Abstract
Commonly used anesthetics induce widespread neuronal degeneration in the developing mammalian brain via the oxidative stress-associated mitochondrial apoptosis pathway. Dysregulation of cytochrome oxidase (CcOX), the terminal oxidase of the electron transport chain, can result in reactive oxygen species (ROS) formation and isoflurane has previously been shown to activate this enzyme. Carbon monoxide (CO), as a modulator of CcOX, is of interest because infants and children are routinely exposed to CO during low-flow anesthesia. We have recently demonstrated that low concentrations of CO limit and prevent isoflurane-induced neurotoxicity in the forebrain of newborn mice in a dose-dependent manner. However, the effect of CO on CcOX in the context of anesthetic-induced oxidative stress is unknown. Seven day old male CD-1 mice underwent 1-hour exposure to 0 ppm (air), 5 ppm, or 100 ppm CO in air with or without isoflurane. Exposure to isoflurane or CO independently increased CcOX kinetic activity and increased ROS within forebrain mitochondria. However, combined exposure to CO with isoflurane paradoxically limited CcOX activation and oxidative stress. There were no changes seen in steady-state levels of CcOX I protein indicating post-translational modification of CcOX as an etiology for changes in enzyme activity. CO exposure led to differential effects on CcOX subunit I tyrosine phosphorylation depending on concentration, while combined exposure to isoflurane with CO markedly increased enzyme phosphorylation state. Phosphorylation of tyrosine 304 of CcOX subunit I has been shown to result in strong enzyme inhibition, and the relative reduction in CcOX kinetics following combined exposure to CO with isoflurane may have been due, in part, to such phosphorylation. Taken together, the data suggest that CO modulates CcOX in the developing brain during isoflurane exposure, thereby limiting oxidative stress. These CO-mediated effects could have implications for the development of low-flow anesthesia in infants and children in order to prevent anesthesia-induced oxidative stress.
Keywords: carbon monoxide, oxidative stress, reactive oxygen species, cytochrome oxidase, phosphorylation, brain, development, anesthesia, isoflurane, neurotoxicity
Introduction
The majority of commonly used anesthetic agents induce widespread neuronal apoptosis in the developing mammalian brain (1–5). Such neurodegeneration has been shown to result in behavioral impairments and cognitive deficits in a variety of newborn animal models (6, 7). Although the exact mechanisms of anesthesia-induced neurotoxicity are not completely understood, evidence indicates that the downstream process is mediated by the oxidative stress-associated mitochondrial pathway of apoptosis (6, 8–11). Reactive oxygen species (ROS), generated as a result of anesthetic exposure, have been shown to arise from mitochondrial sources and accumulate within mitochondria (9, 11).
Cytochrome oxidase (CcOX), or complex IV, is the terminal oxidase of the respiratory chain (12). Dysregulation of CcOX can result in oxidative stress. For example, enhanced CcOX activity can hyperpolarize the mitochondrial membrane potential, resulting in superoxide radical generation by complexes I and III within the electron transport chain (12, 13). ROS production in this setting increases exponentially when the transmembrane potential rises above 140 mV (13). On the other hand, inhibition of CcOX can also lead to free radical generation at complexes I and III by promoting electron leak (12, 14). Thus, modulation of CcOX activity is necessary to prevent oxidative stress. During homeostasis, CcOX regulation occurs by a number of mechanisms including subunit isoform switching, adenosine triphosphate (ATP)-dependent allosteric inhibition, post-translational modification involving reversible subunit phosphorylation, and inhibition via endogenously produced gaseous molecules such as nitric oxide, carbon monoxide (CO), and hydrogen sulfide (12).
Although ROS have been shown to mediate anesthesia-induced neuronal apoptosis, the role of CcOX in this setting has not been well defined (15). CO, as a modulator of CcOX, is of interest because infants and children are routinely exposed to CO during low-flow anesthesia when re-breathing is permitted (16, 17). CO binds to the heme a,a3 binuclear center within the active site of CcOX in a competitive manner with oxygen and can reversibly inhibit the enzyme (18, 19). However, low concentrations of CO have been shown stimulate and increase CcOX activity within minutes of exposure under normoxic conditions (20). Thus, CO has the potential to induce ROS formation by either inhibition or activation of the enzyme (19, 21).
We have recently demonstrated that sub-clinical concentrations of CO limit and prevent isoflurane-induced apoptosis in the forebrain of newborn mice in a dose-dependent manner (22). However, the effect of CO on CcOX in the context of anesthetic-induced oxidative stress is unknown. Thus, in this work we aimed to determine the impact of low concentration CO on CcOX specific activity in the developing murine brain during exposure to isoflurane with a focus on oxidative stress. We demonstrate that isoflurane and CO independently increase CcOX kinetic activity and increase lipid peroxidation within forebrain mitochondria. However, combined exposure to CO and isoflurane paradoxically limited CcOX activation and mitochondrial levels of malondialdehyde adducts below expected levels. Such effects of this combined exposure may be due, in part, to modulation of CcOX kinetic activity via phosphorylation of CcOX subunit I. These CO-mediated effects could have implications for the development of low-flow anesthesia in infants and children in order to prevent anesthesia-induced oxidative stress.
Materials and Methods
Animal exposures
The care of the animals in this study was in accordance with NIH and Institutional Animal Care and Use Committee guidelines. Study approval was granted by the Children’s National Medical Center and Columbia University. Six to eight week old female CD-1 pregnant mice (20–30 grams) were acquired (Charles River, Wilmington MA) to yield newborn pups. CD-1 mice were chosen because newborn pups reliably demonstrate neuronal changes consistent with human neonatal injury in specific experimental models (23). On postnatal day 7, we exposed male CD-1 mouse pups to air (0 ppm CO), 5 ppm CO in air, or 100 ppm CO in air with and without isoflurane (2%) for 1 hour in a 7-liter Plexiglas chamber (25 cm × 20 cm × 14 cm). In a subset of mice, separate cohorts of pups were exposed pups for 20 minutes or 40 minutes. The chamber had a port for fresh gas inlet and a port for gas outlet which was directed to a fume hood exhaust using standard suction tubing. Specific concentrations of CO in air (premixed gas H-cylinders, Air Products, Camden, NJ) were verified using an electrochemical sensing CO detector (Monoxor III, Bacharach, Anderson, CA). Designated CO mixtures were delivered through the variable bypass isoflurane vaporizer and exposure chamber at a flow rate of 3–4 liters/minute. Mice were kept warm with an infrared heating lamp (Cole-Parmer, Court Vernon Hills, IL). Animals were euthanized immediately following exposure with pentobarbital injection (150 mg/kg, ip) and forebrain was harvested. Postnatal day 7 was chosen because synaptogenesis peaks at day 7 in rodents and is completed by the second or third week of life (24, 25). One hour exposure to 2% isoflurane has previously been shown to induce neuronal degeneration in 7 day old mice and represents a brief anesthetic exposure (22, 26).
Mitochondrial isolation
Immediately following exposure, forebrain mitochondria were isolated by differential centrifugation (22). As previously described, forebrain was harvested and homogenized in ice-cold H medium (70 mM sucrose, 220 mM mannitol, 2.5 mM Hepes, pH 7.4 and 2 mM EDTA) (22). The homogenate was spun at 1500 x g for 10 min at 4°C. Supernatant was removed and centrifuged at 10,000 x g for 10 min at 4°C. The pellet was suspended in H medium and centrifuged again at 10,000 x g for 10 min at 4°C. Pellet was again resuspended in H medium and mitochondrial protein concentrations determined using the method of Lowry (22).
