with poverty and health inequality [40, 41].
The number of cases of DF and SD has steadily increased worldwide, and dengue infections have spread to new areas of the world, such as North America and Europe [42]. The disease is endemic in more than 100 countries, and the World Health Organization (WHO) estimated that over 40% of the world's population is at risk of dengue, with 50–100 million dengue infections reported worldwide every year [1]. However, Bhatt et al., 2013 [43], using new modelling approaches, estimated that 390 million dengue infections (apparent and unapparent) occur per year, three times higher than previous WHO estimates.
In the Americas, 1,173,248 suspected dengue cases were reported in 2014, of which 341,192 were confirmed by laboratory tests; 16,008 cases were reported as severe dengue and 684 deaths occurred due to the infection [44]. As demonstrated in a recent review, Brazil presented the fifth highest incidence of DF among Latin American and Caribbean countries from 1995 to 2009 [45]. Between the countries of the Southern Cone—Argentina, Brazil, Chile, Paraguay, and Uruguay—Brazil had the highest incidence rate of dengue: 294.02/100,000 inhabitants in 2014 [44].
Brazil is composed of 5 geographical regions, shown in Figure 1 and Table 1. In 2014, the Southeast region reported the majority of suspected cases in the country (312,318 cases; 52.8%), followed by the Midwest (114,814 cases; 19.4%), Northeast (90,192 cases; 15.3%), North (49,534 cases; 8.4%), and South (24,222 cases; 4.1%) (Table 1; Figure 1). This geographical trending has been previously observed, with the Southeast region presenting the highest number of cases and the South region the lowest ones (Figure 2). However, when comparing incidence/100,000 inhabitants, the Midwest region presents the highest numbers (Figure 2) due to population size (Table 1). Only a few states had an increase in the number of cases in 2014 compared to 2013: Acre, Tocantins, São Paulo, and Distrito Federal. Between the 10 municipalities with higher number of cases reported, 5 are located in the state of São Paulo [46].
Figure 1.
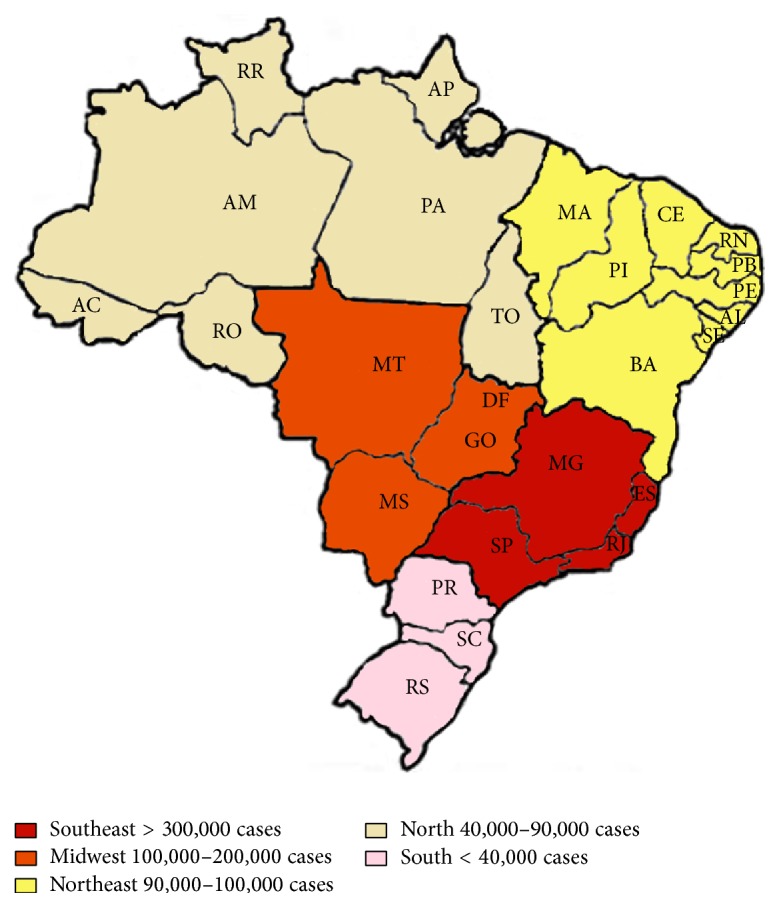
Number of dengue cases reported by the Brazilian Ministry of Health in 2014 distributed by geographical region. Brazil's map is divided according to the regions and number of dengue cases. The numbers were obtained from Brazilian Ministry of Health website, accessed at http://portalsaude.saude.gov.br/. Regions and their states are listed in Table 1.
Table 1.
Dengue cases and DENV serotypes reported in Brazil in 2014.
| Geographical regions and states | Number of inhabitantsa | Number of cases reportedb | Serotypes confirmedb |
|---|---|---|---|
| Southeast region | 85,505,375 | 312,318 | |
| Minas Gerais (MG) | 20,813,172 | 59,222 | DENV-1, DENV-3, and DENV-4 |
| Espírito Santo (ES) | 3,906,648 | 19,223 | DENV-1, DENV-4 |
| Rio de Janeiro (RJ) | 16,520,687 | 7,823 | DENV-1, DENV-4 |
| São Paulo (SP) | 44,264,868 | 226,040 | DENV-1, DENV-2, and DENV-4 |
| Midwest region | 15,344,314 | 114,814 | |
| Mato Grosso do Sul (MS) | 2,638,452 | 3,594 | DENV-1, DENV-2, and DENV-4 |
| Mato Grosso (MT) | 3,247,377 | 7,232 | ND |
| Goiás (GO) | 6,574,219 | 92,311 | DENV-1, DENV-4 |
| Distrito Federal (DF) | 2,884,266 | 11,677 | DENV-1 |
| Northeast region | 41,228,546 | 90,192 | |
| Maranhão (MA) | 6,876,025 | 2,416 | ND |
| Piauí (PI) | 3,200,725 | 7,665 | DENV-1 |
| Ceará (CE) | 8,883,739 | 22,974 | DENV-1, DENV-3, and DENV-4 |
| Rio Grande do Norte (RN) | 3,428,430 | 11,285 | DENV-1, DENV-2, and DENV-4 |
| Paraíba (PB) | 3,957,858 | 5,575 | DENV-1, DENV-2, DENV-3, and DENV-4 |
| Pernambuco (PE) | 9,315,660 | 10,446 | DENV-1, DENV-2, DENV-3, and DENV-4 |
| Alagoas (AL) | 3,332,654 | 13,275 | ND |
| Sergipe (SE) | 2,233,455 | 2,275 | DENV-1, DENV-4 |
| Bahia (BA) | 15,170,625 | 14,281 | DENV-1, DENV-4 |
| North region | 17,367,554 | 49,534 | |
| Rondônia (RO) | 1,760,186 | 2,104 | DENV-1, DENV-4 |
| Acre (AC) | 797,676 | 28,931 | DENV-1 |
| Amazonas (AM) | 3,910,647 | 6,472 | DENV-4 |
| Roraima (RR) | 503,116 | 1,181 | DENV-1, DENV-2, DENV-3, and DENV-4 |
| Pará (PA) | 8,127,744 | 4,833 | DENV-1, DENV-2, and DENV-4 |
| Amapá (AP) | 760,634 | 1,958 | DENV-1 |
| Tocantins (TO) | 1,507,551 | 4,055 | DENV-1, DENV-4 |
| South region | 29,131,203 | 24,222 | |
| Paraná (PR) | 11,133,587 | 23,924 | DENV-1, DENV-4 |
| Santa Catarina (SC) | 6,758,785 | 141 | ND |
| Rio Grande do Sul (RS) | 11,238,831 | 157 | DENV-1, DENV-4 |
Number of dengue cases and DENV serotypes reported in Brazil in 2014 by the Ministry of Health. Data are organized by geographical regions and the states they include. aData obtained in http://www.ibge.gov.br/apps/populacao/projecao/index.html (last accessed on January 30, 2015). bData reported by Brazilian Ministry of Health until the epidemiological week 53—12/28/14 to 01/03/15. Source: http://portalsaude.saude.gov.br (last accessed on May 20, 2015). ND: not determined.
