Abstract
Although an association between air pollution and adverse systemic health effects has been known for years, the effect of pollutants on neurodevelopment has been underappreciated. Recent evidence suggests a possible link between air pollution and neurocognitive impairment and behavioral disorders in children, however, the exact nature of this relationship remains poorly understood. Infants and children are uniquely vulnerable due to the potential for exposure in both the fetal and postnatal environments during critical periods in development. Carbon monoxide (CO), a common component of indoor and outdoor air pollution, can cross the placenta to gain access to the fetal circulation and the developing brain. Thus, CO is of particular interest as a known neurotoxin and a potential public health threat. Here we review overt CO toxicity and the policies regulating CO exposure, detail the evidence suggesting a potential link between CO-associated ambient air pollution, tobacco smoke, and learning and behavioral abnormalities in children, describe the effects of subclinical CO exposure on the brain during development, and provide mechanistic insight into a potential connection between CO exposure and neurodevelopmental outcome. CO can disrupt a number of critical processes in the developing brain, providing a better understanding of how this specific neurotoxin may impair neurodevelopment. However, further investigation is needed to better define the effects of perinatal CO exposure on the immature brain. Current policies regarding CO standards were established based on evidence of cardiovascular risk in adults with pre-existing comorbidities. Thus, recent and emerging data highlighted in this review regarding CO exposure in the fetus and developing child may be important to consider when the standards and guidelines are evaluated and revised in the future.
Keywords: carbon monoxide, air pollution, tobacco smoke, toxicity, policy, brain, development, neurodevelopment, behavior, autism, apoptosis, fetus, infant, children
Introduction
For over fifty years, air pollution has been suspected as an underlying cause of a wide variety of disease processes (Mustafic, 2012). Although commonly encountered indoor and outdoor environmental pollutants have been linked with a range of pulmonary, cardiovascular, and immune system maladies, evidence has only recently emerged to suggest a relationship between air pollution and neurodevelopmental impairment (Block, 2012; Mustafic, 2012; Wang, 2007). It is now believed that indoor and outdoor air pollution may be associated with certain neurocognitive abnormalities and behavioral disorders including the autism spectrum (Block, 2012; Vrijheid, 2012). Infants and children appear to be uniquely vulnerable to the neurotoxicity of air pollution due to the susceptibility of the brain during critical periods in development and the potential for exposure to such neurotoxins in both the fetal milieu and the postnatal environment (Grandjean, 2006).
Air pollution is a heterogeneous mixture of gases and particulate matter (Mustafic, 2012). The main gaseous components of air pollution are ozone, carbon monoxide (CO), nitrogen dioxide, and sulfur dioxide (Mustafic, 2012). As a by-product of incomplete combustion of hydrocarbons, CO is a major component of motor vehicle-related pollution, tobacco smoke, and gas stove pollution (US Environmental Protection Agency, 2012; Vrijheid, 2012). Therefore, CO is a common contaminant of both indoor and outdoor environments. Because it can cross the placenta to gain access to the fetal circulation and the developing brain, CO is of particular interest as a neurotoxin and a public health threat (Greingor, 2001; McGregor, 1998). Here we review overt CO toxicity and the policies regulating CO exposure, detail the evidence suggesting a potential link between CO-associated ambient air pollution, tobacco smoke, and learning and behavioral abnormalities in children, describe the effects of subclinical CO exposure on the brain during development, and provide mechanistic insight into a potential connection between CO exposure and neurodevelopmental outcome.
Environmental CO and overt toxicity
CO poisoning
CO is a colorless and odorless gas that can be poisonous to humans (Iqbal, 2012a; Kao, 2005). It is generated by incomplete combustion of carbonaceous fuels such as oil, gasoline, coal, wood, and tobacco (Bauer, 2009; Kao, 2005). Because CO is non-irritating and imperceptible in the air we breathe, exposure is often not recognized and acute CO toxicity is commonly underappreciated and misdiagnosed (Kao, 2005; Iqbal, 2012a). CO continues to be the leading cause of poison-related mortality in the United States (Kao, 2005; Iqbal, 2012a). Unintentional, non-fire-related CO exposure leads to greater than 20,000 emergency room admissions, more than 2000 hospitalizations, and up to 6000 deaths each year (Centers for Disease Control and Prevention, 2007; Centers for Disease Control and Prevention, 2008; Iqbal, 2012b; Kao, 2005).
Sources of environmental CO
Vehicle exhaust contributes to 75% of all CO emissions in the US and up to 95% of all emissions in US cities (US Environmental Protection Agency, 2012). The remainder is due to steam boilers, industrial processes, solid waste disposal, and miscellaneous other sources (Raub, 1999). Indoor CO sources include tobacco smoke (from cigarettes, cigars, as well as water pipes or hookahs), gas cooking ranges, combustion space and water heaters, coal or wood burning stoves, and improper use of generators and charcoal grills (Daher, 2010; Iqbal, 2012a; Raub, 1999). Although CO exposure occurs year-round, poisonings peak in the winter months due to the increased use of heating devices in closed spaces (Kao, 2005; Iqbal, 2012a).
Global background CO concentrations average between 50 and 120 parts per billion (ppb) in the troposphere and approximately 60% of these levels have been attributed to human activity (Raub, 1999). Although short-term peaks occur each day and demonstrate seasonal variability, CO levels are greatest in the northern hemisphere and over the last decade annual outdoor urban levels in the US have averaged between 2 and 5 parts per million (ppm) (Raub, 1999; US Environmental Protection Agency, 2012). Vehicle exhaust contains up to 100,000 ppm CO and levels can reach between 10 and 12 ppm within passenger compartments of automobiles during heavy traffic (Raub, 2000; US Environmental Protection Agency, 2012). Even higher concentrations are encountered in semi-closed environments routinely exposed to vehicle exhaust such as parking garages, tunnels, and indoor ice skating rinks (Pelham, 2002; US Environmental Protection Agency, 2012). CO toxicity has been reported in children riding in the back of pick-up trucks and exposure commonly occurs with certain recreational activities such as boating (Hampson, 1992). Indoor levels can rise to 100 ppm with use of gas stoves and CO levels can range between 5 and 35 ppm within smoking rooms based on the number of lit cigarettes and the size of the room (Kao, 2005; Gül, 2011; Jo, 2004). Thus, infants and children can be exposed to CO in a variety of commonly encountered environments.
Water pipe (hookah) tobacco smoke deserves special mention. This is because the incidence of hookah smoking has increased dramatically around the world over the last few years (Eissenberg, 2009; Martinasek, 2014). Sidestream hookah smoke contains 30 times the amount of CO as a single cigarette and CO levels within the ambient environment of hookah bars can be as high as 50 ppm (Daher, 1994; Zhou, 2014). In addition, several cases of acute symptomatic CO poisoning have been reported in teenagers and young adults following water pipe use (La Fauci, 2012; Misek, 2014; von Rappard, 2014). CO toxicity in these cases manifested with syncope or loss of consciousness (La Fauci, 2012; Misek, 2014; von Rappard, 2014). Thus, hookah use is rapidly becoming a public health issue and combustion of water pipe tobacco is a significant source of indoor CO pollution in certain environments.
Mechanisms of overt CO toxicity
When inspired, environmental CO diffuses rapidly across the alveolar capillary membrane and binds to hemoglobin, forming carboxyhemoglobin (COHb) (Smithline, 2003). The affinity of hemoglobin for CO is 240 times greater than that for oxygen (Hauck, 1984). High levels of COHb interfere with oxygen binding to and dissociation from hemoglobin resulting in impaired tissue oxygen delivery (Gorman, 2003). In addition, CO binds avidly to cellular hemoproteins such as myoglobin and cytochrome oxidase and can interrupt oxidative phosphorylation (Brown, 1990; Iheagwara, 2007). Thus, overt CO toxicity results from tissue hypoxia and signs and symptoms appear when COHb is greater than 10% (Kao, 2005). The infant and fetus are more susceptible to CO toxicity than adults due to higher rates of metabolism and the presence of fetal hemoglobin, which has a greater affinity for CO than adult hemoglobin (Kao, 2004).
