Abstract
Activation of human pregnane X receptor (hPXR)-regulated expression of cytochrome P450 3A4 (CYP3A4) and multidrug resistance protein 1 (MDR1) plays an important role in mediating adverse drug interactions. Given the common use of natural products as part of adjunct human health behavior, there is a growing concern about natural products for their potential to induce undesired drug interactions through the activation of hPXR-regulated CYP3A4 and MDR1. Here, we studied whether 3,3′-diindolylmethane (DIM), a natural health supplement, could induce hPXR-mediated regulation of CYP3A4 and MDR1 in human hepatocytes and intestinal cells. DIM, at its physiologically relevant concentrations, not only induced hPXR transactivation of CYP3A4 promoter activity but also induced gene expression of CYP3A4 and MDR1. DIM decreased intracellular accumulation of MDR1 substrate rhodamine 123, suggesting that DIM induces the functional expression of MDR1. Pharmacologic inhibition or genetic knockdown of hPXR resulted in attenuation of DIM induced CYP3A4 and MDR1 gene expression, suggesting that DIM induces CYP3A4 and MDR1 in an hPXR-dependent manner. Together, these results support our conclusion that DIM induces hPXR-regulated CYP3A4 and MDR1 gene expression. The inductive effects of DIM on CYP3A4 and MDR1 expression caution the use of DIM in conjunction with other medications metabolized and transported via CYP3A4 and MDR1, respectively.
Keywords: Diindolylmethane, PXR, CYP3A4, MDR1
1. Introduction
Pregnane X receptor (PXR) is a member of the nuclear receptor superfamily of ligand-activated transcription factors. Human PXR (hPXR) plays a major role in activating the expression of drug-metabolizing enzymes and drug-transporting proteins, primarily cytochrome P450 3A4 (CYP3A4) and multidrug resistance protein 1 (MDR1; a.k.a. P-glycoprotein or P-gp) (Chen et al., 2012b). hPXR and its target genes, CYP3A4 and MDR1, are predominantly expressed in the major detoxifying organs such as liver and intestinal tract. CYP3A4 and MDR1 together can contribute to the metabolism and disposition of more than 50% of clinically used drugs (Guengerich, 1999; Veith et al., 2009). Therefore, altered levels of CYP3A4 and MDR1 can significantly affect the therapeutic response of a variety of co-administered drugs, and can cause serious drug interactions.
PXR is activated by various chemically and structurally distinct endobiotics and xenobiotics, including therapeutic drugs and dietary components (Kliewer et al., 1998; Lehmann et al., 1998; Staudinger et al., 2006; Wang et al., 2013b). Activation of hPXR-regulated CYP3A4 and MDR1 expression by xenobiotics can lead to adverse drug-drug or supplement-drug interactions during drug therapy. While the effect of therapeutic drugs on PXR activity has been well known, less is known about natural supplements. Several natural products have been shown to affect gene expression of drug-metabolizing enzymes and drug-transporting proteins (Huang and Lesko, 2004; Kittayaruksakul et al., 2013; NCCAM, 2012; Staudinger et al., 2006; Tsai et al., 2012; Wang et al., 2013b). One common example is St. John’s Wort extract, which is well known to induce expression of CYP3A4 and MDR1 by activating hPXR (Dresser et al., 2003; Moore et al., 2000).
3,3′-Diindolylmethane (DIM) is a natural health supplement and is the major active metabolite of indole-3-carbinol (I3C) (Anderton et al., 2004b), which is also a natural health supplement as well as a naturally-occurring compound in cruciferous vegetables (Bonnesen et al., 2001). DIM is used to treat recurrent respiratory papillomatosis (Auborn, 2002; Wiatrak, 2003). The emerging evidence from several studies indicates that DIM could be used for both the treatment and prevention of a variety of human cancers, including prostate and breast cancers (Azmi et al., 2008; Biersack and Schobert, 2012; Chen et al., 2012a). Additionally, DIM has the potential to promote the antitumor efficacy of chemotherapeutic drugs in combinatorial chemotherapies (Ahmad et al., 2013; Banerjee et al., 2009; Ichite et al., 2009). DIM was also thought to have potential benefits in treating/preventing cardiovascular diseases and metabolic disorders, including obesity and diabetes (Joshipura et al., 1999; Schulze et al., 2005).
DIM can modulate the activity of xenobiotic receptors and xenobiotic receptor-mediated target gene expression (Bhuiyan et al., 2006; Le et al., 2003; Riby et al., 2000). For instance, DIM activates aryl hydrocarbon receptor (AHR)-regulated CYP1A gene expression in human and rat liver and intestine (Chen et al., 1998; Jellinck et al., 1993). Some previous studies suggested that DIM induces other CYPs such as CY2B and CYP3A in rat liver and intestine (Bonnesen et al., 2001; Jellinck et al., 1993; Leibelt et al., 2003; Renwick et al., 1999). CYP3A and CY2B are typical target genes of PXR, but it is unknown whether DIM induces CYP2B and CYP3A by activating PXR. In this study, we show that DIM, at its physiologically relevant concentrations, could activate hPXR and subsequently induce the expression of CYP3A4 and MDR1 in human hepatocytes and intestinal cells.
2. Materials and methods
2.1. Chemicals and plasmids
Dimethyl sulfoxide (DMSO), Rifampicin (RIF), 3,3′-Diindolylmethane (DIM), Ketoconazole (KET), Valspodar (PSC-833) and Rhodamine 123 (R123) were purchased from Sigma–Aldrich. The pcDNA3, pcDNA3-hPXR, FLAG-pcDNA3, FLAG-pcDNA3-hPXR, pEF-rPXR WT, pEF-rPXR F305L, pGL3-CYP3A4-luc and pGL3-CMV-Renilla luciferase plasmids were previously described (Kittayaruksakul et al., 2013; Lin et al., 2008; Mu et al., 2005; Pondugula et al., 2009a, 2010). pcDNA3-mPXR plasmid was provided by Dr. Steven Kliewer (University of Texas Southwestern Medical Center).
2.2. Cells and cultures
HepG2 human liver carcinoma cells and LS174 T and LS180 human intestinal epithelial cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco’s Modified Eagle’s Medium DMEM (Lonza) supplemented with 10% fetal bovine serum (HyClone),100 U/ml penicillin and 100 μg/ml streptomycin (Cellgro), 2 mM L-glutamine (Cellgro), and 1 mM sodium pyruvate (Cellgro). The cells were cultured in an incubator with a humidified atmosphere maintained at 5% CO2 and 95% air at 37°C. The assay media included phenol red-free DMEM (Lonza) supplemented with 5% charcoal/dextran-treated FBS (HyClone) and the other additives. HepG2-FLAG-hPXR cells stably expressing FLAG-hPXR were maintained under the selection of G418 (Cellgro). Cryopreserved human primary hepatocytes (HU1488) and hepatocyte media were purchased from Invitrogen and cultured by following the manufacturer’s protocol. Briefly, the hepatocytes were maintained for 6 h in Williams’ medium E without phenol red, supplemented with 5% fetal bovine serum, cell maintenance cocktail and 500 nM dexamethasone, in collagen coated 24 well culture plates in an atmosphere of 5% CO2 at 37°C. The cells were then incubated in induction media (Williams’ medium E without phenol red, supplemented with cell maintenance cocktail and 100 nM dexamethasone) for 12 h. The cells were then treated with the vehicle or drugs in induction medium for 48 h, unless otherwise stated, before harvesting for gene expression studies.