Determination of lipid peroxidation
Levels of malondialdehyde (MDA) in forebrain mitochondria were determined by measuring thiobarbituric acid reactive substances (TBARS) using a standard colorimetric assay (OxiSelect TBARS Assay Kit, Cell Biolabs, Inc., San Diego, CA) (27). Mitochondria (0.5 mg) were suspended in PBS containing 0.05% butylated hydroxytoluene (BHT) in methanol and homogenized on ice. 100 µL of SDS lysis solution was added and samples were allowed to incubate at room temperature for 5 minutes. 250 µL of thiobarbituric acid (TBA) reagent was then added. Samples were heated to 95°C for 60 min, then cooled on ice for 5 minutes. Following centrifugation at 3000 rpm for 15 min, the supernatant was removed and absorbance measured at 532 nm via a 96-well spectrophotometric plate reader. Levels of TBARS were calculated from a standard curve containing known amounts of TBA–MDA complex and were expressed as µM per milligram of protein. Six animals per cohort were evaluated following 1-hour exposure and 3 animals per cohort were evaluated for the 20 and 40 minute exposures.
Steady-state CcOX kinetics
CcOX kinetics were assayed by the method of Smith in which the rate of oxidation of ferrocytochrome c was measured by following the decrease in absorbance at 550 nm (28, 29). Assays were executed in a 1-mL reaction volume containing 50 mM PO4−2 (pH 7.0), 2% lauryl maltoside, and 1 µg of mitochondrial protein. Ferrocytochrome c was added at a concentration of 40 mM to initiate the reaction. Specific activity was calculated from mean values of three to four measurements using 21.1 mM−1cm−1 as the extinction coefficient of ferrocytochrome c at 550 nm. Six animals per cohort were evaluated following 1-hour exposure and 3 animals per cohort were evaluated for the 20 and 40 minute exposures.
Immunoblot analysis
10µg samples of forebrain mitochondrial protein obtained from animals immediately following 1-hour exposure were subjected to SDS-acrylamide gel electrophoresis and immunoblotting. Blots were labeled with a primary polyclonal antibody to mouse CcOX I (Molecular Probes, Eugene, Oregon, USA), rabbit monoclonal anti-human BCL-xL antibody (Cell Signaling Technology, Beverly, MA), or rabbit polyclonal anti-human BCL-2 antibody (GeneTex Inc., Irvine, California, USA). Blots were secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, California) for CcOX I or goat anti-rabbit IgG (Cell Signaling Technology, Beverly, MA) for BCL-xL and BCL-2. Mitochondrial protein loading was assessed with a primary monoclonal antibody to mouse VDAC (Molecular Probes, Eugene, Oregon, USA) and secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, California.). The signal was detected with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, New Jersey, USA), and density was measured using scanning densitometry. Three animals per group per experiment were evaluated.
CcOX tyrosine phosphorylation
CcOX was extracted from isolated forebrain mitochondria obtained from animals immediately following 1-hour exposure as previously described (30). To preserve phosphorylation state, the phosphatase inhibitors, phenylmethylsulphonyl fluoride (200 mM) and vanadate (1 mM), were added to all isolation buffers. Each step was carried out at 4°C unless otherwise stated. 10% neutralized cholate and 1.67M ammonium sulfate were slowly added to isolated forebrain mitochondria over 30 min maintaining the pH at 7.4. Following 1 hour of incubation, the solution was centrifuged at 20,000 x g for 10 min. The supernatant was brought to 50% saturation with additional ammonium sulfate and centrifuged at 10,000 x g for 10 min. The precipitate was resuspended in 0.1M potassium PO4−2 (pH 6.0) buffer with 2% cholate at a pH of 7.4. Ammonium sulfate was then added to yield 25% saturation. Following incubation for 10 hours at 0°C, the solution was centrifuged at 10,000 x g for 10 min. The supernatant was brought to 35% saturation with additional ammonium sulfate. The precipitate, containing extracted CcOX, was resuspended in 0.1M potassium PO4−2 buffer with 2% cholate at a pH of 7.4. Concentration and purity of CcOX were determined via spectrophotometry (30, 31) (Supplemental figure). Mitochondrial heme a,a3 content was calculated from the difference in spectra (dithionate/ascorbate reduced minus ferricyanide oxidized) of mitochondria solubilized in 10% lauryl maltoside using an absorption coefficient of 24 mM−1cm−1 at 605 to 630 nm as previously described (28, 32).
250 ng of CcOX extracted from forebrain mitochondria was subjected to SDS-acrylamide gel electrophoresis and immunoblotting. Gels were stained with Coomassie to identify CcOX subunits and determine loading (33). Blots were labeled with a primary monoclonal antibody to mouse phosphotyrosine (4G10; Upstate Biotechnology, Inc., Lake Placid, NY) and were secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, California.) (33). The signal was detected with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, New Jersey, USA), and density was measured using scanning densitometry. Three animals per group per experiment were evaluated.
Statistical Analysis
Sample sizes for each endpoint following 1-hour exposure were chosen to detect a 15% difference from air-exposed control values with a power of 80 based on an α of .05. Data are presented as mean plus standard deviation. To assess statistical significance at each time point, we performed two-way ANOVA with post hoc Tukey’s test. We utilized one-way ANOVA to assess for significance within each cohort over time. Linear regression analysis was performed to determine the correlation between levels of TBARS and CcOX kinetic activity at the various time points. Significance was set at P < 0.05.
Results
Combined exposure to CO and isoflurane limits lipid peroxidation within forebrain mitochondria
Isoflurane and a variety of commonly used anesthetic agents have been shown to induce ROS and reactive nitrogen species within developing neurons (9, 10, 34). However, depending on the resultant transmembrane potential and electron flux, CO has the potential to either cause deleterious free radical production within mitochondria or, alternatively, can exert cytoprotective properties by limiting oxidative stress (21). Thus, we assessed the degree of lipid peroxidation within forebrain mitochondria in 7 day old male mice following 1-hour exposure to CO with or without isoflurane using air-exposed mice as a control cohort. Levels of malondialdehyde (MDA), a marker of fatty acid peroxidation, were assessed by colorimetric determination of thiobarbituric acid reactive substances (TBARS) in mitochondria using a standard assay (27, 35).
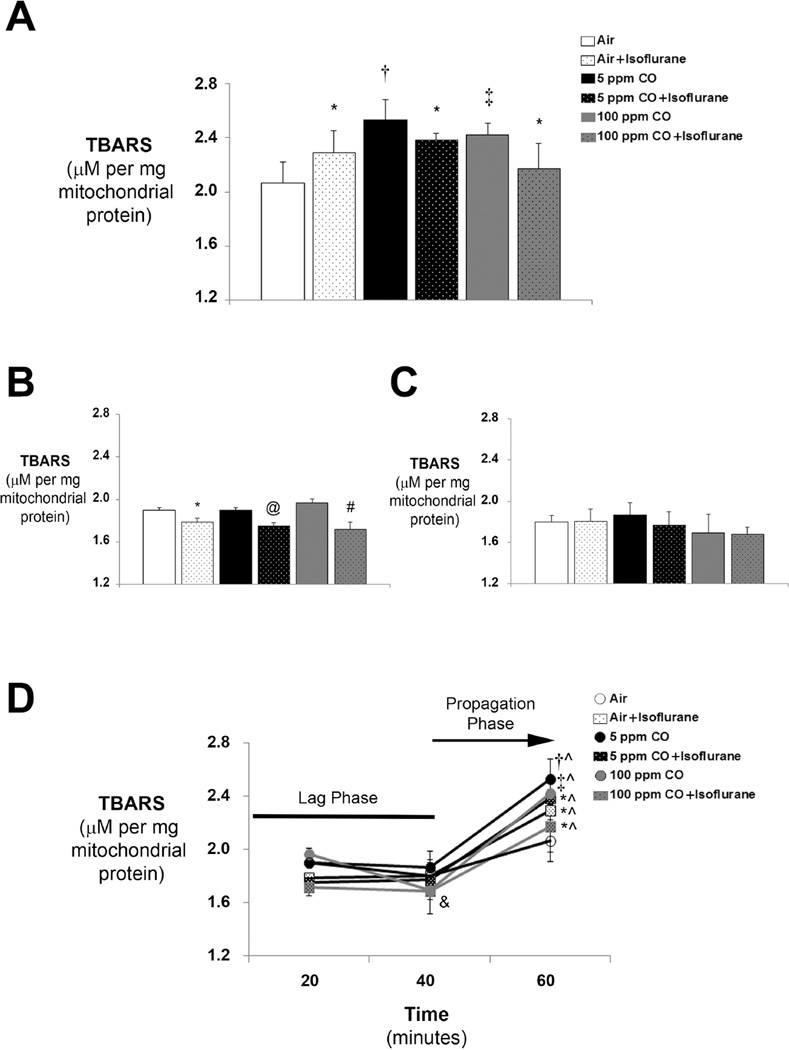
Consistent with prior work demonstrating that anesthetics induce oxidative stress in the developing brain, 1-hour exposure to isoflurane significantly increased TBARS within forebrain mitochondria compared to air exposure (figure 1A). Exposure to 5 ppm and 100 ppm CO also significantly increased mitochondrial TBARS in both CO-exposed cohorts versus exposure to air alone (figure 1A). However, combined exposure to CO with isoflurane resulted in levels of TBARS that were significantly lower than those seen following concentration-matched CO exposure alone (figure 1A). Importantly, exposure to isoflurane with 100 ppm CO resulted in levels of TBARS that approached air-exposed control values (figure 1A). The findings indicate that 1-hour exposure to either isoflurane or low concentration CO independently results in oxidative stress in the developing forebrain, but when inspired simultaneously, the combination limits isoflurane or CO-induced lipid peroxidation within mitochondria.
