Abstract
• Prostate MRI is currently the best diagnostic imaging method for detecting prostate cancer
• Magnetic Resonance Imaging-Ultrasound (MRI/US) fusion allows the sensitivity and specificity of MRI to be combined with real time capabilities of transrectal ultrasound (TRUS).
• Multiple approaches and techniques exist for MRI/US fusion and include (1) direct “in bore” MR biopsies, (2) cognitive fusion, and (3) MRI/US fusion via software-based image co-registration platforms.
Keywords: prostate MRI, MRI/Ultrasound fusion, targeted biopsy, MRI/US fusion platforms
INTRODUCTION
Prostate cancer (PCa) is the second most common malignancy found in men with an estimated 903,500 new cases worldwide per year [1]. In the pre-prostate specific antigen (PSA) era, screening for PCa consisted primarily of the digital rectal exam (DRE). However, inherent in the use of DRE was the understanding that diagnosis was operator-dependent and preferentially detected larger tumors located posteriorly in the gland. Biopsies were then directed to the palpable lesion using finger guides. [2]. However, controlled studies failed to demonstrate a reduction in PCa mortality following routine DRE exam alone [3]. As a consequence, after its discovery as a serum marker, PSA was adopted in the late 1980s as a screening tool. Threshold values of PSA were used to determine the need for random biopsies of the prostate. Since the 1980s, the number of samples obtained per biopsy session has gradually increased.
Following the introduction of PSA testing, the incidence of PCa rose dramatically with the greatest increases seen in local-regional disease with a relative decrease in diagnoses of metastatic disease [4]. Although initially introduced as a potential screening technique, transrectal ultrasound (TRUS) proved to have too many false negatives. Initially TRUS was used to guide biopsies to hypoechoic areas which resulted in a 66% PCa detect rate [5]. Eventually TRUS was adopted as a method to systematically sample the prostate gland using a needle guide coupled to a tranrectal ultrasound probe. Thus, a systematic sextant biopsy technique in conjunction with sampling of hypoechoic lesions has traditionally been the preferred biopsy method, yielding 9% greater detection of PCa compared to biopsy of palpable or sonographic abnormalities alone [6].
Further refinement and evolution of the systematic sextant technique has continued in efforts to improve biopsy yield with schemes that increase the number of systematic cores ranging from ten to eighteen per prostate, and some have even adopted “saturation biopsies” (twenty or more systematic cores per biopsy session) technique [7]. However, there continues to be much debate over the idealized schema for TRUS biopsy as PCa detection rates are low and range anywhere from 33-44% and many of these tumors are not clinically significant [8-10]. Recently concern over the increasing risk of antibiotic resistant infection has prompted a reevaluation of patient preparation, as well as the number and frequency of prostate biopsies [11].
MRI AS A DIAGNOSTIC MODALITY IN PROSTATE CANCER
Magnetic resonance imaging (MRI) was introduced as a staging method for PCa staging in the early1990s, and was primarily used to assess extracapsular extension or seminal vesicles invasion [12, 13]. However, actual detection of prostate cancers within the gland was considered limited. With improved technology, MRI with an endorectal coil was found to be increasingly useful in identifying and characterizing lesions in the prostate as well as detecting recurrent disease after treatment [14, 15]. T2 weighted scans seemed particularly useful and dynamic contrast enhanced (DCE) MRI was also considered helpful in confirming tumors. More recently, the ability of MRI to detect central and anterior prostate cancers has enabled diagnosis of large tumors that went undetected on random biopsies [16]. The addition of MR spectroscopic imaging (MRSI), a functional method that detects relative levels of choline and citrate within tumors, added to the specificity of MRI [17]. Over the past few years, diffusion weighted imaging (DWI) has been added to the list of parameters that are useful in detecting prostate cancer. The inclusion of two or more MRI parameters—T2 weighted, DWI, MRSI, and DCE MRI—became known as multiparametric MRI, and many studies demonstrated improved detection and localization of prostate cancers when two or more of these parameters were positive [18, 19]. However, because each individual MR technique has its own shortcomings, multiparametric MRI (mpMRI) combines the benefits of each individual MRI sequence in order to provide the greatest sensitivity and specificity for cancer foci (Figure 1A-D).
Fig. 1.
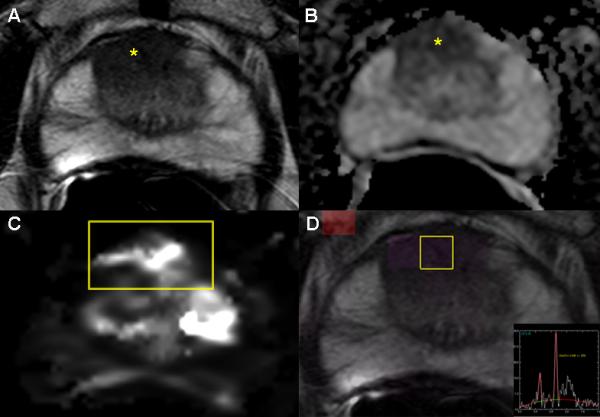
Images from a 65 year old male with serum PSA 8.7 ng/mL and four previously negative TRUS biopsies who underwent a multiparametric MRI(mpMRI).The axial T2W MR image (A) demonstrates an anterior hypointense lesion in the right apical central gland (yellow asterisk); an ADC map of DW-MRI (B) shows a hypointense focus (yellow asterisk) indicating restricted diffusion; quantitative mapping from DCE-MRI (C) localizes the tumor(yellow box); and MRSI (D) (yellow box) demonstrates an increased choline-to-citrine ratio within the lesion. This patient underwent a MRI/US fusion-guided biopsy following mpMRI demonstrating Gleason 4+4 = 8 (90% in 2 targeted cores) in the right anterior lesion.
