Abstract
Objective:
To systematically review and assess the effectiveness and safety of antidepressants for neuropathic pain among individuals with spinal cord injury (SCI).
Methods:
A systematic search was conducted using multiple databases for relevant articles published from 1980 to April 2014. Randomized controlled trials (RCTs) involving antidepressant treatment of neuropathic pain with ≥3 individuals and ≥50% of study population with SCI were included. Two independent reviewers selected studies based on inclusion criteria and then extracted data. Pooled analysis using Cohen’s d to calculate standardized mean difference, standard error, and 95% confidence interval for primary (pain) and other secondary outcomes was conducted.
Results:
Four RCTs met inclusion criteria. Of these, 2 studies assessed amitriptyline, 1 trazadone, and 1 duloxetine among individuals with neuropathic SCI pain. A small effect was seen in the effectiveness of antidepressants in decreasing pain among individuals with SCI (standardized mean difference = 0.34 ± 0.15; 95% CI, 0.05-0.62; P = .02). A number needed to treat of 3.4 for 30% or more pain relief was found by pooling 2 studies. Of these, significantly higher risk of experiencing constipation (risk ratio [RR] = 1.74; 95% CI, 1.09-2.78; P = .02) and dry mouth (RR = 1.39; 95% CI, 1.04-1.85; P = .02) was found amongst individuals receiving antidepressant treatment compared to those in the control group.
Conclusion:
The current meta-analysis demonstrates that antidepressants are effective in reducing neuropathic SCI pain. However, this should be interpreted with caution due to the limited number of studies. Further evaluation of long-term therapeutic options may be required.
Keywords: antidepressants, meta-analysis, neuropathic pain, spinal cord injury
The majority of persons with a spinal cord injury (SCI) experience chronic pain following injury.1 Several treatment options for pain following SCI are available. As many aspects of an individual’s life are affected by pain, optimal pain management requires a multidisciplinary approach.2 Pharmacological treatment, particularly the use of antidepressants, may play an essential role in management of pain following SCI.
Antidepressants have an analgesic effect that has been proven to be effective in relieving neuropathic pain.2 Three classes of antidepressants that have been studied for their pain-relieving qualities are tricyclic antidepressants (TCAs), mixed serotonin noradrenaline reuptake inhibitors (SNRIs), and selective serotonin reuptake inhibitors (SSRI).2 It is speculated that these antidepressants exert their pain-relieving effect by inhibition of serotonin and/or norepinephrine reuptake.3,4 TCAs have been identified as a firstline treatment of post SCI neuropathic pain5 and general neuropathic pain.6–8 The use of TCAs is accompanied by several adverse effects including constipation, dry mouth, nausea, fatigue, and urinary retention.7,9 A different class of antidepressants, SNRIs, have demonstrated efficacy comparable to that of TCAs but are accompanied with fewer or less serious adverse effects.2 SNRIs inhibit noradrenaline and serotonin equally10,11 and are sometimes referred to as balanced inhibitors of serotonin and noradrenaline.11 SSRIs, conversely, inhibit serotonin reuptake while noradrenaline reuptake remains unaffected.7,11 These antidepressants are characterized as selective because they do not inhibit postsynaptic receptors.11 SSRIs are not commonly used to treat SCI-related pain and, due to limited efficacy, are not recommended for neuropathic pain management.7
Considering the extensive use of antidepressants for neuropathic pain post SCI, it is important to examine their efficacy and safety. To our knowledge, however, there has not been a published study reviewing the effectiveness of antidepressants for neuropathic pain in the SCI population specifically. Hence, the current study aims to systematically review and pool data on the effectiveness and safety of antidepressants for neuropathic pain among individuals with SCI.
