Abstract
Poor early conditions have been associated with increasing risks of some chronic diseases during adulthood. Since chronic illnesses are known as important risk factors for disability, it should be the case that poor early conditions are predictive of disability at older ages. In addition, recent literature suggests that poor early conditions may affect the risk of disability even in the absence of chronic illnesses. The aim of the study presented in the paper was to evaluate the magnitude of differentials in the risk of being disabled according to early conditions experienced by elderly populations in Latin America and the Caribbean (LAC), and to identify the group of chronic illnesses responsible for it. We find that poor early conditions exert a strong influence on disability later in life by increasing both the risk of suffering disability-related chronic illnesses and the risks of suffering disabilities given the presence of chronic illnesses.
Keywords: early conditions, chronic diseases, disabilities, aging, Latin America
I. Introduction
Current cohorts of elderly people in Latin America and the Caribbean (LAC) are unique since their survival to old ages is due largely to medical interventions and to a much lesser extent, to ameliorations in standards of living (Preston 1976; Palloni and Wyrick 1981). This implies that poor early nutritional status, growth and development, and most childhood morbidity responsible for the high levels of mortality preceding the deployment of medical improvements continued to affect them albeit with substantially reduced lethality (Palloni et al. 2006). This historical regularity offers an interesting case study of a group of people among which the effect of poor early conditions can be captured at older ages because selection due to premature mortality is significantly reduced. According to new data available for LAC countries (SABE 2000; PREHCO 2002–03), more than one third of the population 60 years and older report having suffered bad economic conditions before age 15 in Bridgetown (Barbados), La Havana (Cuba) and Puerto Rico. More than 20 per cent of the elderly individuals report having gone hungry before age 15 in Mexico City, Santiago de Chile and La Havana, and between 20 per cent and 36 per cent report having suffered from bad health or have experienced important infectious diseases (such as tuberculosis, rheumatic fever, hepatitis, typhus fever, malaria or dengue among others) during their childhood in Puerto Rico, Mexico City, La Havana, and Santiago de Chile.
a. Early conditions
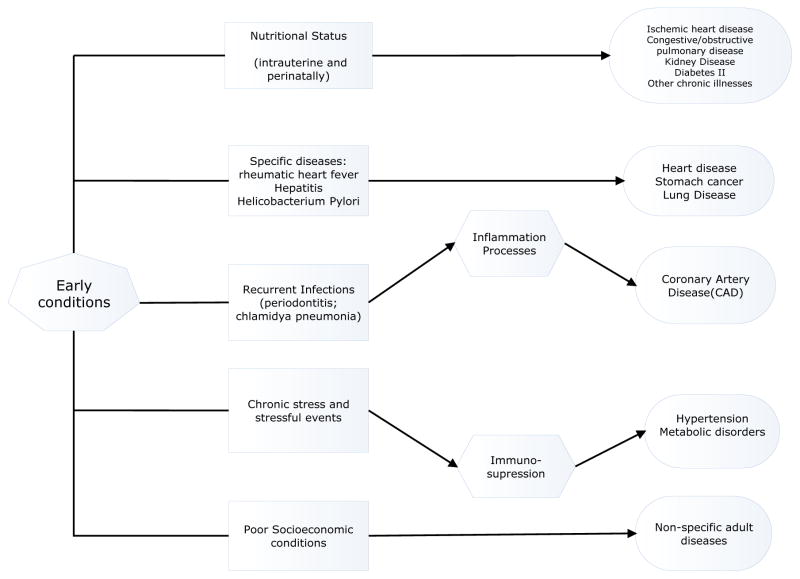
‘Early conditions’ is used broadly in extant literature to refer to a very heterogeneous set of factors that affect nutritional status in utero and perinatally, growth and individual development in early infancy and childhood, exposure to and contraction of infectious and parasitic diseases, experience with stressful environments and, more generally, to experiences associated with family socioeconomic conditions which shape early life environments. Figure 1 is a stylized representation of alternative pathways through which early conditions affect adult health status.
Figure 1.
Pathways linking early conditions (health and SES) and adult health status
The first and most documented pathway in the literature (Barker et al. 1989; Barker 1998; Godfrey 2002; Gluckman and Hanson 2004; Fowden and Forhead 2004) involves nutritional status in utero. The evidence that placental and fetal growth is associated with hypertension, ischemic heart disease, diabetes, obesity, obstructive pulmonary disease, renal failure is mounting (Ravelli et al. 1976; Barker 1995; Edwards et al. 2001; Gluckman and Hanson 2004; Fowden and Forhead 2004). Also, exposure to malnutrition during childhood and early adolescence can influence hip fracture risk late in life (Heaney 1989; Miles and Furner 1991). To date most of the evidence to support the existence of this pathway is from Western Europe and North America. However, there is also some evidence for other countries as well. Thus, in a very recent study among elderly Puerto Ricans it was found that a marker of early growth and stunting (knee height) is associated with the risk of self-reporting diabetes (Palloni et al. 2005).
The second pathway operates through direct relations between well-defined infectious diseases that are common among children and adolescents and a handful of selected adult chronic illnesses (Elo and Preston, 1992). The streptococcal infection known as rheumatic heart fever has been linked to adult heart disease (Elo and Preston 1992; Palloni et al. 2006) and has been shown to be a powerful predictor of adult heart disease in some low income countries (Palloni et al. 2008). Lower respiratory tract infections, most of which are due to streptococcal bacteria, are correlated with a higher risk of suffering obstructive lung disease (such as chronic bronchitis, heart disease with underlying lung deficiency, asthma, and emphysema) in adulthood (Elo and Preston 1992). There is evidence that Helicobacter Pylori infection, a common bacterial infection among children living in crowded and unsanitary conditions in less developed countries, is related to increased risk of stomach cancer later in life (Elo and Preston 1992; Blaser et al. 1995; Go 2002).
The third pathway rests on a relation between inflammation processes triggered by recurrent infections and coronary artery disease (CAD). Some studies show that exposure to systemic infections increases the risk of adult cardiovascular disease, Alzheimer’s disease, and ischemic heart disease and accelerates the aging process (Elo and Preston 1992; Fong 2000; Finch and Vaupel 2001; Finch and Crimmins 2004; Crimmins and Finch 2006). The existence of this pathway, however, remains to be verified as the clinical and epidemiological evidence only supports a connection between inflammation due to Periodontitis and Chlamydia Pneumonia, on the one hand, and CAD, on the other (Fong 2000). Both Periodontitis and Chlamydia Pneumonia are infectious diseases more likely to occur during late childhood and adolescence than during early childhood.