As advancements in prostate mpMRI—such as endorectal coils and high field strength magnets to improve signal-to-noise ratios—continue, there has been growing recognition that mpMRI can risk stratify suspicious lesions prior to biopsy. For instance, MRI results such as the apparent diffusion coefficient (ADC) values calculated from DWI provide quantitative correlation between MRI results and tissue histology. Such prognostication could allow for potentially fewer biopsies if patients could be confidently stratified into low or high-risk disease categories based upon imaging findings [20]. Thus, successful prostate cancer detection requires high specificity in addition to high sensitivity, and MRI provides both.
CONCEPTION OF MRI-GUIDED BIOPSY TECHNIQUES
With the increased recognition of the capabilities of prostate mpMRI for detecting cancers, attempts were made to incorporate MRI into routine prostate biopsies. It has been explored as a sole imaging modality for targeting biopsies or in conjunction with TRUS biopsy, a procedure that is already in the armamentarium of urologists. Three approaches have emerged that utilize MRI information for guiding targeted prostate biopsies — (1) direct “in bore” MR biopsies, (2) cognitive fusion, and (3) MRI/US fusion via software-based image co-registration without requiring the MRI to be physically present. In the sections below, we have reported briefly on the published clinical data for direct “in-bore” MRI and cognitive fusion techniques and then focused in detail on all software fusion platforms that have published clinical data with reporting of all currently available clinical results via a PUBMED, EMBASE, and Cochrane databases.
Direct “in-bore” MRI
Initial attempts to use MRI to guide biopsies involved direct “in bore” approaches. The patient is typically placed prone in the MRI scanner and MRI is performed to localize lesions found previously on a diagnostic MRI. Using either a transrectal or transperineal approach, needles are introduced into the visible lesions and samples are obtained with serial MR scans to confirm biopsy needle placement. For this method, only suspicious lesions are targeted [21-23]. The advantages of this method include a reduction in the number of biopsy cores, precise recording of biopsy needle locations, as well as selecting only those patients with significant lesions. Disadvantages include relatively lengthy procedures that can be uncomfortable for the patient and often require sedation. Moreover, the inability for real-time intervention given the limited space inside a MRI machine, specialized equipment, costs and availability of this technique has limited its use. Additionally, the stringent safety requirements of the magnetic environment place constraints on the type of needles and monitoring equipment that can be used. A high level of awareness regarding the environment must be observed by every member of the team or serious injury can result to the patient.
The number of studies reporting results with direct in-bore biopsies is limited. Many do not incorporate mpMRI for lesion identification or do not use a comparator such as systematic TRUS biopsy. As such, detection rates for clinically-insignificant disease, small-volume Gleason ≤ 6 ranges anywhere from 19.2-78.6% [23-26]. Notable however is work from Hambrock and colleagues [27] which incorporates mpMRI (T2W, DCE, DWI) and a 10-core TRUS comparator and found that in-bore MR-guided biopsies performed significantly better than TRUS-guided biopsies (88% versus 55%, p = 0.001) for PCa detection when assessing radical prostatectomy specimens
Cognitive fusion
Conceptually, cognitive fusion the simplest of the MRI guided biopsy methods. It requires no additional equipment but requires that an experienced operator estimate lesion location based on the MRI. The operator first reviews MR images for suspicious areas and then plans and performs a systematic TRUS-guided biopsy, trying to biopsy the general location of the suspicious lesions identified on diagnostic MRI. Because this technique requires no additional equipment, it can be immediately used in urology offices. However, a primary disadvantage is that it depends on the ability of the operator to translate the MRI findings onto the ultrasound images. This requires experience and training and leads to inaccuracies, subjectivity, variability, and lack of reproducibility. Furthermore, regardless of operator experience, MR images are acquired in an axial plane while 2D TRUS end-fire biopsy is obtained at multiple differing oblique planes, which increases variability and potential inaccuracy of tissue acquisition. Additionally, unlike the other approaches, not all cognitive approaches will have the ability to record and archive biopsy location, which can be important for repeat biopsies and active surveillance.
Initial comparative studies of cognitive fusion versus systematic 10-12 core TRUS-guided biopsy demonstrate that this targeting method increases PCa detection, accuracy, and representation of disease burden as well as Gleason grade identified on biopsy pathology [28-31]. Results of cognitive fusion using a transperineal approach versus a TRUS approach have also demonstrated similar results, but may potentially minimize the oblique sampling plane of TRUS biopsies and allow for biopsy location documentation [32]. Two additional studies have compared cognitive fusion versus software-based MRI/US fusion platforms (Urostation, Koelis; Virtual Navigator, Esaote) and presented conflicting results on performance versus random biopsy [33, 34]—thus indicating the need for further studies.
MRI-US Fusion via a Software Platform
The next step in the evolution of MR-targeted prostate biopsies was to fuse mpMRI to a real time TRUS image. In this way MRI can be used to localize a tumor but TRUS can be used to guide the needle—enabling prostate biopsy to be performed in outpatient centers or doctors’ offices, much like the cognitive technique. Furthermore, because TRUS is already used to guide random biopsies, MRI-US fusion does not alter the normal workflow of urologists who typically perform the biopsy. The patient is in a far more comfortable environment and often only local anesthetic is required.