Figure 1. Thiobarbituric acid reactive substances (TBARS) in forebrain mitochondria following exposure to carbon monoxide (CO) with or without isoflurane.
Levels of TBARS were determined with a colorimetric assay in mitochondria isolated immediately following exposure. Values from the six experimental cohorts are expressed as means plus standard deviation. (A) TBARS following 1-hour exposure. N = 6 animals per cohort. (B) TBARS following 20 minute exposure. N = 3 animals per cohort. (C) TBARS following 40 minute exposure. N = 3 animals per cohort. (D) TBARS over time. Statistical significance was determined at each time point with two-way ANOVA using post hoc Tukey’s test. One-way ANOVA was utilized to assess significance within each experimental cohort over time. *P < 0.05 vs. CO (or air) matched exposure without isoflurane at same time point. †P < 0.001 vs. air-exposed control at same time point. ‡P < 0.005 vs. air-exposed control at same time point. @P < 0.005 vs. CO matched exposure without isoflurane at same time point. #P < 0.01 vs. CO matched exposure without isoflurane at same time point. ^P < 0.01 vs. cohort matched exposure at 20 and 40 minutes. &P < 0.05 100 ppm CO at 40 minutes vs. cohort matched exposure at 20 minutes.
Because formation of MDA is a dynamic process, we next measured the level of TBARS in forebrain mitochondria from separate subgroups of mice in each experimental cohort following 20 or 40 minutes of exposure. No significant change in TBARS was seen following 20 minute exposure to either concentration of CO compared to air exposed controls (figure 1B). Interestingly, exposure to isoflurane with and without CO for 20 minutes significantly decreased the level of TBARS compared to concentration-matched CO (or air) exposure alone (figure 1B). However, levels then returned toward air-control values following 40 minutes of exposure (figure 1C). A similar and significant reduction in TBARS was seen in animals exposed to 100 ppm CO for 40 minutes compared to those exposed to 100 ppm for 20 minutes (figure 1B, 1C). The level of TBARS following 60 minutes of exposure were significantly greater in each experimental group compared to cohort-matched mitochondrial levels measured at 20 and 40 minutes, suggesting a lag phase of lipid peroxidation during the earlier time points (figure 1D).
CO modulates forebrain CcOX kinetic activity in a dose-dependent manner
Isoflurane has previously been shown to increase CcOX activity in the developing subiculum following exposure (36). On the other hand, CO has the potential to either limit CcOX activity as a competitive inhibitor of oxygen or enhance enzyme kinetics following low concentration exposure (20). Thus, we measured steady-state CcOX specific activity in forebrain mitochondria isolated immediately after 1-hour exposure to CO with or without isoflurane.
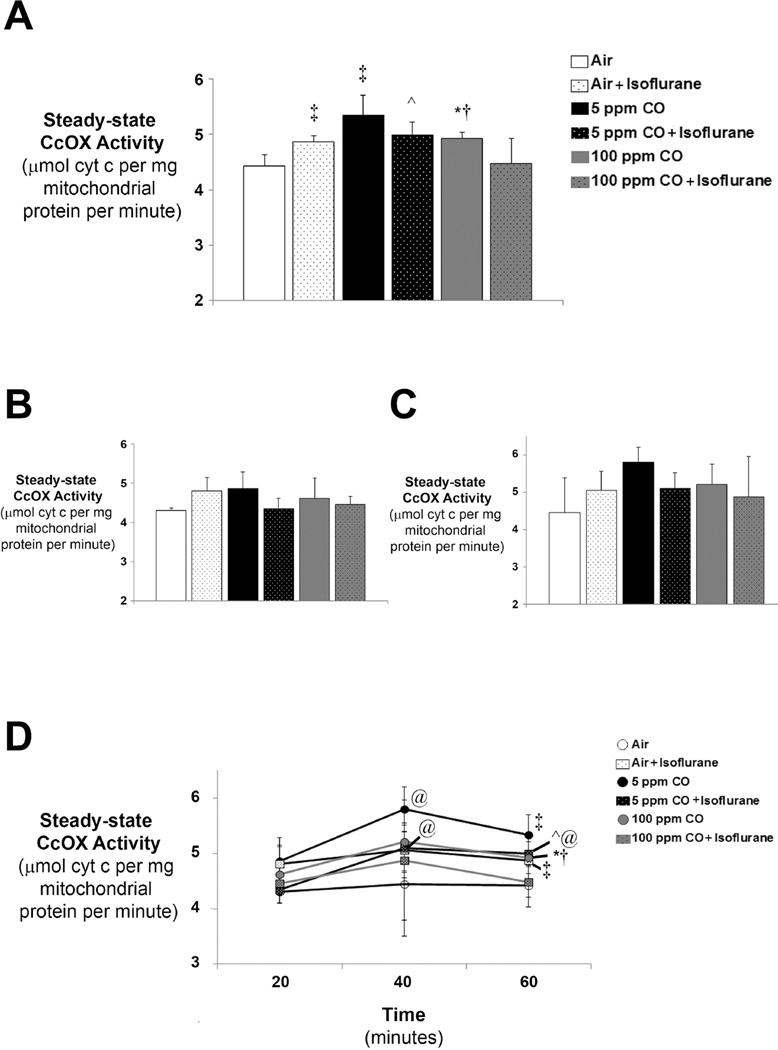
Isoflurane significantly increased steady-state forebrain CcOX activity immediately following 1-hour exposure compared to air-exposed controls (figure 2A). Steady-state CcOX kinetic activity also significantly increased following 1-hour exposure to CO in a dose-dependent fashion (figure 2A). Maximal increases in CcOX kinetics following 1-hour exposure were seen in 5 ppm CO-exposed animals while the resultant increase in enzyme activity following 100 ppm CO exposure was significantly less pronounced (figure 2A). Despite increases in CcOX specific activity following independent exposure to isoflurane or CO, combined exposure resulted in a relative reduction in enzyme kinetics in a concentration-dependent manner for CO (figure 2A). As such, 1-hour exposure to isoflurane with 100 ppm CO resulted in significantly lower steady-state CcOX activity compared to enzyme activities following exposure to 100 ppm CO alone as well as exposure to isoflurane with 5 ppm CO (figure 2A). Importantly, CcOX kinetic activity following combined exposure to isoflurane with 100 ppm CO approximated air-exposed control rates (figure 2A). Although there was a trend toward a decrease in CcOX activity following exposure to isoflurane with 5 ppm CO compared to 5 ppm CO exposure alone, this difference was not statistically significant (P = 0.07) (figure 2A).