Figure 2.
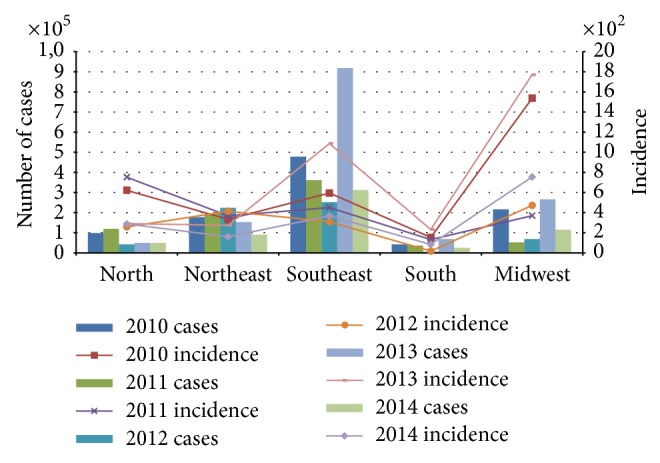
Number of cases and incidence/100,000 inhabitants reported by the Brazilian Ministry of Health in the last 5 years (2010–2014). The numbers were obtained from Brazilian Ministry of Health website, accessed at http://portalsaude.saude.gov.br/.
Brazil is considered a tropical country in its entirety because of its hot and humid climate which provides a receptive and highly favorable environment for proliferation of the dengue vector. The different climate zones have differences in the rain dynamics in the areas of the coastal strip, altitude variation, and so forth. DENV activity occurs throughout the year, but the majority of outbreaks and the highest levels of vector infestation show a marked seasonal pattern, occurring during the Brazilian rainy season, from December to May, which are also the hottest months of the year [47].
The scope of this review includes the epidemiological situation of dengue in Brazil, the temporal-spatial distribution of outbreaks, serotype and genotype distribution, geographical distribution of vector, and an analysis of measures that have been or could be taken to address this problem in the country.
2. Dengue in Brazil
2.1. Epidemiology and Outbreaks
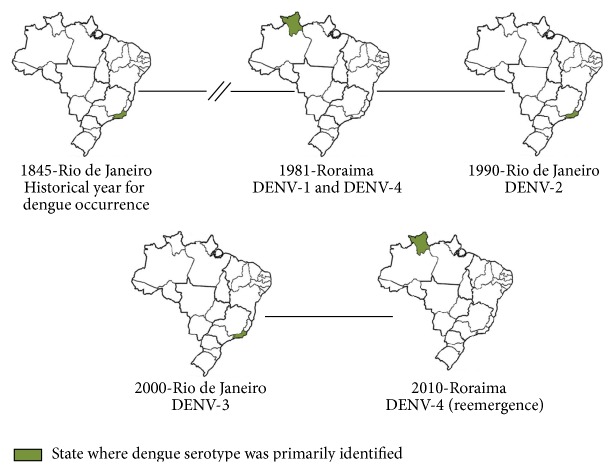
Dengue has been present in Brazil since 1845, when the first epidemic was reported in the state of Rio de Janeiro (Figure 3) [48]. Other epidemics were registered during the years 1851–1853 and 1916–1923 [49]. The mosquito eradication program to prevent urban yellow fever, coordinated by the Pan American Health Organization (PAHO), functioned successfully to keep Brazil free of Aedes aegypti until 1976. After that, the first evidence of a dengue epidemic was in 1981, when dengue cases occurred due to reinfestation of urban areas of Brazil by Ae. aegypti [47, 48]. This epidemic took place in the state of Roraima (Figure 3) and was caused by DENV-1 and DENV-4 (Table 2) [50]. It was the first laboratory and clinically reported dengue outbreak in Brazil with the presence of both serotypes [48, 50]. Nevertheless, the disease only received proper attention in 1986 and 1987, after DENV-1 was introduced into Rio de Janeiro [6, 47, 51, 52] (Figure 3). More than a million individuals from Rio de Janeiro were infected with DENV-1 [52]. DENV-1 was also responsible for epidemics in Ceará and Alagoas states in 1986 and in Pernambuco state in 1987 [53].
Figure 3.
Timeline of introduction of dengue serotypes in Brazil. The states where dengue serotypes were primarily identified are highlighted in green. The figure was assembled with information collected from literature as described in the paper.
Table 2.
Dengue activity in Brazil between 1845 and 2010.
| Year (s) | Activity reported | Dengue serotype | Location | Reference |
|---|---|---|---|---|
| 1845 | 1st dengue epidemic was reported | Unknown | Rio de Janeiro | [46] |
|
| ||||
| 1981 | 1st dengue epidemic in Brazil after Ae. aegypti reinfestation | DENV-1 DENV-4 |
Roraima | [46, 50] |
|
| ||||
| 1986-1987 | Epidemic | DENV-1 | Rio de Janeiro | [6, 48, 49, 51] |
|
| ||||
| 1990 | First identification of DENV-2 | DENV-2 | Rio de Janeiro | [26, 48, 49, 54] |
|
| ||||
| 1990–2000 | DENV spread intensified contributing to several outbreaks | DENV-1 DENV-2 |
Southeast and northeast region | [47, 55, 56] |
|
| ||||
| 2000 | 1st appearance of DENV-3 in Brazil | DENV-3 | Rio de Janeiro | [48–51, 53] |
|
| ||||
| 2002 | One of the largest dengue outbreaks in Brazil since the virus emergence | DENV-3 | Rio de Janeiro | [48–51] |
|
| ||||
| 2000–2007 | Brazil reported >60% of the cases registered in the world | DENV-1 DENV-2 DENV-3 |
All Brazilian states | [47, 54, 55] |
|
| ||||
| 2007-2008 | Intense outbreak with high number of severe cases and fatalities | DENV-2 | Rio de Janeiro | [6, 55] |
|
| ||||
| 2009 | Large outbreak | DENV-2 | Espírito Santo | [56] |
|
| ||||
| 2010 | Several outbreaks | DENV-1 DENV-2 DENV-3 DENV-4 |
21 Brazilian states | [26] |
|
| ||||
| 2010 | Reemergence of DENV-4 | DENV-4 | Roraima, Amazonas, Amapá, Pará, São Paulo, and Rio de Janeiro | [57, 58, 65, 68] |
The paper text contains more detailed information about the outbreaks and other epidemics description as well.
DENV-2 spread across the country after its first identification in 1990 in the state of Rio de Janeiro (Figure 3), where the first cases of SD were documented [26, 47, 52, 54] and 8 deaths due to SD were registered [46]. Both DENV-1 and DENV-2 have been possibly introduced in Brazil from Africa [49].
During the 1990s, the spread of DENV-1 and DENV-2 intensified and outbreaks affected Southeast and Northeast states [55–57] (Table 2). A total of 1,696 and 7,374 cases of dengue were reported in 1992 and 1993, respectively, but no deaths were attributed to dengue in these years. All cases occurred in the Southeast region with exception of the state of Espírito Santo [46].
In 1994, 12 states of Brazil documented cases of the disease: Tocantins, Piauí, Ceará, Rio Grande do Norte, Alagoas, Bahia, Rio de Janeiro, São Paulo, Mato Grosso do Sul, Mato Grosso, Goiás, and Distrito Federal, with the highest incidence rate in the Northeast region (112.2 cases/100,000 inhabitants). During the 1990s, the highest incidence rate of dengue (313.8 cases/100,000 inhabitants) occurred in the year 1998 [46]. In 1999, 50% of Brazilian municipalities had already reported DF cases and Ae. aegypti had been detected in 64% of them and only the states of Acre and Distrito Federal did not document cases of dengue [52].