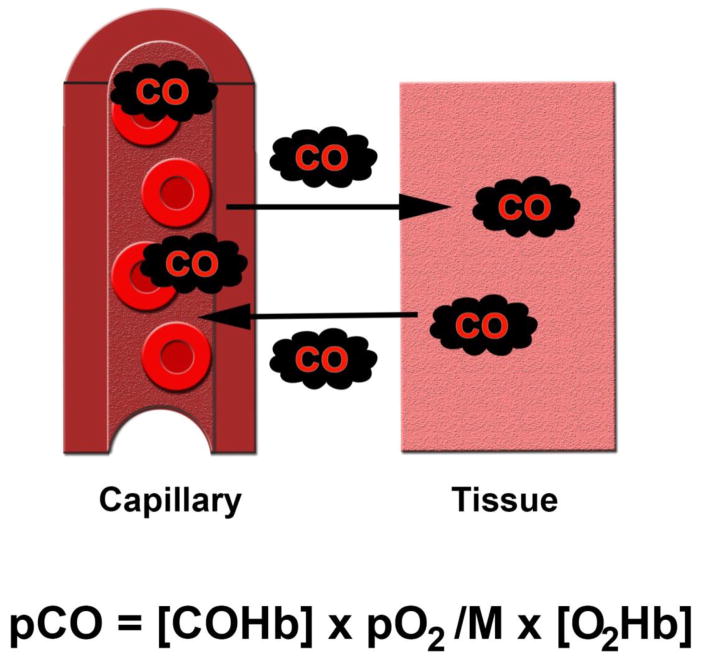
Although symptoms usually appear when COHb is greater than 10%, it is important to note that COHb levels do not necessarily correlate with the severity of clinical presentation and do not predict outcome (Hampson, 2012; Kao, 2005). This is because formation of COHb, although critical to the manifestation of overt toxicity, is not the major mechanism of such toxicity (Hampson, 2012). Rather, accumulation of CO within the tissues following unloading of CO from hemoglobin is the key factor that determines the degree of cellular toxicity (Hampson, 2012). Diffusion of CO into the various tissue compartments following exposure is dictated by the Haldane equation and is based on both COHb level and CO tension within the tissue (Figure 1) (Jain, 2009). Thus, resultant COHb concentrations are of central importance and higher levels will lead to greater tissue CO delivery. For these reasons, COHb levels are utilized clinically as biomarkers of CO exposure in order to confirm acute poisoning but are not used to guide prognosis (Hampson, 2012).
Figure 1. Diffusion of carbon monoxide (CO) into tissue is dictated by the Haldane equation (Bissonnette, 1977a; Jain, 2009).
Following formation of carboxyhemoglobin (COHb), CO diffuses from the capillaries into the tissue compartments. Tissue tension of CO (pCO) is determined by COHb concentration, partial pressure of oxygen (pO2), the affinity of hemoglobin for CO (M), and the concentration of oxyhemoglobin (O2Hb). The Haldane equation also dictates transplacental CO diffusion.
In addition to tissue hypoxia, a number of other mechanisms of CO-induced cellular toxicity have recently been identified. These include oxidative stress, peroxynitrite formation, apoptosis, inflammation, and immune-mediated injury (Hampson, 2012). With regard to inflammation, CO poisoning has been shown to activate microglia within the brain following exposure in a manner similar to that of other pollutants (Allen, 2014; Bolton, 2013; Wang, 2011). Thus, overt CO toxicity is caused by both tissue hypoxia and direct cellular effects (Hampson, 2012). Unlike tissue hypoxia, however, it is less clear to what degree these other mechanisms contribute to symptomatic CO toxicity (Hampson, 2012).
Organs with the greatest aerobic activity, such as the heart and brain, are the most vulnerable (Kao, 2005). Cardiovascular manifestations of acute CO toxicity include hypotension, arrhythmia, ischemia, infarction, and cardiac arrest while neurologic involvement may manifest as headache, dizziness, impaired judgment, altered mental status, confusion, syncope, seizure, stroke, and coma (Kao, 2005; Winter, 1976). Symptoms of CO toxicity are often nonspecific in children and poisoned patients commonly present with nausea, vomiting, and lethargy (Baker, 1988; Foster, 1999). Thus, in the pediatric population, CO exposure is often misdiagnosed as a viral illness (Kao, 2005).
Overt CO toxicity is a function of concentration and duration of exposure
The formation of COHb and the degree of toxicity relate to the concentration and duration of CO exposure (Raub, 1999; Winter, 1976). Coburn, Forster, and Kane used this time-weighted relationship to model CO inhalation and calculate resultant COHb levels (Winter, 1976). The equation they developed remains in use today. Exposure to between 70 and 120 ppm CO for approximately 4 hours, for example, results in COHb levels between 10 and 20% and is usually asymptomatic (Raub, 2002; Tomaszewski, 2002; Winter, 1976). Such an exposure is generally regarded as subclinical (Winter, 1976). On the other hand, inspiring more than 200 ppm CO results in COHb levels of approximately 30% and causes headache, dizziness, and impaired judgment (Winter, 1976). Exposure to greater than 800 ppm CO can result in COHb levels above 60% and rapidly leads to seizures, coma, and death (Winter, 1976). Due to its diverse clinical presentation, CO toxicity continues to be a diagnostic challenge for clinicians.
Fetal CO exposure
Gas transfer from the maternal circulation to the fetus is determined by uterine and umbilical blood flow, maternal and fetal capacity and affinity for the specific gas, oxygen consumption of the placenta, and placental diffusing capacity (Bissonnette, 1977b; Carter, 1999). Placental diffusing capacity is the quantity of gas that crosses the placenta over time and is influenced by surface area, thickness and solubility of the tissue membranes, diffusion properties of the gas, and distribution of fetal and maternal blood within the placental capillaries (Carter, 1999). Following maternal exposure to CO and formation of maternal COHb, CO readily diffuses across the placenta and binds to fetal hemoglobin (Bissonnette, 1977b; McGregor, 1998). There is also evidence that transplacental CO diffusion may be facilitated by a carrier mediated process (Bissonnette, 1977b). Placental diffusing capacity for CO has been found to increase during gestation, paralleling growth of the fetus (Bissonnette, 1977a). Although fetal hemoglobin has a higher affinity for CO than maternal hemoglobin, the resultant concentration of fetal COHb following exposure varies as a function of maternal COHb (Longo, 1970). The relationship between fetal COHb and maternal COHb can be determined by the Haldane equation and in humans the ratio of fetal-to-maternal COHb following exposure is approximately 1.1 (Longo, 1970).
CO Policy
As a result of the accumulation of visible dense smog within many US cities in the 1960s, the United States Congress established the basis of the Clean Air Act in 1970 (US Environmental Protection Agency, 2012). Section 109 of the Clean Air Act gave the Environmental Protection Agency (EPA) the authority to set and revise emissions standards for on- and off-road motor vehicles (US Environmental Protection Agency, 2012). In 1971, the EPA set the United States National Ambient Air Quality Standard (NAAQS) for CO limiting time-weighted average exposure to 9 ppm for 8 hours and 35 ppm for 1 hour in an outdoor environment, not to be exceeded more than once per year (US Environmental Protection Agency, 2012). The initial policy established this primary standard to protect public health as well as an identical secondary standard to protect public welfare (US Environmental Protection Agency, 2012). Following review in 1985, the EPA decided to maintain the primary standard without revision, however revoked the secondary standard due to lack of evidence demonstrating a direct effect of ambient CO levels on public welfare (US Environmental Protection Agency, 2012). Although the EPA reviews the NAAQS for CO periodically, the primary standard has never been revised and the original maximum allowable set-points remain in effect today.
The NAAQS was developed via a synthetic process that critically evaluated and assessed a multitude of peer-reviewed studies in a large body of literature. The NAAQS established for CO was based on investigational evidence that demonstrated a correlation between post-exposure rise in COHb (2.9–5.9%) and decreased time to the onset of angina during exercise in adult subjects with coronary artery disease following an acute CO exposure (Adams, 1988; Allred, 1989; Allred, 1991; Anderson, 1973; Aronow, 1973; Kleinman, 1989; Kleinman, 1998; Sheps, 1987). The EPA currently works closely with state, local, and tribal agencies to meet the standard (US Environmental Protection Agency, 2012). Agencies are responsible for monitoring air levels, calculating air quality to predict future trends, taking emissions inventories, establishing emission control strategies, formally adopting measures to achieve emissions reductions, and must undergo periodic review (US Environmental Protection Agency, 2012). The World Health Organization (WHO) has also established guidelines for time-weighted CO exposure in order to prevent toxicity (Raub, 1999). The WHO recommends limiting exposure to 9 ppm CO for 8 hours, 26 ppm CO for 1 hour, 52 ppm CO for 30 minutes, and 87 ppm CO for 15 minutes (Raub, 1999). These limits were set based on the prediction that COHb levels would not exceed 2.5% when a healthy adult engaged in light to moderate exertion during exposure (Raub, 1999).