Figure 2. Cytochrome oxidase (CcOX) kinetic activity following exposure to CO with or without isoflurane.
Steady-state CcOX specific activity was measured in isolated mitochondria immediately after exposure. Values are expressed as means plus standard deviation. (A) CcOX kinetics following 1-hour exposure. N = 6 animals per cohort. (B) CcOX kinetics following 20 minute exposure. N = 3 animals per cohort. (C) CcOX kinetics following 40 minute exposure. N = 3 animals per cohort. Statistical significance was determined at each time point with two-way ANOVA using post hoc Tukey’s test. One-way ANOVA was utilized to assess significance within each experimental cohort over time. *P < 0.05 vs. 100 ppm CO + isoflurane, 5 ppm CO at the same time point. ^P < 0.05 vs. 100 ppm CO + isoflurane at the same time point. †P < 0.01 vs. air-exposed control at the same time point. ‡P < 0.001 vs. air-exposed control at the same time point. @P < 0.05 vs. cohort matched exposure at 20 minutes.
As with our assessment of TBARS, we next measured steady-state CcOX activity in forebrain mitochondria isolated from separate subgroups of mice following exposure for 20 or 40 minutes. Changes in CcOX kinetics began 20 minutes following exposure to isoflurane and CO alone, though, did not reach statistical significance between groups until the 1-hour time point (figure 2A–C). However, within exposure cohorts, CcOX activity significantly increased at 40 minutes compared to the 20 minute time point following exposure to 5 ppm CO and significantly increased at 40 minutes and 1-hour compared to the 20 minute time point following exposure to 5 ppm CO with isoflurane (figure 2D). The findings indicate that independent exposure to either isoflurane or low concentration CO stimulates and activates forebrain CcOX. However, combined exposure paradoxically limits CcOX activation in a dose-dependent manner for CO.
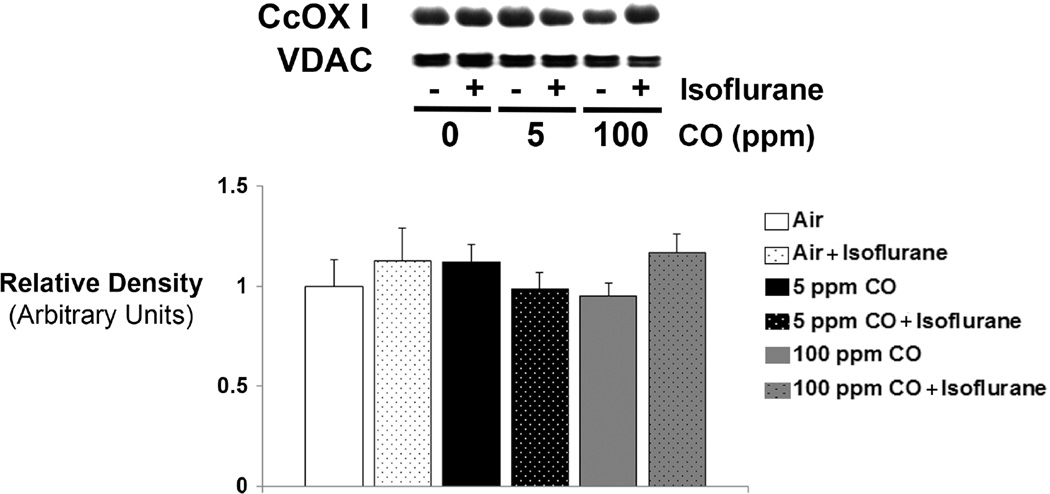
In order to determine if changes in enzyme activity following 1-hour exposure were on the basis of protein content, we performed immunoblot analysis for steady-state levels of forebrain CcOX subunit I, the active site. Although steady-state levels of CcOX subunit I fluctuated between groups, these differences were not statistically significant (figure 3). Thus, changes in enzyme activity following exposure must have been due to post-translational modification of CcOX.
Figure 3. Cytochrome oxidase (CcOX) expression following 1-hour exposure to CO with or without isoflurane.
A representative immunoblot of steady-state levels CcOX subunit I protein is depicted. Concentration of CO exposure (or air [0 ppm]) with (+) or without (−) isoflurane is indicated. VDAC was used as the mitochondrial protein loading control. Graphical representation of relative densities is shown below the blot. Values were normalized to VDAC density and are expressed as means plus standard deviation. Air-exposed control values were set arbitrarily to 1. N = 3 animals per cohort. Statistical significance was determined with two-way ANOVA using post hoc Tukey’s test.
Oxidative stress following exposure to CO with or with isoflurane correlates strongly with CcOX activity
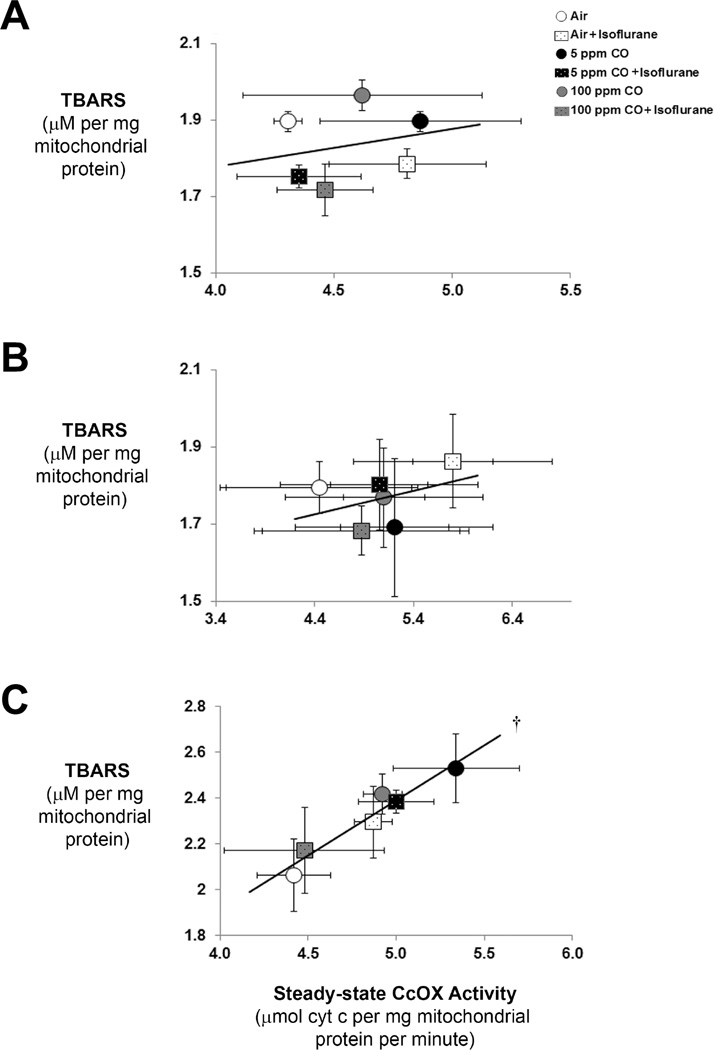
Mitochondria are the major source of ROS production within cells (12). Furthermore, CcOX dysregulation can result in oxidative stress. Thus, we performed linear regression analysis to determine the relationship between mitochondrial lipid peroxidation and CcOX kinetics at the 20 minute, 40 minute, and 1-hour time points. Although there was no significant correlation at the earlier time points, the level of TBARS in forebrain mitochondria following 1-hour exposure correlated strongly in a highly significant manner with steady-state CcOX specific activity (r = 0.97) (figure 4). Although the lack of correlation between TBARS and CcOX kinetics at the 20 and 40 minute time points may reflect the dynamics of lipid peroxidation and the lag phase, the findings at the 1-hour time point suggest a potential role for CcOX dysregulation as an etiology for oxidative stress induced by either exposure to isoflurane or CO. Furthermore, the relationship suggests the importance of CcOX modulation in limiting oxidative stress following combined exposure to isoflurane with CO.