DENV-3 first appeared and caused an outbreak in the state of Rio de Janeiro in December 2000 [58] (Figure 3), with 3,220 dengue cases reported. In 2002, DENV-3 caused one of the largest outbreaks ever reported in Brazil (696.472 cases/100,000 inhabitants) in the state of Rio de Janeiro, with 288,245 reported cases and 91 deaths [47, 52, 53, 59, 60].
Between 1981 and 2006, 4,243,049 dengue cases were reported in Brazil, including 5,817 cases of SD and 338 fatalities. The highest number of reports came from the Northeast and Southeast regions [59]. One of the states of the Northeast region, Pernambuco, had from 1995 to 2006 approximately 380,000 DF cases, 612 DHF cases, and 33 deaths reported [61].
From 2002 to 2006, the most prevalent serotype in Brazil was DENV-3. In the following years, between 2007 and 2009, DENV-2 was responsible for the majority of cases [62]. From 2000 to 2007, Brazil alone accounted for more than 60% of the world reported cases of dengue. Dengue epidemics occurred in different regions of the country and cases of DENV-1, DENV-2, and DENV-3 were reported in all states [57, 63, 64].
Between 2007 and 2008, DENV-2 caused an intense outbreak in the state of Rio de Janeiro with a higher number of severe cases (954 and 15,730, resp.) and fatalities (41 and 263, resp.) than previous outbreaks, primarily among children and adolescents [6, 64]. In these 2 years, 851 deaths due to severe dengue were registered in the country [46]. In 2008, approximately 80% of the cases reported in the country occurred in the Southeast and the Northeast regions [62]. In 2009-2010, over a million suspected cases of DF and 665 deaths were reported in Brazil, with DENV-1 accounting for most of the cases [62]. However, in 2009 in the state of Espírito Santo in the Southeast region, DENV-2 was the predominant type between the 53,708 cases notified, followed by DENV-1 [65].
The 2010 dengue epidemic in Brazil was characterized by several outbreaks in 21 states, Rio Grande do Sul, Santa Catarina, Paraná, São Paulo, Minas Gerais, Rio de Janeiro, Espírito Santo, Bahia, Mato Grosso, Mato Grosso do Sul, Tocantins, Acre, Pará, Roraima, Goiás, Rondônia, Alagoas, Pernambuco, Rio Grande do Norte, Piauí, and Ceará, and by cocirculation of all serotypes, with DENV-4 reemerging in the northern region of the country after 28 years of absence [66] (Figure 3, Table 2). Thereafter, DENV-4 was reported in the states of Amazonas, Amapá and Pará [67], São Paulo [68], and Rio de Janeiro [69]. Currently, 100% of dengue cases in the states of Rondônia, Amazonas, Piauí, and Paraíba, all with incidence rate over 100 cases/100,000 inhabitants, are due to DENV-4. Overall, in Brazil the current most prevalent DENV types are DENV-1 (83.3%), followed by DENV-4 (15.1%), DENV-2 (1.3%), and DENV-3 (0.3%) [46].
2.2. Current Situation
Epidemiologic studies performed in Brazil during 2005 and 2008 demonstrated that dengue affected predominantly adults and usually occurred in cities with more than 500,000 inhabitants [47, 60]. Nevertheless, the profile of dengue outbreaks in Brazil is changing and current data underscore a different epidemiological pattern, wherein an increased proportion of severe cases occur among children [70, 71] and in low population density areas [52]. During the 2007 epidemic, 40% of dengue cases came from municipalities with fewer than 100,000 inhabitants [52], and more than 50% of cases were in children younger than 15 years of age [70]. The same pattern occurred in Ceará state: in 2007 the majority of hospitalized cases due to SD were in children <15 years of age; in 2008, DF incidence was highest (599.4 cases/1000,000 inhabitants) among children <10 years of age [71].
Between the years 2000 and 2006, the ratio of DF/SD cases reported in Brazil was 467.7, considerably higher than that reported during the same period in Honduras, Venezuela, Colombia, and Mexico, becoming the highest ratio in the continent [72]. However, the lethality ratio of dengue in Brazil was 0.01% and mortality per 100,000 inhabitants was 0.03, both from 1995 to 2009 [45]. Still, the diagnoses of severe dengue, the number of hospitalized cases, and the number of deaths attributed to dengue have increased during the past 10 years [47] (Figure 4).
Figure 4.
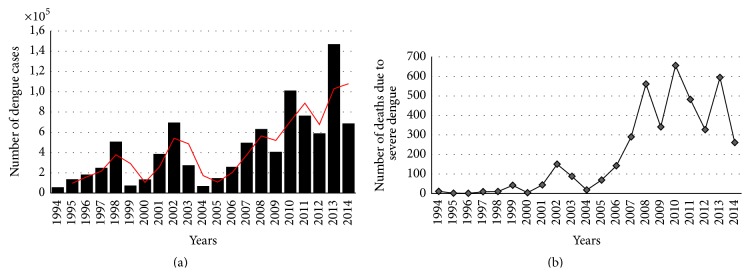
Number of cases (a) and deaths (b) due to confirmed dengue cases in Brazil from 1994 to 2014. The numbers were obtained from Brazilian Ministry of Health website, accessed at http://portalsaude.saude.gov.br/. Dengue cases were confirmed by laboratory tests as described in Introduction. Red line represents two periods moving average and was set up with the Spreadsheet Software Program Microsoft Excel.
When compared to 2012, there was a decrease in the number of cases compared with 2011 but in 2013 the incidence increased by 190% [73]. According to this data, in 2013, 1,468,873 cases were reported in Brazil, of which 6,969 were severe cases, with 545 deaths [46]. In 2014, the Brazilian Ministry of Health had reported to PAHO 591,080 cases of dengue for an incidence rate of 291.5/100,000 inhabitants and 410 deaths. It represents a decrease of 39% of deaths in relation to the same period in 2013 [44, 46]. As of April 2015, the Brazilian Ministry of Health has registered 1,254,907 notified cases of dengue, representing an increase which is more than twofold compared to that of 2014, and it is being considered a new epidemic in the country, with 530 deaths due to SD (53% more compared to the same period of 2014) [46].
The observed clinical manifestations of dengue may include neurological, hepatic, and cardiac involvement as described in Brazil and other countries of the Americas and in Southeast Asia [52]. Neurological manifestations of DENV include reduced levels of consciousness, severe headaches, neck stiffness, focal neurological signs, tense fontanels, and convulsions [74]. The pathophysiology is attributed to factors, such as cerebral edema, cerebral hemorrhage, hyponatremia, and fulminant hepatic failure with encephalopathy, cerebral anoxia, microcapillary hemorrhage, and release of toxic products [23]. Some authors state that dengue infection should be considered a possible cause of encephalitis in endemic regions [74, 75]. During the outbreaks of 1997 and 2002, there were reports of 41 cases with neurological manifestations in the state of Pernambuco. Encephalitis, Guillain-Barré syndrome, convulsions, meningoencephalitis, and reduced levels of consciousness were the most common manifestations [76].
Severe dengue is not necessarily associated with secondary infections. Disease severity appears to be determined by many risk factors, including the strain virulence and host immunity [10, 52, 60]. Concerning the differences of severity between Brazilian individuals, the human leukocyte antigen (HLA) gene, which mainly encodes the major histocompatibility complex (MHC) class I and class II molecules, has been appointed as a possible marker of susceptibility to dengue disease. A recent study of dengue patients in Recife, Brazil, showed a significant association of MHC I and MHC II molecules encoding the alleles HLA-B∗44, -B∗50, and -DR∗16 with increased susceptibility to SD [77]. The link between HLA class I alleles and SD was found among patients from Rio de Janeiro with association of SD with the HLA-A∗01 allele and a potential protective role of the HLA-A∗ allele in SD [78].
2.3. The Different Genotypes of Dengue Virus in Brazil
In addition to frequent outbreaks, an increased genetic diversity of DENV may have contributed to severe consequences such as an increase in pathogenicity, transmissibility, and virulence properties [79]. Genetic diversity allows enhancement of viral replication following heterologous infections due to limited cross-reactive immunity [80]. The four DENV types are known to be genetically distinct from each other, and each one has genetic variations classified as subtypes or genotypes [81].