Although there are currently no legal standards for indoor air levels of CO, several US government agencies have put forward guidelines for permissible exposure limits for CO. For example, the Occupational Safety and Health Administration (OSHA) recommends limiting exposure to 50 ppm CO for 8 hours, The National Institute for Occupational Safety and Health (NIOSH) recommends a time-weighted limit of 35 ppm CO for 8 hours with a concentration ceiling of 200 ppm, and The American Conference of Governmental Industrial Hygienists (ACGIH) recommends a limit of 25 ppm CO for 8 hours (US Environmental Protection Agency, 2012). In 1992, the Underwriters Laboratories voluntarily established standards for indoor CO detectors (Raub, 1999). These standards were revised in 1995 and currently designate that the detector’s alarm will be triggered within 90 minutes of exposure to 100 ppm CO, within 35 minutes of exposure to 200 ppm CO, and within 15 minutes of exposure to 400 ppm CO (Raub, 1999). These set points were determined based on calculations that predict a COHb level of 10% following a specific time-weighted exposure (Raub, 1999).
These standards and guidelines relied on cardiovascular risk in adults given a predicted rise in COHb following a known time-weighted average CO exposure (US Environmental Protection Agency, 2012; Raub, 1999). The amount of COHb formed was calculated based on the known binding properties of CO to adult hemoglobin. However, the neurodevelopmental risk of CO exposure to the fetus, infant, and child in the context of fetal hemoglobin has never been stratified. Therefore, we review current evidence detailing the effects of CO exposure on neurodevelopment in the fetus and developing child. These emerging data and concepts in addition to existing understanding may serve to inform regulatory agencies when considering the impact of CO on the developing brain in future reviews of the standards. In the next section we will highlight findings that suggest a potential link between CO pollution and impaired neurodevelopment. Given the vulnerability of the developing brain to neurotoxicity, such findings should provoke thought about the gaps in our understanding and raise questions about the need to explore scientific investigation that could be used to support policy development designed to protect infants and children from CO exposure.
Air pollution and neurodevelopment
The association between air pollution and adverse pulmonary and cardiovascular health effects has been widely recognized for many years (Block, 2012). However, only recently have concerns been raised about the impact of environmental toxins on neurologic well-being (Block, 2012). Through epidemiological and experimental toxicology studies, investigators now recognize that air pollution is associated with chronic brain inflammation, microglia activation, and white matter abnormalities (Block, 2012). Recent studies indicate that pollutants are associated with adverse effects on neurocognitive development that include disorders of behavior, providing evidence for a potential link between air pollution and neurodevelopmental abnormalities in children (Block, 2012; Vrijheid, 2012).
The components of air pollution that have been implicated in being associated with deleterious neurologic sequelae include particulate matter, polycyclic aromatic hydrocarbons, black carbon, heavy metals, volatile organic compounds, environmental tobacco smoke, ozone, and CO (Block, 2012). Because air pollution is a complicated mixture of toxins, teasing out the biologic effects of individual agents is inherently challenging (Block, 2012). Furthermore, different pollutants may act via a variety of different pathways and may exert their neurotoxic effects via synergistic activity with other agents (Block, 2012). Thus, pinpointing the role and responsibility of a single pollutant with regard to neurotoxicity has its limitations. Given the obvious caveats, however, we will highlight the scientific evidence that has demonstrated a potential association between air pollution and neurocognitive impairment and behavioral abnormalities in children with a focus on CO. It should be noted that a direct causal relationship has yet to be shown due to the lack of controlled studies. In the following discussion, we refer to any heterogeneous mixture of pollutants as “air pollution” and delineate the findings that have been specifically attributed to CO.
Prenatal CO exposure adversely affects perinatal outcomes
Prenatal exposure to air pollution may negatively impact fetal development. Evidence indicates that there is an association between fetal exposure to air pollution and intrauterine growth restriction (IUGR), low and very low birth weight, and prematurity (Wang, 2007). With regard to CO, a number of studies have identified a significant association between in utero fetal exposure and development of IUGR following maternal exposure in either the first or third trimester of pregnancy (Ha, 2001; Maisonet, 2001; Ritz, 2000; Salam, 2005; Wang, 2007). The odds ratio for developing IUGR was calculated to be 1.22 [95% confidence interval, 1.03–1.44] for a fetus exposed to more than 5.5 ppm CO during the last trimester of gestation compared to those exposed to less than 2 ppm (Ritz, 1999). In a study of infants born in southern California between 1975 and 1987, a 1.4 ppm increase in CO exposure during the first trimester of pregnancy was associated with a 21.7 gram reduction in birth weight [95% confidence interval, 1.1–42.3 grams] (Salam, 2005). In addition, other work demonstrated an association between CO exposure and prematurity when maternal exposure occurred during the last month of pregnancy (Liu, 2003). Furthermore, exposure to outdoor air pollution in the early postnatal period has been identified as a contributor to infant mortality (Wang, 2007). For example, postnatal exposure to outdoor air pollution 2 weeks prior to death was associated with a 16% increase in the risk of respiratory demise for each 1 ppm increase in ambient CO in infants in the South Coast Air Basin of California (Ritz, 2006). Taken together, these studies have led researchers to conclude that environmental CO exposure may be an important cause of adverse perinatal outcomes (Wang, 2007).
Prenatal CO exposure may adversely affect neurodevelopment
Prenatal exposure to levels of CO that exceed ambient outdoor concentrations may impair the developing brain. With regard to neurodevelopment, an acute, non-lethal maternal CO exposure at 20 weeks gestation due to use of a defective indoor gas heater, resulted in dystonia in the exposed infant at 2 months of age (Alehan, 2007). Abnormal signal changes in the globus pallidus and basal ganglia were seen with brain imaging and, within the first few years of life, the affected child manifested severe gross motor retardation, spasticity, severe dystonia, and mild mental retardation (Alehan, 2007). In other work, use of gas cookers during pregnancy was found to be associated with decreased cognitive development in exposed children tested beyond 14 months of age (Vrijheid, 2012). These findings were independent of social class, maternal education level, and other potential confounders (Vrijheid, 2012). In a study of Guatemalan children exposed to chronic indoor woodsmoke, exposure to an average of 3.8 ppm CO in the third trimester of pregnancy was associated with impaired neuropsychological performance tested at 6 to 7 years of age (Dix-Cooper, 2012). Thus, CO pollution in both indoor and outdoor environments may be associated with adverse neurodevelopmental outcome.
Tobacco smoke and neurodevelopment
Tobacco smoke contains approximately 5000 chemical compounds and is another important source of CO (Rauh, 2010). Smokers are exposed to between 400 and 500 ppm of CO with each cigarette and their baseline COHb levels may be as high as 10% (Raub, 2000; Tomaszewski, 2002). Active cigarette smoking is known to acutely increase a smoker’s COHb content to even higher levels (Morris, 1995). Hookah smokers, on the other hand, are exposed to even greater concentrations of CO (Daher, 1994). Active hookah smoking increases COHb levels to three times that of conventional cigarette smokers and COHb levels as high as 33.8% have been reported (Martinasek, 2014; Misek, 2014). In addition, following waterpipe use, CO concentrations in a smoker’s exhaled breath may be as high as 58 ppm (Martinasek, 2014).
Prenatal exposure to tobacco smoke affects fetal growth
Maternal smoking during pregnancy has been shown to reduce birth weight and increases the risk of prematurity, placenta previa, placental abruption, and perinatal mortality (Higgins, 2002; Hofhuis, 2003; Wisborg, 2001). It has been estimated that infant birth weight is reduced by 5% for each pack of cigarettes smoked per day in a dose-dependent manner with an average reduction of 25–40 grams in weight (Herrmann, 2008; Lindbohm, 2002). Furthermore, a 79 gram reduction in birth weight has been found in infants born to mothers exposed to second hand smoke during their pregnancy (Hegaard, 2006). One mechanism by which tobacco smoke has been proposed to reduce birth weight is chronic fetal hypoxia (Herrmann, 2008). Such hypoxia may manifest from increased placental vascular resistance, reduced uterine blood flow, and elevated maternal and fetal COHb levels (Herrmann, 2008).
Maternal smoking has also been shown to affect fetal brain growth (Herrmann, 2008). Fetal head circumference demonstrated a growth reduction of 0.13 mm per week [95% confidence interval, -0.18-(-0.09)] in pregnant tobacco smokers and maternal exhaled concentrations of greater than 5 ppm CO during pregnancy have been associated with significantly decreased infant head circumference (Gomez, 2005; Roza, 2007). Although children exposed to smoking during gestation can catch up to their peers with regard to weight and body length, their relatively small head circumferences persist throughout early childhood (Vik, 1996). Importantly, cessation of smoking during pregnancy has been shown spare the fetus from tobacco smoke-induced effects on head size (Lindley, 2000). It is important to note that fetal growth impairments seen with maternal smoking may result from other non-CO related factors.