Figure 4. Correlation between thiobarbituric acid reactive substances (TBARS) and cytochrome oxidase (CcOX) kinetic activity.
Linear regression analysis of level of TBARS with CcOX specific activity was performed. Pearson correlation coefficients and r-values were calculated. Values are expressed as means +/− standard deviation. (A) Correlation following 20 minute exposure. N = 3 animals per cohort. Pearson correlation coefficient is 0.06 and r-value is 0.24. P = 0.65. (B) Correlation following 40 minute exposure. N = 3 animals per cohort. Pearson correlation coefficient is 0.15 and r-value is 0.38. P = 0.45. (C) Correlation following 1-hour exposure. N = 6 animals per cohort. Pearson correlation coefficient is 0.94 and r-value is 0.97. †P < 0.002.
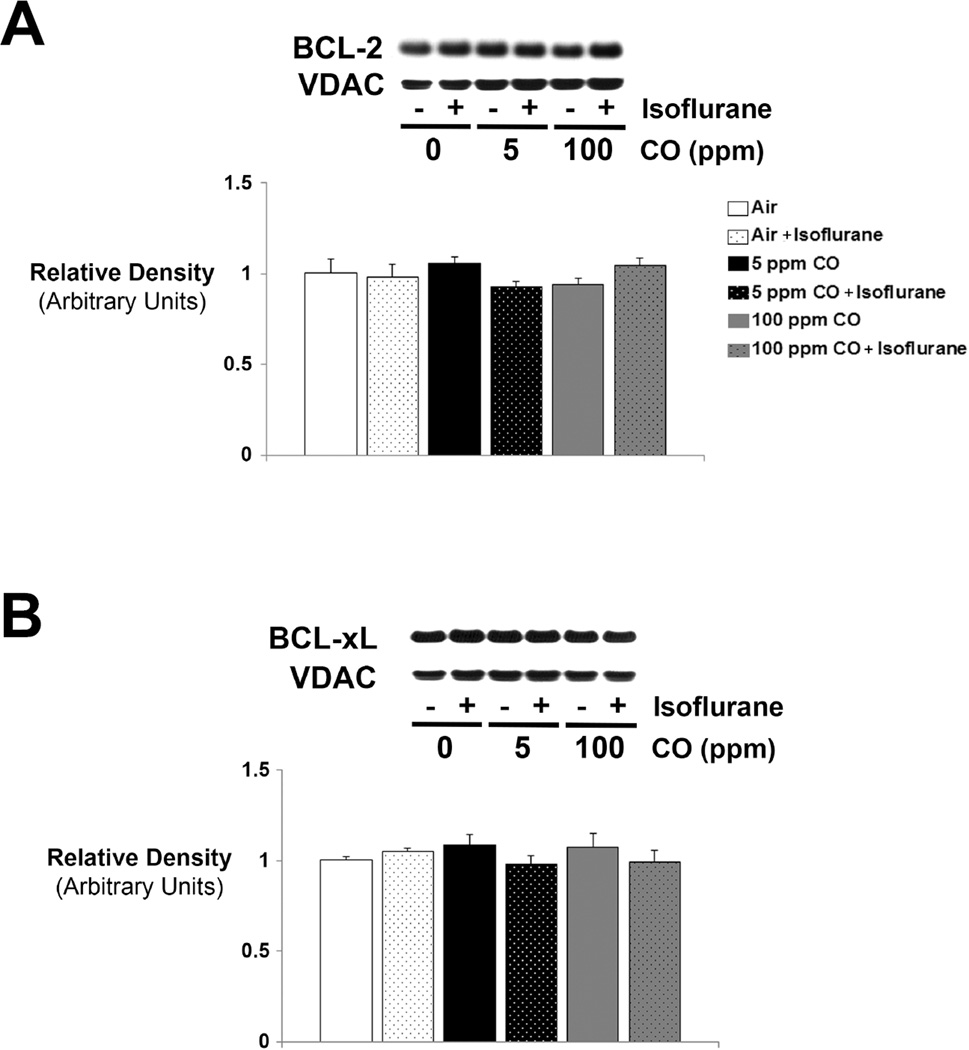
Brief exposure to isoflurane or CO does not affect protein levels of BCL-2 or BCL-xL
In addition to oxidative stress, anesthesia-induced activation of the mitochondrial apoptosis pathway also involves downregulation of the anti-apoptotic proteins, BCL-2 and BCL-xL (6, 8). In contrast, CO exposure has been shown to induce BCL-2 expression (20). BCL-2 has the potential to regulate CcOX activity and is necessary for CO-induced modulation of oxidative phosphorylation and cytoprotection (20). Likewise, BCL-xL enhances bioenergetic efficiency by reducing excess ion flux across the mitochondrial inner membrane (37, 38). Therefore, we determined steady-state levels of BCL-2 and BCL-xL protein with immunoblot analysis in forebrain mitochondria immediately following 1-hour exposure to CO with or without isoflurane. We found no differences in steady-state levels of either protein between any of the exposed cohorts (figure 5). Thus, changes in lipid peroxidation and CcOX activity following 1-hour exposure to isoflurane, CO, or their combination were not associated with acute changes in BCL-2 or BCL-xL expression.
Figure 5. Expression of BCL-2 and BCL-xL following exposure to CO with or without isoflurane.
Steady-state levels of (A) BCL-2 and (B) BCL-xL protein were determined. Representative immunoblots are depicted. Concentration of CO exposure (or air [0 ppm]) with (+) or without (−) isoflurane is indicated. VDAC was used as the mitochondrial protein loading control. Graphical representations of relative densities are shown below the blots. Values were normalized to VDAC density and are expressed as means plus standard deviation. Air-exposed control values were set arbitrarily to 1. N = 3 animals per cohort. Statistical significance was determined with two-way ANOVA using post hoc Tukey’s test.
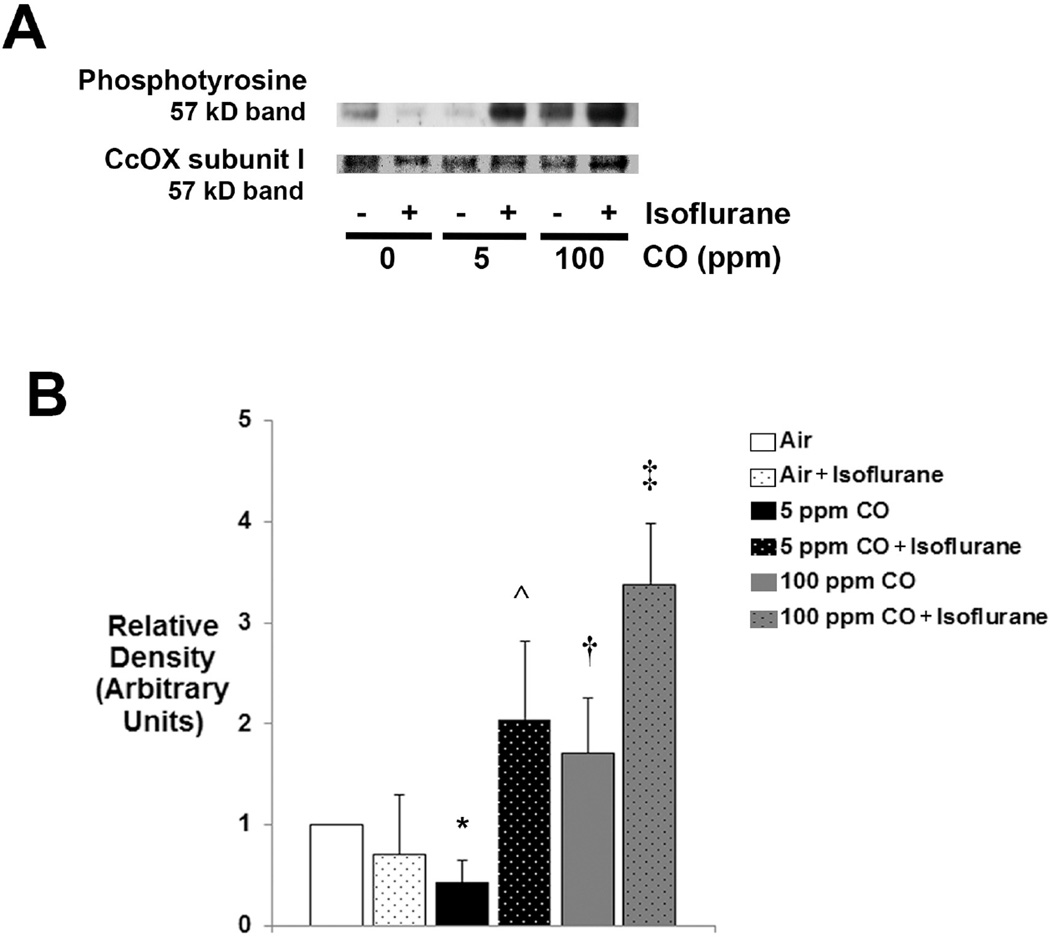
Combined exposure to CO and isoflurane increases tyrosine phosphorylation of CcOX subunit I
CcOX is regulated by a number of mechanisms. Phosphorylation of tyrosine 304 of CcOX subunit I, the active site, has been shown to result in strong enzyme inhibition (39). Although additional CcOX phosphorylation sites have been identified, their functional roles have not been defined (40). Therefore we assessed for steady-state levels of phosphotyrosine in CcOX extracted from isolated forebrain mitochondria using immunoblot analysis immediately following 1-hour exposure to CO with or without isoflurane.