The fourth pathway relates stress either in utero (Couzin 2002, Camacho 2008) or early childhood (Dowd et al. 2007) to some chronic conditions such as hypertension (Langley-Evans, 2004) and metabolic disorders (Seckl 1997; Couzin 2002), to low birth weight (Camacho 2008), or, through immunosuppressive disorders and inflammation to poor adult health (Shanks and Stafford 2001; McDade and Kuzawa 2004; Danese et al. 2007).
The final pathway in Figure 1 is the least specific as it simply postulates that poor SES during childhood affects adult health. No clear mechanism has been formulated apart from those identified above that could be captured by indicators of poor childhood SES (Blackwell et al. 2001; Kuh et al. 2002; Galobardes et al. 2004; Guralnik et al. 2006).
In this paper we focus on the combined effects of poor early childhood health and poor early childhood SES. Although our work also suggests the independent effects of early childhood health (while controlling for early childhood SES) and of poor early childhood SES (while controlling for early childhood health), in this paper we only show the results of using a combined indicator. We chose this strategy because the measures available to us were far from being precise enough to isolate thoroughly one or the other dimension of early childhood. Thus it is somewhat deceptive to use them separately as if one were in a position to identify cleanly the pathways depicted in Figure 1. We preferred to use the combined indicator and thus attempt to capture one or more of the pathways without testing hypotheses about the relative contribution of each one of them.
b. Disability and chronic illnesses
A separate strand of literature makes a compelling case supporting the hypothesis that some chronic diseases are among the main causes of disability and that the risk of being disabled increases rapidly in the presence of chronic illnesses (Verbrugge et al. 1989; Fried et al. 1999). Similarly, the severity of disability is associated with the number of illnesses, and there is evidence for the existence of mechanisms whereby interactions between specific diseases are the root cause of functional limitations (Fried et al. 1999).
If adverse early health conditions do indeed increase the risk of chronic illnesses and if these are the main cause of disability, it follows per force that there must be a relation between early health conditions and adult and old age disability. This relation could be amplified by two additional linkages. The first is that the risk of disability given the presence of chronic illness is higher among those who experience poor early conditions than among those who do not. The second linkage could be important if there are mediating pathways operating through conditions, co-morbidities or chronic illnesses that have not yet been identified. Indeed, recent research offers hints about a connection between early socioeconomic conditions and early developmental indicators (assessed by birth weight, physical growth, advanced childhood motor skills, cognitive abilities, etc.) and disability quite independently of chronic illnesses (Guralnick et al. 2006, Kuh et al. 2006). The most likely mechanism is one whereby the growth of muscle mass among adults is constrained by conditions experienced early on, during a period of time when the architecture of muscle fibers is being created (Bailey et al 2001; Kuh et al. 2002; Kuh et al. 2006; Ridler et al. 2006). In turn, deficiency in muscle mass in adulthood may precipitate disability.
The main goals of the study presented in the paper were (a) to estimate the total (gross) effect of poor early health conditions on the probability of being disabled among elderly in Latin America and the Caribbean (LAC) countries, and (b) to evaluate the fraction of the total effect associated with well-identified chronic diseases. The latter quantity is a result of only two possible pathways or combination thereof. The first is one that increases the risk of experiencing chronic illnesses among those who experience poor early conditions. The second is one that increases the risk of disability after the onset of the chronic condition.
II. Data, Model and Measurement
a. Nature of Database
The data used are from the Puerto Rican Elderly: Health Conditions survey - PREHCO I, 2002–03 - (PREHCO, 2002–03) and from the survey of Salud, Bienestar y Envejecimiento en América Latina y El Caribe - SABE, 2000 (Peláez et al. 2003). The PREHCO study is a panel of a nationally representative sample of Puerto Rican adults over 60 years old. We used the first wave of the PREHCO study with a sample size of 4,293 individuals.
The SABE survey is a cross-sectional study of people aged 60 years and over which was carried out in seven cities of Latin America and the Caribbean (Bridgetown, Barbados; Buenos Aires, Argentina; La Havana, Cuba; Mexico City, Mexico; Montevideo, Uruguay; Santiago de Chile, Chile; Sao Paulo, Brazil), in 1999–2000. Our analysis pooled the seven cities but we included dummy variables for each city in the estimated models. The pooled SABE sample size is 10,602 individuals.
b. Model
Let us assume that there are K well-identified disability-related chronic illnesses. The probabilities of experiencing disability among those with and without poor early conditions are algebraically defined by the expressions (1) and (2) respectively:
| (1) |
and
| (2) |
where:
prx(d/pec) =probability of being disabled (d) at age x, conditional on having experienced poor early conditions (pec);
prx(Īi/pec) = probability of not experiencing any disability-related illness (Īi) at age x conditional on having experienced poor early conditions (pec).
prx(d/pec, Īi)= probability of being disabled (d) at age x, conditional on having experienced poor early conditions (pec) but no current disability-related diseases (Īi);
prx(Ii/pec) = probability of experiencing disability-related disease (Ii) at age x, conditional on having experienced poor early conditions (pec);
prx(d/pec, Ii) = probability of being disabled (d) at age x, conditional on having experienced poor early conditions (pec), and disability-related diseases (Ii);
Analogous definitions hold for all terms included in equation (2) for those who do not experience poor early conditions (pēc). The product [prx(Īi/pec) × prx(d/pec, Īi)] in equation (1) is the probability of being disabled among those who experienced poor early conditions but who did not experience any of the disability-related chronic diseases at older ages. Instead the quantity: is the overall probability of being disabled among those who experience poor early conditions given that they experience one of K disabling diseases. Analogous definitions apply to equation (2). If we assign the subscript 0 whenever there is no disability-related chronic illness (that is, when Īi= 0 ), we can write compactly the difference between the probabilities defined in (1) and (2) as:
| (3) |
Note that (3) is analogous to the difference between crude rates in two populations. In our case the terms prx(d/pec, Ii)and prx(d/pēc, Ii) are the ‘rates’ of occurrence, and the terms prx(Ii/pec), prx(Ii/pēc) are measures of ‘population composition’ (in this case composition by chronic illness) where and . A standard result of rate decomposition analysis (Kitagawa 1955; Das Gupta 1978) is that equation (3) can be expressed in a more revealing way as:
| (4) |
where wx and Wx are “standard” weights defined as 0.5×(prx(Ii/pec) + prx(Ii/pēc)) and 0.5×(prx(d/pec, Ii) + prx(d/pēc, Ii)), respectively. By separating terms involving chronic illnesses (i=1,K) from those denoting absence of chronic condition (i=0) we obtain the following expression:
| (5) |
The term associated with the first summation sign is a measure of the effects operating through excess risk of disability given a chronic illness and we will call it a disability component. The term associated with the second summation sign is a measure of the excess risk of experiencing a disability-related chronic illness and we will call it a chronic disease component. Taken together these two terms constitute a measure of the effects of early conditions operating through the set of K chronic illnesses. The remaining two terms prx(d/pec, Ī0) − prx(d/pēc, Ī0)]×wx0+ [prx (Ī0/pec) − prx(Ī0/pēc)]×Wx0 are a measure of the effects that operate in the absence of the K disability-related illnesses. This is a residual component that captures one of two things: either errors due to, for example, faulty measurement of one or more of the K chronic illnesses or, alternatively, effects that pass through chronic illnesses, conditions or comorbidities that are not explicitly considered in the analysis.