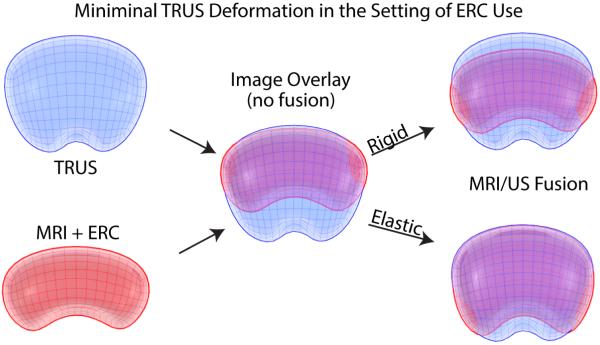
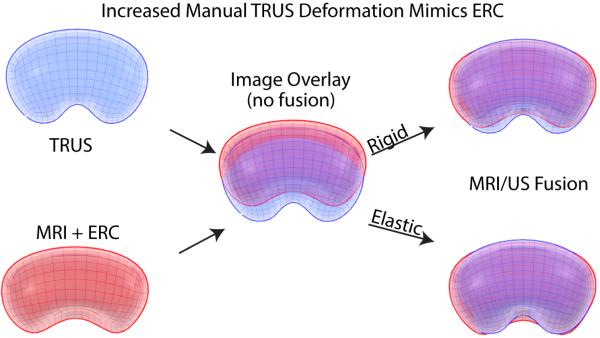
This method is rapidly evolving, with the major technical hurdle involving the “registration” of the MRI to the ultrasound image. Because the prostate on MRI (with or without an endorectal coil) often differs in shape and deformation from the same prostate on TRUS, some method of image registration must take place for successful fusion. This process can involve the identification of landmarks (e.g. points, curves, surfaces) which can be recognized on both corresponding images, thereby allowing the two images to become aligned through either a “rigid” or “elastic” transformation. Rigid transformations do not change the images themselves, but allow for translation and rotational variations between images, while elastic transformations account for the addition of local deformation, warping, or scale changes as well (Figure 2A). It is important to note that elastic methods stretch or warp one of the image volumes, thus data is also stretched and moved. Therefore, rigid registration-derived images may look less pleasing to the eye when looking at image borders, but the data integrity is greater, because anatomy is not artificially altered by the computer in order to create the appearance of a “match.” Further, operator input may often adjust or correct for rigid registration error or offset, with either manual correction, or manual adjustment of targeting, or even adjustment of the manual insertion depth or pressure from the TRUS transducer (Figure 2B). The registration step is probably the biggest opportunity for operator error.
Fig. 2.
Elastic and Rigid Software Image Registration Methods. Pre-biopsy MR data is registered with real-time TRUS images by aligning landmarks(e.g. points, curves, surfaces,) in corresponding images via rigid or elastic transformations. (A) represents MRI/US registration when there is minimal TRUS deformation and use of an endorectal coil (ERC) for MR images, and (B) demonstrates increased manual TRUS deformation that can mimic ERC deformation. As seen above, a simple overlay of TRUS and MRI models (middle images in panels A and B) results in reduced correlation between imaging modalities. A rigid registration method can account for translational and rotational differences between models while an elastic registration method has the additional ability to account for local deformations (e.g. caused by an endorectal coil or TRUS probe). However, elastic warping can move or alter relative anatomic location despite more matched borders. ERC, endorectal coil.
Kaplan and colleagues [35] described a transperineal biopsy utilizing a rigid stereotactic stepper device, commonly employed for deployment of brachytherapy seeds in 2002. Since then, multiple MRI/US software platforms have been developed. The existing platforms with published clinical data to date are summarized in Table 1. The general work flow of all platforms first requires a pre-biopsy diagnostic MRI to identify and annotate lesions suspicious for cancer based on imaging characteristics, with interpretation by a prostate-trained radiologist. Then, depending on the particular platform, targets are delineated before or after MR data has been loaded onto the software platform. TRUS-guided biopsy of the prostate is performed and MRI and real-time TRUS images are superimposed and displayed side- by-side, thus creating an easily navigable 3D prostate reconstruction. Because MRI and ultrasound images have been co-localized and co-registered, allowing blending back and forth between MRI and TRUS.
Table 1.
Summary of MRI/US Fusion Platform Specifications
| MRI |
ULTRASOUND |
BIOPSY |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fusion System – Trade Name (Manufacturer) |
Principle Investigator Location |
MRI | Parameters Used to Define Target |
Endorectal/ Pelvic Coil |
Ultrasound Image Acquisition |
Method
of Image Registration |
Tracking Mechanism |
Biopsy Route |
# Cores per MR Lesion |
Comparator |
| UroNav (In Vivo/Philips) | Bethesda, USA [36-39] | 3T Philips | T2W, DCE, DWI, Spectroscopy | Y/Y | Manual US 2D sweep. Freehand manipulation of US probe. | Rigid | Electromagnetic tracking US | Transrectal | Minimum 2 | 12 core TRUS |
| Artemis (Eigen) | Los Angeles, USA [40,41] | 3T Siemens | T2W, DCE, DWI | N/N | Manual rotation along a fixed axis (US probe on a tracking arm) | Elastic | Mechanical arm with encoded joints | Transrectal | Mean 2.2 | 12 core TRUS |
| Urostation (Koelis) | Paris, France [34] Oslo, Norway [43] Grenoble, France [44] |
1.5T Siemens 1.5 Siemens 3T Philips |
T2W, DCE, DWI T2W, DWI T2W, DCE, DWI |
Y/Y Y/N N/N |
Automatic US probe rotation, 3 different volumes elastically registered | Elastic | Image-based registration | Transrectal | Minimum 2 Minimum 2 2 |
10-12 core TRUS 12 core TRUS 12 core TRUS |
| BiopSee (Pi Medical/MedCom) | Heidelberg, Germany [45] | 3T Siemens | T2W, DCE, DWI | N/N | Custom-made biplane Transrectal US probe mounted on a stepper |
Rigid | Stepper with 2 built-in encoders | Transperineal | Median 4 | 12 core TRUS |
| Virtual Navigator (Esaote) | Paris, France [34] Lille, France [33] |
1.5T Siemens 1.5T Philips |
T2W, DCE, DWI T2W, DCE, DWI |
Y/Y N/Y |
Manual US sweep. Freehand rotation of US probe. | Rigid | Electromagnetic tracking US and needle | Transrectal | Minimum 2 Minimum 2 |
10-12 core TRUS 12 core TRUS |
| HI RVS/Real-Time Virtual Sonography (Hitachi) | Chiba, Japan [46] | 1.5T Philips | T2W, DCE, DWI | N/Y | Real-time biplanar TRUS | Rigid | Electromagnetic tracking | Transrectal or transperineal | 1-2 | 10 core TRUS |
The major differences between fusion platforms are the registration method, operator input, and their original intended use. Electromagnetic tracking fusion was primarily designed for prospectively navigating a needle to an MRI target, and later adapted to archive locations or prior biopsies. Image based fusion was originally designed to track, document, and archive the location of biopsy, and was later adapted to prospectively target MRI-defined targets. Image-processing based fusion may have limitations in speed or accuracy of prospective needle guidance, but is significantly less cumbersome. Cognitive fusion is also limited in accuracy which means it may be difficult for small targets. Learning curves and degree of automation may vary. Automatic organ edge detection, automatic segmentation (outlining of the organ), and motion compensation are facilitating tools to help account for differences in TRUS insertion depth. Some image-based registration platforms may require a several second pause with the needle in place, in order to track and archive the location. The platforms also differ greatly in the degree to which they are seamlessly integrated to the MRI workstations. Less human input translates into faster with less room for error.