A 57 kD band was readily detected in each experimental cohort suggesting tyrosine phosphorylation of CcOX subunit I (figure 6). Steady-state levels of phosphotyrosine significantly decreased following exposure to 5 ppm CO compared to air-exposed controls (figure 5). However, exposure to 100 ppm CO significantly increased tyrosine phosphorylation of CcOX when compared to the 5 ppm CO-exposed cohort (figure 6). Furthermore, steady-state levels of phosphotyrosine significantly increased following combined exposure to 5 ppm CO with isoflurane compared to 5 ppm CO exposure alone and significantly increased following 100 ppm CO exposure with isoflurane compared to isoflurane exposure alone (figure 6). The findings indicate differential effects of CO on tyrosine phosphorylation of CcOX depending on concentration and a synergistic effect of combined exposure to CO with isoflurane.
Figure 6. Tyrosine phosphorylation of cytochrome oxidase (CcOX) subunit I.
CcOX was extracted from isolated mitochondria and steady-state levels of phosphotyrosine were determined. (A) A representative immunoblot of the 57 kD band is depicted. Concentration of CO exposure (or air [0 ppm]) with (+) or without (−) isoflurane is indicated. CcOX subunit I (57 kD) staining with Coomassie dye was used as the loading control. (B) Graphical representation of relative densities is shown. Values are expressed as means plus standard deviation. Air-exposed control values were set arbitrarily to 1. N = 3 animals per cohort. Statistical significance was determined with two-way ANOVA using post hoc Tukey’s test. *P < 0.05 vs. air-exposed control. ^P < 0.025 vs. 5 ppm CO. †P < 0.005 vs. 5 ppm CO. ‡P < 0.005 vs. air + isoflurane.
Discussion
Oxidative stress is an important mediator of anesthesia-induced neurotoxicity in the developing brain (10). However, the underlying mechanisms of free radical production caused by anesthetics are not well understood. Consistent with prior work, we demonstrate that 1-hour exposure to isoflurane causes lipid peroxidation and increases CcOX activity in the forebrain of 7 day old male mice (9, 10, 34, 36). CO, unlike isoflurane, has the potential to either cause or limit ROS production depending on the mitochondrial transmembrane potential, and stimulates or reversibly inhibits CcOX depending on the concentration of exposure (18–21). Here we found that both concentrations of CO increased lipid peroxidation within forebrain mitochondria and independently stimulated CcOX following the 1-hour exposure. The CO-induced increases in CcOX kinetics are consistent with known effects of low concentration CO exposure (20). Finding increased levels of lipid peroxidation in both CO-exposed cohorts suggests that enhanced CcOX activity following exposure likely led to hyperpolarization of the mitochondrial membrane potential, resulting in superoxide radical generation (12, 13).
Importantly, isoflurane- and CO-induced increases in lipid peroxidation were not seen until the 1-hour time point. MDA levels initially declined at the 20 minute time point following initiation of isoflurane exposure, increased toward control values at the 40 minute time point, and then rose significantly by 1 hour. A similar pattern was seen following exposure to 100 ppm CO, however, the relative decrease in MDA levels was not seen until the 40 minute time point. The delay in appearance of markers of lipid peroxidation likely reflects a lag phase following initiation of oxidative stress due to endogenous anti-oxidant defenses and the time necessary for MDA formation (41, 42). In addition, the decrease in TBARS at 20 minutes in the isoflurane-exposed cohorts and at 40 minutes in the 100 ppm CO-exposed animals suggests potential activation of detoxification processes and rapid metabolism of MDA via oxidation and decarboxylation, for example (43). However, the acute increase in markers of lipid peroxidation at the 1-hour time point suggests the onset of the propagation phase and is consistent with the previously reported timeline of TBARS formation during hydrogen peroxide induced oxidative stress (42).
Changes in CcOX activity began early during isoflurane or CO exposure, with maximal and significant increases seen at the 40 and 60 minute time points (coincident with the propagation phase of lipid peroxidation). Although both isoflurane and CO independently enhanced CcOX activity and induced oxidative stress in the developing brain following exposure alone, combined exposure to both gases relatively reduced CcOX activity in a concentration-dependent manner for CO and paradoxically decreased forebrain lipid peroxidation. These findings are somewhat surprising given that each agent induced oxidative stress on its own. The data indicate that simultaneous exposure somehow limited the activation of CcOX, partially inhibited the enzyme (or both) and resulted in a relative anti-oxidant state compared with single gas exposure. The strong and tight correlation between lipid peroxidation and CcOX specific activity at the 1-hour time point suggests that CcOX dysregulation and stimulation may play a role in isoflurane- and CO-induced oxidative stress and that CcOX modulation may limit ROS production during combined exposure. We, however, cannot definitively conclude that changes in CcOX activity caused or prevented lipid peroxidation as part of this study because we did not specifically attempt to uncouple the two processes. Such an approach will need to be performed in future work to establish a causative role for CcOX with regard to oxidative stress in this context. Furthermore, our assumption that the lack of correlation between TBARS and CcOX kinetics in the early time points was due to the lag phase will need to be further tested and confirmed.
The increases in CcOX kinetics seen following 1-hour 100 ppm CO exposure are of interest because they were relatively less than those seen following 5 ppm CO exposure and suggest a lesser degree of CcOX activation, a relative inhibition of the enzyme, or both. Unfortunately, as part of this work we did not expose cohorts to concentrations beyond 100 ppm CO to better define this relationship. However, previous work has demonstrated that exposure to 1000 ppm CO for 30 minutes inhibited CcOX within rodent forebrain mitochondria (44). Thus, by way of extrapolation, we postulate that CcOX activation lessens as the concentration of CO increases and that enzyme inhibition becomes a more prominent feature following exposure to concentrations greater than 100 ppm CO. Such a hypothesis will need to be tested in future work and will need to include measures of mitochondrial transmembrane potential to provide a better understanding of the effects on electron flux.