Perinatal tobacco smoke exposure leads to abnormal behavior
There is a wealth of literature demonstrating an increased incidence of behavioral abnormalities in children who were exposed to tobacco smoke in either the prenatal or postnatal periods (Herrmann, 2008). Exposed children often exhibit oppositional defiance, conduct disorders, delinquency, and attention deficit hyperactivity disorder (ADHD) later in life (Herrmann, 2008). A clear dose-dependent relationship has been demonstrated between quantity of cigarettes smoked during pregnancy and such negative behaviors (Herrmann, 2008). With regard to intelligence and school performance, there is a suggestion that prenatal tobacco smoke exposure is associated with subsequent cognitive deficits in children (Herrmann, 2008). However, maternal education level and intelligence have been found to be major confounders (Herrmann, 2008).
Air pollution and autism
Autism and autism spectrum disorder (ASD) are a group of developmental disorders characterized by abnormalities in social interaction and communication along with repetitive patterns of behavior (Volk, 2011). Overt signs and symptoms of autism often present prior to the age of 3 (Autism Speaks, 2014). The prevalence of ASD has increased ten-fold over the last 40 years and autism currently affects 1 in 68 children (Center for Disease Control and Prevention, 2014; Volk, 2011). Although revised diagnostic criteria and heightened awareness have contributed to the increased incidence, investigators suspect environmental factors along with a strong genetic predisposition as a likely etiology of autism (Herbert, 2010). Residing in an urban setting was recognized as a risk factor for developing ASD in the mid-1990s, however, the role of outdoor air pollution as an etiology of autism was only first evaluated in 2011 (Rosenberg, 2009, Volk, 2011).
In the last few years there have been five key studies that have demonstrated a link between outdoor air pollution and autism (Becerra, 2013; Jung, 2013; Roberts, 2013; Volk, 2011; Volk, 2013). These works identified that traffic-related air pollution exposure in the prenatal period, at the time of birth, and in the first year of life was associated with a diagnosis of autism (Becerra, 2013; Jung, 2013; Roberts, 2013; Volk, 2011; Volk, 2013). It should be noted, however, that the pollutants in these studies were characterized as being mostly related to vehicle emissions and not CO, specifically. The most recently published research, on the other hand, assessed the risk of developing ASD in Taiwanese children and found a significant relationship between yearly average exposure to outdoor environmental CO and a new diagnosis of ASD (Jung, 2013). Based on values obtained from several EPA monitoring stations in Taiwan, a 37% risk increase per 10 ppb increase in CO [95% confidence interval, 1.31–1.44] was identified (Jung, 2013). Taken together, these findings indicate that outdoor air pollution may play a role in the development of autism and ASD. Although these studies provide evidence for an association between specific environmental toxins and autism, no study to date has demonstrated a causal relationship. Furthermore, it is currently unclear how air pollution interacts with specific genetic susceptibilities to manifest the autistic phenotype.
Vulnerability of the developing brain
The developing brain is uniquely vulnerable to environmental insults during critical periods of development (Grandjean, 2006; Rice, 2000). Susceptibility is dependent on the timing of exposure as well as the ability of specific toxins to gain access to the developing nervous system (Rice, 2000). Adverse effects of individual neurotoxins relate to the specific developmental processes that are disrupted (Rice, 2000). In this respect, it is generally recognized that neurotoxicity in the developing brain differs from that of the mature nervous system and alteration of developmental processes can have significant consequences (Rice, 2000).
Interruption of critical processes within the developing brain
Precursors of the central nervous system originate early in embryogenesis and specific areas of the brain begin to develop within the first month of gestation (Rice, 2000). Normal brain development requires careful coordination of a number of critical processes including proliferation, differentiation, migration, synaptogenesis, apoptosis, and myelination (Rice, 2000). Neuronal proliferation occurs via a tightly controlled process during fetal development with variable timing and degree of neurogenesis depending on brain region (Grandjean, 2006; Rice, 2000). Following proliferation, neuroblasts begin to differentiate into their terminal phenotype and migrate from the germinal layers in a radial and tangential fashion (Rice, 2000). In the neocortex, migration is coordinated such that neurons populate the layers of the cortical plate in an inside-out manner (Rice, 2000). Interruption of any of these processes can lead to functional developmental abnormalities (Rice, 2000). Because proliferation, differentiation, and migration are interdependent, disruption of one process often affects the others (Rice, 2000). For example, exposure to agents such as ethanol and methyl mercury during gestation has been shown to impair all three processes in the developing brain (Rice, 2000).
Synaptogenesis is the development of cell to cell communication between neurons (Rice, 2000). This process occurs over the first 3 postnatal weeks of life in rodents and through adolescence in humans (Rice, 2000). Synaptic junctions in the mature brain are predominantly located on dendritic spines and mature synaptic structures do not appear until day of life 7 in mice (Li, 2010). Thereafter, synaptic structures are easily identified (Li, 2010). Neurogenesis, neurite outgrowth, and formation of synapses are critical for establishing neuronal connections in the developing brain (Li, 2010). Subsequently, excess and aberrant synapses are eliminated via cell death and axon pruning during development to ensure proper circuitry and functioning (Vanderhaeghen, 2010). A defect in synapse elimination and dendrite maturation has been found to be associated with certain neurodevelopmental disorders and evidence suggests that such impairments contribute to neurocognitive and behavioral abnormalities (Pfeiffer, 2009). With regard to environmental toxins, exposure to methylazoxymethanol, ethanol, lead, and methyl mercury, for example, during critical periods of postnatal development has been shown to disrupt synaptogenesis (Rice, 2000). In other work, vulnerability of the brain to postnatal neurotoxicity has been demonstrated to coincide with the peak in synaptogenesis (Rizzi, 2010).
Apoptosis is a widespread and natural phenomenon that occurs within the central nervous system (Chan, 2002). In the postnatal brain, developmental apoptosis peaks between day of life 5 and 7 in rodents, coincident with the peak in synaptogenesis (Sanno, 2010). Developmental programmed cell death occurs in the brain in two phases (Rice, 2000). The early embryonic phase coincides with neurogenesis, migration, and differentiation while the postnatal phase is important for selective elimination of aberrant connections and matching of the input size with the target field (Chan, 2002). Apoptosis occurs in approximately 2% of cells in the developing cortex at any time during neurogenesis and in 24–30% of all cortical neurons in the postnatal period (Jiang, 2005; Sanno, 2010). As with synaptogenesis, apoptosis occurs over the first 2 postnatal weeks of life in the mouse and is completed by the third week (Vecino, 2004). A number of environmental agents have been shown to affect apoptosis in the developing brain (Rice, 2000). For example, postnatal exposure to ethanol and a variety of clinically used anesthetic agents, has been shown to lead to widespread apoptosis in the brain (Patel, 2009). The mechanism of neurotoxicity appears to be related to activation of the mitochondrial pathway of apoptosis and dysregulation of neurotrophic factor pathways within the brain, leading to progressive loss of neurons and functional neurodevelopmental deficits later in life (Olney, 2004; Rice, 2000).
Because the human brain begins to develop in utero and continues to mature in the postnatal period, vulnerability to various environmental toxins begins in the fetal period and extends for years into childhood (Grandjean, 2006). The neurodevelopmental risk of infants and children is increased due to the fact that they are commonly exposed to a variety of pollutants in both indoor and outdoor environments, they can rapidly absorb toxins which readily gain access to the developing brain, and they have reduced ability to detoxify certain poisons (Grandjean, 2006). Although it is recognized that a number of environmental agents are associated with subclinical toxicity in children, the mechanisms by which these chemicals result in neurodevelopmental disorders remain unknown (Grandjean, 2006).
Low concentration CO exposure and subclinical neurotoxicity
Much is known about the overt toxicity of exposure to high concentrations of CO. However, little is understood about the neurotoxicity of a subclinical CO exposure (< 200 ppm), as is encountered in a number of ambient environments. Because evidence suggests a potential association between air pollution, tobacco smoke, CO and neurodevelopmental deficits, understanding the possible adverse neurologic effects of such low concentration exposures during critical time periods in development is important. Furthermore, a complete understanding of the specific mechanisms and pathophysiology is necessary given that infants and children are commonly exposed to low levels of CO in a variety of environments.