Methods
Literature search strategy
A systematic review was conducted in April 2014 utilizing the following electronic databases: CINAHL (1982-present), Cochrane Database of Systematic Reviews (1991-present), EMBASE (1947-present), MEDLINE (1946-present), and PsycINFO (1806-present). A combination of MeSH, keywords, subject headings, and EMTREE terms were used to represent the 3 main concepts: antidepressant medications, pain, and spinal cord injury. This search was limited to published material in English with human subjects only. Reference lists of retrieved articles were searched for potentially relevant articles. In addition, EMBASE provided access to conference proceedings that might be relevant. Journals were not searched by hand, as the databases used captured those journals of particular interest.
Inclusion/exclusion criteria
Published studies were selected for analysis if the following criteria were met: (1) a pharmacological intervention utilizing antidepressants for the treatment of pain was described, (2) 50% or more of the population had an SCI, (3) the study was designed as a randomized controlled trial, and (4) participants were 18 years or older. Review articles were excluded, although reference lists of the articles were consulted for missing studies. To pool data from the studies using a meta-analytic process, we included only RCTs. Furthermore, to ensure that a more stringent methodology was utilized in the studies that were assessed, we selected only those studies that were published in a peer-review journal.
Selection of relevant studies
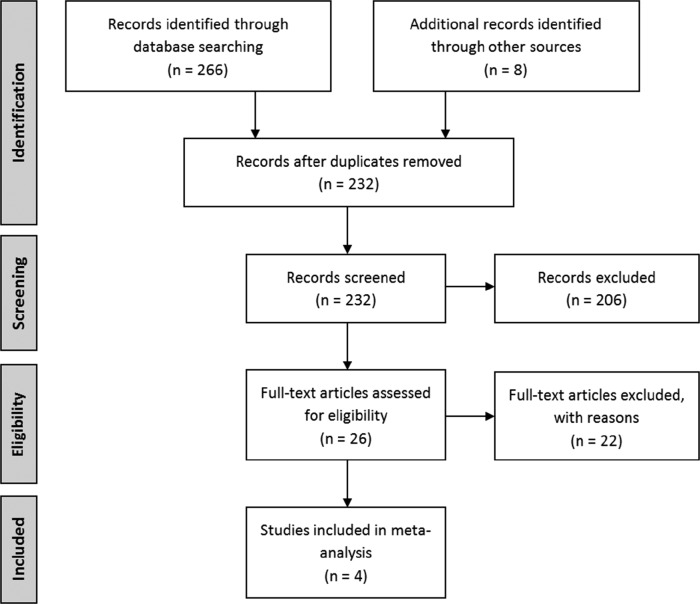
The titles and abstracts of retrieved articles were reviewed by 2 authors (S.G. and S.M.) following the removal of duplicates. A third reviewer (E.L.) resolved any conflicts. Full papers were retrieved for eligible studies. Figure 1 outlines the retrieval and selection of studies.
Figure 1. Study selection flow chart.
Study appraisal and data synthesis
Information pertaining to the following areas was extracted by 2 authors (S.M. and S.G.) using a data extraction form including intervention (allocation of participants, duration, control versus treatment protocol), study population (level of injury, demographics, duration of pain, start of pain), drug used, outcome measures, and results. Methodological quality of RCTs was assessed using the 3-item Jadad quality assessment tool.12
Data analysis
Pooled analyses were conducted for the primary outcome of pain along with adverse events (AEs). Treatment effect was reported as a standardized mean difference (SMD), standard error (SE), and 95% confidence interval (CI); adverse events were pooled based on risk ratio (RR) using the software Comprehensive Meta-Analysis v2 (Biostat, Englewood, NJ). A random effects model was used in pooling data from all studies. The criteria used by Cohen13 were used to interpret the resulting effect sizes: small ≥ 0.2; moderate ≥ 0.5; and large ≥ 0.8. All studies were pooled for the primary outcome measure. Only studies reporting the secondary outcome measures were pooled.