DENV-1 has five distinct genotypes, designated I to V [62, 80]. The genotype V of DENV-1 was identified in the years 2009-2010 in the states of Rio de Janeiro and Espírito Santo, in Southeast of Brazil. The circulating strains of genotype V were grouped into a clade (lineage II) that was distinct from the clade represented by earlier Brazilian DENV-1 strains (lineage I). Another distinct clade (lineage III), identified in samples from Rio de Janeiro in 2010 and 2011, showed similarity to strains isolated in 2007 and 2008 in Colombia, Venezuela, and Mexico [62].
DENV-2 comprises six genotypes: Asian I, Asian II, Southeast Asian/American, Cosmopolitan, American, and Sylvatic genotypes [62, 65, 81]. The Sylvatic genotype represents strains from humans, arboreal mosquitoes, and nonhuman primates collected in West Africa and Southeast Asia [80]. Strains from the Southeast Asian/American genotype were isolated during epidemics periods in 1990 and 1998 in Rio de Janeiro [26, 82]. In 2010, DENV-2 isolates circulating in the cities Guarujá and Santos, in the state of São Paulo, clustered within the Southeast Asian/American genotype. This is the same genotype that circulated in Rio de Janeiro in 2007-2008 and in Espírito Santo in 2009 [26, 65, 82, 83]. These observations suggest that the genetically different viruses detected in Rio de Janeiro could have resulted from local evolution of DENV-2 since its introduction in 1990 [82, 83]. Indeed, DENV-2 strains circulating in Brazil belong to separate Southeast Asian/American genotype lineages: lineage I, circulating from 1990 to 2003, and lineage II for strains isolated after 2007 [83].
DENV-3 has five genotypes designated I to V by phylogenetic analysis based on different viral gene regions [80, 84]. The majority of Brazilian samples are grouped with genotype III [85] but genotype I was isolated between 2002 and 2004 in the state of Minas Gerais and later detected in Ae. aegypti mosquitoes and eggs [86]. This genotype was associated with a fatal case [87]. DENV-3 strains isolated during 2006 and 2007 outbreaks in the city of São José do Rio Preto in the state of São Paulo fell within genotype III and grouped with Brazilian isolates from different regions in different years, including samples from Acre in 2004 and Rio de Janeiro in 2002 [85]. Four distinct lineages of DENV-3 genotype III have been identified in Brazil (I to IV); however only lineages I and II seem to have become effectively established and disseminated in the country [88].
DENV-4 has four genotypes: I to IV, where genotype IV is the only Sylvatic DENV-4 strain isolated from sentinel monkeys in Malaysia [80, 89]. Genotype II predominates in Brazil. It was introduced into the Americas (Caribbean region) around 1978 [67]. Sequence analyses performed with samples of DENV-4 from São Paulo and Rio Grande do Sul revealed that all samples were of genotype II and grouped with samples from the Caribbean and northern South America [90]. In addition, genomic and envelope protein phylogeographic analyses showed that DENV-4 genotype I was isolated in 2011 in the city of Salvador, in the state of Bahia, and appeared to originate from mainland Southeast Asia [67]. DENV-4 genotype I infecting Aedes aegypti have been also described in the city of Manaus, in the state of Amazonas [91].
Introduction of new DENV genotypes may have facilitated the increase in clinical severity of dengue infections observed in more recent dengue epidemics [81]. Table 3 shows the DENV genotypes known to circulate in Brazil.
Table 3.
DENV genotypes identified in Brazil.
2.4. Dengue Vectors in Brazil
Dengue is transmitted mainly by Ae. aegypti and Ae. albopictus. Both vectors are adapted to the peridomestic environment where they feed on humans and domestic animals and oviposit in a variety of natural and artificial water holding containers [86, 92–94].
The efficiency of transmission depends on vector competence which is defined as the susceptibility of a mosquito to become infected and subsequently transmit the virus through the bite [95]. Ae. albopictus is more susceptible than Ae. aegypti to midgut infection by DENV. However, a smaller proportion of Ae. albopictus develops disseminated infection when compared to Ae. aegypti, suggesting that DENV dissemination by Ae. albopictus is less efficient [93].
Between the 1950s, 1960s, and most of the 1970s, epidemic dengue was rare in Central and South America because Ae. aegypti had been eliminated from most of the countries in the continent. The eradication program organized by PAHO was discontinued in the early 1970s, and the mosquito was reintroduced in countries from which it had been previously eradicated [96].
In Brazil, Ae. aegypti has been responsible for dengue transmission since the early 1980s. Ae. albopictus was introduced in Brazil in 1986 [93, 94] and is present in all Brazilian states [97]. Ae. albopictus is not considered as a vector of DENV in the country and has not been associated with dengue epidemics [93, 97, 98]. However, the occurrence of vertical transmission of DENV-2 and DENV-3 in Ae. aegypti and Ae. albopictus has already been observed in Fortaleza [94] and raises questions about the potential for transmission of DENV by Ae. albopictus in Brazil. Indeed, studies in the state of Rio de Janeiro have shown that Ae. albopictus was the dominant species in discarded tires used as traps [99, 100].
The continuous global expansion of Ae. albopictus is a serious concern as it may play a role in the maintenance of DENV in nature [95] and alter the transmission dynamics of many arboviral diseases increasing the risk of mosquito-borne viral infections among humans [101].
A major example is Chikungunya fever (CHIK), a disease caused by an arthropod-borne virus, the Chikungunya virus (CHIKV), which often cocirculates with DENV in their respective endemic regions. CHIKV reached the Americas in December 2013, causing outbreaks that now affect 44 territories throughout the Americas with more than 1.2 million suspected cases reported to PAHO [102, 103]. More than 1,000,000 suspected cases, 24,375 laboratory-confirmed cases, and 178 deaths were reported to PAHO between 2013 and 2014 [102]. The Brazilian government has reported three imported cases of CHIK in 2010 [104]. 3,195 CHIK autochthonous cases have been reported in Brazil from which 2,196 were confirmed as of January 2015 [46].
Dengue epidemics causing mostly dengue fever attributed to transmission by Ae. albopictus have occurred in Asia (Japan, China, Maldives Islands, and northern Taiwan), Africa (Seychelles Islands), Oceania (La Reunion Island), and Hawaii. Major epidemics of severe dengue have only occurred in areas where Ae. aegypti is found [93].
Many factors support proliferation of Ae. aegypti and consequently the sustained transmission of mosquito-borne diseases in Brazil, including the climate, high human population density in large cities, precarious socioeconomic status, and lack of infrastructure, particularly adequate sanitation [52, 72]. Meteorological conditions and seasonal variations may affect the distribution and abundance of the vector [72]. As an example, rainfall affects mosquito abundance positively through the creation of new breeding sites [105]. The vector in turn is able to adapt to new environmental situations that affect dengue epidemiology [52].
An initial report of Ae. aegypti occurrence in Brazil in 2014 shows the following percentage of municipalities at risk, based on the Breteau Index (density of mosquito larvae): 32.7% in the North, 34.3% in the Northeast, 8.4% in the Southeast, 5.8% in the Midwest, and 32.5% in the South region [46]. It is important to observe that, even with a low percentage of municipalities at risk, the Southeast region presented a dengue incidence rate of 366.9 cases/100,000 inhabitants in 2014 (Figure 2). The appropriateness of larval indices for population monitoring has been questioned because their relationship with adult Aedes densities heavily depends on larval mortality [106, 107], but the Breteau Index continues to be used by the Brazilian Ministry of Health.
2.5. Prevention and Research
No safe and effective vaccine for dengue is currently available. Therefore, the control of DENV infections relies solely on vector control. In this context, some prevention measures have been adopted in Brazil, such as development and implementation of public awareness campaigns to educate the population with the aim of reducing the availability of Ae. aegypti breeding sites. Insecticide application [52] and monitoring systems of Aedes larvae and eggs, as well as mosquito adults, have also been used [108, 109].