Prenatal low concentration CO exposure impacts rodent fetal growth and neurodevelopment
A number of experimental studies in rodents have demonstrated that chronic prenatal CO exposure yielding up to 16% COHb results in impaired memory, learning, and behavior in offspring (De Salvia, 1995; Fechter, 1980; Giustino, 1999). Such investigations were designed to mimic fetal CO exposure encountered with maternal smoking during pregnancy as well as gestational exposure to industrial and ambient air pollution (De Salvia, 1995; Fechter, 1980; Giustino, 1999). As with cigarette smoking during pregnancy in humans, exposure to 150 ppm CO during gestation yielded maternal COHb levels of 15% and resulted in lower birth weight and reduced rates of growth in newborn rats (Fechter, 1980). Prenatal CO exposure also impaired behavior in these animals as assessed with negative geotaxis and homing tests (Fechter, 1980). Maternal inhalation of 75 or 150 ppm CO during gestation was shown to increase COHb in pregnant rat dams to levels that approximate those seen in cigarette smokers (means of 7.3% and 16.08%, respectively) and resulted in abnormal habituation and working memory in prenatally exposed juvenile male rats while sparing motor activity (Giustino, 1999). Furthermore, exposure to 150 ppm CO during pregnancy (resulting in maternal COHb levels of 15%) led to impaired acquisition of a two-way active avoidance task in male rats tested at 3 months of age (De Salvia, 1995). Importantly, this defect in learning and memory was permanent and persisted into late adulthood (De Salvia, 1995).
Postnatal low concentration CO exposure impairs rodent neurodevelopment
Postnatal subclinical CO exposure has also been shown to impact rodent neurodevelopment (Cheng, 2012). Exposure to 5 ppm and 100 ppm CO for three hours on postnatal day 10 resulted in average COHb levels of 1.7% and 9.4% immediately following exposure, respectively, and was shown to impair reference memory, memory retention, and spatial working memory in a dose-dependent manner several weeks after exposure in mice (Cheng, 2012). CO-exposed mice also demonstrated greater avoidance activity indicating abnormal social behavior compared to air-exposed controls (Cheng, 2012). Thus, low concentration prenatal and postnatal CO exposures may result in abnormalities in neurodevelopment that persist throughout life.
CO exposure and rodent neuro-ototoxicity
Chronic mild postnatal CO exposure has also been shown to impair the developing auditory system (Lopez, 2003; Stockard-Sullivan, 2003; Webber, 2003). Expression of c-Fos, an indicator of neuronal activation following sound stimulation, was found to be significantly decreased in the central nucleus of the inferior colliculus in 27 day old rats following chronic postnatal exposure to between 12 and 50 ppm CO beginning on the eighth day of life (Webber, 2003). In addition, chronic postnatal exposure to 50 ppm CO beginning at the onset of myelination was shown to attenuate the amplitude of conduction of the eighth cranial nerve in newborn rats (Stockard-Sullivan, 2003). Interestingly, exposure to 100 ppm CO, yielding a mean COHb level of 10.2%, had no effect on eighth cranial nerve conduction suggesting that lower levels of CO exposure were responsible for the pathologic effects (Stockard-Sullivan, 2003). In both of these studies, CO-induced defects persisted into adulthood and failed to recover completely, however, the exact mechanisms remain unknown (Stockard-Sullivan, 2003; Webber, 2003). The authors of these investigations concluded that their results may have implications for auditory neuropathy acquired by children born to tobacco smoking mothers (Stockard-Sullivan, 2003). Acute CO exposure has also been shown to potentiate rodent noise-induced auditory threshold shifts, indicating a chronic hearing impairment following exposure (Fechter, 1988; Young, 1987). Such findings suggest a role of CO pollution in noise-related hearing loss.
CO exposure impairs critical developmental processes
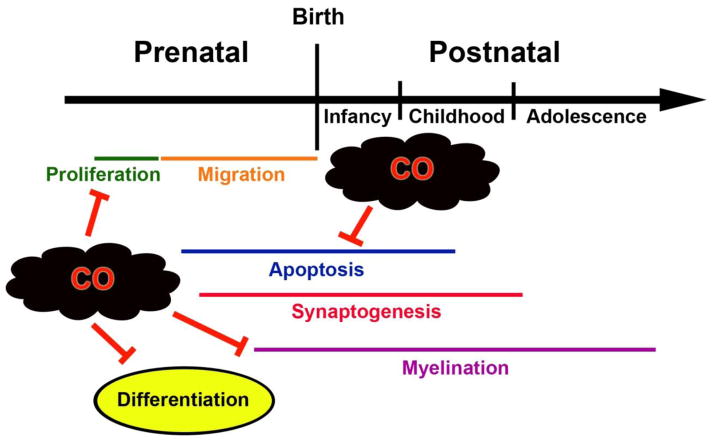
Perinatal subclinical CO exposure interrupts many of the developmental processes that are vital for normal brain maturation. Proliferation, differentiation, myelination, and natural apoptosis in the brain and nervous tissue have all been shown to be adversely affected by low concentration CO (Benagiano, 2005; Carratù, 2000; Fechter, 1987a; Fechter, 1987b) (Figure 2). In one study, perinatal exposure to 75, 150, or 300 ppm CO, beginning early in gestation, yielded mean maternal COHb levels of 11.4%, 18.5%, and 26.8%, respectively, and resulted in a dose-dependent reduction in cerebellar weight and the number of γ-aminobutyric acid (GABA)-ergic neurons in the cerebellum of exposed rat pups, indicating CO-mediated disruption of neuronal proliferation in utero (Fechter, 1987a). In other work, maintaining such perinatal CO exposure until postnatal day 10 resulted in a significant increase in deoxyribonucleic acid (DNA) content and cell number in the neostriatum compared to prenatal exposure alone (Fechter, 1987b). The investigators concluded that, because postnatal CO exposure occurred beyond the period of primary neurogenesis, the increase in DNA and number of cells likely reflected enhanced glial proliferation in response to neuronal loss in the neostriatum (Fechter, 1987b).
Figure 2. Developmental processes in the brain impaired by carbon monoxide (CO).
Animal models have demonstrated that perinatal subclinical CO exposure interrupts many of the processes that are critical for normal brain development. Inhibition due to prenatal or postnatal CO exposure is indicated. Specific mechanisms remain largely unknown and are likely not due to tissue hypoxia.
With regard to differentiation, prenatal exposure to 75 ppm CO yielding 7% maternal COHb levels, was shown to decrease the number of glutamic acid decarboxylase/GABA positive neuronal bodies and axon terminals in the molecular layer and Purkinje neuron layer of the cerebellar cortex in prenatally exposed adult rats (Benagiano, 2005). The authors suggested that CO-mediated interruption of differentiation of GABA synthesizing cerebellar neurons could contribute to the behavioral disorders seen in rats following a similar CO exposure (Benagiano, 2005). Myelination has also been shown to be impaired by CO. Adult rats prenatally exposed to either 75 or 150 ppm CO for the entire period of gestation yielding maternal COHb levels of 7.2–7.4% and 14.4–16.1%, respectively, demonstrated a significant decrease in myelin sheath thickness in their sciatic nerves compared to control animals (Carratù, 2000). This defect was only seen during peak myelination and was not evident during early development (Carratù, 2000). Although CO has been shown to impair proliferation, differentiation, and myelination during development, the exact mechanisms have not been elucidated. Because COHb levels were well below the threshold necessary to impact oxygen delivery, it is likely that CO-mediated inhibition of these critical processes was not due to tissue hypoxia.