c. Measurement
While the algebra of decomposition above is quite routine, we faced important measurement problems that need to be resolved. What follows is a brief sketch of the choices made to assess disability, early conditions, and chronic illnesses.
i) Alternative measures of disability
We classified as disabled all individuals who self-report at least one limitation in Activities of Daily Life (ADL). The ADLs considered were the standard ones, namely, moving across the room, dressing oneself, taking a shower, eating, going to the bed, and using the toilet. According to these definitions, the proportion of people 60 years and older with a limitation in at least one ADL is 15 per cent in Puerto Rico, and 16 per cent in SABE cities. The percentage of missing values in both samples is less than one per cent. One may reasonably argue that results are sensitive to the definition of disability. To test the robustness of our inferences to different ways of operationalizing disability we examined results using two quite different criteria to classify people as disabled. The first includes joint consideration of Instrumental Activities of Daily Living (IADLs) and ADLs. The IADLs are: preparing food, handling own money, going out, buying food and clothes, using telephone, accomplishing home tasks, and taking medications. The second combines difficulties in ADLs with impairments (such as poor vision including blindness, and poor hearing including deafness). However, after comparing the results, we noted that the main inferences remained unchanged irrespective of the operationalization of disability. Thus, to simplify presentation we only discuss results associated with the definition of disability that relies only on ADLs.
One caveat is still in order. The degree to which the measure(s) of disability we used actually corresponds to existing indicators of functional limitations must be at least approximately assessed. In the PREHCO survey, interviewers were directed to ask the interviewee to perform exercises to detect the existence of limitations and their severity. In particular, we chose the so-called ‘get-up-and-go’ routine whereby the individual was requested to sit on a chair, get up, and walk a certain distance. The interviewer then calculated the time it took for the respondent to perform the exercise. Similar exercises have been used by other researchers to ‘validate’ self reported disability (Guralnik et al. 2006). Here, as well as in other research, we find a satisfyingly high degree of concordance between the two. Among those who did not report any difficulties in ADLs, almost 50 per cent could perform the exercise in less than the median time, whereas among who did report suffering from problems with at least one ADL, only 16 per cent performed the exercise in less than the median time. This finding bolsters our confidence about the use of the ADL- based definition of functional limitation. Unlike performance measures the ADL-based indicator can be employed very generally as the component items are collected routinely in surveys of the elderly.
In the case of SABE, information on exercises was not collected. However, we could test the internal consistency between self reported ADLs and other self reported measures (such as if the individual was capable picking up a coin; extending arms above shoulder level, and getting up from a chair). The percentage of people who self-reported problems performing those activities among those who also reported having difficulties in ADLs is three to four times higher than the percentage among those who did not report any ADL problems.
In summary, it is unquestionable that the conjectures we examined in the study are better served if one employs more ‘objective’ measures of individual disability. But the state of the art in population-based surveys of the elderly can only provide us with the indicators we used. In the absence of more objective measures, we relied on the definition of disability as a function of ADLs only, a definition that appears to produce results robust to changes in criteria for classification of disability and that is highly concordant with more objective criteria to identify functional limitations.
ii) Alternative measures of early conditions
Table 1 displays the variables used to assess various dimensions of early conditions. All these variables are measures of adult retrospective reports of conditions experienced during the first 15 years of life. Although a more nuanced representation of early conditions would have been desirable, we opted to use a very simple design: we defined a dummy variable to flag individuals who either report poor early socio-economic status (SES) or episodes of poor health status early on. We used this coarse measure in order to reduce the problems associated with omitted variables (for example some specific infectious diseases or malnutrition) that are highly correlated with other dimensions of the early environments (such as SES).
Table 1.
Variables1 used to assess various dimensions of early conditions likely to be predictive of disability among contemporary cohorts of the elderly in Latin America and the Caribbean
| Poor early condition during the first 15 years of life | Socio-economic status | SABE cities2 | Bad family economic condition before age 15 years |
| Puerto Rico | Bad household economic situation before age 15 years | ||
| Health Status | SABE cities2 | Poor early general health: confined to a bed for a month or more OR Experienced at least one of the following diseases: hepatitis, tuberculosis, rheumatic fever, chronic bronchitis, nephritis | |
| Puerto Rico | Poor general health: was often sick or often had activities restricted OR Experienced at least one of the following diseases: hepatitis, tuberculosis, rheumatic fever, chronic bronchitis, typhus fever, polio, malaria, dengue, pneumonia, asthma. |
All variables are measured by retrospective reports by adults of conditions experienced during the first 15 years of life.
Bridgetown, Buenos Aries, Havana, Mexico City, Montevideo, Santiago, Sao Paulo
The proportion of individuals who experienced poor early conditions is higher among Puerto Rican elderly than in SABE cities. In Puerto Rico, 53 per cent of individuals over 60 years old had experienced poor early conditions, whereas among SABE cities this percentage is 40 per cent. The percentage of missing values using this general definition of early condition is remarkably low (less than three per cent in both studies).