Health professionals in the country are being trained to improve early diagnosis and treatment of severe dengue [52]. Indeed, an effective diagnostic test contributes significantly to the clinical treatment, etiologic investigation, and control of DENV infections [42]. Since 2002, Brazil has adopted a clinical protocol looking at the clinical progression of disease. It is based on the recognition of clinical and laboratory data and conditions related to severity, with the goal of appropriate treatment and avoidance of deaths. In this context, the Brazilian Ministry of Health recommends virus isolation and serology as the main diagnostic tests [110].
Currently, a new vector control strategy has been developed, which consists of the introduction of Wolbachia bacteria into target vector populations, which directly inhibits the ability of several pathogens to infect Ae. aegypti [111–113]. Another advanced technique developed by Oxitec is the releasing of transgenic mosquitoes to reduce wild mosquitos numbers [114, 115]. In Brazil, the evaluation of Oxitec mosquitoes is called “Project Aedes Transgenico” (PAT) [116]. Around 11 million male mosquitoes have been released in the country from February 2011 through February 2012 [116, 117]. Results of both strategies have been encouraging. However, more studies are needed before considering these approaches safe and effective.
National and international efforts have been applied to the development of a dengue vaccine. The major challenge is to create a safe and effective tetravalent vaccine that generates immunity to all four serotypes. Patients that recover from dengue infection by one serotype are at risk of developing severe dengue when infected subsequently by a different serotype. The concern about a vaccine unable to raise immunity to the four serotypes simultaneously is that it may increase the risk of severe dengue when the immunized individual is infected by a serotype for which the individual is not immune [52, 118]. One obstacle that hampers research in this area is the lack of an adequate animal model for SD [15].
Several vaccine candidates are currently being evaluated in clinical trials and vaccines are likely to be available within the next several years [119–121]. A recent cost-effectiveness study of dengue vaccines in Brazil showed that herd-immunity may be achieved by vaccinating 82% of the population at a vaccine efficacy of 70%. At this efficacy, vaccination would cost up to US$534 per vaccinated individual with cost-savings of up to $204 [120]. These values indicate that, even at a relatively low efficacy, vaccination would still be cost-effective since the total vaccination cost would be sufficiently low [120]. Indeed, the vaccination in low- and middle-income countries brings important economic benefits and cost-effectiveness studies suggest that vaccines are an efficient public health investment [122]. Another detailed economic analysis of the steady-state production of 60 million doses per year at the Instituto Butantan in the state of São Paulo showed that the vaccine, if it proves to be safe and effective, can be available at a price that most ministries of health in developing countries could afford [123].
3. Conclusions
Dengue is considered the most important mosquito-borne viral disease in the world by the World Health Organization [42] and an ongoing threat to the Brazilian population. Because of the lack of vaccines and specific therapies, vector control is currently the only effective measure available to control the spread of the disease. Many studies are being conducted to develop and to improve technologies that are able to reduce or eliminate Ae. aegypti populations on a large scale. However, the constant occurrence of dengue epidemics in Brazil demonstrates that sustained dengue control and surveillance policies at the local level (municipalities) are still needed. It will avoid the constant reestablishment of foci of active mosquito breeding and transmission of the infection giving rise to new cases of the disease.
The tropical climate makes Brazil susceptive to cocirculation of different arboviruses, such as DENV, CHIKV, and the recently introduced Zika virus, already reported in several Brazilian states. Those viral infections are oligosymptomatic and clinically similar, hampering the differential diagnosis. Furthermore, there is still a need of improvement of laboratory diagnosis for dengue, since serologic tests available often take several days to be completed and present high cross-reactivity among DENV serotypes. Beyond the differentiation between the arboviruses cocirculating, efficient diagnosis allows appropriate patient care, generation of accurate epidemiological data, and implementation of effective public health interventions.
Disclosure
Katia P. R. Souza is currently working at Fundação Oswaldo Cruz, Brazil, and Germán Añez is currently working at Sanofi Pasteur, USA.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.World Health Organization (WHO) Fact sheet Nº117. 2013, http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Gubler D. J., Kuno G., Markoff L. Flaviviruses. In: Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., editors. Fields Virology. 5th. Philadelphia, Pa, USA: Lippincott-Raven Publishers; 2007. pp. 1497–1526. [Google Scholar]
- 3.Levi J. E., Nishiya A., Félix A. C., et al. Real-time symptomatic case of transfusion-transmitted dengue. Transfusion. 2015;55(5):961–964. doi: 10.1111/trf.12944. [DOI] [PubMed] [Google Scholar]
- 4.Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 5.Halstead S. B. Dengue. The Lancet. 2007;370(9599):1644–1652. doi: 10.1016/s0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 6.Dick O. B., San Martín J. L., Montoya R. H., del Diego J., Zambrano B., Dayan G. H. The history of dengue outbreaks in the Americas. American Journal of Tropical Medicine and Hygiene. 2012;87(4):584–593. doi: 10.4269/ajtmh.2012.11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Añez G., Rios M. Dengue in the United States of America: a worsening scenario? BioMed Research International. 2013;2013:13. doi: 10.1155/2013/678645.678645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 9.Gurugama P., Garg P., Perera J., Wijewickrama A., Seneviratne S. Dengue viral infections. Indian Journal of Dermatology. 2010;55(1):68–78. doi: 10.4103/0019-5154.60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraes G. H., Duarte E. D. F., Duarte E. C. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. The American Journal of Tropical Medicine and Hygiene. 2013;88(4):670–676. doi: 10.4269/ajtmh.11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yacoub S., Wills B. Predicting outcome from dengue. BMC Medicine. 2014;12(1, article 147) doi: 10.1186/s12916-014-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hottz E. D., Oliveira M. F., Nunes P. C. G., et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. Journal of Thrombosis and Haemostasis. 2013;11(5):951–962. doi: 10.1111/jth.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordignon J., Strottmann D. M., Mosimann A. L. P., et al. Dengue neurovirulence in mice: identification of molecular signatures in the E and NS3 helicase domains. Journal of Medical Virology. 2007;79(10):1506–1517. doi: 10.1002/jmv.20958. [DOI] [PubMed] [Google Scholar]
- 14.Tang K. F., Ooi E. E. Diagnosis of dengue: an update. Expert Review of Anti-Infective Therapy. 2012;10(8):895–907. doi: 10.1586/eri.12.76. [DOI] [PubMed] [Google Scholar]
- 15.Shu P. Y., Huang J. H. Current advances in dengue diagnosis. Clinical and Diagnostic Laboratory Immunology. 2004;11(4):642–650. doi: 10.1128/CDLI.11.4.642-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchy P., Yoksan S., Peeling R. W., Hunsperger E. Working paper for the Scientific Working Group on Dengue Research. Geneva, Switzerland: Special Program for Research and Training in Tropical Diseases; 2006. Laboratory tests for the diagnosis of dengue virus infection. [Google Scholar]