Results
Study selection and design
Four studies met inclusion criteria.14–17 All included studies were of high quality based on the Jadad scale. All 4 studies were randomized double-blind controlled trials. Most studies used a parallel group design14,15,17; Rintala et al16 utilized a triple crossover design comparing the effect of amitriptyline, gabapentin, and a placebo. For the purpose of the current study, the gabapentin data were not assessed; instead, data were collected from outcomes relating to amitriptyline and control group outcomes. Two studies used a placebo control.15,17 Cardenas et al14 and Rintala et al16 administered an active placebo, benztropine mesylate, and diphenhydramine, respectively. Amitriptyline was assessed in 2 studies14,16; trazadone15 and duloxetine17 were assessed in the other studies. Table 1 provides details on treatment protocol and dosages.
Table 1. Study characteristics.
|
Author Study design Jadad score |
N Drug Comparator Type of pain Duration of pain |
Dosage Treatment duration |
| Cardenas et al14 |
N: 84 Drug: Amitriptyline |
Dosage:
|
| RCT | Comparator: Benztropine mesylate | |
| Jadad: 5 | Type of pain: Chronic pain | Treatment duration: 6 weeks |
| Duration of pain: At least 3 months | ||
| Davidoff et al15 |
N: 18 Drug: Trazodone |
Dosage:
|
| RCT | Comparator: Placebo | |
| Jadad: 5 | Type of pain: Dyesthetic pain | Treatment duration: 8 weeks |
| Duration of pain: At least 1 month | ||
| Rintala et al16 |
N: 38 Drug: Amitriptyline |
Dosage:
|
| RCT | Comparator: Diphenhydramine | |
| Jadad: 5 | Type of pain: Chronic neuropathic pain | Treatment duration: 8 weeks |
| Duration of pain: At least 6 months | ||
| Vranken et al17 |
N: 48 Drug: Duloxetine |
Dosage:
|
| RCT | Comparator: Placebo | |
| Jadad: 5 | Type of pain: Central neuropathic pain | Treatment duration: 8 weeks |
| Duration of pain: At least 6 months |
Note: RCT = randomized controlled trial.
Patients with a history of depression or currently on antidepressant treatment to manage depression were excluded from Vranken et al.17 Cardenas et al14 excluded individuals meeting psychiatric diagnostic criteria for a major depressive disorder; however, they included individuals presenting with depressive symptoms. To ensure balance between the groups, randomization was stratified by level of depressive symptoms. Rintala et al16 included individuals with depressive symptoms. Their study conducted a subanalysis examining the effect of treatment relative to individuals’ depression scores on the Center for Epidemiologic Studies Depression Scale (CESD).
Two studies allowed for concomitant pain management treatments. Rintala et al16 required all participants to discontinue current pain management medications. All participants in the study, however, were provided with 8 tablets of a combination of 5 mg of oxycodone and 325 mg of acetaminophen for breakthrough pain. Vranken et al17 asked participants to maintain a stable regimen of their concomitant analgesic medication and to discontinue any antidepressant treatment at least 30 days prior to initiating the study medication. The remaining 2 studies did not provide information on concomitant treatment protocols.14,15
Compliance to treatment was assessed in 2 studies. Davidoff et al15 found that at 6 weeks, both groups had similar levels of compliance. By the eighth week, however, the placebo group had significantly more individuals compliant to treatment than the trazodone group (P < .01). Cardenas et al.14 found that 45.5% of individuals on amitriptyline and 45% of individuals on placebo were compliant to their treatment protocol. Rintala et al16 and Vranken et al17 did not examine compliance among study participants.