As for an explanation of the paradoxical reduction in lipid peroxidation and CcOX activity following combined exposure, there is no guidance provided by previous investigation. CcOX is known to be regulated by a number of mechanisms. The anti-apoptotic proteins, BCL-2 and BCL-xL, for example, are known modulators of CcOX and oxidative phosphorylation and both have been shown to be downregulated by long duration anesthetic exposures (6, 8, 20, 37, 38). However, in this work, we found no change in steady-state levels of either protein following brief exposure to CO with or without isoflurane. This was likely due to the short duration of experimental exposure and the fact that we evaluated for protein expression immediately following the 1-hour exposure. Furthermore, changes in CcOX kinetics were also not associated with altered levels of CcOX subunit I. Thus, differences in CcOX activity must have been due to post-translational modification of CcOX following exposure. Therefore, the two regulatory mechanisms most likely responsible for the modulation of CcOX activity seen following combined exposure to isoflurane with CO in this study are changes in ATP-dependent allosteric inhibition of the enzyme or reversible subunit phosphorylation.
Phosphorylation sites have been identified on all of the electron transport chain enzyme complexes, however, the majority of kinases and phosphatases regulating their phosphorylation state remain unknown (45). Phosphorylation of CcOX is thought to rapidly modulate enzyme function and permit adaptation to meet cellular needs (45). CcOX has at least 14 known phosphorylation sites located on the amino acids of several different subunits (40, 45). For the majority of these sites, the functional consequences of phosphorylation remain undefined (40). However, for tyrosine 304 of CcOX subunit I, phosphorylation has been clearly shown to result in strong enzyme inhibition (39). Therefore, we focused on assessing the degree of tyrosine phosphorylation of CcOX extracted from isolated forebrain mitochondria immediately following 1-hour exposure to CO with or without isoflurane.
A readily detectable phosphotyrosine band was seen in the air-exposed cohort, suggesting that CcOX may be under tonic inhibition in the developing forebrain at baseline. Although we did not confirm that the 57 kD band specifically represented phosphorylated tyrosine 304, the associated directionality of change in CcOX activity in each cohort was consistent with the state of phosphorylation given the known inhibitory effect of tyrosine 304 phosphorylation. For example, exposure to 5 ppm CO significantly decreased tyrosine phosphorylation of forebrain CcOX and was associated with an increase in CcOX specific activity. On the other hand, exposure to 100 ppm CO increased CcOX phosphotyrosine levels relative to the 5 ppm CO-exposed cohort and, as would be expected, was associated with a relative decrease in CcOX activity. Combined exposure to CO with isoflurane increased the degree of tyrosine phosphorylation of CcOX in both CO-exposed cohorts and was associated with enzyme kinetics that tended to be slower than those seen following exposure to concentration-matched CO alone. Taken together, the data suggest that tyrosine phosphorylation of CcOX subunit I might contribute, in part, to the modulation of CcOX kinetic activity following exposure to CO with or without isoflurane. However, hyperphosphorylation of CcOX did not inhibit the enzyme relative to air-exposed controls. Thus, control of CcOX kinetics under these conditions is likely a complex process and may involve a variety of other regulatory mechanisms which may become activated or inhibited by isoflurane and CO.
Tyrosine 304 is phosphorylated by a cyclic adenosine monophosphate (cAMP)-dependent mechanism (33). Although the exact kinase involved is unknown, elevated cAMP levels have been shown to result in phosphorylation of tyrosine 304 and subsequent CcOX inhibition (33). Exogenous CO has the potential to activate adenylate cyclase and increase cAMP content in exposed cells (46, 47). Thus, it is possible that exposure to 100 ppm CO enhanced adenylate cyclase activity and increased cAMP levels in the forebrain of exposed mice, leading to tyrosine phosphorylation of CcOX and partial inhibition of the enzyme relative to the 5 ppm CO exposure. Although it is unclear why the lower concentration of CO led to dephosphorylation of CcOX, the differential effects of each concentration of CO on tyrosine phosphorylation may underlie the differential effects seen with CcOX activity following exposure.
It is less clear why combined exposure to CO with isoflurane resulted in robust tyrosine phosphorylation. Although not well studied, volatile anesthetics have been shown to inhibit adenylate cyclase and cAMP production and isoflurane has been shown to prevent the increases in cardiomyocyte cAMP seen following stimulation with isoproterenol (48, 49). Such properties could potentially explain the relatively unchanged CcOX phosphotyrosine levels seen following isoflurane exposure alone, but do not provide insight into the significant increases in phosphorylation seen following combined exposure to isoflurane with either concentration of CO. Thus, the mechanism of enhanced phosphorylation during such combined exposure is not obvious and will need to be explored in future work.
Conclusions
During low-flow general anesthesia, when re-breathing is permitted, infants and children routinely inspire low concentrations of CO (16, 17). A common source of CO in this clinical setting is exhaled CO generated via endogenous heme catabolism (17). Because exhaled CO is not scavenged or removed from the anesthesia breathing circuit, patients are simultaneously exposed to both low concentration CO and inhaled anesthetic agents (17, 50). In prior work we demonstrated that clinically relevant concentrations of CO limit and prevent isoflurane-induced apoptosis in the forebrain of newborn mice in a dose-dependent manner (22). Here we found that combined exposure to CO with isoflurane limited CcOX activation and oxidative stress in the developing brain. Consistent with our previous findings, exposure to 100 ppm CO with isoflurane yielded outcome measures that approximated air-exposed control values. Thus, higher concentrations of CO may be required to offset the pro-oxidant effects of volatile anesthetics. Although further work is necessary, these CO-mediated cellular protective effects could have implications for the development of low-flow anesthesia and CO re-breathing in infants and children in order to prevent anesthesia-induced oxidative stress.
Supplementary Material
The absorbance spectra between 500 and 650 nm were determined via spectrophotometer (31). Mitochondrial heme aa3 content (the heme moiety of CcOX subunit I) was calculated from the difference in spectra (dithionate/ascorbate reduced minus ferricyanide oxidized) of mitochondria solubilized in 10% lauryl maltoside using an absorption coefficient of 24 mM−1cm−1 at 605 to 630 nm as previously described (28, 32). (A) A representative absorbance spectrum from CcOX extracted from forebrain mitochondria of an air-exposed mouse is depicted. A single peak at ~603 nm, representing the α absorption band of heme aa3 is visible. (B) A representative spectrum obtained from intact mitochondria is depicted. The peak at ~520 nm corresponds to the β band of hemes b and c and the peak at ~560 corresponds to the heme b of the bc1 complex (31). The peak at ~603 nm, representing the α absorption band of heme aa3 is also visible. The lack of absorption peaks at ~520 and ~560 in (A) indicates CcOX purity. (C) Isolated CcOX was further assessed with immunoblot analysis for CcOX subunit I. A representative blot from mice exposed to air (0 ppm CO), 5 ppm CO, or 100 ppm is shown. A 57 kD CcOX I band was readily visible.
Highlights.