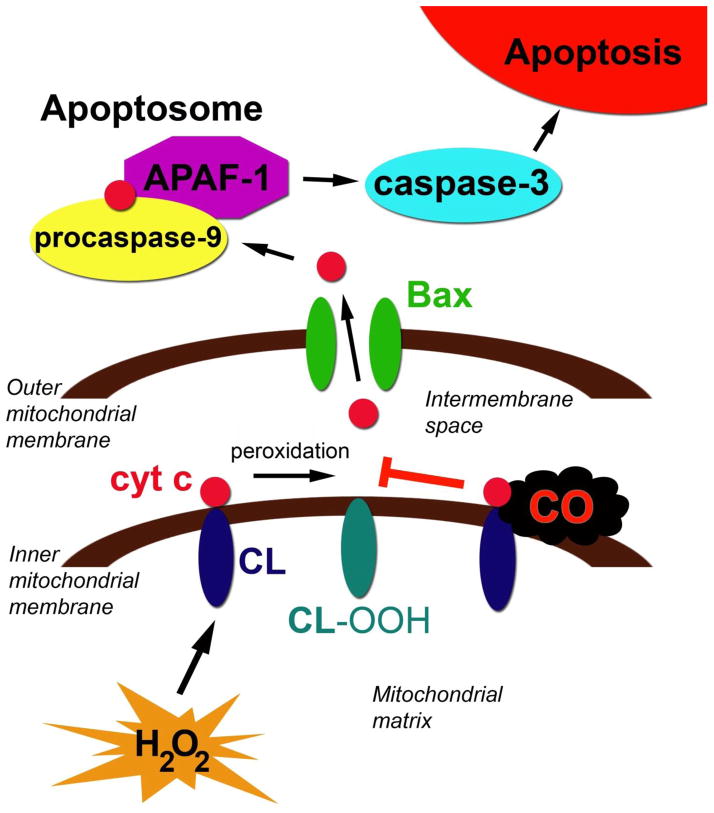
On the other hand, the mechanism of CO-mediated inhibition of developmental programmed cell death in the developing murine brain has been fairly well defined (Cheng, 2012). Postnatal exposure to 5 ppm or 100 ppm CO for three hours, resulting in average COHb levels of 1.7% and 9.4%, respectively, impaired cytochrome c release from forebrain mitochondria, decreased caspase-3 activity, reduced the number of activated caspase-3 positive cells in the neocortex and hippocampus, and decreased forebrain apoptosis in a dose-dependent manner in 10 day old mouse pups (Cheng, 2012). Upstream from cytochrome c release, CO inhibited the peroxidase activity of cytochrome c (Cheng, 2012). Peroxidation of cardiolipin permits mobilization of cytochrome c from the inner mitochondrial membrane and is an important step for cytochrome c release following activation of the intrinsic apoptosis pathway (Cheng, 2012). Nanomolar concentrations of CO are known to bind to the cytochrome c-cardiolipin complex within mitochondria and inhibit cytochrome c peroxidase activity (Kapetanaki, 2009) (Figure 3). In vivo work demonstrated that postnatal CO exposure significantly decreased the peroxidase activity of brain cytochrome c in a concentration-dependent manner, suggesting a potential mechanism for the anti-apoptotic effects of subclinical CO in the developing brain (Cheng, 2012).
Figure 3. Mechanism of carbon monoxide (CO)-mediated inhibition of developmental apoptosis.
The mitochondrial pathway of apoptosis is depicted. Cytochrome c (cyt c), can bind to cardiolipin (CL) on the inner mitochondrial membrane via hydrophobic and electrostatic interactions. Cyt c has peroxidase activity and, in the presence of hydrogen peroxide (H2O2), peroxidizes CL to hydroperoxycardiolipin (CL-OOH). This mobilizes cyt c from the inner mitochondrial membrane and allows cyt c to be released following permeabilization of the outer mitochondrial membrane by Bax. Subsequently, cyt c forms the apoptosome along with procaspase-9 and APAF-1. Caspase-9 then becomes activated and, in turn, activates caspase-3, resulting in apoptosis. CO readily diffuses across the outer mitochondrial membrane and binds to the cyt c-CL complex. CO inhibits the peroxidase activity of cyt c, preventing CL peroxidation, cyt c mobilization, cyt c release, and subsequent caspase activation.
Consistent with impaired neuron elimination, CO-mediated inhibition of apoptosis in the newborn mouse pup brain led to increased neuron specific antigen content, greater number of neurons, and megalencephaly one week following exposure (Cheng, 2012). Because caspase-3 and -9 deficiencies are known to result in morphologic consequences and neuronal disorganization, the investigators suggested that interruption of programmed cell death in the brain at a critical time during development may underlie the impairments in neurocognition and behavior seen in mice briefly exposed to CO in the postnatal period (Hakem, 1998; Kuida, 1996).
The experimental evidence suggests that perinatal exposure to low levels of CO impairs neurodevelopment. The degree of impairment has been shown to be concentration dependent and vulnerability of the developing brain probably also relates to the timing and duration of CO exposure. Although it is likely that CO exerts its neurotoxic effects via several different mechanisms, CO-mediated inhibition of developmental apoptosis is the only pathway that has been well delineated thus far. Therefore, future work will need to more clearly define the effects of perinatal CO exposure on additional critical developmental processes in the immature brain and focus on identifying other potential mechanisms of CO-induced neurotoxicity.
Conclusions
Exposure to indoor and outdoor air pollution including tobacco smoke may negatively impact brain development. It needs to be reiterated, however, that air pollution is a complex mixture of heterogeneous gaseous and particulate neurotoxins (Block, 2012). Therefore, attributing pathologic effects to an individual pollutant is fraught with limitations. Recognizing this caveat, there is a suggestion that CO, a major component of air pollution, is associated with impaired neurodevelopmental outcome and experimental perinatal exposure has been shown to disrupt a number of critical processes in the developing brain. Although much is known about the toxic effects of CO at symptomatic levels, the potential public health impact of subclinical CO exposure has, by-and-large, been underappreciated. Thus, future work should focus specifically on low concentration CO as a potential neurotoxicant. Because the developing auditory system has been shown to be sensitive to the effects of low CO concentration exposure in preclinical studies, future investigation could utilize easily obtainable audiological endpoints to stratify risk and quantify toxic effect of such exposure in children. Furthermore, as our understanding about the risks of CO exposure during critical times in development increases, emerging data should guide and inform future revision of the standards and guidelines regulating CO exposure to account for the vulnerabilities and nuances of the fetus and developing child. Ultimately, enhanced knowledge and consideration will beneficially impact the safety and well-being of infants and children around the world.
Supplementary Material
Highlights.
Carbon monoxide (CO) is a common contaminant of indoor and outdoor environments
CO-induced neurotoxicity can be overt or subclinical
CO can disrupt critical processes within the developing brain
CO exposure may impair neurodevelopment in infants and children
Acknowledgments
This work is supported by NIH/NIGMS R01GM103842-01.
Footnotes
Declaration of Interest
The author has sole responsibility for the writing and content of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KF, Koch G, Chatterjee B, Goldstein GM, O’Neil JJ, Bromberg PA, Sheps DS, McAllister S, Price CJ, Bissette J. Acute elevation of blood carboxyhemoglobin to 6% impairs exercise performance and aggravates symptoms in patients with ischemic heart disease. J Am Coll Cardiol. 1988;12:900–909. doi: 10.1016/0735-1097(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Alehan F, Erol I, Onay OS. Cerebral palsy due to nonlethal maternal carbon monoxide intoxication. Birth Defects Res A Clin Mol Teratol. 2007;79:614–6. doi: 10.1002/bdra.20379. [DOI] [PubMed] [Google Scholar]
- Allen JL, Liu X, Weston D, Prince L, Oberdörster G, Finkelstein JN, Johnston CJ, Cory-Slechta DA. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140:160–78. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989;321:1426–1432. doi: 10.1056/NEJM198911233212102. [DOI] [PubMed] [Google Scholar]
- Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Effects of carbon monoxide on myocardial ischemia. Environ Health Perspect. 1991;91:89–132. doi: 10.1289/ehp.919189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EW, Andelman RJ, Strauch JM, Fortuin NJ, Knelson JH. Effect of low-level carbon monoxide exposure on onset and duration of angina pectoris. A study in ten patients with ischemic heart disease. Ann Intern Med. 1973;79:46–50. doi: 10.7326/0003-4819-79-1-46. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Isbell MW. Carbon monoxide effect on exercise-induced angina pectoris. Ann Intern Med. 1973;79:392–5. doi: 10.7326/0003-4819-79-3-392. [DOI] [PubMed] [Google Scholar]
- Autism Speaks. [Accessed on May 27, 2014];What is autism? [Online] Available at: http://www.autismspeaks.org/what-autism.
- Baker MD, Henretig FM, Ludwig S. Carboxyhemoglobin levels in children with nonspecific flu-like symptoms. J Pediatr. 1988;113:501–4. doi: 10.1016/s0022-3476(88)80638-3. [DOI] [PubMed] [Google Scholar]
- Bauer I, Pannen BH. Bench-to-bedside review: Carbon monoxide--from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect. 2013;121:380–6. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano V, Lorusso L, Coluccia A, Tarullo A, Flace P, Girolamo F, et al. Glutamic acid decarboxylase and GABA immunoreactivities in the cerebellar cortex of adult rat after prenatal exposure to a low concentration of carbon monoxide. Neuroscience. 2005;135:897–905. doi: 10.1016/j.neuroscience.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Wickham WK. Placental diffusing capacity for carbon monoxide in unanesthetized guinea pigs. Respir Physiol. 1977a;31:161–8. doi: 10.1016/0034-5687(77)90099-8. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Wickham WK, Drummond WH. Placental diffusing capacities at varied carbon monoxide tensions. J Clin Invest. 1977b;59:1038–44. doi: 10.1172/JCI108726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–84. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, Bilbo SD. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect. 2013;121:1075–82. doi: 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain, J. Appl Physiol. 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- Carratù MR, Cagiano R, Desantis S, Labate M, Tattoli M, Trabace L, et al. Prenatal exposure to low levels of carbon monoxide alters sciatic nerve myelination in rat offspring. Life Sci. 2000;67:1759–72. doi: 10.1016/s0024-3205(00)00761-x. [DOI] [PubMed] [Google Scholar]
- Carter AM. Placental oxygen transfer and the oxygen supply to the fetus. Fetal and Maternal Medicine. 1999;11:151–161. [Google Scholar]
- Center for Disease Control and Prevention. [Accessed on May 27, 2014];Autism Spectrum Disorder. [Online] Available at: http://www.cdc.gov/ncbddd/autism/data.html.