2.3. PXR transactivation assays
The cells were transfected with pcDNA3, pcDNA3-hPXR, pcDNA3-mPXR, pEF-rPXR WT, pEF-rPXR F305L, CYP3A4-luc and CMV-renilla luciferase plasmids using FuGENE 6 (Promega). Twenty-four hours after transfection in growth media, approximately 10,000 live cells were plated in each well of a 96-well culture plate (PerkinElmer), and treated with DMSO, RIF, DIM, KET, RIF + KET or DIM + KET for an additional 24 h in the assay media. Forty-eight hours after transfection, a luciferase assay was performed to measure luminescence using the Neolite Reporter Gene Assay System (PerkinElmer) or the Dual-Glo Luciferase Assay System (Promega) and FLUOstar Optima microplate reader (BMG Labtech). The firefly luciferase activity was normalized to either the renilla luciferase activity or the number of liver cells. Cell viability was determined using the CellTiter-Glo luminescent cell viability assays (Promega). The normalized promoter activity was shown as relative luminescence units or fold activation.
2.4. RT-PCR analysis
Total RNA was extracted from the primary hepatocytes, LS174 T and LS180 cells by using the RNeasy Mini Kit (Qiagen; Valencia, CA). The quality and quantity of the total RNA were determined using NanoVue Plus Spectrophotometer (GE Healthcare). Reverse transcription was performed with the QuantiTect Reverse Transcription Kit (Qiagen) and quantitative PCR was performed by using the QuantiTect SYBR Green Kit (Qiagen) and iCycler iQ Real-Time PCR Detection System (Bio-Rad; Hercules, CA) according to the manufacturer’s protocol. Transcripts of 18 S small subunit ribosomal RNA (18 S rRNA) housekeeping gene, hPXR, CYP3A4 and MDR1 were amplified using gene-specific primers (Table 1). The comparative Ct method was used for relative quantification for gene expression with the following formula: ΔCt = Ct (test gene) − Ct (18 S rRNA); ΔΔCt (test gene) = ΔCt (test gene in treatment group) −ΔCt (test gene in vehicle control group); the fold changes of mRNA = 2−ΔΔCt, which indicated the relative mRNA level of the corresponding transcript to the control samples.
Table 1.
Forward (F) and reverse (R) Primers used for quantitative RT-PCR of 18 S rRNA, hPXR, CYP3A4 and MDR1.
| Gene/Primer sequence | Amplified segment (bp) | Gene bank accession no | Reference |
|---|---|---|---|
| 18S rRNA | 315 | BK000964 | (Pondugula et al., 2010) |
| F: 50-GAGGTTCGAAGACGATCAGA-3′ | |||
| R: 5′-TCGCTCCACCAACTAAGAAC-3′ | |||
| hPXR | 307 | AF061056 | (Pondugula et al., 2010) |
| F: 5′-TGTTCAAAGGCATCATCAGC-3′ | |||
| R: 5′-TCTGGGGAGAAGAGGGAGAT-3′ | |||
| CYP3A4 | 265 | NM017460 | (Sporstol et al., 2005) |
| F: 5′-TTGGAAGTGGACCCAGAAAC-3′ | |||
| R: 5′-CTGGTGTTCTCAGGCACAGA-3′ | |||
| MDR1 | 322 | NM000927 | (Stephens et al., 2001) |
| F: 5′-GATCTTGAAGGGGACCGCAA-3′ | |||
| R: 5′-TCATGAAGAACCCTGTAT-3′ |
2.5. Western blotting analysis
Total cell lysates were collected in RIPA buffer containing a cocktail of protease inhibitors, and protein concentration was determined by Bio-Rad protein assay (Bio-Rad). Equal amounts of protein were separated on a SDS-polyacrylamide gel electrophoresis and were transferred on to nitrocellulose membranes. The membranes were then blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20, incubated with Anti-FLAG (Sigma) or anti-actin (Santa Cruz) antibodies in blocking buffer, washed with Tris-buffered saline, and finally incubated with HRP-conjugated secondary antibodies (Santa Cruz) in blocking buffer. The proteins were visualized using HyGLO Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific).
2.6. Small interfering RNA transfection
HepG2-FLAG-hPXR and LS174 T cells were transfected with 200 nM of ON-TARGETplus SMARTpool siRNA targeting PXR (Dharmacon) or Nontargeting Pool (Dharmacon) using Lipofectamine RNAiMAX (Invitrogen). Efficiency of hPXR knockdown was confirmed in HepG2-FLAG-hPXR and LS174 T cells using western blots and RT-PCR, respectively. hPXR target gene expression was analyzed in the siRNA transfected LS174 T cells after treating with DMSO, RIF or DIM for 24 h in the assay media. hPXR transactivation function was assessed in the siRNA transfected HepG2-FLAG-hPXR cells by transiently transfecting with CYP3A4-LUC and CMV-Renilla luciferase plasmids.
2.7. Intracellular rhodamine 123 accumulation assays
The efflux activity of MDR1 was determined by measuring the intracellular accumulation of the fluorescent MDR1 probe rhodamine 123 (Chan et al., 2013; Harmsen et al., 2010; Ishikawa et al., 2010; Wang et al., 2013a). In brief, LS174 T and LS180 cells were treated with vehicle control DMSO, RIF or DIM for 48 h in the assay media. The cells were then washed with PBS and incubated at 37°C for 15 min with or without MDR1-specific inhibitor Valspodar (PSC-833) (10 μM) (Keller et al., 1992; Zandvliet et al., 2013) in phenol red-free DMEM. Rhodamine 123 (5 μM) was added later to the cells in the presence or absence of PSC-833 and incubated for another 45 min. After washing with ice-cold PBS, the cells were solubilized in 0.1% triton-PBS. To determine the intracellular concentration of rhodamine 123, the fluorescence was measured using Infinite microplate reader (TECAN) at an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The ratio of intracellular rhodamine 123 concentrations in the absence and presence of PSC will indicate the efflux activity of MDR1 (Collett et al., 2004; Harmsen et al., 2010, 2013; Kota et al., 2010).
2.8. Statistical analysis
Data were analyzed with Student’s t test by using GraphPad Prism 6 software. Differences were considered significant (*) for P < 0.05 and not significant for p > 0.05.
3. Results
3.1. DIM induces hPXR transactivation of CYP3A4 promoter activity
It has been shown that hPXR target gene expression in liver and intestine is modulated by a broad variety of xenobiotics, including therapeutic drugs and dietary components (Kliewer et al., 1998; Lehmann et al., 1998; Staudinger et al., 2006; Wang et al., 2013b). To identify natural therapeutic supplements that modulate hPXR function, we sought a small-scale cell-based screening approach using hPXR transactivation assays. We identified DIM as one of the natural supplements that activates hPXR, leading to our hypothesis that DIM activates hPXR-regulated gene expression.