CO and isoflurane independently caused oxidative stress and activated CcOX
Combined CO and isoflurane exposure limited ROS and prevented CcOX activation
CO exposure with isoflurane synergistically phosphorylated forebrain CcOX
CO may limit ROS via tyrosine phosphorylation of CcOX during isoflurane exposure
Acknowledgments
Supported by NIH/NIGMS R01GM103842-01 (RJL)
Abbreviations
- CO
Carbon monoxide
- CcOX
cytochrome oxidase
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 3.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–373. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 4.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced Neuroapoptosis in the Neonatal Rhesus Macaque Brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the Neuroapoptotic Properties of Equipotent Anesthetic Concentrations of Desflurane, Isoflurane, or Sevoflurane in Neonatal Mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 6.Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V. Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology. 2004;101:273–275. doi: 10.1097/00000542-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010;1199:43–51. doi: 10.1111/j.1749-6632.2009.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ. Ketamine Enhances Human Neural Stem Cell Proliferation and Induces Neuronal Apoptosis via Reactive Oxygen Species-Mediated Mitochondrial Pathway. Anesth Analg. 2013;116:869–880. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boscolo A, Milanovic D, Starr JA, Sanchez V, Oklopcic A, Moy L, Ori CC, Erisir A, Jevtovic-Todorovic V. Early Exposure to General Anesthesia Disturbs Mitochondrial Fission and Fusion in the Developing Rat Brain. Anesthesiology. 2013;118:1086–1097. doi: 10.1097/ALN.0b013e318289bc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee I, Bender E, Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol Cell Biochem. 2002;234–235:63–70. [PubMed] [Google Scholar]
- 14.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–3631. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 15.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116:869–880. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy RJ, Nasr VG, Rivera O, Roberts R, Slack M, Kanter JP, Ratnayaka K, Kaplan RF, McGowan FX., Jr Detection of carbon monoxide during routine anesthetics in infants and children. Anesth Analg. 2010;110:747–753. doi: 10.1213/ANE.0b013e3181cc4b9f. [DOI] [PubMed] [Google Scholar]
- 17.Nasr V, Emmanuel J, Deutsch N, Slack M, Kanter J, Ratnayaka K, Levy R. Carbon monoxide re-breathing during low-flow anaesthesia in infants and children. Br J Anaesth. 2010;105:836–841. doi: 10.1093/bja/aeq271. [DOI] [PubMed] [Google Scholar]
- 18.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitricoxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 19.Choi YK, Por ED, Kwon YG, Kim YM. Regulation of ROS production and vascular function by carbon monoxide. Oxid Med Cell Longev. 2012;2012:794237. doi: 10.1155/2012/794237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida AS, Queiroga CS, Sousa MF, Alves PM, Vieira HL. Carbon monoxide modulates apoptosis by reinforcing oxidative metabolism in astrocytes: role of Bcl-2. J Biol Chem. 2012;287:10761–10770. doi: 10.1074/jbc.M111.306738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Queiroga CS, Almeida AS, Vieira HL. Carbon monoxide targeting mitochondria. Biochem Res Int. 2012;2012:749845. doi: 10.1155/2012/749845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Levy RJ. Sub-clinical carbon monoxide limits apoptosis in the developing brain after isoflurane exposure. Anesth Analg. 2014;118:1284–1292. doi: 10.1213/ANE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43:20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 24.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–4231. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson SA, Young C, Olney JW. Isoflurane-induced Neuroapoptosis in the Developing Brain of Nonhypoglycemic Mice. J Neurosurg Anesthesiol. 2008;20:21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 27.Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. J Toxicol Pathol. 2013;26:293–299. doi: 10.1293/tox.26.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome C oxidase in sepsis. Shock. 2004;21:110–114. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- 29.Smith L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- 30.Cytochrome oxidase: beef heart. In: Yonetani T, editor; Estabrook RW, Pullman ME, editors. Oxidation and Phosphorylation. Methods in Enzymology. X. Orlando: Academic Press; 1967. pp. 332–335. [Google Scholar]
- 31.Lee I, Salomon AR, Yu K, Samavati L, Pecina P, Pecinova A, Hüttemann M. Isolation of regulatory-competent, phosphorylated cytochrome C oxidase. Methods Enzymol. 2009;457:193–210. doi: 10.1016/S0076-6879(09)05011-3. [DOI] [PubMed] [Google Scholar]
- 32.Vijayasarathy C, Biunno I, Lenka N, Yang M, Basu A, Hall IP, Avadhani NG. Variations in the subunit content and catalytic activity of the cytochrome c oxidase complex from different tissues and different cardiac compartments. Biochimica et Biophysica Acta. 1998;1371:71–82. doi: 10.1016/s0005-2736(97)00278-2. [DOI] [PubMed] [Google Scholar]
- 33.Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka H, Watanabe Y, Isshiki A, Quock RM. Increased production of nitric oxide metabolites in the hippocampus under isoflurane anaesthesia in rats. Eur J Anaesthesiol. 1999;16:216–224. doi: 10.1046/j.1365-2346.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 35.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, Barrera G. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez V, Feinstein SD, Lunardi N, Joksovic PM, Boscolo A, Todorovic SM, Jevtovic-Todorovic V. General Anesthesia Causes Long-term Impairment of Mitochondrial Morphogenesis and Synaptic Transmission in Developing Rat Brain. Anesthesiology. 2011;115:992–1002. doi: 10.1097/ALN.0b013e3182303a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408–420. doi: 10.1038/cdd.2009.132. [DOI] [PubMed] [Google Scholar]
- 38.Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012;1817:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cadenas E, Seis H. The lag phase. Free Radic Res. 1998;28:601–609. doi: 10.3109/10715769809065816. [DOI] [PubMed] [Google Scholar]
- 42.Hermes-Lima M. Oxygen in biology and biochemistry: Role of free radicals. In: Storey Kenneth B., editor. Functional Metabolism. Regulation and Adaptation. New Jersey: Wiley and Sons, Inc; 2004. pp. 319–366. [Google Scholar]
- 43.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taskiran D, Nesil T, Alkan K. Mitochondrial oxidative stress in female and male rat brain after ex vivo carbon monoxide treatment. Hum Exp Toxicol. 2007;26:645–651. doi: 10.1177/0960327107076882. [DOI] [PubMed] [Google Scholar]
- 45.Hüttemann M, Lee I, Grossman LI, Doan JW, Sanderson TH. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: respiration, apoptosis, and human disease. Adv Exp Med Biol. 2012;748:237–264. doi: 10.1007/978-1-4614-3573-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitdikova GF, Islamov RR, Mukhamedyarov MA, Permyakova VV, Zefirov AL, Palotás A. Modulation of neurotransmitter release by carbon monoxide at the frog neuro-muscular junction. Curr Drug Metab. 2007;8:177–184. doi: 10.2174/138920007779815940. [DOI] [PubMed] [Google Scholar]
- 47.Mosén H, Salehi A, Henningsson R, Lundquist I. Nitric oxide inhibits, and carbon monoxide activates, islet acid alpha-glucoside hydrolase activities in parallel with glucose-stimulated insulin secretion. J Endocrinol. 2006;190:681–693. doi: 10.1677/joe.1.06890. [DOI] [PubMed] [Google Scholar]
- 48.Ohlson KB, Shabalina IG, Lennström K, Backlund EC, Mohell N, Bronnikov GE, Lindahl SG, Cannon B, Nedergaard J. Inhibitory effects of halothane on the thermogenic pathway in brown adipocytes: localization to adenylyl cyclase and mitochondrial fatty acid oxidation. Biochem Pharmacol. 2004;68:463–477. doi: 10.1016/j.bcp.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Peña JR, Wolska BM. Differential effects of isoflurane and ketamine/inactin anesthesia on cAMP and cardiac function in FVB/N mice during basal state and beta-adrenergic stimulation. Basic Res Cardiol. 2005;100:147–153. doi: 10.1007/s00395-004-0503-6. [DOI] [PubMed] [Google Scholar]
- 50.Woehlck HJ. Carbon monoxide rebreathing during low flow anaesthesia. Anesth Analg. 2001;93:516–517. doi: 10.1097/00000539-200108000-00058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The absorbance spectra between 500 and 650 nm were determined via spectrophotometer (31). Mitochondrial heme aa3 content (the heme moiety of CcOX subunit I) was calculated from the difference in spectra (dithionate/ascorbate reduced minus ferricyanide oxidized) of mitochondria solubilized in 10% lauryl maltoside using an absorption coefficient of 24 mM−1cm−1 at 605 to 630 nm as previously described (28, 32). (A) A representative absorbance spectrum from CcOX extracted from forebrain mitochondria of an air-exposed mouse is depicted. A single peak at ~603 nm, representing the α absorption band of heme aa3 is visible. (B) A representative spectrum obtained from intact mitochondria is depicted. The peak at ~520 nm corresponds to the β band of hemes b and c and the peak at ~560 corresponds to the heme b of the bc1 complex (31). The peak at ~603 nm, representing the α absorption band of heme aa3 is also visible. The lack of absorption peaks at ~520 and ~560 in (A) indicates CcOX purity. (C) Isolated CcOX was further assessed with immunoblot analysis for CcOX subunit I. A representative blot from mice exposed to air (0 ppm CO), 5 ppm CO, or 100 ppm is shown. A 57 kD CcOX I band was readily visible.