- 17.Honda E. R., Zanchi F., Rios K., et al. Design and heterologous expression of dengue virus envelope protein (E) peptides and their use for serological diagnosis. Journal of Virological Methods. 2012;186(1-2):55–61. doi: 10.1016/j.jviromet.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Peeling R. W., Artsob H., Pelegrino J. L., et al. Evaluation of diagnostic tests: dengue. Nature Reviews Microbiology. 2010;8(supplement 12):S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 19.Guzman M. G., Jaenisch T., Gaczkowski R., et al. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Neglected Tropical Diseases. 2010;4(8, article e811) doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima M. D. R. Q., Nogueira R. M. R., Schatzmayr H. G., dos Santos F. B. Comparison of three commercially available dengue NS1 antigen capture assays for acute diagnosis of Dengue in Brazil. PLoS Neglected Tropical Diseases. 2010;4(7, article e738) doi: 10.1371/journal.pntd.0000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo T. E., Vedovello D., Araki C. S., et al. Dengue-4 false negative results by Panbio Dengue Early ELISA assay in Brazil. Journal of Clinical Virology. 2013;58(4):710–712. doi: 10.1016/j.jcv.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Sea V. R. F., Cruz A. C. R., Gurgel R. Q., et al. Underreporting of Dengue-4 in Brazil due to low sensitivity of the NS1 Ag test in routine control programs. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064056.e64056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balmaseda A., Hammond S. N., Pérez L., et al. Serotype-specific differences in clinical manifestations of dengue. American Journal of Tropical Medicine and Hygiene. 2006;74(3):449–456. [PubMed] [Google Scholar]
- 24.Sierra B. D. L. C., Kourí G., Guzmán M. G. Race: a risk factor for dengue hemorrhagic fever. Archives of Virology. 2007;152(3):533–542. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 25.Costa R. L., Voloch C. M., Schrago C. G. Comparative evolutionary epidemiology of dengue virus serotypes. Infection, Genetics and Evolution. 2012;12(2):309–314. doi: 10.1016/j.meegid.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Romano C. M., de Matos A. M., Araújo E. S. A., et al. Characterization of Dengue virus type 2: new insights on the 2010 Brazilian epidemic. PLoS ONE. 2010;5(7) doi: 10.1371/journal.pone.0011811.e11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan G. K., Alonso S. Pathogenesis and prevention of dengue virus infection: state-of-the-art. Current Opinion in Infectious Diseases. 2009;22(3):302–308. doi: 10.1097/qco.0b013e328329ae32. [DOI] [PubMed] [Google Scholar]
- 28.Noisakran S., Perng G. C. Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection. Experimental Biology and Medicine. 2008;233(4):401–408. doi: 10.3181/0707-mr-198. [DOI] [PubMed] [Google Scholar]
- 29.Pang T., Cardosa M. J., Guzman M. G. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunology and Cell Biology. 2007;85(1):43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- 30.Zulkarnain E., Hotta S., Takegami T. Molecular comparison of dengue type 1 Mochizuki strain virus and other selected viruses concerning nucleotide and amino acid sequences of genomic RNA: a consideration of viral epidemiology and variation. Microbiology and Immunology. 1994;38(7):581–585. doi: 10.1111/j.1348-0421.1994.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 31.Diamond M. S., Edgil D., Roberts T. G., Lu B., Harris E. Infection of human cells by dengue virus is modulated by different cell types and viral strains. Journal of Virology. 2000;74(17):7814–7823. doi: 10.1128/JVI.74.17.7814-7823.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira G. P., Figueiredo L. B., Coelho L. F. L., et al. Dengue virus 3 clinical isolates show different patterns of virulence in experimental mice infection. Microbes and Infection. 2010;12(7):546–554. doi: 10.1016/j.micinf.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Gubler D. J., Suharyono W., Tan R., Abidin M., Sie A. Viraemia in patients with naturally acquired dengue infection. Bulletin of the World Health Organization. 1981;59(4):623–630. [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughn D. W., Green S., Kalayanarooj S., et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. Journal of Infectious Diseases. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 35.Thisyakorn U., Nimmannitya S. Nutritional status of children with dengue hemorrhagic fever. Clinical Infectious Diseases. 1993;16(2):295–297. doi: 10.1093/clind/16.2.295. [DOI] [PubMed] [Google Scholar]
- 36.Acosta E. G., Castilla V., Damonte E. B. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cellular Microbiology. 2009;11(10):1533–1549. doi: 10.1111/j.1462-5822.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C.-F., Chiu S.-C., Hsiao Y.-L., et al. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. The Journal of Immunology. 2005;174(1):395–403. doi: 10.4049/jimmunol.174.1.395. [DOI] [PubMed] [Google Scholar]
- 38.Gubler D. J., Clark G. G. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerging Infectious Diseases. 1995;1(2) doi: 10.3201/eid0102.952004. http://wwwnc.cdc.gov/eid/article/1/2/95-0204_article?commit=GO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gubler D. J. Dengue and dengue hemorrhagic fever; its history and resurgence as a global public health problem. In: Gubler D. J., Kuno G., editors. Dengue and Dengue Hemorrhagic Fever. 1997. pp. 1–22. [Google Scholar]
- 40.Gubler D. J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in Microbiology. 2002;10(2):100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 41.Guzman M. G., Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. Journal of Clinical Virology. 2003;27(1):1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 43.Bhatt S., Gething P. W., Brady O. J., et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan American Health Organization/WHO. Dengue Cases, Americas. 2014. http://www.paho.org/ [Google Scholar]
- 45.Cafferata M. L., Bardach A., Rey-Ares L., et al. Dengue epidemiology and burden of disease in Latin America and the Caribbean: a systematic review of the literature and meta-analysis. Value in Health Regional Issues. 2013;2(3):347–356. doi: 10.1016/j.vhri.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Brazilian Ministry of Health. http://portalsaude.saude.gov.br.
- 47.Siqueira J. B., Jr., Martelli C. M. T., Coelho G. E., da Rocha Simplício A. C., Hatch D. L. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerging Infectious Diseases. 2005;11(1):48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider J., Droll D. A timeline for dengue in the Americas to December 31, 2000 and noted first occurrences. 2001, http://www.paho.org/English/HCP/HCT/dengue_timeline.xls.
- 49.Pinheiro F., Nelson M. Re-emergence of dengue and emergence of dengue hemorrhagic fever in the Americas. Dengue Bulletin. 1997;21:16–24. [Google Scholar]
- 50.Osanai C. H., Travassos da Rosa A. P., Tang A. T., do Amaral R. S., Passos A. D., Tauil P. L. Dengue outbreak in Boa Vista, Roraima. Preliminary report. Revista do Instituto de Medicina Tropical de São Paulo. 1983;25(1):53–54. [PubMed] [Google Scholar]
- 51.Schatzmayr H. G., Nogueira R. M., Travassos da Rosa A. P. An outbreak of dengue virus at Rio de Janeiro—1986. Memorias do Instituto Oswaldo Cruz. 1986;81(2):245–246. doi: 10.1590/s0074-02761986000200019. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira M. G., Costa M. D. C. N., Barreto F., Barreto M. L. Dengue: twenty-five years since reemergence in Brazil. Cadernos de Saude Publica. 2009;25(supplement 1):S7–S18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- 53.Barreto M. L., Teixeira M. G. Dengue no Brasil: situação epidemiológica e contribuições para uma agenda de pesquisa. Estudos Avancados. 2008;22(64):53–72. doi: 10.1590/s0103-40142008000300005. [DOI] [Google Scholar]
- 54.Nogueira R. M. R., Miagostovich M. P., Lampe E., Schatzmayr H. G. Isolation of dengue virus type 2 in Rio de Janeiro. Memorias do Instituto Oswaldo Cruz. 1990;85(2):p. 253. doi: 10.1590/s0074-02761990000200022. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira R. M., Zagner S. M., Martins I. S., Lampe E., Miagostovich M. P., Schatzmayr H. G. Dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) caused by serotype 2 in Brazil. Memórias do Instituto Oswaldo Cruz. 1991;86(2, article 269) doi: 10.1590/s0074-02761991000200018. [DOI] [PubMed] [Google Scholar]