Effectiveness and adverse events of antidepressants
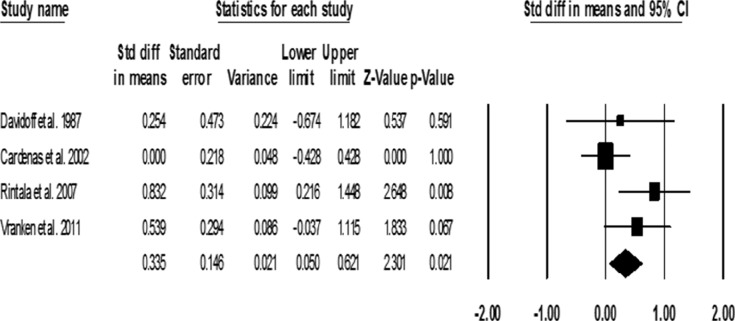
In a pooled analysis, a small effect was seen in the effectiveness of antidepressants in decreasing pain among individuals with SCI (SMD = 0.34 ± 0.15; 95% CI, 0.05-0.62; P = .02) (Figure 2). Trazadone was not found to significantly decrease pain among the treatment group compared to the control group (SMD = 0.25 ± 0.47; 95% CI, -0.67-1.182; P = .591). Conflicting evidence is seen between the 2 studies examining the effect of amitriptyline: Cardenas et al.14 found no significant reduction in pain (SMD = 0.0 ± 0.22; 95% CI, -0.428-0.428; P = 1.00), whereas Rintala et al16 found a large effect size (SMD = 0.832 ± 0.31; 95% CI, 0.10-1.45; P = .008). Vranken et al17 found a trend toward reduction of pain symptoms post duloxetine treatment (SMD = 0.54 ± 0.30; 95% CI, -0.04-1.115; P = .067). A number need to treat (NNT) of 3.4 for 30% or more pain relief was found by pooling 2 studies.16,17
Figure 2. Pooled standard mean differences (SMDs) of pain outcome post treatment.
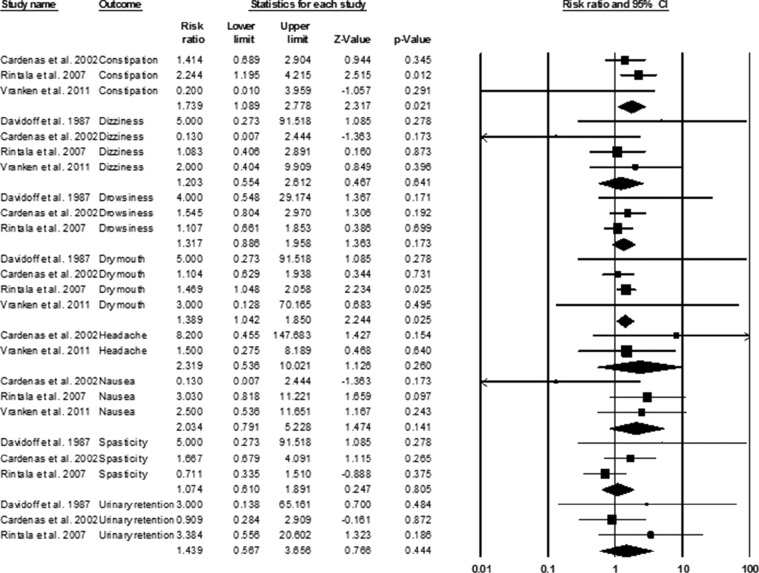
Pooled assessment was possible for 8 adverse events. Of these, significantly higher risk of experiencing constipation (RR = 1.74; 95% CI, 1.09-2.78; P = .02) and dry mouth (RR = 1.39; 95% CI, 1.04-1.85; P = .02) were found amongst individuals receiving antidepressant treatment compared to those in the control group (Figure 3).
Figure 3. Adverse events pooled risk ratios.
Discussion
The current meta-analysis found a small effect size in improving pain after SCI with antidepressant treatment. Similar ranges in effect size have previously been reported for treatment of pain with antidepressants in other conditions. Chan et al18 found that effect size for treating fibromyalgia pain was largest for TCAs, specifically amitriptyline; SNRIs (eg, duloxetine) were likely to have smaller effect sizes. The current study found a pooled NNT of 3.4 for a reduction in 30% or more pain. A previous Cochrane review on the effect of various antidepressants (9 TCAs, 5 SSRIs/SNRIs, 5 other antidepressant drugs, and St. John’s wort) on undifferentiated neuropathic pain found a similar NNT of 3.6.19 A review on chronic pain due to diabetic neuropathy found the overall effectiveness of antidepressants in general to be 1.3 in terms of NNTs.18
The 2 included studies that allowed for concomitant pain management treatment were also the 2 studies that demonstrated significance or trend towards reduction in neuropathic pain.16,17 Hence, it would be important to examine whether it is the synergistic effect of the treatments that reduced pain or the effect of the treatment of interest itself. Furthermore, in these studies there may also be a psychological effect influencing the effectiveness of the treatment. Because these individuals were either given extra treatment options or allowed to continue their previous treatment, this may have resulted in a greater locus of control for these individuals over their pain, thereby increasing their perception of pain reduction.