The platforms also differ in regards to steps for manual input (or operator-refinement or “tweaking”) of an automatic registration, which is the art of fusion biopsy. Will all platforms be reproducible, standardized, and able to normalize the procedure? The platforms differ also in terms of how the three dimensional ultrasound volumes are built. Some build a 3D volume from a 2D “sweep” with the TRUS transducer fanning out sequentially obtaining 2D images from known perspectives defined by electromagnetic tracking, for example. Others are able to use a biplane probe or a 3D probe that can acquire the data in 3D, rather than reconstruct it. At present, all systems also vary in terms of their ultrasound vendor requirements, with some limited to one vendor, and others relatively vendor-agnostic to varying degrees.
The graphical user interfaces also differ markedly, with some displaying side by side “co-displayed” MRI and US separately, and others displaying a blended fusion image where the ultrasound and MRI can both be seen on the same image in different colors or grey scales. The TRUS guidance can be primarily relied upon with modifications based upon fusion information, or alternatively the fusion can be relied upon with TRUS input for modifications. Needle depth can be estimated based upon visual feedback from TRUS, and automatic needle detection algorithms are available that automatically detect the distal-most tip of the biopsy needle.
URONAV/IN VIVO
The UroNav platform (In Vivo, USA) was the first office-based fusion biopsy platform and was developed at the National Institutes of Health in Bethesda, Maryland, USA in collaboration with Philips Healthcare. Patient recruitment began in 2004 and has since continued to undergo clinical testing and development. Research done at the NCI has largely employed a diagnostic mpMRI performed at 3T (Philips Achieva MRI) using four MR parameters (T2W, DCE, DWI, Spectroscopy) to identify lesions and individually assign them as low, moderate, or high suspicion for prostate cancer based on their imaging characteristics and abnormal MR parameters [36]. Biopsy needle localization and tracking data is recorded via an external magnetic field generator but the biopsy uses existing freehand ultrasound technology. The biopsies are performed transrectally. Once the MR data is loaded onto the software platform and an initial TRUS sweep is performed, rigid image fusion is performed and clinicians are able to see both the MRI and US images move in real time. This allows for a lesion to be targeted on MRI but monitored via TRUS for the course and depth of the needle to ensure that it enters the suspicious area. Because biopsy still uses familiar freehand TRUS technology, training for this platform is primarily software-based and can be gained after only a few biopsy sessions.
Initial data for the first 101 men who underwent biopsy on a research-based iteration of UroNav demonstrated that 89.5% of men with high suspicion lesions on MR were diagnosed with PCa, with targeted cores detecting more PCa than standard 12-core TRUS cores [37]. Findings have since been updated to more closely assess the utility of the MR lesion suspicion scoring for cancer detection; results of 582 patients have demonstrated an increasing correlation between mpMRI suspicion and Gleason score with detection for Gleason ≥ 8 PCa showing a 98% sensitivity at the low-moderate cutoff and a 91% negative predictive value (NPV) at the moderate-high cutoff [38]. Overall cancer detection rates are nearly equivalent for targeted versus systematic (80% vs 81%) (Table 2), but the addition of targeted cores to systematic cores markedly increased the detection rates of intermediate-high risk disease with 32% of patients upgraded after targeted biopsy. Furthermore, targeted cores detected clinically significant disease (biopsy Gleason Score (bGS) ≥ 4+3) in 18% of patients with negative systematic biopsies, while systematic cores detected 8% of Gleason ≥ 4+3 cases missed by targeted biopsy [39].
Table 2.