- Centers for Disease Control and Prevention (CDC) Nonfatal, unintentional, non-fire-related carbon monoxide exposures---United States, 2004---2006. MMWR Morb Mortal Wkly Rep. 2008;57:896–899. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Unintentional Poisoning deaths— United States 1999---2004. MMWR Morb Mortal Wkly Rep. 2007;56:93–96. [PubMed] [Google Scholar]
- Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267:261–76. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Thomas A, Mardini F, Bianchi SL, Tang JX, Peng J, et al. Neurodevelopmental Consequences of Sub-clinical Carbon Monoxide Exposure in Newborn Mice. PLoS One. 2012;7:e32029. doi: 10.1371/journal.pone.0032029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher N, Saleh R, Jaroudi E, Sheheitli H, Badr T, Sepetdjian E, Al Rashidi M, Saliba N, Shihadeh A. Comparison of carcinogen, carbon monoxide, and ultrafine particle emissions from narghile waterpipe and cigarette smoking: Sidestream smoke measurements and assessment of second-hand smoke emission factors. Atmos Environ. 1994;44:8–14. doi: 10.1016/j.atmosenv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Salvia MA, Cagiano R, Carratù MR, Di Giovanni V, Trabace L, Cuomo V. Irreversible impairment of active avoidance behavior in rats prenatally exposed to mild concentrations of carbon monoxide. Psychopharmacology (Berl) 1995;122:66–71. doi: 10.1007/BF02246443. [DOI] [PubMed] [Google Scholar]
- Dix-Cooper L, Eskenazi B, Romero C, Balmes J, Smith KR. Neurodevelopmental performance among school age children in rural Guatemala is associated with prenatal and postnatal exposure to carbon monoxide, a marker for exposure to woodsmoke. Neurotoxicology. 2012;33:246–54. doi: 10.1016/j.neuro.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37:518–23. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechter LD. Neurotoxicity of prenatal carbon monoxide exposure. Res Rep Health Eff Inst. 1987a;12:3–22. [PubMed] [Google Scholar]
- Fechter LD, Annau Z. Prenatal carbon monoxide exposure alters behavioral development. Neurobehav Toxicol. 1980;2:7–11. [PubMed] [Google Scholar]
- Fechter LD, Karpa MD, Proctor B, Lee AG, Storm JE. Disruption of neostriatal development in rats following perinatal exposure to mild, but chronic carbon monoxide. Neurotoxicol Teratol. 1987b;9:277–81. doi: 10.1016/0892-0362(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Young JS, Carlisle L. Potentiation of noise induced threshold shifts and hair cell loss by carbon monoxide. Hear Res. 1988;34:39–47. doi: 10.1016/0378-5955(88)90049-4. [DOI] [PubMed] [Google Scholar]
- Foster M, Goodwin SR, Williams C, Loeffler J. Recurrent acute life-threatening events and lactic acidosis caused by chronic carbon monoxide poisoning in an infant. Pediatrics. 1999;104:e34. doi: 10.1542/peds.104.3.e34. [DOI] [PubMed] [Google Scholar]
- Giustino A, Cagiano R, Carratù MR, Cassano T, Tattoli M, Cuomo V. Prenatal exposure to low concentrations of carbon monoxide alters habituation and non-spatial working memory in rat offspring. Brain Res. 1999;844:201–5. doi: 10.1016/s0006-8993(99)01832-6. [DOI] [PubMed] [Google Scholar]
- Gomez C, Berlin I, Marquis P, Delcroix M. Expired air carbon monoxide concentration in mothers and their spouses above 5 ppm is associated with decreased fetal growth. Prev Med. 2005;40:10–5. doi: 10.1016/j.ypmed.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Gorman D, Drewry A, Huang YL, Sames C. The clinical toxicology of carbon monoxide. Toxicology. 2003;187:25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Greingor JL, Tosi JM, Ruhlmann S, Aussedat M. Acute carbon monoxide intoxication during pregnancy. One case report and review of the literature. Emerg Med J. 2001;18:399–401. doi: 10.1136/emj.18.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül I, Karapinar H, Yarlioglues M, Ozdogru I, Kaya MG, Yilmaz A, et al. Acute effects of passive smoking on endothelial function. Angiology. 2011;62:245–7. doi: 10.1177/0003319710377077. [DOI] [PubMed] [Google Scholar]
- Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–52. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Hampson NB, Norkool DM. Carbon monoxide poisoning in children riding in the back of pickup trucks. JAMA. 1992;267:538–40. [PubMed] [Google Scholar]
- Hampson NB, Piantadosi CA, Thom SR, Weaver LK. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095–101. doi: 10.1164/rccm.201207-1284CI. [DOI] [PubMed] [Google Scholar]
- Hauck H, Neuberger M. Carbon monoxide uptake and the resulting carboxyhemoglobin in man. Eur J Appl Physiol Occup Physiol. 1984;53:186–90. doi: 10.1007/BF00422585. [DOI] [PubMed] [Google Scholar]
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. The effect of environmental tobacco smoke during pregnancy on birth weight. Acta Obstet Gynecol Scand. 2006;85:675–681. doi: 10.1080/00016340600607032. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–10. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20:184–90. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Higgins S. Smoking in pregnancy. Curr Opin Obstet Gynecol. 2002;14:145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–1090. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iheagwara KN, Thom SR, Deutschman CS, Levy RJ. Myocardial cytochrome oxidase activity is decreased following carbon monoxide exposure. Biochim Biophys Acta. 2007;1772:1112–6. doi: 10.1016/j.bbadis.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Clower JH, Hernandez SA, Damon SA, Yip FY. A review of disaster-related carbon monoxide poisoning: surveillance, epidemiology, and opportunities for prevention. Am J Public Health. 2012a;102:1957–63. doi: 10.2105/AJPH.2012.300674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Law HZ, Clower JH, Yip FY, Elixhauser A. Hospital burden of unintentional carbon monoxide poisoning in the United States, 2007. American Journal of Emergency Medicine. 2012b;30:657–664. doi: 10.1016/j.ajem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Jain KK. Carbon Monoxide and Other Tissue Poisons. In: Jain KK, editor. Textbook of Hyperbaric Medicine. Cambridge, MA: Hogrefe Publishing; 2009. pp. 111–133. [Google Scholar]
- Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J, Conway EM, et al. Essential role for survivin in early brain development. J Neurosci. 2005;25:6962–70. doi: 10.1523/JNEUROSCI.1446-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo WK, Oh JW, Dong JI. Evaluation of exposure to carbon monoxide associated with passive smoking. Environ Res. 2004;94:309–18. doi: 10.1016/S0013-9351(03)00135-X. [DOI] [PubMed] [Google Scholar]
- Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8:e75510. doi: 10.1371/journal.pone.0075510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LW, Nañagas KA. Carbon monoxide poisoning. Emerg Med Clin North Am. 2004;22:985–1018. doi: 10.1016/j.emc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kao LW, Nañagas KA. Carbon monoxide poisoning. Med Clin N Am. 2005;89:1161–1194. doi: 10.1016/j.mcna.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kapetanaki SM, Silkstone G, Husu I, Liebl U, Wilson MT, Vos MH. Interaction of carbon monoxide with the apoptosis-inducing cytochrome c-cardiolipin complex. Biochemistry. 2009;48:1613–9. doi: 10.1021/bi801817v. [DOI] [PubMed] [Google Scholar]
- Kleinman MT, Davidson DM, Vandagriff RB, Caiozzo VJ, Whittenberger JL. Effects of short-term exposure to carbon monoxide in subjects with coronary artery disease. Arch Environ Health. 1989;44:361–369. doi: 10.1080/00039896.1989.9935908. [DOI] [PubMed] [Google Scholar]
- Kleinman MT, Leaf DA, Kelly E, Caiozzo V, Osann K, O’Niell T. Urban angina in the mountains: effects of carbon monoxide and mild hypoxemia on subjects with chronic stable angina. Arch Environ Health. 1998;53:388–397. doi: 10.1080/00039899809605726. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–72. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- La Fauci G, Weiser G, Steiner IP, Shavit I. Carbon monoxide poisoning in narghile (water pipe) tobacco smokers. CJEM. 2012;14:57–9. doi: 10.2310/8000.2011.110431. [DOI] [PubMed] [Google Scholar]