iii) Identification of disability related chronic illnesses
Our aim is to include only chronic illnesses, and combinations thereof, that are known to be related to the onset of functional limitations. We tested all chronic diseases purportedly related to both poor early conditions and disability status. Cancer was the only chronic condition whose effect on disability is not statistically significant in both samples and it is not significantly affected by conditions experienced early in life. Therefore, this disease was not included in the analysis. We grouped the selected chronic illnesses in four clusters:
-
c.1
Diseases of the circulatory system: stroke, coronary disease, hypertension, and diabetes. We included diabetes in this cluster because its most important effect is to impair the circulatory system and to increase the propensity of vascular diseases. The prevalence of these diseases among people 60 years and older in SABE cities is 61 per cent (SABE, 2000), and in Puerto Rico 71 per cent (PREHCO, 2002–03);
-
c.2
Mental illnesses: this group includes psychological problems, depression, and anxiety disorders. The prevalence of these diseases among people 60 years and older in SABE cities is 22 per cent (SABE, 2000), and in Puerto Rico 27 per cent (PREHCO, 2002–03);
-
c.3
Diseases of the respiratory system: this group includes chronic bronchitis, emphysema, and asthma. The prevalence of these diseases among people 60 years and older in SABE cities is 10 per cent (SABE, 2000), and in Puerto Rico 18 per cent (PREHCO, 2002–03);
-
c.4
Diseases of the musculoskeletal system: this group includes rheumatism, arthritis, osteoarthritis, and osteoporosis. The prevalence among people 60 years and older in SABE cities is 45 per cent (SABE, 2000), and in Puerto Rico 54 per cent (PREHCO, 2002–03);
We then considered all possible combinations of these four groups. Of the sixteen possible combinations some were very rare and we proceed to reclassify them as other combinations of diseases. Finally, the residual group (absence of disease) includes people who do not suffer from any of the four groups of chronic diseases aforementioned and those who report only conditions unrelated to both disability and poor early conditions (e.g. cancer). The final groups of illnesses used in our analysis are defined in Table 2.
Table 2.
Chronic diseases believed to be related both to poor early conditions and disability status and the percentage of the elderly suffering from them in SABE cities1 (2000) and Puerto Rico (2002–03)
| Groups of diseases | SABE cities1 Per cent |
Puerto Rico Per cent |
|---|---|---|
| Total number of cases | 9,403 | 3,715 |
| Residual group (Absence of disease) | 17 | 12 |
| Respiratory | 1 | - |
| Mental | 3 | - |
| Circulatory | 19 | 20 |
| Circulatory and Respiratory | 2 | - |
| Circulatory and Mental | 5 | 5 |
| Musculoskeletal | 9 | 9 |
| Musculoskeletal and Mental | 3 | - |
| Musculoskeletal and Circulatory | 16 | 21 |
| Musculoskeletal, Circulatory, and Respiratory | 2 | 5 |
| Musculoskeletal, Circulatory, and Mental | 7 | 9 |
| Musculoskeletal, Circulatory, Mental, and Respiratory | 2 | 5 |
| Other combinations of diseases 2 | 2 | 12 |
| Missing Value | 12 | 1 |
See Table 1, footnote 2
In SABE cities, the group ‘other combinations of diseases’ comprises individuals who suffer from respiratory diseases combined with others diseases. In Puerto Rico, this group comprises individuals who suffer from either musculoskeletal diseases combined with mental disorders or respiratory diseases combined with others diseases.
Source: Pelaez et al (2003), PREHCO (2002–03).
The only means of identification of disability-related illnesses we have at our disposal is based on self reports of medical diagnosis. Although it is known that self-reported illnesses are not the best tools to identify existing chronic illnesses, there is mounting evidence that, despite inaccuracies, some of them at least remain very useful. A recent analysis of ELSA and HRS confirms that results obtained with self reported hypertension and diabetes corresponds closely with those obtained from biomarkers (Banks et al, 2006). Similarly, a study of elicited symptoms for diabetes and circulatory illness confirms a high degree of correspondence with self reports for the PREHCO sample (Palloni and White, 2006). Finally, in a study of a large national sample of Mexican adults (ENSA) and comparable national sample of adults in the United States (NHANES) it was shown that SES gradients estimated using self reported diabetes and hypertension are remarkably similar to those obtained using biomarkers for both conditions (Wong et al., 2008).
But even if self-reports of illnesses are in error, our estimates will be affected only to the extent that they are correlated with errors in self-reported early conditions. There is no reason to suspect that people who misreport early conditions are more (or less) likely to also misreport chronic illnesses. Indeed, in a preliminary report on the quality of reporting of the Puerto Rican Elderly study it is shown that variability in reporting of early conditions across two waves (which should be ideally nil) is unrelated to variability in reporting chronic illnesses (Palloni and Garcia, 2008)
iv) Estimation of conditional probabilities
The conditional probabilities included in equations (1) and (2) above were estimated by means of two groups of regressions. The first group of regressions (logistic models) estimates the probabilities of being disabled conditional on experiencing each group of disability-related chronic diseases and on having experienced poor or good early conditions. The dependent variable is a dummy variable that equals one if the individual is disabled according to the first definition described before. In order to take into account the interaction effect between early conditions and chronic diseases on disability, this model was estimated separately for individuals who experienced poor and good conditions early in life. The second group of regressions consists of Multinomial Logistic Regressions that estimate the probabilities of experiencing each group of chronic illness (see Table 2) conditional on early conditions. Both groups of regressions were adjusted for socio-economic status (level of education measured as a set of three dummies variables), behavioral variables (smoking, a dichotomous variable for ever smoked), age (a continuous variable), and sex (a dichotomous dummy variable).
We used the estimated parameters of both set of models, one for the probability of experiencing disability-related chronic illnesses and the other for the probability of experiencing ADL limitations, to generate predicted probabilities of a chronic illness and of being disabled given a chronic illness for two subpopulations: those who experienced poor early conditions and those who did not. We then defined a matrix where each cell represents one of the twelve possible combinations of values of the control variables used in the models (sex, education and ever smoked). For each cell, we calculated the predicted probabilities of experiencing one of the (groups) of chronic illnesses and the predicted probabilities of experiencing disability given chronic illnesses by single age. Those probabilities were estimated separately for individuals who experienced poor and good early conditions. Final values were then calculated averaging over cells in the matrix and then over age (in the case of SABE cities we also averaged over countries). The averaging uses as weight the proportion of individuals in each cell, age, and countries. The distribution of each variable was derived from the entire sample and thus the weight of each cell was the same for both samples: individuals who experienced poor early conditions and those who did not.
Since in the PREHCO database individuals who needed proxies for the administration of the questionnaire did not answer the section about early conditions, we estimated the models excluding those cases. They represent about 11 per cent of the SABE sample, and 13 per cent of the PREHCO sample. In SABE cities it was possible to estimate the models with and without individuals who needed proxies but the results obtained are very similar (results not shown here). Thus, to ensure comparability between SABE cities and Puerto Rico, we only showed results excluding individuals who needed proxies.