Patient-Based Histologic Biopsy Outcomes by Fusion Platform
| MRI |
Biopsy Results |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fusion System – Trade Name (Manufacturer) |
Principle Investigator Location |
Patient Inclusion Criteria |
Defining Lesion Suspicion for PCa |
# of Patients with MR Suspicious Lesions |
Cancer Detection in Patients with MR Lesion (# of Patients) |
Cancer Detection (TB) |
Cancer Detection (SB) |
p- value |
Additional/Notable Study Conclusions |
| UroNav (In Vivo/Philips) | Bethesda, USA [36-39] | August 2007-August 2012 | Low, moderate, or high | Low - 123 (21%); Moderate - 370 (64%); High - 89 (15%) | 315/582 (54%) | 253/315 (80%) pts | 255/315 (81%) pts | --- | TB upgrades and detects higher Gleason score in 36% of patients compared with TRUS. Lesions with high suspicion on mpMRI associated with higher rate of Gleason upgrading on target vs 12 core biopsy |
| Artemis (Eigen) | Los Angeles, USA [40-41] | March 2010-September 2011. | Score 1 to 5 (normal to highly suspicious) | 151/171 (88%) | 84/151 (56%) | 101/486 (20.8%) cores. | 127/174 1 (7.3%) cores | 0.001 | TB is three times more likely to identify disease than SB (20.8% vs 7.3%, p=0.001) with greater detection of intermediate to high risk disease (36% vs 24%, p=0.037)Biopsy findings correlate with level of MR suspicion. |
| Urostation (Koelis) | Paris, France [34] | January 2011 - March 2012. | Yes/No | 82/133 (62%) | 71/82 (87%) | 62/82 (76%) patients | 44/133 (33%) patients | --- | TB cancer detection was sig higher than SB (p = 0.0016). Cognitive TB no diff from SB (= 0.66) |
| Oslo, Norway [43] | December 2010 - May 2011. | Low, medium, or high | 80/90 (89%) patients. Lesions - High (55), Medium (6), Low (4) | 54/80 (67.5%) | 60/115 (52%) Targets. | 6/42 (14%) patients | --- | 10 pts neg MRI all neg RB. 112/115 (97%) MR targets successful, 50/115 (52%) positive for cancer. | |
| Grenoble, France [44] | November 2011 - August 2012. | Prostate Imaging-Reporting and Data System (PI-RADS) | 20/30 (67%) | 11/20 (55%) | --- | --- | --- | Urostation has good accuracy for targeting suspicious areas on MRI | |
| BiopSee (Pi Medical/MedCom) | Heidelberg, Germany [45] | June 2010 - December 2011. | Highly suspicious, Questionable, or not suspicious | Highly suspicious -104/347 (30%); Questionable - 149 (43%) ; Not suspicious -94 (27%) | Overall - 200/347 (58% pts). Highly suspicious -(82.6% pts). Questionable - (67% pts). Not suspicious (14.8% pts). | 386/1281 (30%) cores | 523/632 6 (8.2%) cores | 0.01 | TB detect significantly more cancer than SB (30% vs 8.2%) p = 0.01 and of greater significance (41% had significant PCa).Patients without cancer-suspicious MR lesions, 11.7% (11/94) were diagnosed with intermediate risk disease. 50.6% pts (152/300) patients reported mild hematuria, 26% temporary erectile dysfunction. |
| Virtual Navigator (Esaote) | Paris, France [34] | January 2011 - March 2012. | Yes/No | 78/131 (59%) | 78/78 (100%) | 64/78 (82%) patients | 60/131 (46%) patients | --- | Rigid system TB were sig higher than RB (p = 0.0065). Cognitive TB no diff from RB (= 0.66) |
| Lille, France [33] | May 2009 - January 2011 | 1-5 (Unlikely to highly likely) | 95/95 (100%) | --- | 66/95 (69%) patients *combination of cognitive and Vnav fusion cores | 56/95 (56%) patients | 0.033 | Clinically significant PCa 49/95 (52%) SB patients, 64/95 (67%) TB patients (p = 0.0011). Cognitive vs Platform fusion no significant different in cancer detection or Gleason score assessment (47% vs 53%, p = 0.16) | |
| HI RVS/Real-Time Virtual Sonography (Hitachi) | Chiba, Japan [46] | February 2007-August 2009. | Yes/No | 85/85 (100%) | 52/85 (61%) | 62/192 (32%) cores | 75/833 (9%) cores | <0.01 | TB cores revealed more cancer than SB (32% vs 9%, p < 0.01) |
Thus, stratification of low-risk versus high-risk PCa patients is possible and may help minimize the number of biopsy sessions a patient undergoes while also strengthening confidence in biopsy results, also important for active surveillance. However, more research is warranted to assess patients with low-moderate MR lesion suspicions as well as further clarifying whether the improved performance of targeted biopsy cores in diagnosing PCa is due to improved sampling techniques or as a result of improved localization from the imaging findings.
ARTEMIS/EIGEN
The Artemis platform (Eigen, Grass Valley, California, USA) received FDA approval in 2008 with patient recruitment beginning in September 2009 at the University of California, Los Angeles (UCLA). The general software features of the Artemis system are similar to the other platforms mentioned (Table 1) however ultrasound images are acquired along a fixed axis using an articulated mechanical arm. Therefore, the biopsy is limited by the rotation of the articulated arm. Needle tracking information is recorded based on encoders at each joint of the mechanical arm. A 3T MRI (Siemens Somatom Trio) using T2W, DCE, and DWI parameters was used to identify MRI lesions and define the suspicion for each lesion on a 1-5 (normal to highly suspicious) scale [40]. Image registration is done via an elastic method. Training for use of this platform requires not only familiarity with the software, but also time to acclimate to TRUS biopsy using manual rotation of the mechanical arm as opposed to freehand techniques commonly used in urologic practice.
Results of 171 men undergoing fusion biopsy from March 2010 to September 2011 at UCLA have shown that 94% (16 of 17 patients) with MR lesion suspicion of grade 5 have had biopsy positive PCa, with targeted cores three times more likely to detect disease versus systematic cores (20.8% vs 7.3%, p = 0.001) . Furthermore, a 38% detection rate was found for intermediate-high risk PCa detected only on targeted biopsy, with targeted cores more likely to detect intermediate to high risk disease versus systematic biopsy (36% vs 24%, p = 0.037), as well as a correlation between MR suspicion and biopsy findings (Table 2) [41].