We examined the effect of DIM on hPXR-regulated CYP3A4 promoter activity in human HepG2 liver and LS174 T intestinal cells (Fig. 1). The cells were transiently transfected with CYP3A4-luc and pcDNA, hPXR or mouse PXR (mPXR), and untreated or treated with DMSO, RIF or DIM. DIM was used at its nearly physiologically relevant concentrations reported in the serum and/or tissues of rodents/humans (Fig. 1A–C) (Anderton et al., 2004a; Anderton et al., 2004b; Fan et al., 2009; Moiseeva et al., 2007; Reed et al., 2006, 2008; Stresser et al., 1995). DIM, similar to RIF, significantly induced CYP3A4 promoter activity in an hPXR-dependent manner in both HepG2 and LS174 T cells (Fig. 1A and B). Likewise, DIM, similar to mPXR agonist pregnenolone 16α-carbonitrile (PCN), induced mPXR transactivation of CYP3A4 promoter activity (Fig. 1C), suggesting that DIM also activates mPXR. To confirm the effect of DIM on CYP3A4 promoter, a concentration-response experiment was conducted, and the half-maximal effective concentration (EC50) was determined (Fig. 1D and E). DIM induced hPXR-mediated CYP3A4 promoter activity at EC50 values ranging from 8–11 μM (Fig. 1F) in the cell lines, and the maximal induction occurred at varied concentrations depending on the cell line. These results suggest that the effect of DIM was mediated through hPXR activation. The ranges of EC50 values of DIM were significantly higher than RIF under the same conditions (Fig. 1F), suggesting that DIM is less potent than RIF to activate hPXR. However, EC50 values of DIM are within the range of physiologically relevant concentrations (Anderton et al., 2004a; Anderton et al., 2004b; Fan et al., 2009; Moiseeva et al., 2007; Reed et al., 2006, 2008; Stresser et al., 1995). These results confirm that DIM activates hPXR function, and led us to hypothesize that DIM modulates hPXR-regulated gene expression in hepatocytes and intestinal cells.
Fig. 1.
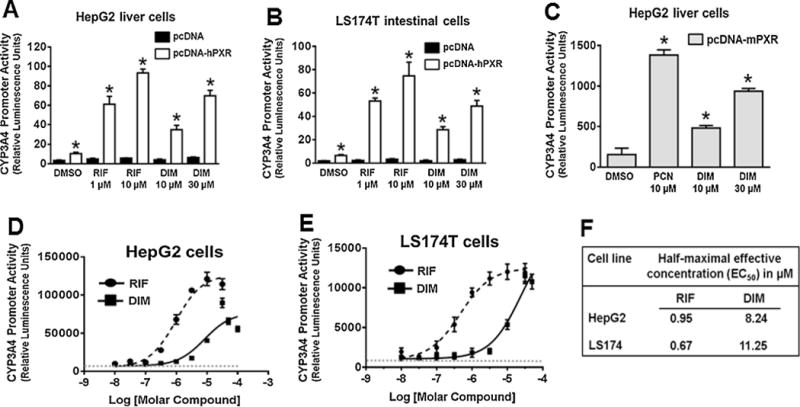
DIM induces PXR transactivation of CYP3A4 promoter activity: (A, B & C) CYP3A4 promoter activity was determined in HepG2 and LS174 T cells. The cells were transiently cotransfected with pGL3-CYP3A4-luc and either pcDNA3 (empty vector) or pcDNA3-hPXR or pcDNA3-mPXR plasmids. After 24 h of transfection, the cells were treated with the vehicle control DMSO, RIF, PCN or DIM as indicated for another 24 h. CYP3A4 promoter activity was determined by measuring the firefly luciferase activity 24 h after the treatments. The firefly luciferase activity was normalized to number of live cells measured using the CellTiter-Glo Luminescent Cell Viability Assay and presented as Relative Luminescence Units. DIM did not exert a noticeable cytotoxicity at the tested concentrations (data not shown). The values represent the means of eight independent experiments, and the bars denote the standard deviation. *, p < 0.05; compared with the vector or DMSO by unpaired Students t test. (D & E) DIM induces hPXR-mediated CYP3A4 promoter activity in a concentration-dependent manner. The cells were transfected as described above and treated with increasing concentrations of RIF or DIM as indicated for 24 h. DMSO was used as a negative control and RIF and PCN as a positive controls. The normalized CYP3A4 promoter activity is expressed as Relative Luminescence Units, and drug concentration is expressed in a log scale. Data at each point represent mean ± SEM from quadruplicate measurements. (F) Potency of DIM on hPXR-mediated CYP3A4 promoter activity in human liver and intestinal cells. Half-maximal effective concentration (EC50) values of DIM and RIF were determined by using curve-fitting software GraphPad Prism 6.0.
We next determined whether DIM-activated PXR function is affected by specific amino acids in the ligand binding pocket of PXR. We used the rPXR wild-type (WT) and rPXR F305L mutant (MT) to determine whether F305 affects DIM-activated rPXR transactivating function. It has been reported that F305 in the ligand binding pocket of rPXR is important for its activation by agonists such as PCN, and that mutation of F to L significantly alters rPXR activation by PCN in HepG2 cells (Tirona et al., 2004). As expected, fold activation of rPXR by PCN was significantly higher in F305L transfected HepG2 cells (Tirona et al., 2004) (Fig. 2). Likewise, DIM activation of rPXR was also higher in F305L transfected cells (Fig. 2), suggesting that DIM induction of PXR transcriptional activity is affected by specific amino acids in the ligand binding pocket. These results also point to the likely scenario of DIM being a PXR agonist, although ligand binding studies are required to confirm whether DIM binds to PXR to activate PXR.
Fig. 2.
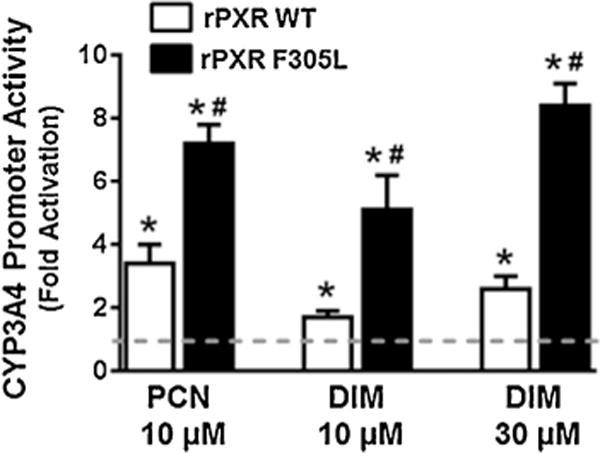
DIM activation of rPXR WT and rPXR F305L: HepG2 cells were transiently cotransfected with pGL3-CYP3A4-luc, CMV-Renilla and pEF-rPXR or pEF-rPXR F305L plasmids. After 24 h transfection, the cells were treated with the vehicle control DMSO, PCN or DIM as indicated for another 24 h. The firefly and renilla luciferase activities were measured 24 h after the treatments using Dual-Glo luciferase assay system. The normalized CYP3A4 promoter activity is presented as fold activation over the DMSO control and represent the average from triplicate assays. P < 0.05, vs the vehicle (*) or vs the rPXR WT (#).