III. Results
Table 3 displays the estimated coefficients for the disability and the chronic illness models. The first panel shows the effects of having poor early conditions on the probability of suffering the diseases (Multinomial Logistic model’s coefficients). The second panel shows the estimated effects of chronic diseases on the probability of being disabled (Logistic model’s coefficient). All the coefficients are in the expected direction and most of them are also statistically significant at less than one per cent.
Table 3.
Effects of early conditions on probability of suffering chronic disease and effects of chronic diseases on probability of being disabled among the elderly in SABE cities1 (2000) and Puerto Rico (2002–03)2.
| I. Effect of poor early conditions on chronic disease (Multinomial Logit)
| ||||
|---|---|---|---|---|
| Groups of chronic diseases Reference category: absence of disease |
SABE cities1 (2000) (Coefficients) | Puerto Rico (2002–03) (Coefficients) | ||
| Respiratory | 0.69** | |||
| Mental | 0.61** | |||
| Circulatory | 0.04 ns | 0.12 ns | ||
| Circulatory and Respiratory | 0.49** | |||
| Circulatory and Mental | 0.59** | 0.51** | ||
| Musculoskeletal | 0.26** | 0.16 ns | ||
| Musculoskeletal and Mental | 0.84** | |||
| Musculoskeletal and Circulatory | 0.36** | 0.44 ** | ||
| Musculoskeletal, Circulatory, and Respiratory | 1.21** | 1.18 ** | ||
| Musculoskeletal, Circulatory, and Mental | 0.74** | 0.74 ** | ||
| Musculoskeletal, Circulatory, Mental, and Respiratory | 1.36** | 1.68 ** | ||
| Other combinations of diseases | 1.03** | 0.82 ** | ||
|
| ||||
|
II. Effect of chronic disease on disability3
(Logit)
| ||||
| Groups of chronic diseases Reference category: absence of disease |
SABE cities1 (2000) (Coefficients) | Puerto Rico (2002–03) (Coefficients) | ||
|
| ||||
| Poor early conditions | Good early conditions | Poor early conditions | Good early conditions | |
| Respiratory | −0.38 ns | 1.15 ** | ||
| Mental | 0.94 ** | 1.28 ** | ||
| Circulatory | 0.29 ns | 0.71 ** | 0.39 ns | 2.73** |
| Circulatory and Respiratory | 1.17 ** | 1.13 ** | ||
| Circulatory and Mental | 1.35 ** | 1.56 ** | 1.14** | 3.71** |
| Musculoskeletal | 0.90 ** | 1.29 ** | 0.86* | 2.83** |
| Musculoskeletal and Mental | 1.82 ** | 1.66 ** | ||
| Musculoskeletal and Circulatory | 1.30 ** | 1.42 ** | 1.40** | 3.46** |
| Musculoskeletal, Circulatory, and Respiratory | 1.76 ** | 2.07 ** | 1.86** | 3.50** |
| Musculoskeletal, Circulatory, and Mental | 2.02 ** | 2.31 ** | 2.32** | 4.16** |
| Musculoskeletal, Circulatory, Mental, and Respiratory | 1.71 ** | 3.04 ** | 2.28** | 4.92** |
| Other combinations of diseases | 1.22 ** | 1.98 ** | 1.48** | 3.55** |
| Constant | −6.33 ** | −6.33 ** | −3.54** | −8.75** |
See Table 1, footnote 2
ns: not significant,
p < 5%,
p < 1%
The Chronic Disease Model and the Disability Model were estimated controlling for sex, age (continuous variable starting at age 60), three dummy variables representing years of schooling (0, 1–6 and 7+), dummy variable if individual ever smoked and, in the case of SABE cities, dummies variables for each country. The values of these coefficients are not shown here.
Model estimated separately for individuals who experienced poor and good early conditions
Source: As for Table 2
a. Excess risks of chronic illnesses
In both Puerto Rico and SABE cities, poor early conditions increases the probability of experiencing each group of diseases, even after controlling for level of education, smoking, age, and sex (Table 3, top panel). Remarkably despite the fact that measures of early conditions are not strictly comparable between the two samples analyzed here, their effects on the probability of experiencing chronic diseases are very close to each other. In both samples, the effects of poor early conditions are larger on the probabilities of suffering from some comorbidities than a single disease.
b. Excess risk of disability given the chronic illnesses
In both Puerto Rico and SABE cities, the presence of chronic diseases increases the probability of being disabled, even after controlling for level of education, smoking, age, and sex. This result is observed among individuals who experienced both good and poor early conditions (Table 3, bottom panel). Although not immediately clear from the coefficients in the table, examination of predicted probabilities (see below) reveals that these effects are larger among those who experienced poor early conditions. We also note higher effects on the probability of being disabled among individuals who suffer from more than one chronic disease (e.g. musculoskeletal diseases combined with circulatory diseases and others). Finally, the odds of having a functional limitation are higher among women, people who smoke, and individuals whose level of schooling is lower (results are not shown).
c. Decomposition of effects
Table 4 displays the results of the decomposition for Puerto Rico and SABE cities. It shows the contribution of each component (expressed in percentages) to the total difference in the probability of being disabled between those who did and did not experience poor early conditions (see formula 5, section II). The first panel displays the magnitude of the contribution of the effects operating through increases in the risk of experiencing a chronic condition. The second panel displays the magnitude of the contribution of the effects operating through increases in the risk of disability given a chronic condition. Both the first and second panels are broken down by groups of chronic illnesses. The third panel shows the residual component, namely, an estimate of the effect in the absence of chronic conditions identified in our analysis. Finally, in the last panel we show the total predicted probabilities of being disabled (for individuals who experienced poor and good early conditions) and the difference between those probabilities.
Table 4.