- Li M, Cui Z, Niu Y, Liu B, Fan W, Yu D, Deng J. Synaptogenesis in the developing mouse visual cortex. Brain Res Bull. 2010;81:107–13. doi: 10.1016/j.brainresbull.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Lindbohm ML, Sallmen M, Taskinen H. Effects of exposure to environmental tobacco smoke on reproductive health. Scand J Work Environ Health. 2002;28(Suppl 2):84–96. [PubMed] [Google Scholar]
- Lindley A, Becker S, Gray R, Herman A. Effect of continuing or stopping smoking during pregnancy on infant birth weight, crown-heel length, head circumference, ponderal index, and brain: body weight ratio. Am J Epidemiol. 2000;152:219–225. doi: 10.1093/aje/152.3.219. [DOI] [PubMed] [Google Scholar]
- Liu S, Knewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, British Columbia. Environ Health Perspect. 2003;111:1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LD. Carbon monoxide in the pregnant mother and fetus and its exchange across the placenta. Ann N Y Acad Sci. 1970;174:312–41. doi: 10.1111/j.1749-6632.1970.tb49798.x. [DOI] [PubMed] [Google Scholar]
- Lopez I, Acuna D, Webber DS, Korsak RA, Edmond J. Mild carbon monoxide exposure diminishes selectively the integrity of the cochlea of the developing rat. J Neurosci Res. 2003;74:666–75. doi: 10.1002/jnr.10813. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Bush TJ, Correa A, Jaakkola JJ. Relation between ambient air pollution and low birth weight in the northeastern United States. Environ Health Perspect. 2001;109:351–356. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinasek MP, Ward KD, Calvanese AV. Change in carbon monoxide exposure among waterpipe bar patrons. Nicotine Tob Res. 2014;16:1014–9. doi: 10.1093/ntr/ntu041. [DOI] [PubMed] [Google Scholar]
- McGregor HP, Westcott K, Walker DW. The effect of prenatal exposure to carbon monoxide on breathing and growth of the newborn guinea pig. Pediatr Res. 1998;43:126–31. doi: 10.1203/00006450-199801000-00019. [DOI] [PubMed] [Google Scholar]
- Misek R, Patte C. Carbon monoxide toxicity after lighting coals at a hookah bar. J Med Toxicol. 2014;10:295–8. doi: 10.1007/s13181-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RD, Naumova EN, Munasinghe RL. Ambient air pollution and hospitalization for congestive heart failure among elderly people in seven large US cities. Am J Public Health. 1995;85:1361–5. doi: 10.2105/ajph.85.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–21. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V. Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology. 2004;101:273–5. doi: 10.1097/00000542-200408000-00004. [DOI] [PubMed] [Google Scholar]
- Patel P, Sun L. Update on neonatal anesthetic neurotoxicity: insight into molecular mechanisms and relevance to humans. Anesthesiology. 2009;110:703–8. doi: 10.1097/ALN.0b013e31819c42a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham TW, Holt LE, Moss MA. Exposure to carbon monoxide and nitrogen dioxide in enclosed ice arenas. Occup Environ Med. 2002;59:224–33. doi: 10.1136/oem.59.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–67. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub J. Carbon Monoxide (2nd Edition) Environmental Health Criteria 213. World Health Organization; 1999. [Accessed May 27, 2014]. [Online] Available at: http://whqlibdoc.who.int/ehc/WHO_EHC_213.pdf. [Google Scholar]
- Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev. 2002;26:925–40. doi: 10.1016/s0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide poisoning a public health perspective. Toxicology. 2000;145:1–14. doi: 10.1016/s0300-483x(99)00217-6. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Horton MK, Miller RL, Whyatt RM, Perera F. Neonatology and the Environment: Impact of Early Exposure to Airborne Environmental Toxicants on Infant and Child Neurodevelopment. Neoreviews. 2010;11:363–369. doi: 10.1542/neo.11-7-e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, Zhao Y. Air pollution and infant death in southern California, 1989–2000. Pediatrics. 2006;118:493–502. doi: 10.1542/peds.2006-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ Health Perspect. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010;1199:43–51. doi: 10.1111/j.1749-6632.2009.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121:978–84. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RE, Daniels AM, Law JK, Law PA, Kaufmann WE. Trends in autism spectrum disorder diagnoses: 1994–2007. J Autism Dev Disord. 2009;39:1099–111. doi: 10.1007/s10803-009-0723-6. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur J Neurosci. 2007;25:611–7. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Salam MT, Millstein J, Li YF, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the children’s health study. Environ Health Perspect. 2005;113:1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, et al. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–31. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheps DS, Adams KF, Jr, Bromberg PA, Goldstein GM, O’Neil JJ, Horstman D, Koch G. Lack of effect of low levels of carboxyhemoglobin on cardiovascular function in patients with ischemic heart disease. Arch Environ Health. 1987;42:108–116. doi: 10.1080/00039896.1987.9935805. [DOI] [PubMed] [Google Scholar]
- Smithline HA, Ward KR, Chiulli DA, Blake HC, Rivers EP. Whole body oxygen consumption and critical oxygen delivery in response to prolonged and severe carbon monoxide poisoning. Resuscitation. 2003;56:97–104. doi: 10.1016/s0300-9572(02)00272-1. [DOI] [PubMed] [Google Scholar]
- Stockard-Sullivan JE, Korsak RA, Webber DS, Edmond J. Mild carbon monoxide exposure and auditory function in the developing rat. J Neurosci Res. 2003;74:644–54. doi: 10.1002/jnr.10808. [DOI] [PubMed] [Google Scholar]
- Tomaszewski C. Carbon monoxide. In: Goldfrank LR, Flomenbaum NE, Lewin NA, et al., editors. Goldfrank’s toxicologic emergencies. 7. New York: McGraw-Hill; 2002. pp. 1478–97. [Google Scholar]
- US Environmental Protection Agency. [Accessed May 27, 2014];Carbon monoxide. 2012 [Online]. Available at: http://www.epa.gov/airquality/carbonmonoxide/
- Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Hernández M, García M. Cell death in the developing vertebrate retina. Int J Dev Biol. 2004;48:965–74. doi: 10.1387/ijdb.041891ev. [DOI] [PubMed] [Google Scholar]
- Vik T, Jacobsen G, Vatten L, Bakketeig L. Pre and postnatal growth in children of women who smoked in pregnancy. Early Hum Dev. 1996;45:245–255. doi: 10.1016/0378-3782(96)01735-5. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–7. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–7. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rappard J, Schönenberger M, Bärlocher L. Carbon monoxide poisoning following use of a water pipe/hookah. Dtsch Arztebl Int. 2014;111:674–9. doi: 10.3238/arztebl.2014.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Aguilera I, Bustamante M, Ballester F, Estarlich M, et al. INMA Project. Indoor air pollution from gas cooking and infant neurodevelopment. Epidemiology. 2012;23:23–32. doi: 10.1097/EDE.0b013e31823a4023. [DOI] [PubMed] [Google Scholar]
- Wang L, Pinkerton KE. Air pollutant effects on fetal and early postnatal development. Birth Defects Res C Embryo Today. 2007;81:144–54. doi: 10.1002/bdrc.20097. [DOI] [PubMed] [Google Scholar]
- Wang W, Li J, Chang Y, Xie X, Ren J, Wang X, Li Y. Effects of immune reaction in rats after acute carbon monoxide poisoning. Undersea Hyperb Med. 2011;38:239–46. [PubMed] [Google Scholar]
- Webber DS, Korsak RA, Sininger LK, Sampogna SL, Edmond J. Mild carbon monoxide exposure impairs the developing auditory system of the rat. J Neurosci Res. 2003;74:655–65. doi: 10.1002/jnr.10809. [DOI] [PubMed] [Google Scholar]
- Winter PM, Miller JN. Carbon monoxide poisoning. JAMA. 1976;236:1502. [PubMed] [Google Scholar]
- Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol. 2001;154:322–327. doi: 10.1093/aje/154.4.322. [DOI] [PubMed] [Google Scholar]
- Young JS, Upchurch MB, Kaufman MJ, Fechter LD. Carbon monoxide exposure potentiates high-frequency auditory threshold shifts induced by noise. Hear Res. 1987;26:37–43. doi: 10.1016/0378-5955(87)90034-7. [DOI] [PubMed] [Google Scholar]
- Zhou S, Weitzman M, Vilcassim R, Wilson J, Legrand N, Saunders E, Travers M, Chen LC, Peltier R, Gordon T. Air quality in New York City hookah bars. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051763. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.