3.2. DIM induces the expression of endogenous CYP3A4 and MDR1 in human primary hepatocytes and intestinal cells
We examined the effect of DIM on endogenous CYP3A4 and MDR1 gene expression in human primary hepatocytes and LS174 T cells, as liver and intestine are the major organs with highly hPXR inducible expression of CYP3A4 and MDR1 in response to uptake of xenobiotics. Both primary hepatocytes and LS174 T cells express endogenous hPXR with inducible activity, and have been used as liver and intestine cell models to study the function of hPXR and hPXR-regulated gene expression of CYP3A4 and MDR1 (Geick et al., 2001; Kota et al., 2010; Wang et al., 2013b). The cells were untreated or treated for 48 h with DMSO, DIM or RIF and relative mRNA levels were quantified (Fig. 3). DIM induced CYP3A4 mRNA in a concentration-dependent manner in both human primary hepatocytes and LS174 T cells (Fig. 3A and C), although the magnitude of DIM induction was significantly less when compared to RIF induction. Under the same experimental conditions, MDR1 mRNA levels were slightly increased by DIM, similar to RIF, in human primary hepatocytes (Fig. 3B). However, MDR1 mRNA induction by both DIM and RIF was significantly higher in LS174 T cells compared to the hepatocytes (Fig. 3D). These results show that DIM induces CYP3A4 and MDR1 gene expression in human primary hepatocytes and LS174 T cells.
Fig. 3.
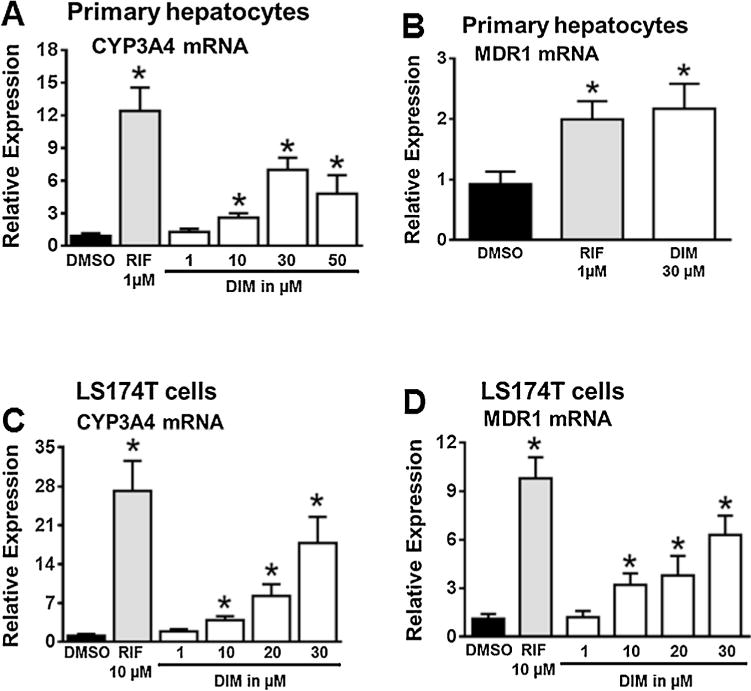
DIM induces CYP3A4 and MDR1 gene expression in primary human hepatocytes and LS174 T cells: CYP3A4 and MDR1 mRNA expression was analyzed by quantitative RT-PCR in primary human hepatocytes (HU1488, Invitrogen) (A & B) and LS174 T cells (C & D) after treatment with vehicle DMSO, RIF, or increasing concentrations of DIM as indicated for 48 h. Results are presented as fold increase over the untreated control. Data represent mean ± SEM from three independent experiments: *, p < 0.05; compared with the untreated control by unpaired Students t test.
3.3. DIM decreases intracellular accumulation of MDR1 substrate rhodamine 123
To determine the functional relevance of DIM-induced MDR1 expression, we measured the intracellular accumulation of rhodamine 123, a typical MDR1 substrate, in LS174 T and LS180 cells that were pretreated for 48 h with DMSO, RIF or DIM. The measurements were performed in the presence or absence of the MDR1-specific inhibitor Valspodar (PSC-833) (Keller et al., 1992; Zandvliet et al., 2013). The ratio of intracellular rhodamine 123 concentrations in the absence and presence of 10 μM PSC-833 is indicative of the efflux activity of MDR1 (Collett et al., 2004; Harmsen et al., 2010, 2013; Kota et al., 2010). As shown in Fig. 4, DIM, similar to RIF, significantly decreased rhodamine 123 accumulation, suggesting that DIM increased the functional expression of MDR1 protein in these cells. In LS180 cells, DIM (20 μM) significantly induced hPXR transactivation of CYP3A4 promoter activity as well as endogenous CYP3A4 and MDR1 gene expression (data not shown).
Fig. 4.
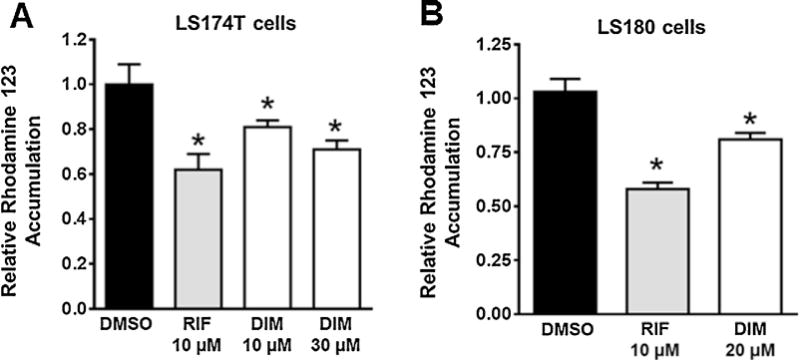
DIM affects MDR1 substrate accumulation in LS174 T and LS180 cells: LS174 T (A) and LS180 (B) cells were treated with DMSO, RIF or DIM as indicated for 48 h. Rhodamine 123 accumulation was then determined in the absence or presence of the MDR1 specific inhibitor Valspodar (PSC-833). The ratio of intracellular rhodamine 123 fluorescence in the absence or presence of PSC-833 indicates the functionality of MDR1. The data are normalized to the DMSO controls and are presented as mean ± SD of three independent experiments (*P < 0.05 determined by Students unpaired t test).
3.4. Genetic knockdown of hPXR diminishes DIM induced hPXR target gene expression
Overexpression of hPXR significantly increased DIM induced CYP3A4 promoter activity, suggesting that DIM induces CYP3A4 promoter in an hPXR dependent manner (Fig. 1). To confirm that DIM induces hPXR-mediated CYP3A4 promoter activity, the effect of DIM on CYP3A4 promoter activity was tested after genetically knocking down the expression of hPXR in HepG2-FLAG-hPXR cells stably expressing FLAG-hPXR. hPXR siRNA, but not control non-silencing siRNA, significantly knocked down the protein expression of FLAG-hPXR (Fig. 5A). The siRNA transfected HepG2-FLAG-hPXR cells were transiently transfected with CYP3A4-luc and CMV-Renilla luciferase, and treated with DMSO, DIM or RIF. DIM and RIF significantly induced CYP3A4 promoter activity in the cells treated with control siRNA (Fig. 5B). In contrast, knockdown of hPXR with hPXR siRNA resulted in a significant attenuation of DIM and RIF induced CYP3A4 promoter activity (Fig. 5B). We also examined gene expression of CYP3A4 and MDR1 in LS174 T cells after genetically knocking down the expression of endogenous hPXR. Treatment with hPXR siRNA, but not control non-silencing siRNA, resulted in significant knockdown in mRNA levels of hPXR (Fig. 5C) as well as DIM and RIF induction of CYP3A4 and MDR1 gene expression (Fig. 5D and E). Together, these results demonstrate that DIM induces CYP3A4 and MDR1 in an hPXR-dependent manner.