One notable difference in the UCLA approach to fusion biopsy is their 5-point semi-quantitative scoring system for assessment of MRI identified lesion suspicion for PCa. Scores are based on levels of variation in T2 characteristics, quantitative ADC maps of the DWI parameter, and DCE curve analysis as opposed to a binary evaluation of abnormal versus normal for individual parameters for a given lesion. The utility of the different scoring systems in direct comparison with one another has yet to be investigated and fully defined.
UROSTATION/KOELIS
Urostation (Koelis, Grenoble, France) has been studied clinically at centers in France and Norway as well as preclinically at the University of Southern California in Los Angeles, California, USA (Table 1) [34, 42-44]. Because there are multiple independent centers using this platform, they have each chosen different MR parameters and thus varying definitions of what qualifies as a suspicious lesion on MR (Table 1, 2). The fusion process is performed via elastic registration and similar to the UroNav platform with the exception that confirmation of biopsy needle placement is done retrospectively and requires a 3-5 second delay for each needle for 3D TRUS-acquisition. Training on this system is otherwise software-based as the biopsy guidance uses a standard freehand approach.
Experience with the Urostation platform has shown targeting accuracy to be as high as 97% (112/115 MR targets) in clinical models [43] with cancer detection rates for MR suspicious lesions that vary from 55-87% of patients [34, 43, 44]. Furthermore, Delongchamps and colleagues [34] have demonstrated improved cancer detection in targeted cores versus 12-core systematic TRUS biopsy (76% vs 33% patients, p = 0.0016) with greater clinically significant disease (bGS > 6) detected in targeted cores (33% vs 14% patients, p = 0.01). Results by Rud and colleagues also highlight findings that higher suspicion MR lesions have a greater propensity to contain PCa on targeted biopsy sampling (91% high suspicion lesions were positive for PCa versus 10% positivity rate for low suspicion lesion targets) [43] (Table 2).
BIOPSEE/MEDCOM
The BiopSee Platform (Pi Medical, Athens, Greece) is a system whose main clinical development has been performed at University Hospital Heidelberg in Heidelberg, Germany. Unlike other fusion platforms (Table 1), BiopSee is the only platform in which the prostate biopsy is performed via a transperineal route. An endorectal ultrasound probe is still employed for guidance and is attached to a custom-made mechanical stepper fixed to the operating table. This TRUS probe has two degrees of freedom that allow for adjustments in probe depth and rotation along the main axis. These movements and rotations are tracked by two built-in encoders. Biopsy needles are guided through a grid mounted to the mechanical stepper.
MRI was obtained at 3T (Magnetom Trio) with T2W, DWI, and DCE sequences to define lesion suspicion for PCa based on a three point scale (not suspicious, questionably suspicious or highly suspicious). A rigid image registration process is employed. Training with this system requires not only familiarity with the software aspect of BiopSee, but also experience in handling the US probe along fixed degrees of movement and rotation while also aligning it via software prompts (virtual needle insertion lines) to ensure correct needle placement and penetration as designed by the system. Per Hadaschik and colleagues [45], the learning curve for platform has been estimated at approximately 10 patients.
Patient recruitment for BiopSee began in June 2010 and by March 2012 had enrolled 347 patients [45]. Overall cancer detection was 58% of patients with targeted biopsy cores accounting for 51% of cases, of which 41% had clinically significant disease. When looking at performance on a per core analysis, targeted cores detected significantly more cancer than 12-core systematic TRUS biopsy (30% vs 8.2%, p = 0.01). A correlation between higher lesion suspicion and PCa detection was found, with 82.6% of highly suspicious lesions demonstrating PCa (72% of which demonstrated bGS ≥ 7), while questionably suspicious lesions had a PCa detection rate of 67%.
BiopSee data appears comparable to other methods, however the study design created biases favoring targeted cores and sometimes omitted sampling of systematic cores and thus data should be interpreted cautiously.
VIRTUAL NAVIGATOR/ESAOTE
Virtual Navigator (Esaote, Italy) is a fusion platform that was initially released in 2004 for percutaneous interventional-guidance procedures and thus was capable of image fusion between real-time ultrasound and either prior diagnostic CT or MRI studies. Its use for prostate biopsy has only recently been explored, particularly in France, and is currently not commercialized in the United States. MR targets are selected after uploading MRI studies into the software platform and then via rigid registration fused to real-time, free hand TRUS images. Studies reporting the use of Virtual Navigator suspicious lesions have been defined by 1.5 T MRI using T2W, DCE, and DWI parameters.
Delongchamps and colleagues [34] reported that this platform was 100% accurate for PCa detection in 78 patients with a suspicious MR lesion, of which targeted biopsy detected 82% (64/78 patients). When assessing targeted versus systematic biopsy performance, targeted cores detected an additional 9% (7/78) of patients with intermediate to high risk disease while systematic biopsy detected an additional 18% (14/78) of Gleason 6 patients. Puech and colleagues [33] have also published results with the Virtual Navigator (VNav) platform demonstrating significant differences in overall PCa in favor of targeted biopsy (69% versus 59% patients, p = 0.033) with more clinically significant disease detected by targeted biopsy versus systematic biopsy (67% versus 52% patients, p = 0.0011). However, it is important to note that their evaluation of targeted cores includes results from both cognitive targeting as well as VNav targeting, with not all patients receiving both targeting modalities.
Overall, results such as these concur with the other platforms mentioned herein (UroNav, Artemis, Koelis, BiopSee) [34, 39, 41, 45] and indicate targeted biopsy has greater utility in not only detecting PCa, but in avoiding the diagnosis of non significant disease.