- 56.Vasconcelos P. F., de Menezes D. B., Melo L. P., et al. A large epidemic of dengue fever with dengue hemorrhagic cases in Ceará State, Brazil, 1994. Revista do Instituto de Medicina Tropical de São Paulo. 1995;37(3):253–255. doi: 10.1590/s0036-46651995000300012. [DOI] [PubMed] [Google Scholar]
- 57.Duarte H. H. P., França E. B. Data quality of dengue epidemiological surveillance in Belo Horizonte, Southeastern Brazil. Revista de Saude Publica. 2006;40(1):134–142. doi: 10.1590/s0034-89102006000100021. [DOI] [PubMed] [Google Scholar]
- 58.Nogueira R. M. R., Miagostovich M. P., Filippis A. M. B., Pereira M. A. S., Schatzmayr H. G. Dengue type 3 in Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz. 2001;96:925–926. doi: 10.1590/s0074-02762001000700007. [DOI] [PubMed] [Google Scholar]
- 59.Nogueira R. M. R., de Araújo J. M. G., Schatzmayr H. G. Dengue viruses in Brazil, 1986-2006. Revista Panamericana de Salud Publica. 2007;22(5):358–363. doi: 10.1590/s1020-49892007001000009. [DOI] [PubMed] [Google Scholar]
- 60.Guilarde A. O., Turchi M. D., Siqueira J. B., Jr., et al. Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes, and antibody response. Journal of Infectious Diseases. 2008;197(6):817–824. doi: 10.1086/528805. [DOI] [PubMed] [Google Scholar]
- 61.Cordeiro M. T., Schatzmayr H. G., Nogueira R. M. R., De Oliveira V. F., De Melo W. T., De Carvalho E. F. Dengue and dengue hemorrhagic fever in the State of Pernambuco, 1995–2006. Revista da Sociedade Brasileira de Medicina Tropical. 2007;40(6):605–611. doi: 10.1590/s0037-86822007000600001. [DOI] [PubMed] [Google Scholar]
- 62.dos Santos F. B., Nogueira F. B., Castro M. G., et al. First report of multiple lineages of dengue viruses type 1 in Rio de Janeiro, Brazil. Virology Journal. 2011;8, article 387 doi: 10.1186/1743-422x-8-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.San Martín J. L., Brathwaite O., Zambrano B., et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. The American Journal of Tropical Medicine and Hygiene. 2010;82(1):128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daumas R. P., Passos S. R. L., Oliveira R. V. C., et al. Clinical and laboratory features that discriminate dengue from other febrile illnesses: a diagnostic accuracy study in Rio de Janeiro, Brazil. BMC Infectious Diseases. 2013;13, article 77 doi: 10.1186/1471-2334-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dettogni R. S., Louro I. D. Phylogenetic characterization of dengue virus type 2 in Espírito Santo, Brazil. Molecular Biology Reports. 2012;39(1):71–80. doi: 10.1007/s11033-011-0711-8. [DOI] [PubMed] [Google Scholar]
- 66.Temporão J. G., Penna G. O., Carmo E. H., et al. Dengue virus serotype 4, Roraima State, Brazil. Emerging Infectious Diseases. 2011;17(5):938–940. doi: 10.3201/eid1705.101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunes M. R. T., Faria N. R., Vasconcelos H. B., et al. Phylogeography of dengue virus serotype 4, Brazil, 2010-2011. Emerging Infectious Diseases. 2012;18(11):1858–1864. doi: 10.3201/eid1811.120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rocco I. M., Silveira V. R., Maeda A. Y., et al. First isolation of dengue 4 in the state of São Paulo, Brazil, 2011. Revista do Instituto de Medicina Tropical de Sao Paulo. 2012;54(1):49–51. doi: 10.1590/s0036-46652012000100009. [DOI] [PubMed] [Google Scholar]
- 69.Nogueira R. M. R., Eppinghaus A. L. F. Dengue virus type 4 arrives in the state of Rio de Janeiro: a challenge for epidemiological surveillance and control. Memorias do Instituto Oswaldo Cruz. 2011;106(3):255–256. doi: 10.1590/s0074-02762011000300001. [DOI] [PubMed] [Google Scholar]
- 70.Teixeira M. G., Costa M. C. N., Coelho G., Barreto M. L. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. Emerging Infectious Diseases. 2008;14(10, article 1663) doi: 10.3201/eid1410.071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavalcanti L. P., Vilar D., Souza-Santos R., Teixeira M. G. Change in age pattern of persons with dengue, Northeastern Brazil. Emerging Infectious Diseases. 2011;17(1):132–134. doi: 10.3201/eid1701.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gómez-Dantés H., Willoquet J. R. Dengue in the Americas: challenges for prevention and control. Cadernos de Saude Publica. 2009;25(1):S19–S31. doi: 10.1590/s0102-311x2009001300003. [DOI] [PubMed] [Google Scholar]
- 73.Pan American Health Organization. Dengue Cases, Americas. 2013, http://www.paho.org/
- 74.Solomon T., Dung N. M., Vaughn D. W., et al. Neurological manifestations of dengue infection. The Lancet. 2000;355(9209):1053–1059. doi: 10.1016/s0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 75.Araújo F., Nogueira R., de Sousa Araújo M., et al. Dengue in patients with central nervous system manifestations, Brazil. Emerging Infectious Diseases. 2012;18(4):677–679. doi: 10.3201/eid1804.111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferreira M. L. B., Cavalcanti C. G., Coelho C. A., Mesquita S. D. Neurological manifestations of dengue: study of 41 cases. Arquivos de Neuro-Psiquiatria. 2005;63(2):488–493. doi: 10.1590/s0004-282x2005000300023. [DOI] [PubMed] [Google Scholar]
- 77.de Alencar L. X. E., de Mendonça Braga-Neto U., do Nascimento E. J. M., et al. HLA-B∗44 is associated with dengue severity caused by DENV-3 in a Brazilian population. Journal of Tropical Medicine. 2013;2013:11. doi: 10.1155/2013/648475.648475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monteiro S. P., do Brasil P. E. A. A., Cabello G. M. K., et al. HLA-A*01 allele: a risk factor for dengue haemorrhagic fever in Brazil's population. Memorias do Instituto Oswaldo Cruz. 2012;107(2):224–230. doi: 10.1590/s0074-02762012000200012. [DOI] [PubMed] [Google Scholar]
- 79.Figueiredo L. T. Dengue in Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2012;45(3, article 285) doi: 10.1590/s0037-86822012000300001. [DOI] [PubMed] [Google Scholar]
- 80.Chen R., Vasilakis N. Dengue—quo tu et quo vadis? Viruses. 2011;3(9):1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Añez G., Morales-Betoulle M. E., Rios M. Circulation of different lineages of dengue virus type 2 in Central America, their evolutionary time-scale and selection pressure analysis. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027459.e27459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliveira M. F., Araújo J. M. G., Ferreira O. C., Jr., et al. Two lineages of dengue virus type 2, Brazil. Emerging Infectious Diseases. 2010;16(3):576–578. doi: 10.3201/eid1603.090996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faria N. R. D. C., Nogueira R. M. R., de Filippis A. M. B., et al. Twenty years of DENV-2 activity in Brazil: molecular characterization and phylogeny of strains isolated from 1990 to 2010. PLoS Neglected Tropical Diseases. 2013;7(3) doi: 10.1371/journal.pntd.0002095.e2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tung Y.-C., Lin K.-H., Chang K., et al. Phylogenetic study of dengue-3 virus in Taiwan with sequence analysis of the core gene. Kaohsiung Journal of Medical Sciences. 2008;24(2):55–62. doi: 10.1016/s1607-551x(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 85.Villabona-Arenas C. J., Mondini A., Bosch I., et al. Dengue virus type 3 adaptive changes during epidemics in São Jose de Rio Preto, Brazil, 2006-2007. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063496.e63496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vilela A. P. P., Figueiredo L. B., dos Santos J. R., et al. Dengue virus 3 genotype I in Aedes aegypti mosquitoes and eggs, Brazil, 2005-2006. Emerging Infectious Diseases. 2010;16(6):989–992. doi: 10.3201/eid1606.091000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Figueiredo L. B., Cecílio A. B., Ferreira G. P., et al. Dengue virus 3 genotype 1 associated with dengue fever and dengue hemorrhagic fever, Brazil. Emerging Infectious Diseases. 2008;14(2):314–316. doi: 10.3201/eid1402.070278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Araújo J. M. G., Bello G., Romero H., Nogueira R. M. R. Origin and evolution of dengue virus type 3 in Brazil. PLoS Neglected Tropical Diseases. 2012;6(9) doi: 10.1371/journal.pntd.0001784.e1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Añez G., Heisey D. A. R., Espina L. M., Stramer S. L., Rios M. Phylogenetic analysis of dengue virus types 1 and 4 circulating in Puerto Rico and Key West, Florida, during 2010 epidemics. The American Journal of Tropical Medicine and Hygiene. 2012;87(3):548–553. doi: 10.4269/ajtmh.2012.12-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Souza R. P., Rocco I. M., Maeda A. Y., et al. Dengue virus type 4 phylogenetics in brazil 2011: looking beyond the veil. PLoS Neglected Tropical Diseases. 2011;5(12) doi: 10.1371/journal.pntd.0001439.e1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Figueiredo M. L. G. D., Alfonso H. L., Amarilla A. A., et al. Detection of DENV-4 genotype I from mosquitoes collected in the city of Manaus, Brazil. Virology journal. 2013;10, article 60 doi: 10.1186/1743-422X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yap H. H., Chong N. L., Foo A. E., Lee C. Y. Dengue vector control: present status and future prospects. Kaohsiung Journal of Medical Sciences. 1994;10:S102–S108. [PubMed] [Google Scholar]