Percentage contribution of early conditions to the total difference in the probability of being disabled among the elderly in SABE cities1 (2000) and Puerto Rico (2002–03) between those who did and those who did not experience poor early conditions
| A. Contribution of the effect of early conditions in the presence of chronic diseases
| ||
|---|---|---|
| A.1 Chronic disease component (per cent)
| ||
| SABE cities1 (2000) | Puerto Rico (2002–2003) | |
| Respiratory | 0.67 | - |
| Mental | 2.13 | - |
| Circulatory | −8.64 | −6.54 |
| Circulatory and Respiratory | 0.76 | - |
| Circulatory and Mental | 4.33 | 0.51 |
| Musculoskeletal | −2.50 | −4.03 |
| Musculoskeletal and Mental | 5.93 | - |
| Musculoskeletal and Circulatory | 0.21 | −1.66 |
| Musculoskeletal, Circulatory, and Respiratory | 7.61 | 10.29 |
| Musculoskeletal, Circulatory, and Mental | 14.82 | 9.82 |
| Musculoskeletal, Circulatory, Mental, and Respiratory | 10.97 | 25.91 |
| Other combinations of diseases | 6.58 | 9.77 |
| Total chronic disease component (A1) | 42.88 | 44.07 |
|
| ||
| A.2 Disability component (per cent)
| ||
| Respiratory | −1.44 | - |
| Mental | 1.90 | - |
| Circulatory | 5.71 | 1.20 |
| Circulatory and Respiratory | 2.58 | - |
| Circulatory and Mental | 5.81 | −0.57 |
| Musculoskeletal | 4.91 | 4.04 |
| Musculoskeletal and Mental | 7.26 | - |
| Musculoskeletal and Circulatory | 21.58 | 10.62 |
| Musculoskeletal, Circulatory, and Respiratory | 1.91 | 7.97 |
| Musculoskeletal, Circulatory, and Mental | 8.67 | 17.69 |
| Musculoskeletal, Circulatory, Mental, and Respiratory | −5.29 | −1.31 |
| Other combinations of diseases | −1.12 | 10.06 |
| Total disability component (A2) | 52.46 | 49.70 |
|
| ||
| TOTAL “A” (A1+A2) | 95.34 | 93.77 |
|
| ||
|
B. Contribution of the effect of early conditions in the absence of chronic diseases (per cent)
| ||
| Absence of diseases (B1) | −5.54 | −2.13 |
| Disability given absence of diseases (B2) | 10.20 | 8.36 |
|
| ||
| TOTAL “B” (B1+ B2) | 4.66 | 6.23 |
|
| ||
|
C. Predicted probabilities
| ||
| Predicted probability of being disabled among those who experienced poor early conditions (C1) | 0.20 | 0.18 |
| Predicted probability of being disabled among those who did not experience poor early conditions (C2) | 0.14 | 0.11 |
|
| ||
| Total difference (C1 – C2) | 0.06 | 0.07 |
For some components we observe negative values suggesting that poor early conditions may sometimes attenuate the overall probability of disability. Almost all the negative values are associated with the pathway that operates by increasing the risk of experiencing a chronic condition. But in all these cases the magnitudes of effects are very low and they pale relative to the other components.
A remarkable feature of our results is the similarity across estimates in the two samples. First, the difference in the probability of being disabled among those who experienced poor and good early conditions is almost identical in both studies (0.06 for SABE and 0.07 for PREHCO). Second, in both cases the disability component and the chronic diseases components are equally important to explain the higher vulnerability to being disabled among those with poor early conditions. The percentage contributions are 52.5 per cent and 42.9 per cent in SABE cities, and 49.7 per cent and 44.1 per cent in Puerto Rico, respectively. Third, the residual effect (or the effect in absence of the K chronic illnesses recognized in our analysis) is very similar, about five per cent in SABE cities and about six per cent in Puerto Rico. In both datasets most of the effects of EC on disability take place through the chronic diseases included in the analysis. Finally, note that in both samples the groups of chronic illnesses that matter the most are those that include the joint occurrence of musculoskeletal, vascular and mental illnesses. The total contribution of this group of diseases (adding up both the disability and the chronic disease components) is about 24 per cent and 28 per cent in SABE cities and Puerto Rico, respectively. None of the groups of illnesses in isolation is able to contribute more than a few points to the total difference.
IV. Discussion
Our results suggest that the experience of poor early conditions (EC) increases significantly the probability of being disabled at older ages. These findings are consistent with conjectures about the nature and characteristics of elderly populations in Latin America and Caribbean countries. Current cohorts of elderly people in these countries have survived to old ages due largely to improvements in medical interventions and, to a much lesser extent, to ameliorations in standards of living. They represent a large proportion of individuals, whose birth and subsequent growth and development were significantly impaired by poor early conditions, including precarious nutrition and substantial exposure to infectious diseases such as tuberculosis, rheumatic fever, typhus fever, polio, and malaria among others. Therefore, it is among these cohorts where poor early conditions have a greater potential to express themselves and to induce effects on functional limitations later in life. Because Puerto Rico and other countries of the Latin American and Caribbean region experience demographic regimes that are quite similar, at least up to 1950 or 1960, the growth of the aging population and its composition by past experiences ought to be quite similar in both settings.
The main pathway through which poor early conditions could affect functional status later in life is through disability-inducing illnesses perhaps associated with musculoskeletal diseases, circulatory, and mental rather than through single diseases. Do poor early conditions increase the vulnerability of elderly by increasing the probability of suffering chronic conditions or, alternatively, by increasing the probability of being disabled (given the chronic illnesses)?
Results from the decomposition analysis reveal that both these mechanisms are by and large similar in importance in both Puerto Rico and SABE cities. One exception to the general pattern is the group with higher number of comorbidities (musculoskeletal, circulatory, mental, and respiratory). In this case, having experienced poor early conditions does not increase the risk of being disabled even though the probability of suffering from these diseases is higher among who suffer poor early conditions. Those who experience this group of illnesses are likely to be composed of very fragile individuals whose chronic conditions are more than enough to increase the risk of disability, independently of other characteristics including their early life experiences. Alternatively, this result could reflect selection effects that offset the influence that poor early conditions may have on the probabilities of being disabled.
We also find that there is a residual effect, albeit quite low, of poor early conditions on the probability of being disabled that remains even after controlling for chronic diseases (five per cent for SABE cities and six per cent for Puerto Rico). This may be consistent with recent literature that postulates a physio-developmental mechanism connecting EC and adult disability (Bailey et al 2001; Kuh et al. 2002; Ridler et al. 2005; Kuh et al. 2006). But it could also be a reflection of measurement errors or of chronic illnesses that were altogether ignored in our analysis since they were not included among the battery to be self-reported.
Some caveats are in order. First, it is very difficult to distinguish clearly the pathways through which EC affects disability or susceptibility to chronic illness. In order to shed some light on this issue we performed two exercises. The first considered only early socioeconomic conditions (ESES) to classify the individuals between poor and good conditions. The second considered only early health conditions (EHC). EHC is more likely to express effects associated with the first four pathways in Figure 1. ESES is more likely to express effects of the fourth and the fifth pathways in Figure 1. The joint indicator (which we use in this study) is more likely to express the operation of a combination of pathways without signaling which one is dominant. These two exercises, however, generate results that are very similar to the ones we obtain with the more coarse definition of EC. This outcome is somewhat disappointing since it does not confirm the idea that each of the two dimensions of measured EC are distinguishable from each other at least with the items available to us.