Real-time Virtual Sonography (HI RVS)/HITACHI
Real-time Virtual Sonography (RVS) (Hitachi, Japan) is another general fusion platform that has been customized for prostate biopsy and as such, has image fusion capabilities between US and MRI as well as between US and CT. The HI RVS platform was developed in Japan and uses a freehand TRUS with a electromagnetic sensor for motion-tracking. Research on this platform at Hitachi General Hospital utilized a 1.5 T MRI (Philips Interna) with T2W, DCE, and DWI parameters to define MR lesions as positive or negative for PCa suspicion (Table 1) after MR data was loaded on the RVS biopsy platform. Rigid registration is then utilized between MRI and TRUS images.
In 2010, results from an 85 patient study conducted between February 2007 and August 2009 on the HI RVS platform showed an overall PCa detection rate of 61% (52/85) of patients, of which 87% (45/52) were detected by targeted cores. Per core analysis revealed that targeted cores detected significantly more cancer than systematic cores (32% versus 9%, p <0.01) [46]. These results are again congruent with other platform detection rates. However no further comments were made about the ability of this biopsy platform to track needle cores or histologic correlation between targets versus systematic biopsy locations at the current time.
FUSION BIOSPY: HOW DOES IT COMPARE TO CURRENT STANDARDS?
Given the relative novelty of these fusion biopsy platforms, there have been few studies that have performed comparisons between the different platforms, much less between MR-guided biopsy techniques [33, 34]. However, as seen on a per platform analysis, targeted biopsy appears to have improved PCa detection rates compared to systematic TRUS biopsies alone and demonstrates a greater detection rate of clinically relevant disease (Table 2). A systematic review by Moore and colleagues [47] assessed the accuracy of all three MRI-guided biopsy techniques (in-bore, cognitive, fusion platform) compared to systematic TRUS-guided biopsy for the detection of clinically significant disease and concluded that MRI guidance versus systematic TRUS biopsy detected the same amount of cancer. However, further analysis revealed that targeted cores resulted in less tissue sampling (7% (368/5441) systematic cores positive versus 30% (375/1252) targeted cores positive) with an additional one-third of men detected on targeted biopsy versus systematic biopsy (48% for targeted biopsy versus 36% for systematic biopsy). Additionally, targeted biopsy missed a diagnosis of clinically insignificant disease in roughly 10% (53/555) of men in their investigation.
While this systematic review provides an excellent overall perspective on MRI-guided techniques, further studies are needed to elucidate the roles of individual targeted biopsy techniques compared to current standards. Also unclear is whether new techniques with new sensitivities will alter the core criteria for “significant disease” Additionally, as described previously, due to the lack of standardization in MRI parameters (e.g. 1.5 versus 3T, use of functional parameters including spectroscopy, use of endorectal and/or surface coils) and variations in definitions for MR lesion suspicion, it is difficult to form consensus guidelines for MRI at this time. Finally, since targeted biopsies typically yield a higher tumor-positive percentage of the core new guidelines will need to be established for the management of cancers detected by MR-US fusion technology.
FUTURE DIRECTIONS OF PROSTATE FUSION BIOPSY
As the use of fusion biopsy platforms becomes more widespread, the implications for how it will change current diagnostic and management decisions is yet to be fully understood. Perhaps one of the most potentially promising outcomes is higher PCa detection rates, particularly of clinically significant disease, along with the potential to minimize the number of repeat biopsy sessions and cores sampled per session. With earlier and more accurate diagnosis of PCa, not only does this have the potential to improve patient quality of life but could also potentially could affect mortality outcomes for men with intermediate to high-risk disease at the time of initial diagnosis. As present studies indicate, while there is certainly measurable utility in the use of targeted cores, systematic biopsy methods cannot be dismissed because they continue to diagnose a small but measureable number of significant lesions that are missed on targeted biopsy [39, 41, 45, 46]. Whether this is due to limitations of MRI to detect lesions below a certain size threshold, non-imageable cancer foci, or misplaced targeted cores due to poor co-registration of MRI with the TRUS used for guidance is unclear at this point. However, MR technology improvements may potentially shed light on these questions, as MRI strives to shed light on prostate cancer detection and characterization.
Another important downstream effect of MRI/US fusion technology is the improved ability to correlate histologic outcomes with radiologic findings, both qualitatively and quantitatively. Lesions visible on MRI can be linked to their corresponding biopsy cores with greater confidence because fusion platforms are able to track needle placement and create a biopsy map (“mapping”) for post-biopsy session reference (“archiving”). This mapping will be increasingly vital for active surveillance patients who receive repeat imaging +/− biopsy. The radiology to pathology correlation is a powerful tool for validation of imaging screening tools, such as research MRI sequences, contrast agents, or specific indications. Work such as this has been pursued with small patient cohorts such as findings from Turkbey and colleagues [48] which demonstrated a significant negative correlation between ADC values, Gleason score, and D'Amico clinical risk scores. However, there is an urgent need for standardizing reporting of MR suspicious lesions so that risk assessments do not suffer from wide inter-observer variations (Table 1). In an effort to accomplish this, an international working group has recently released recommendations for MRI-targeted biopsy results which may prove helpful in the future to allow for interpretation of data from a variety of different centers worldwide [49].
Perhaps one of the most interesting effects of fusion platforms and the use of targeted cores is how it will affect the management of PCa—both from the perspective of active surveillance as well as focal therapy.. Not only should needle tracking allow for more precise re-biopsy of previously diagnosed, known cancer foci, but it should increase confidence that MR-visible lesions can be histologically and radiographically followed for disease progression. One question however that remains unanswered is the potential effect of targeted cores upon current biopsy interpretation, which are based upon less efficient TRUS guided sampling. More “efficient” targeted cores are more likely to have a greater proportion of positive cores (more cancer represented per core due to their targeted nature). Since current guidelines with regard to the “per cent of core” involved assume a “tip of the iceberg” underestimation of disease extent, this new, more accurate information, will need to be incorporated into decision algorithms for prostate cancer management and prognosis. Biopsy needle tracking may also prove beneficial in the arena of focal therapy for image-able and localized PCa. Although further investigations are warranted in regards to appropriate patient-selection for focal therapy, the ability to confirm and follow foci of PCa becomes critical when undergoing lesion-directed or partial gland therapies versus the classic radiation whole gland surgical and radiation treatments currently in use today.