Fig. 5.
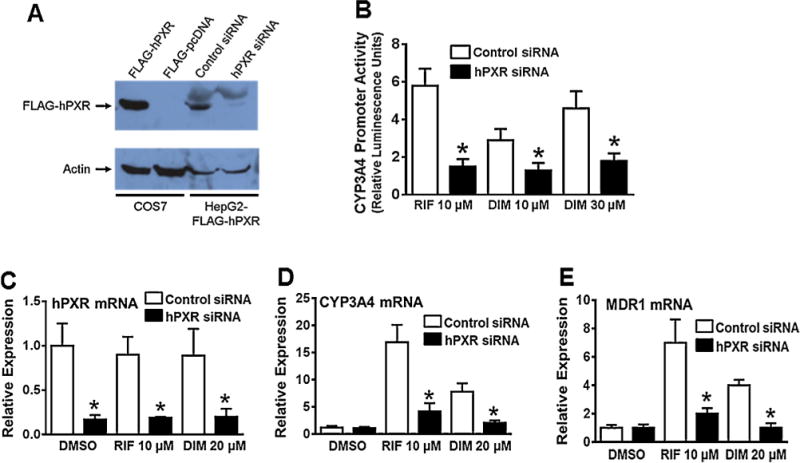
Knockdown of hPXR expression attenuates DIM-induced hPXR target gene expression in HepG2 and LS174 T cells. (A) HepG2 cells stably expressing FLAG-hPXR were transfected with control non-silencing siRNA or hPXR siRNA. Whole-cell lysates were collected 72 h after siRNA transfections and subjected to western blot analysis using anti-FLAG and anti-actin antibodies (as a loading control). COS7 cells transiently transfected with FLAG-hPXR or FLAG-pcDNA served as positive or negative controls, respectively, for hPXR expression. Data shown are from a representative experiment. (B) 48 h after siRNA transfections, the cells were transiently cotransfected with CYP3A4-luc and CMV-Renilla (transfection control) plasmids, and 24 h after transfection, the cells were treated for another 24 h with DMSO, RIF, or DIM, as indicated. The firefly and renilla luciferase activities were measured 24 h after the treatments using Dual-Glo luciferase assay system. The normalized CYP3A4 promoter activity is expressed as relative luciferase units and presented as fold change over the DMSO control. Data represent mean ± SD from three independent experiments. Statistical significance (*, p < 0.05) was determined using Students t test by comparing hPXR siRNA samples with control siRNA in each drug treatment group. (C, D & E) LS174 Tcells were transiently transfected with hPXR siRNA or control non-silencing siRNA. 48 h after transfection, the cells were treated for another 24 h with DMSO, RIF or DIM, as indicated, and mRNA expression of hPXR (C), CYP3A4 (D) and MDR1 (E) was determined. The induction of each gene mRNA level by the treatment was normalized as fold increase over the untreated control. Data represent mean ± SEM from three independent experiments. Statistical significance (*, p < 0.05) was determined using Students t test by comparing hPXR siRNA samples with control siRNA in vehicle or each drug treatment group.
3.5. Pharmacological inhibition of hPXR attenuates DIM induced hPXR target gene expression
To further confirm DIM activation of hPXR activity, we tested the effect of DIM on hPXR transactivation as well as hPXR target gene expression when hPXR activity was pharmacologically inhibited. In HepG2 and LS174 T cells transiently cotransfected with CYP3A4-luc and hPXR, when hPXR activity was inhibited with a known hPXR inhibitor ketoconazole (KET) (Huang et al., 2007; Li et al., 2013; Mani et al., 2013; Venkatesh et al., 2011; Wang et al., 2007), the induction of CYP3A4 promoter activity by DIM and RIF was significantly diminished (Fig. 6A and B). Similarly, in HepG2-FLAG-hPXR cells cotransfected with CYP3A4-luc and CMV renilla luciferase, KET significantly blunted DIM and RIF induction of CYP3A4 promoter (Fig. 6C). Notably, in both human primary hepatocytes and LS174 T cells, the induction of CYP3A4 and MDR1 mRNA expression by DIM and RIF was also significantly reduced by hPXR inhibition (Fig. 6D–G). These results suggest that hPXR was required for DIM to induce the promoter activity of CYP3A4 and gene expression of CYP3A4 and MDR1.
Fig. 6.
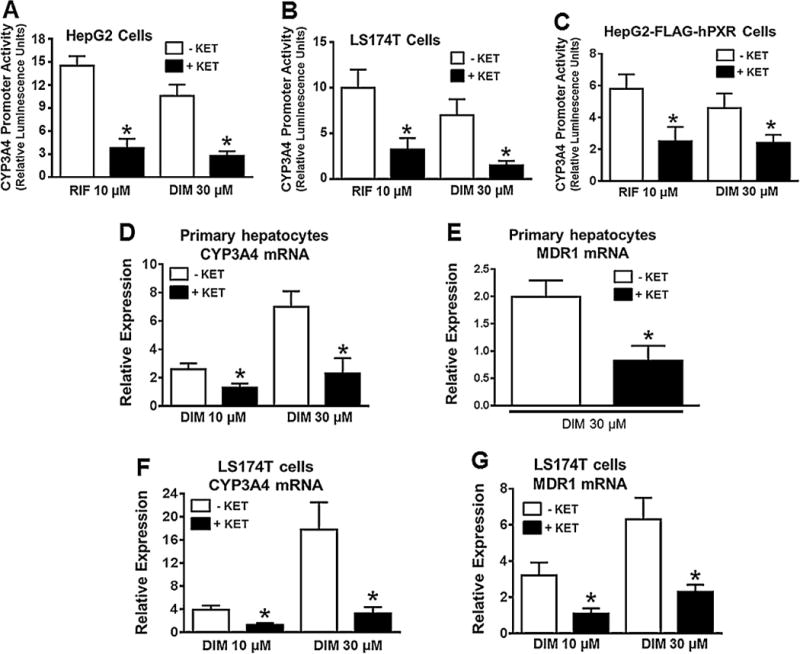
Pharmacologic inhibition of hPXR activity attenuates DIM-induced hPXR target gene expression in human primary hepatocytes, HepG2 and LS174 T cells. CYP3A4 prompter activity was determined in HepG2 (A) and LS174 T (B) cells after transiently co-transfecting the cells with pGL3-CYP3A4-luc reporter and pcDNA3-hPXR plasmids for 24 h, followed by treatment with DMSO, RIF or DIM in the presence and absence of PXR antagonist ketoconazole (KET) at 25 μM, as indicated for another 24 h. Similarly, CYP3A4 promoter activity was determined in HepG2-FLAG-hPXR cells (C) after transiently co-transfecting the cells with pGL3-CYP3A4-luc and CMV-Renilla. The induction of CYP3A4 promoter activity by the treatment was normalized as fold increase over the DMSO control. Data represent mean ± SD from three independent experiments. Statistical significance (*, p < 0.05) was determined using Students t test by comparing RIF or DIM alone with the combined RIF + KET or DIM + KET. mRNA expression of CYP3A4 and MDR1 was determined by quantitative RT-PCR in primary human hepatocytes (HU1488) (D & E) and LS174 T cells (F & G) after treatment with DMSO, RIF or DIM in the presence and absence of 25 μM KET, as indicated for 48 h. Results are presented as fold increase over the DMSO control. In all graphs, data represent mean ± SEM from three independent experiments. Statistical significance (*, p < 0.05) was determined using Students t test by comparing RIF or DIM alone with the combined RIF + KET or DIM + KET.