Among the various antidepressants, amitriptyline has been shown to be the most commonly administered in an SCI rehabilitation setting.20 In a recent Cochrane report on amitriptyline for the treatment of neuropathic pain and fibromyalgia, Moore et al21 reported an NNT of 4.6. However, the review found a relative risk of 1.5 for developing AEs in individuals receiving amitriptyline compared to those in the control group with a number needed to harm (NNH) of 4.1. The current study was unable to calculate NNH in the SCI population due to the lack of reported data in the included studies. Based on the increased incidence of AEs reported in the Cochrane review, however, amitriptyline should be used with caution. Since issues such as urinary retention and constipation may already be concerns in the SCI population; examination of the long-term effects of amitriptyline use among individuals with SCI is warranted.
The use of duloxetine is considered first line of treatment for management of neuropathic pain by the Neuropathic Pain Special Interest Group.22 Watson et al23 found an NNT of 5.3 for 50% pain relief with duloxetine (60 mg/day) among various populations including knee osteoarthritis, fibromyalgia, painful diabetic neuropathy (PDN), and low back pain. A review by Verdu et al24 found that providing 60 mg duloxetine twice a day resulted in an NNT of 4.9 in those with chronic pain. Although duloxetine showed a trend toward improvement in pain symptoms in the current study, it did not reach statistical significance. This may be due to the lack of power in the study to show a treatment difference. Another factor for the lack of observed effect may be due to the low rates of compliance reported in the Davidoff et al15 study. Examination of potential deterrents for adhering to a treatment protocol should be evaluated. Further study into the efficacy of duloxetine would be beneficial.
Limitations
There are several limitations of this review. The current study was limited by the number of RCTs available. In addition, there was a lack of reporting details to calculate NNH on the AEs experienced by the participants in the study. Furthermore, each study had a relatively low number of participants (<25 individuals per group), except for Cardenas et al,14 with 44 individuals per group. Future studies with larger sample sizes and long-term follow-up may be necessary to examine the effect in the SCI population. Another significant limitation to the current study was the presence of participants with depressive symptoms. Only one study16 examined the effect of antidepressants among individuals with highly depressive symptoms and found that those individuals who presented with depressive symptoms were more likely to improve their pain. Hence, it is difficult to ascertain the effect depression may have on the treatment of neuropathic pain. Furthermore, due to the similarity between the AEs experienced among participants on antidepressant treatment and those experienced by SCI individuals in general, it may be important to differentiate between the 2 and determine whether antidepressant treatments may accentuate the risk of AEs that SCI individuals are already likely to face.
The treatment compliance rate among the studies was low. This can result in the studies being underpowered to detect significant difference between the 2 groups. The low compliance could be due to the increased levels of adverse events. The use of dose titration techniques may help to decrease the effect of adverse events. Furthermore, the use of motivational models for self-managing pain among participants may help increase treatment adherence. Jensen et al25 developed a model to help engage and motivate participants in adhering to chronic pain treatments. The model incorporates perceived importance, self-efficacy, and readiness to change to help individuals improve their self-management behaviors.
Conclusion
The current review suggests that antidepressants are effective in reducing neuropathic SCI pain. Individual antidepressants have varying ranges of effects, with amitriptyline demonstrating the largest effect sizes followed by duloxetine. However, due to the increased AEs reported among those on amitriptyline and the low rates of adherence to treatment, it may not be an optimal treatment for individuals with SCI. The effects of treatments were also potentially enhanced by concomitant treatment. Therefore, the examination of multimodal treatment plans may be warranted. Duloxetine may be an important emerging treatment for neuropathic pain post SCI due to its increasing use among other pain populations. Future studies into the use of other novel antidepressants may be required to increase the therapeutic options available to individuals with neuropathic SCI pain.