- 93.Lambrechts L., Scott T. W., Gubler D. J. Consequences of the expanding global distribution of aedes albopictus for dengue virus transmission. PLoS Neglected Tropical Diseases. 2010;4(5, article e646) doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martins V. E. P., Alencar C. H., Kamimura M. T., et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041386.e41386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castro M. G., Nogueira R. M. R., Schatzmayr H. G., Miagostovich M. P., Lourenço-de-Oliveira R. Dengue virus detection by using reverse transcription-polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Memorias do Instituto Oswaldo Cruz. 2004;99(8):809–814. doi: 10.1590/s0074-02762004000800005. [DOI] [PubMed] [Google Scholar]
- 96.Díaz-Nieto L. M., Maciá A., Perotti M. A., Berón C. M., O'Neill S. L. Geographical limits of the Southeastern distribution of Aedes aegypti (Diptera, Culicidae) in Argentina. PLoS Neglected Tropical Diseases. 2013;7(1) doi: 10.1371/journal.pntd.0001963.e1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gratz N. G. Critical review of the vector status of Aedes albopictus . Medical and Veterinary Entomology. 2004;18(3):215–227. doi: 10.1111/j.0269-283x.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 98.Degallier N., Sócrates Teixeira J. M., da Silva Soares S., et al. Aedes albopictus may not be vector of dengue virus in human epidemics in Brazil. Revista de Saude Publica. 2003;37(3):386–387. doi: 10.1590/S0034-89102003000300019. [DOI] [PubMed] [Google Scholar]
- 99.Honório N. A., Lourenço-De-Oliveira R. Frequency of Aedes aegypti and Aedes albopictus larvae and pupae in traps, Brazil. Revista de Saude Publica. 2001;35(4):385–391. doi: 10.1590/s0034-89102001000400009. [DOI] [PubMed] [Google Scholar]
- 100.Honório N. A., Cabello P. H., Codeço C. T., Lourenço-De-Oliveira R. Preliminary data on the performance of Aedes aegypti and Aedes albopictus immatures developing in water-filled tires in Rio de Janeiro. Memorias do Instituto Oswaldo Cruz. 2006;101(2):225–228. doi: 10.1590/s0074-02762006000200017. [DOI] [PubMed] [Google Scholar]
- 101.Wong P.-S. J., Li M.-Z. I., Chong C.-S., Ng L.-C., Tan C.-H. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Neglected Tropical Diseases. 2013;7(8) doi: 10.1371/journal.pntd.0002348.e2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan American Health Organization/WHO. Number of Reported Cases of Chikungunya fever in the Americas. 2014. http://www.paho.org/ [Google Scholar]
- 103.Centers for Disease Control and Prevention (CDC) Geographic Distribution of Chikungunya. Centers for Disease Control and Prevention (CDC); 2015. http://www.cdc.gov/chikungunya/geo/index.html. [Google Scholar]
- 104.Ministry of Health. Preparação e Resposta à Introdução do Vírus Chikungunya no Brasil. Brasília, Brazil: Ministério da Saúde; 2014, (Portuguese) [Google Scholar]
- 105.Simões T. C., Codeço C. T., Nobre A. A., Eiras Á. E., Ryan S. J. Modeling the non-stationary climate dependent temporal dynamics of Aedes aegypti . PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0064773.e64773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Focks D. A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 107.Sanchez L., Cortinas J., Pelaez O., Gutierrez H., Concepción D., Van Der Stuyft P. Breteau Index threshold levels indicating risk for dengue transmission in areas with low Aedes infestation. Tropical Medicine and International Health. 2010;15(2):173–175. doi: 10.1111/j.1365-3156.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 108.Pepin K. M., Marques-Toledo C., Scherer L., Morais M. M., Ellis B., Eiras A. E. Cost-effectiveness of novel system of mosquito surveillance and control, Brazil. Emerging Infectious Diseases. 2013;19(4):542–550. doi: 10.3201/eid1904.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Regis L. N., Acioli R. V., Silveira J. C., Jr., et al. Sustained reduction of the dengue vector population resulting from an integrated control strategy applied in two Brazilian cities. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0067682.e67682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brazilian Ministry of Health. Health Surveillance Secretariat: SES/SINAN. 2013. http://portalsaude.saude.gov.br/ [Google Scholar]
- 111.Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 112.Mousson L., Zouache K., Arias-Goeta C., Raquin V., Mavingui P., Failloux A.-B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus . PLoS Neglected Tropical Diseases. 2012;6(12) doi: 10.1371/journal.pntd.0001989.e1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baton L. A., Pacidônio E. C., Gonçalves D. D. S., Moreira L. A. wFlu: characterization and evaluation of a native Wolbachia from the Mosquito Aedes fluviatilis as a potential Vector Control Agent. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059619.e59619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Subbaraman N. Science snipes at Oxitec transgenic-mosquito trial. Nature Biotechnology. 2011;29(1):9–11. doi: 10.1038/nbt0111-9a. [DOI] [PubMed] [Google Scholar]
- 115.Bargielowski I., Nimmo D., Alphey L., Koella J. C. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti . PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020699.e20699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Press Release: Moscamed prepares for next phase in the development of Oxitec's transgenic mosquitoes in Brazil, http://www.oxitec.com/press-release-moscamed-prepares-next-phase-development-oxitecs-transgenic-mosquitoes-brazil/
- 117.Carvalho D. O., Nimmo D., Naish N., et al. Mass production of genetically modified Aedes aegypti for field releases in Brazil. Journal of Visualized Experiments. 2014;(83) doi: 10.3791/3579.e3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barreto M. L., Teixeira M. G. Dengue fever: a call for local, national, and international action. The Lancet. 2008;372(9634):p. 205. doi: 10.1016/s0140-6736(08)61069-7. [DOI] [PubMed] [Google Scholar]
- 119.Chokephaibulkit K., Perng G. C. Challenges for the formulation of a universal vaccine against dengue. Experimental Biology and Medicine. 2013;238(5):566–578. doi: 10.1177/1535370212473703. [DOI] [PubMed] [Google Scholar]
- 120.Durham D. P., Ndeffo Mbah M. L., Medlock J., et al. Dengue dynamics and vaccine cost-effectiveness in Brazil. Vaccine. 2013;31(37):3957–3961. doi: 10.1016/j.vaccine.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Villar L., Dayan G. H., Arredondo-García J. L., et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. The New England Journal of Medicine. 2015;372(2):113–123. doi: 10.1056/nejmoa1411037. [DOI] [PubMed] [Google Scholar]
- 122.Ozawa S., Mirelman A., Stack M. L., Walker D. G., Levine O. S. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine. 2012;31(1):96–108. doi: 10.1016/j.vaccine.2012.10.103. [DOI] [PubMed] [Google Scholar]
- 123.Mahoney R. T., Francis D. P., Frazatti-Gallina N. M., et al. Cost of production of live attenuated dengue vaccines: a case study of the Instituto Butantan, Sao Paulo, Brazil. Vaccine. 2012;30(32):4892–4896. doi: 10.1016/j.vaccine.2012.02.064. [DOI] [PubMed] [Google Scholar]