4. Discussion
To our knowledge, our study is the first report that DIM, at physiologically relevant levels, could induce CYP3A4 and MDR1 expression in an hPXR-dependent manner in human hepatocytes and intestinal cells.
DIM has been shown to modulate the transcriptional activity of xenobiotic receptors (Bhuiyan et al., 2006; Le et al., 2003; Riby et al., 2000; Vivar et al., 2010). Notably, DIM activates AHR-regulated CYP1A gene expression (Chen et al., 1998; Jellinck et al., 1993). While previous studies also show DIM induction of other CYPs such as CYP2B/CYP3A in human/rodent liver and intestine (Bonnesen et al., 2001; Gross-Steinmeyer et al., 2004, 2009; Jellinck et al., 1993; Lake et al., 1998; Leibelt et al., 2003; Renwick et al., 1999), the induction was found to be inconsistent and varied depending on the species, gender and dosage (Lake et al., 1998; Leibelt et al., 2003; Renwick et al., 1999). In addition, DIM induction of drug-transporting proteins has never been reported, and most importantly, whether PXR is the mediator for the effects of DIM on the expression of drug metabolizing enzymes and drug-transporting proteins is unknown. Our study shows that DIM, at its physiologically relevant concentrations, induces CYP3A4 and MDR1 by activating hPXR.
DIM is not only a health supplement but also the major active metabolite of I3C, which is also a health supplement and naturally-occurring compound enriched in cruciferous vegetables such as broccoli, cabbage and cauliflower. It is noteworthy that the acidic environment of the stomach fosters the non-enzymatic self-condensation of about 20% I3C to form DIM (Spande, 1979; Stresser et al., 1995). Therefore, DIM could be taken as a direct supplement or indirectly in the form of I3C as a supplement or through I3C-rich cruciferous vegetables. Although broccoli has been shown to induce the expression of CYPs in rat liver and colon tissues (Vang et al., 1991), it is unknown how much of DIM can be found in human plasma after consuming cruciferous vegetables. While DIM derived from I3C in the acidic stomach or pure DIM is poorly absorbed through the gastrointestinal tract, Bioresponse-DIM (BR-DIM), the absorption-enhanced DIM formulation, exhibits higher bioavailability (Anderton et al., 2004a; Reed et al., 2006, 2008). It was shown in healthy humans that the peak plasma concentration of BR-DIM was 200–300% higher compared to DIM derived from I3C when normalized to administered dose (Reed et al., 2008). Similarly, the peak plasma concentration of BR-DIM was 50% higher in mice compared to an equivalent dosage of pure DIM (Anderton et al., 2004a).
While its therapeutic potential has been well emphasized, the plasma concentration of DIM in humans is not fully evaluated. In a phase I trial conducted in humans, the level of DIM in plasma reached to approximately 2.5 μM after a single oral dose of 1000 mg I3C (Reed et al., 2006). However, it was later shown in healthy humans that the peak plasma concentration of BR-DIM was 200–300% higher compared to DIM derived from I3C when normalized to administered dose (Reed et al., 2008). Notably, the distribution of DIM between blood and other tissues has been reported to be remarkably different in animal studies. After a single oral administration of I3C in rats, concentration of DIM in blood and tissues was significantly different, with liver having 5–10 times higher concentration of DIM than in blood (Stresser et al., 1995). Similar observations were made in mice after dosing with 250 mg/kg I3C (Anderton et al., 2004b). The peak levels of DIM at 2 h were about 5 times higher in the liver than plasma. The concentration of DIM in liver was found to be even higher in mice after dosing with 250 mg/kg DIM (Anderton et al., 2004a). The levels of DIM peaked at about 1 h in the liver and plasma at 160 μM and 6 μM, respectively. While DIM was being tested in several in vitro studies at concentrations ranging from 1–100 μM, up to 20 μM has been reported as physiological concentration of DIM as these concentrations would be comparable with serum or tissue concentrations achievable in vivo (Fan et al., 2009; Moiseeva et al., 2007). Together, based on published in vitro and in vivo studies, our results show that DIM can induce hPXR-regulated CYP3A4 and MDR1 in liver and intestine at its physiologically relevant concentrations.
Many xenobiotics, including natural products such as St John’s Wort, activate PXR by directly binding to it (Dresser et al., 2003; Kliewer et al., 1998; Lehmann et al., 1998; Moore et al., 2000; Staudinger et al., 2006; Wang et al., 2013b). DIM can bind to nuclear receptors such as AHR and modulate AHR transcriptional activity (Chen et al., 1998; Jellinck et al., 1993). It remains to be determined whether DIM binds to PXR to activate PXR transcriptional activity. Some xenobiotics indirectly activate PXR by modulating cellular signaling pathways (Pondugula et al., 2009b; Staudinger et al., 2011). Therefore, it is also possible that DIM modulates cellular signaling pathways to activate PXR. For example, while NF-kB and CDK2 inhibit PXR activity (Gu et al., 2006; Lin et al., 2008), DIM inhibits NF-kB and CDK2 activity (Rahman et al., 2007; Vivar et al., 2009). It is unknown whether DIM modulates signaling pathways such as NF-kB and CDK2 to activate PXR. How DIM activates PXR warrants further investigation.
It was recently reported that about 38 million adults in the US use natural supplements, but only one-third tell their physician about this use (Kennedy et al., 2008). Recent surveys also found that multiple drug use is common in the US (Kaufman et al., 2002), and more importantly, the use of multiple drugs and supplements is more prevalent in patients (Richardson et al., 2000). Another survey indicated that 15% of patients receiving drugs also take natural supplements and, among these, potential adverse supplement-drug interactions were observed in 40% of patients (Bush et al., 2007). Therefore, while the use of natural supplements along with multiple drugs is very common, an increasingly important concern regarding natural supplements is that their potential to induce undesired drug interactions by inducing changes in drug-metabolizing enzymes and drug-transporting proteins (Huang and Lesko, 2004; Izzo and Ernst, 2009; NCCAM, 2012).
DIM effects on CYP3A4 and MDR1 could have important clinical implications in supplement-drug interactions. The altered activity of CYP3A4 and MDR1, subsequent to altered expression, by DIM may lead to undesired pharmacokinetic interactions during drug therapy. Such undesired effects by supplements have received attention in clinical and preclinical studies, such as hyperforin in St. John’s Wort (Moore et al., 2000). In some preclinical/clinical studies, cotreatment of St. John’s Wort with different drugs, such as cyclosporine A, indinavir, oral contraceptives, tacrolimus, warfarin, verapamil and fexofenadine, led to undertreatment and failure of the therapeutic drugs, because the plasma concentrations of coadministrated drugs were significantly reduced by St. John’s Wort due to PXR target gene induction (Izzo, 2012; Izzo and Ernst, 2009). Our study suggested that the physiologically relevant level of DIM was sufficient to change CYP3A4 and MDR1 gene expression in human liver and intestine and could potentially affect the absorption and metabolism and subsequently the efficacy of co-administered therapeutic drugs, as seen in the case of St. John’s Wort.