Acknowledgments
The authors declare no conflicts of interest.
References
- 1.Cardenas DD, Felix ER. Pain after spinal cord injury: A review of classification, treatment approaches, and treatment assessment. PM R. 2009;1:1077–1090. [DOI] [PubMed] [Google Scholar]
- 2.Demarin V, Basic-Kes V, Zavoreo I, et al. Ad hoc committee of the Croatian Society of Neurovascular Disorders, Croatian Medical Association: Recommendations for neuropathic pain treatment. Acta Clin Croat. 2008;47:181–191. [PubMed] [Google Scholar]
- 3.Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. [DOI] [PubMed] [Google Scholar]
- 4.Attal N, Mazaltarine G, Perrouin-Verbe B, Albert T. Chronic neuropathic pain management in spinal cord injury patients. What is the efficacy of pharmacological treatments with a general mode of administration ? Ann Phys Rehabil Med. 2009;52:124–141. [DOI] [PubMed] [Google Scholar]
- 5.Bryce TN, Ragnarsson KT. Pain after spinal cord injury. Top Spinal Cord Injury Rehabil. 2000;11:157–168. [PubMed] [Google Scholar]
- 6.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: Evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25:81–104. [DOI] [PubMed] [Google Scholar]
- 7.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. [DOI] [PubMed] [Google Scholar]
- 8.Teasell RW, Mehta S, Aubut JL, et al. ; the Spinal Cord Rehabilitation Evidence Research Team. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010;91:816–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnerup NB, Jensen TS. Spinal cord injury pain - mechanisms and treatment. Eur J Neurol. 2004;11:73–82. [DOI] [PubMed] [Google Scholar]
- 10.Irving GA. Contemporar y assessment and management of neuropathic pain. Neurology. 2005;64:S21–S27. [DOI] [PubMed] [Google Scholar]
- 11.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96:399–409. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized 282 clinical trials: Is blinding necessary ? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Academic Press; 1988. [Google Scholar]
- 14.Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: Results of a randomized controlled trial. Pain. 2002;96:365–373. [DOI] [PubMed] [Google Scholar]
- 15.Davidoff G, Guarracini M, Roth E, Sliwa J, Yarkony G. Trazodone hydrochloride in the treatment of dysesthetic pain in traumatic myelopathy: A randomized, double-blind placebo-controlled study. Pain. 1987;29:151–161. [DOI] [PubMed] [Google Scholar]
- 16.Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547–1560. [DOI] [PubMed] [Google Scholar]
- 17.Vranken JH, Hollmann MW, van der Vegt MH, et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: A randomized, double-blind, placebo-controlled trial. Pain. 2011;152:267–273. [DOI] [PubMed] [Google Scholar]
- 18.Chan HN, Fam J, Ng BY. Use of antidepressants in the treatment of chronic pain. Ann Acad Med Singapore. 2009;38:974–979. [PubMed] [Google Scholar]
- 19.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Systematic Rev. 2007;4:CD005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janzen S, Mehta S, McIntyre A, Teasell R. Antidepressant prescription patterns in SCI and ABI rehabilitation. Arch Phys Med. 2012;93:e47. [Google Scholar]
- 21.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Systematic Rev. 2012;12:CD008242. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clinic Proceed. 2010;85:S3–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson CPN, Gilron I, Sawynok J, Lynch ME. Nontricyclic antidepressants analgesics and pain: Are serotonin norepinephrine reuptake inhibitors (SNRIs) any better ? Pain. 2011;152:2206–2210. [DOI] [PubMed]
- 24.Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–2632. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Nielson WR, Kerns RD. Toward the development of a motivational model of pain self-management. J Pain. 2003;4:477–492. [DOI] [PubMed] [Google Scholar]