Second, our results cannot be interpreted as if they confirmed the existence of a “critical period” linking early conditions with health and disability later in life (Barker 1998) instead of alternative mechanisms. Even though our variables reflect well the conditions during a very important period (first 15 years of life) for the physical and cognitive development of the individual, we cannot identify separately what part of the effects are attributable to incidents and events that occurred during selected critical periods. Neither are we able to follow up the entire trajectory of the individuals who we study. Since we are controlling for current characteristics which are, in part at least, consequences of these trajectories, we are, to some extent, isolating the biological effects that take place before age 15. Therefore, our analysis focused on the biological pathways linking events experienced early in life, chronic diseases and disability (as depicted by figure 1).
Third, although our findings seem to be highly consistent with the literature about early conditions and morbidity profiles of disabled people, they are vulnerable to errors in self reports and recall errors. As is well known, the main problem with self reports of childhood events is that they are subject to recall bias which, in most cases, will induce attenuation of estimated effects. There could be systematic biases when accurate recall is a function of the intensity of the episode being recalled. For example, if recalls of illnesses that affect the individual during early life are more likely when typical symptomatology is more acute and visible or was the origin of traumatic experiences or both, then we will systematically bias downwards the estimates of effects since we will not be able to capture the occurrence of influential but non-acute episodes. Thus, for example, biases in self reported experiences with polio or rheumatic fever are less likely than in self-reports of more recurrent disorders and ailments such as digestive illnesses, influenza or dysentery. Self-reported conditions (health status and illnesses) are also distorted by perceptions as well as by lack of information (in many cases these are the result of conditions that predispose individuals to higher risk of diseases). However, systematic biases will creep through our analysis only if there is a correlation between biases in self reported chronic illnesses and self reported childhood conditions
Fourth, an important limitation of the study is that we are not able to ascertain the order of occurrence of chronic conditions and disability. All the analyses herein are predicated on the assumption that the latter follow the former but obviously this may not be the case among some individuals. In this case we are downplaying the role of the residual component and biasing upwards the estimates of the effects in presence of chronic diseases.
The findings in this paper shed some light on the nature of the relation between EC and disability and on the importance of mechanisms that work through well-identified chronic illnesses. They reveal that the residual component is of muted importance and that the bulk of the association is due to either increased risks of chronic illness or increased risks of disability given the experience of chronic illnesses. This is somewhat at odds with recent literature on the theme that emphasizes more generic mechanisms, those not linked to well-known disability-related chronic illnesses. Guralnik et al. (2006) shows a negative association between children’s social environment and objectively measured functional status at midlife. The relation persist after controlling for adult behavioral risk factors, middle-age SES and for disability-related chronic diseases such as cardiovascular diseases, cancer, diabetes, respiratory problems, neurological disease, and musculoskeletal symptoms. Kuh et al. (2006) find that physical growth, as a marker of early development of muscle fibers, and advanced childhood motor and cognitive abilities, as markers of central nervous system development, are positively related to midlife balance and chair rising, independently of later life experiences and health status. According to the authors, their findings may reflect the effect of early conditions on the number of muscle fibers, growth and tracking of muscle size which will be an alternative mechanism connecting early experiences and later physical performance, one that takes place in the absence of manifest chronic illnesses. To our knowledge all findings connecting early conditions and later disability come from high income countries (Sayer et al. 1998; Kuh et al. 2002; Guralnik et al. 2006; Kuh et al. 2006; Harkonmaki et al. 2007) and it is not clear at all that they could apply also to low income countries with their peculiar patterns of aging.
What is the significance of these results for future conditions of elderly in the LAC region? Elsewhere, it has been shown that the changing composition of the new elderly cohorts in terms of early experiences is likely to have an impact on mortality (Palloni et al., 2008). This impact is substantial enough that it can bring to a halt the linear increase in life expectancy at age 60 experienced since 1950. The implication of the results we obtain in this paper is that an upward progression of the fraction of elderly who have experienced poor early conditions should increase the prevalence of chronic illness and of disability, possibly compromising the future progress in healthy life expectancy. Whether healthy life expectancy stops increasing, begins to increase at a slower rate or actually declines will depend on the strength of exogenous macro forces (medical technology, access to and use of health care services, etc.) and of their power to offset the sheer momentum implied by the higher prevalence of elderly individuals who experienced poor early conditions.
Acknowledgments
The study was supported by grants from the National Institute of Aging (R01 AG016209 and R37 AG025216) and Fogarty International Center (FIC) training program (5D43TW001586) to the Center for Demography and Ecology (CDE) and the Center for Demography of Health and Aging (CDHA), University of Wisconsin-Madison. CDE is funded by the NICHD Center Grant 5R24HD04783; CDHA by the NIA Center Grant 5P30AG017266. We are very grateful to the reviewers of Population Studies for their valuable criticisms that helped us to improve the first version of this manuscript.