In summary, DIM induced CYP3A4 and MDR1 gene expression by activating hPXR in human hepatocytes and intestinal cells. Given that DIM is a natural health supplement and an active metabolite of I3C, our observations indicate that there may be an increased chance of undesired DIM-drug interactions caused by the uptake of DIM or I3C supplements or food rich in I3C during multiple drug therapy.
Supplementary Material
Highlights.
Diindolylmethane activates PXR.
Diindolylmethane induces CYP3A4 and MDR1 expression.
Caution is advised when using diindolylmethane in conjunction with other medications during multidrug therapy.
Acknowledgments
We thank Drs. Tao and Pinkert for sharing their microplate readers. This work was supported by the Animal Health and Disease Research Grant, Auburn University Research Initiative in Cancer and Auburn University Startup Funds to Pondugula SR.
Abbreviations
- DIM
3,3′-diindolylmethane
- PXR
pregnane X receptor
- hPXR
human pregnane X receptor
- mPXR
mouse pregnane X receptor
- rPXR
rat pregnane X receptor
- CYP
cytochrome p450
- CYP3A4
cytochrome p450 3A4
- MDR1
multidrug resistance protein 1
- DMSO
dimethyl sulfoxide
- RIF
rifampicin
- KET
ketoconazole
- R123
rhodamine 123
- PSC-833
Valspodar
- I3C
indole-3-carbinol
- BR-DIM
bioresponse-DIM
- AHR
aryl hydrocarbon receptor
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- CDK2
cyclin-dependent kinase 2
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2014.12.015.
References
- Ahmad A, Ali S, Ahmed A, Ali AS, Raz A, Sakr WA, Rahman KM. 3, 3′-Diindolylmethane enhances the effectiveness of herceptin against HER-2/neu-expressing breast cancer cells. PLoS One. 2013;8:e54657. doi: 10.1371/journal.pone.0054657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE. Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004a;32:632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004b;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- Auborn KJ. Therapy for recurrent respiratory papillomatosis. Antivir Ther. 2002;7:1–9. [PubMed] [Google Scholar]
- Azmi AS, Ahmad A, Banerjee S, Rangnekar VM, Mohammad RM, Sarkar FH. Chemoprevention of pancreatic cancer: characterization of Par-4 and its modulation by 3,3′ diindolylmethane (DIM) Pharm Res. 2008;25:2117–2124. doi: 10.1007/s11095-008-9581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Wang Z, Kong D, Sarkar FH. 3,3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 2009;69:5592–5600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4–2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- Biersack B, Schobert R. Indole compounds against breast cancer: recent developments. Curr Drug Targets. 2012;13:1705–1719. doi: 10.2174/138945012804545551. [DOI] [PubMed] [Google Scholar]
- Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- Bush TM, Rayburn KS, Holloway SW, Sanchez-Yamamoto DS, Allen BL, Lam T, So BK, Tran de H, Greyber ER, Kantor S, Roth LW. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Alternat Ther Health Med. 2007;13:30–35. [PubMed] [Google Scholar]
- Chan GN, Patel R, Cummins CL, Bendayan R. Induction of p-glycoprotein by antiretroviral drugs in human brain microvessel endothelial cells. Antimicrob Agents Chemother. 2013;57:4481–4488. doi: 10.1128/AAC.00486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Banerjee S, Cui QC, Kong D, Sarkar FH, Dou QP. Activation of AMP-activated protein kinase by 3,3′-diindolylmethane (DIM) is associated with human prostate cancer cell death in vitro and in vivo. PLoS One. 2012a;7:e47186. doi: 10.1371/journal.pone.0047186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem Pharmacol. 2012b;83:1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett A, Tanianis-Hughes J, Warhurst G. Rapid induction of P-glycoprotein expression by high permeability compounds in colonic cells in vitro: a possible source of transporter mediated drug interactions? Biochem Pharmacol. 2004;68:783–790. doi: 10.1016/j.bcp.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Schwarz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- Fan S, Meng Q, Saha T, Sarkar FH, Rosen EM. Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res. 2009;69:6083–6091. doi: 10.1158/0008-5472.CAN-08-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Stapleton PL, Liu F, Tracy JH, Bammler TK, Quigley SD, Farin FM, Buhler DR, Safe SH, Strom SC, Eaton DL. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004;34:619–632. doi: 10.1080/00498250412331285481. [DOI] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Stapleton PL, Tracy JH, Bammler TK, Strom SC, Buhler DR, Eaton DL. Modulation of aflatoxin B1-mediated genotoxicity in primary cultures of human hepatocytes by diindolylmethane, curcumin, and xanthohumols. Toxicol Sci. 2009;112:303–310. doi: 10.1093/toxsci/kfp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated P-glycoprotein induction by small molecule tyrosine kinase inhibitors. Eur J Pharm Sci. 2013;48:644–649. doi: 10.1016/j.ejps.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV, Mani S. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- Huang SM, Lesko LJ. Drug–drug, drug–dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol. 2004;44:559–569. doi: 10.1177/0091270004265367. [DOI] [PubMed] [Google Scholar]
- Ichite N, Chougule MB, Jackson T, Fulzele SV, Safe S, Singh M. Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non-small cell lung cancer. Clin Cancer Res. 2009;15:543–552. doi: 10.1158/1078-0432.CCR-08-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nagai J, Okada Y, Sato K, Yumoto R, Takano M. Function and expression of ATP-binding cassette transporters in cultured human Y79 retinoblastoma cells. Biol Pharm Bull. 2010;33:504–511. doi: 10.1248/bpb.33.504. [DOI] [PubMed] [Google Scholar]
- Izzo AA. Interactions between herbs and conventional drugs: overview of the clinical data Medical principles and practice: international journal of the Kuwait University. Health Sci Centre. 2012;21:404–428. doi: 10.1159/000334488. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- Keller RP, Altermatt HJ, Nooter K, Poschmann G, Laissue JA, Bollinger P, Hiestand PC. SDZ PSC 833, a non-immunosuppressive cyclosporine: its potency in overcoming P-glycoprotein-mediated multidrug resistance of murine leukemia. Int J Cancer J Int du cancer. 1992;50:593–597. doi: 10.1002/ijc.2910500418. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Wang CC, Wu CH. Patient Disclosure about Herb and Supplement Use among Adults in the US. Evid Based Complement Alternat Med. 2008;5:451–456. doi: 10.1093/ecam/nem045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittayaruksakul S, Zhao W, Xu M, Ren S, Lu J, Wang J, Downes M, Evans RM, Venkataramanan R, Chatsudthipong V, Xie W. Identification of three novel natural product compounds that activate PXR and CAR and inhibit inflammation. Pharm Res. 2013;30:2199–2208. doi: 10.1007/s11095-013-1101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kota BP, Tran VH, Allen J, Bebawy M, Roufogalis BD. Characterization of PXR mediated P-glycoprotein regulation in intestinal LS174T cells. Pharm Res. 2010;62:426–431. doi: 10.1016/j.phrs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Lake BG, Tredger JM, Renwick AB, Barton PT, Price RJ. 3,3′-Diindolylmethane induces CYP1A2 in cultured precision-cut human liver slices. Xenobiotica. 1998;28:803–811. doi: 10.1080/004982598239227. [DOI] [PubMed] [Google Scholar]
- Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE. Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3′-diindolylmethane in sprague-dawley rats. Toxicol Sci. 2003;74:10–21. doi: 10.1093/toxsci/kfg103. [DOI] [PubMed] [Google Scholar]
- Li H, Redinbo MR, Venkatesh M, Ekins S, Chaudhry A, Bloch N, Negassa A, Mukherjee P, Kalpana G, Mani S. Novel yeast-based strategy unveils antagonist binding regions on the nuclear xenobiotic receptor PXR. J Biol Chem. 2013;288:13655–13668. doi: 10.1074/jbc.M113.455485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem. 2008;283:30650–30657. doi: 10.1074/jbc.M806132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45:60–72. doi: 10.3109/03602532.2012.746363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva EP, Almeida GM, Jones GD, Manson MM. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007;6:3071–3079. doi: 10.1158/1535-7163.MCT-07-0117. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor Proc Natl Acad Sci U S A. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Stephenson CR, Kendall C, Saini SP, Toma D, Ren S, Cai H, Strom SC, Day BW, Wipf P, Xie W. A pregnane X receptor agonist with unique species-dependent stereoselectivity and its implications in drug development. Mol Pharmacol. 2005;68:403–413. doi: 10.1124/mol.105.013292. [DOI] [PubMed] [Google Scholar]
- NCCAM. Summary of Roundtable Meeting on Dietary Supplement-Drug Interactions. The National Center for Complementary and Alternative Medicine 2012 [Google Scholar]
- Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab Dispos. 2009a;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula SR, Dong H, Chen T. Phosphorylation and protein–protein interactions in PXR-mediated CYP3A repression. Expert Opin Drug Metab Toxicol. 2009b;5:861–873. doi: 10.1517/17425250903012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula SR, Tong AA, Wu J, Cui J, Chen T. Protein phosphatase 2Cbetal regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. Drug Metab Dispos. 2010;38:1411–1416. doi: 10.1124/dmd.110.032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman KM, Ali S, Aboukameel A, Sarkar SH, Wang Z, Philip PA, Sakr WA, Raz A. Inactivation of NF-kappaB by 3,3′-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther. 2007;6:2757–2765. doi: 10.1158/1535-7163.MCT-07-0336. [DOI] [PubMed] [Google Scholar]
- Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,30-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–2624. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick AB, Mistry H, Barton PT, Mallet F, Price RJ, Beamand JA, Lake BG. Effect of some indole derivatives on xenobiotic metabolism and xenobiotic-induced toxicity in cultured rat liver slices. Food Chem Toxicol. 1999;37:609–618. doi: 10.1016/s0278-6915(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Riby JE, Chang GH, Firestone GL, Bjeldanes LF. Ligand-independent activation of estrogen receptor function by 3,3′-diindolylmethane in human breast cancer cells. Biochem Pharmacol. 2000;60:167–177. doi: 10.1016/s0006-2952(00)00307-5. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–684. 714–675. doi: 10.1093/ajcn.82.3.675. quiz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spande T. Hydroxyindoles, indoles, alcohols and indolethiols. In: Houlihan WJ, editor. Indoles, part 3. John Wiley & Sons, Inc; New York: 1979. pp. 1–355. [Google Scholar]
- Sporstol M, Tapia G, Malerod L, Mousavi SA, Berg T. Pregnane X. receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun. 2005;331:1533–1541. doi: 10.1016/j.bbrc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Ding X, Lichti K. Pregnane X. receptor and natural products: beyond drug-drug interactions. Expert opinion on drug metabolism & Toxicology. 2006;2:847–857. doi: 10.1517/17425255.2.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Xu C, Biswas A, Mani S. Post-translational modification of pregnane x receptor. Pharmacol Res. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RH, O’Neill CA, Warhurst A, Carlson GL, Rowland M, Warhurst G. Kinetic profiling of P-glycoprotein-mediated drug efflux in rat and human intestinal epithelia. J Pharmacol Exp Ther. 2001;296:584–591. [PubMed] [Google Scholar]
- Stresser DM, Williams DE, Griffin DA, Bailey GS. Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion in male Fischer 344 rats. Drug Metab Dispos. 1995;23:965–975. [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Podust LM, Kim RB. Identification of amino acids in rat pregnane X receptor that determine species-specific activation. Mol Pharmacol. 2004;65:36–44. doi: 10.1124/mol.65.1.36. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Lin HW, Simon Pickard A, Tsai HY, Mahady GB. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: a systematic literature review. Int J Clin Pract. 2012;66:1056–1078. doi: 10.1111/j.1742-1241.2012.03008.x. [DOI] [PubMed] [Google Scholar]
- Vang O, Jensen H, Autrup H. Induction of cytochrome P-450IA1, IA2, IIB1, IIB2 and IIE1 by broccoli in rat liver and colon. Chem -Biol Interact. 1991;78:85–96. doi: 10.1016/0009-2797(91)90105-g. [DOI] [PubMed] [Google Scholar]
- Veith H, Southall N, Huang R, James T, Fayne D, Artemenko N, Shen M, Inglese J, Austin CP, Lloyd DG, Auld DS. Comprehensive characterization of cytochrome P450 isozyme selectivity across chemical libraries. Nature Biotechnol. 2009;27:1050–1055. doi: 10.1038/nbt.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Wang H, Cayer J, Leroux M, Salvail D, Das B, Wrobel JE, Mani S. In vivo and in vitro characterization of a first-in-class novel azole analog that targets pregnane X receptor activation. Mol Pharmacol. 2011;80:124–135. doi: 10.1124/mol.111.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar OI, Lin CL, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane induces a G(1) arrest in human prostate cancer cells irrespective of androgen receptor and p53 status. Biochem Pharmacol. 2009;78:469–476. doi: 10.1016/j.bcp.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar OI, Saunier EF, Leitman DC, Firestone GL, Bjeldanes LF. Selective activation of estrogen receptor-beta target genes by 3,3′-diindolylmethane. Endocrinology. 2010;151:1662–1667. doi: 10.1210/en.2009-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang H, Li H, Teotico DG, Sinz M, Baker SD, Staudinger J, Kalpana G, Redinbo MR, Mani S. Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin Cancer Res. 2007;13:2488–2495. doi: 10.1158/1078-0432.CCR-06-1592. [DOI] [PubMed] [Google Scholar]
- Wang X, Fang X, Zhou J, Chen Z, Zhao B, Xiao L, Liu A, Li YS, Shyy JY, Guan Y, Chien S, Wang N. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc Natl Acad Sci U S A. 2013a;110:13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Lin W, Chai SC, Wu J, Ong SS, Schuetz EG, Chen T. Piperine activates human pregnane X receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol Appl Pharmacol. 2013b;272:96–107. doi: 10.1016/j.taap.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiatrak BJ. Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg. 2003;11:433–441. doi: 10.1097/00020840-200312000-00005. [DOI] [PubMed] [Google Scholar]
- Zandvliet M, Teske E, Chapuis T, Fink-Gremmels J, Schrickx JA. Masitinib reverses doxorubicin resistance in canine lymphoid cells by inhibiting the function of P-glycoprotein. J Veter Pharmacol Ther. 2013 doi: 10.1111/jvp.12039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