V. References
- Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13:679–89. doi: 10.1016/s0955-0674(00)00271-4. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and Disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The Welcome Fundation Lecture, 1994. The fetal origins of adult diseases. Proc Biol Sci. 1995;262(1363):37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Winter PD, Magaretts B, Simmonds SJ. Weight in infancy and death from ischemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker D. Mothers, Babies and Health in Later Life. 2. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Blackwell DL, Hayward MD, Crimmins EM. Does childhood health affect chronic morbidity in later life? Social Science & Medicine. 2001;52(8):1269–1284. doi: 10.1016/s0277-9536(00)00230-6. [DOI] [PubMed] [Google Scholar]
- Blaser MJ, Chyou PH, Nomura A. Age at Establishment of Helicobacter pylori Infection and Gastric Carcinoma, Gastric Ulcer, and Duodenal Ulcer Risk. Cancer Research. 1995;55:562–565. [PubMed] [Google Scholar]
- Camacho A. Stress and Birth Weight: Evidence from Terrorist Attacks. American Economic Review: Papers & Proceedings. 2008;98(2):511–515. [PubMed] [Google Scholar]
- Couzin J. Quirks of Fetal Environment Felt Decades Later. SCIENCE. 2002;296(21):2167–2169. doi: 10.1126/science.296.5576.2167. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. PNAS. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Gupta P. A General Method of Decomposing a Difference Between Two Rates into Several Components. Demography. 1978;15(1):99–112. [PubMed] [Google Scholar]
- Dowd JB. Early childhood origins of the income/health gradient: The role of maternal health behaviors. Social Science & Medicine. 2007;65:1202–1213. doi: 10.1016/j.socscimed.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Coulter CL, Symonds ME, McMillen C. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clinical and Experimental Pharmacology and Physiology. 2001;28:938–941. doi: 10.1046/j.1440-1681.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- Elo I, Preston S. Effects of Early-Life Conditions on Adult Mortality: A Review. Population Index. 1992;58(2):186–212. [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. SCIENCE. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Vaupel JW. Collecting Biological Indicators in Household Surveys. In: Finch C, Vaupel J, Kinsella K, editors. Cells and Surveys. Should Biological Measures Be Included in Social Science Research? Washington, D.C: National Academy Press; 2001. pp. 1–8. [PubMed] [Google Scholar]
- Fong IW. Emerging relations between infectious diseases and coronary artery disease and atherosclerosis. CMAJ. 2000;163(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of Comorbidity with Disability in Older Women: The Women’s Health and Aging Study. Journal of Clinical Epidemiology. 1999;52(1):27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MM. Maternal constraint of fetal growth and its consequences. Seminars in Neonatology. 2004:1–7. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Go MF. Review article: Natural history and epidemiology of Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics. 2002;16S:3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- Godfrey KM. The Role of the Placenta in Fetal Programming—A Review. Placenta. 2002;23(16):S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Butterworth BS, Wadsworth MEJ, Kuh D. Childhood Socioeconomic Status Predicts Physical Functioning a Half Century Later. Journal of Gerontology: MEDICAL SCIENCES. 2006;61A(7):694–701. doi: 10.1093/gerona/61.7.694. [DOI] [PubMed] [Google Scholar]
- Harkonmaki K, Korkeila K, Vahtera J, Kivimaki M, Suominen S, Sillanmaki L, Koskenvuo M. Childhood adversities as a predictor of disability retirement. J Epidemiol Community Health. 2007;61:479–484. doi: 10.1136/jech.2006.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney RP. Nutritional in bone health in elderly subjects: methodological and contextual problems. Am J Cli Nutr. 1989;50:1182–1189. doi: 10.1093/ajcn/50.5.1182. [DOI] [PubMed] [Google Scholar]
- Kitagawa EM. Components of a Difference between the Two Rates. Journal of American Statistical Association. 1955;50:1168–l194. [Google Scholar]
- Kuh D, Bassey J, Hardy R, Sayer AA, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002;156:627–633. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- Kuh D, Hardy R, Butterworth S, Okell L, Richards M, Wadsworth M, Cooper C, Sayer AA. Developmental origins of midlife physical performance: evidence from a British birth cohort. Am J Epidemio. 2006;164:110–121. doi: 10.1093/aje/kwj193. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition. CAB International; UK, USA: 2004. [Google Scholar]
- McDade TW, Kuzawa CW. Fetal programming of immune function: The early origins of immunity in Filipino adolescents. In: Langley-Evans SC, editor. Fetal nutrition and adult disease: Programming of chronic disease through fetal exposure to undernutrition. 2004. pp. 311–332. [Google Scholar]
- Miles TP, Furner SE. Early life Malnutrition and risk of hip fracture: identifying populations at risk. Age & Nutrition. 1991;2(3):137–140. [Google Scholar]
- Palloni A, Garcia A. The reliability of self reported conditions in early childhood: the case of Puerto Rico. 2008. (unpublished manuscript) [Google Scholar]
- Palloni A, McEniry M, Davila AL, Garcia A. A Contrarian View: is the Room for Improvements in Life Expectancy in Latin America and the Caribbean Shrinking?. Paper presented at the Population Association of America meeting; New Orleans. April 17–19.2008. [Google Scholar]
- Palloni A, McEniry M, Davila AL, Garcia Gurucharri A. The influence of early conditions on health status among elderly Puerto Ricans. Social Biology. 2005;52(3–4):132–163. doi: 10.1080/19485565.2005.9989106. [DOI] [PubMed] [Google Scholar]
- Palloni A, McEniry M, Wong R, Peláez M. The Tide to Come: Elderly Health in Latin America and the Caribbean. Journal of Aging and Health. 2006;18:180–206. doi: 10.1177/0898264305285664. [DOI] [PubMed] [Google Scholar]
- Palloni A, White R. Comparison of prevalence of chronic conditions among the elderly using self-reports and identification of symptoms: Puerto Rico 2000. 2006. (unpublished draft) [Google Scholar]
- Palloni A, Wyrick R. Mortality decline in Latin America: Changes in the structures of causes of deaths, 1950–1975. Social Biology. 1981;28(3–4):187–216. doi: 10.1080/19485565.1981.9988458. [DOI] [PubMed] [Google Scholar]
- Peláez M, Palloni A, Albala C, Alfonso JC, Ham-Chande R, Hennis A, Lebrao ML, Leon-Diaz E, Pantelides E, Pratts O. Encuesta Salud, Bienestar y Envejecimiento, 2000: Organización Panamericana de la Salud (OPS/OMS) 2003. [DOI] [PubMed] [Google Scholar]
- PREHCO. Puerto Rican Elderly: Health Conditions. 2002–03 [CD-ROM]. http://prehco.rcm.upr.edu/index.html.
- Preston SH. Mortality patterns in national populations with special reference to recorded causes of death. New York: Academic Press; 1976. [Google Scholar]
- Ravelli G-P, Stein ZA, Susser MW. Obesity in young men after adulthood appeared to rise linearly throughout childhood. One famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, Murray GK, Haapea M, Jones PB, Isohanni MK, Bullmore ET. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. PNAS. 2006;103(42):15651–15656. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. The Milbank Quarterly. 1989;67(3/4):450–484. [PubMed] [Google Scholar]
- Sayer AA, Cooper C, Evans JR, Rauf A, Wormald RP, Osmond C, Barker DJ. Are rates of ageing determined in utero? Age and Ageing. 1998;27:579–583. doi: 10.1093/ageing/27.5.579. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, feto-placental 11B-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 1997;62:89–94. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Shanks N, Stafford LL. The maternal-neonatal neuro-immune interface: Are there long-term implications for inflammatory or stress-related disease? J Clin Invest. 2001;108:1567–1573. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Palloni A, Riosmena F. Using self-reported chronic conditions and biomarkers to identify chronic conditions: sensitivity analyses. 2008. (unpublished draft) [Google Scholar]