CONCLUSIONS
Improvements in imaging technology and screening methods have changed the way clinicians approach not only prostate cancer, but solid tumors in general. Resulting expanded capabilities for real-time tumor targeting and risk stratification may minimize unnecessary intervention. Each commercial platform has its own optimal application, workflow, strengths & weaknesses. Awareness of the differences of each is vital to understanding how to optimally use these tools. The fact that there are many commercial options for fusion biopsy will drive future applications and refinement of the technology. However, the strengths and weaknesses of each platform remain opinions and largely anecdotal, and there is no clear consensus on which methodology is optimal for screening, detection, or surveillance, nor on the specific indications (versus standard TRUS-guidance or MRI in-bore guidance). When fusion is useful in any specific clinical setting also remains somewhat speculative. What is clearer now, more than ever, is that the era of fusion biopsy is here, and this will involve radiologists and urologists working in multidisciplinary teams, in order to fully realize the potential of this powerful approach.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program.
Footnotes
Disclosures:
J.Logan has nothing to disclose.
Dr. Rais-Bahrami has nothing to disclose.
Dr. Turkbey has nothing to disclose.
A.Gomella has nothing to disclose.
Dr. Amalou has nothing to disclose.
Dr. Choyke has nothing to disclose.
Dr. Wood has nothing to disclose.
Dr. Pinto has nothing to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61:69–90. doi: 10.3322/caac.20107. 2011. [DOI] [PubMed] [Google Scholar]
- 2.Krahn MD, Mahoney JE, Eckman MH, Trachtenberg J, Pauker SG, Detsky AS. Screening for prostate cancer. A decision analytic view. JAMA. 1994 Sep;272:773–80. [PubMed] [Google Scholar]
- 3.Chodak GW KP, Schoenberg HW. Assessment of screening for prostate cancer using the digital rectal examination. J Urol. 1989;141(5):1136. doi: 10.1016/s0022-5347(17)41192-x. [DOI] [PubMed] [Google Scholar]
- 4.Newcomer LM, Stanford JL, Blumenstein BA, Brawer MK. Temporal trends in rates of prostate cancer: declining incidence of advanced stage disease, 1974 to 1994. J Urol. 1997 Oct;158:1427–30. doi: 10.1016/s0022-5347(01)64231-9. [DOI] [PubMed] [Google Scholar]
- 5.Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989 Jul;142:66–70. doi: 10.1016/s0022-5347(17)38663-9. [DOI] [PubMed] [Google Scholar]
- 6.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989 Jul;142:71–4. doi: 10.1016/s0022-5347(17)38664-0. discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 7.Yeo L, Patel D, Bach C, Papatsoris A, Buchholz N, Junaid I, Masood J. Bissada DNK, editor. The Development of the Modern Prostate Biopsy. Prostate Biopsy. 2011 http://www.intechopen.com/books/prostate-biopsy/the-development-of-the-modern-prostate-biopsy InTech.
- 8.Rabets JC, Jones JS, Patel A, Zippe CD. Prostate cancer detection with office based saturation biopsy in a repeat biopsy population. J Urol. 2004 Jul;172:94–7. doi: 10.1097/01.ju.0000132134.10470.75. [DOI] [PubMed] [Google Scholar]
- 9.Ravery V, Goldblatt L, Royer B, Blanc E, Toublanc M, Boccon-Gibod L. Extensive biopsy protocol improves the detection rate of prostate cancer. J Urol. 2000 Aug;164:393–6. [PubMed] [Google Scholar]
- 10.Presti JC, O'Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003 Jan;169:125–9. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 11.Wagenlehner FM, van Oostrum E, Tenke P, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013 Mar;63:521–7. doi: 10.1016/j.eururo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Dahms SE, Hohenfellner M, Linn JF, Eggersmann C, Haupt G, Thüroff JW. Retrovesical mass in men: pitfalls of differential diagnosis. J Urol. 1999 Apr;161:1244–8. doi: 10.1016/s0022-5347(01)61647-1. [DOI] [PubMed] [Google Scholar]
- 13.D'Amico AV, Schnall M, Whittington R, et al. Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology. 1998 Mar;51:449–54. doi: 10.1016/s0090-4295(97)00630-4. [DOI] [PubMed] [Google Scholar]
- 14.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004 May;231:379–85. doi: 10.1148/radiol.2312030011. [DOI] [PubMed] [Google Scholar]
- 15.Tatli S, Mortele KJ, Breen EL, Bleday R, Silverman SG. Local staging of rectal cancer using combined pelvic phased-array and endorectal coil MRI. J Magn Reson Imaging. 2006 Apr;23:534–40. doi: 10.1002/jmri.20533. [DOI] [PubMed] [Google Scholar]
- 16.Komai Y, Numao N, Yoshida S, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol. 2013 Sep;190:867–73. doi: 10.1016/j.juro.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Zakian KL, Eberhardt S, Hricak H, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging--initial results. Radiology. 2003 Oct;229:241–7. doi: 10.1148/radiol.2291021383. [DOI] [PubMed] [Google Scholar]
- 18.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011 Nov;186:1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013 May;37:1035–54. doi: 10.1002/jmri.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkbey B, Choyke PL. Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol. 2012 Jul;22:310–5. doi: 10.1097/MOU.0b013e32835481c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005 Feb;234:576–81. doi: 10.1148/radiol.2342031887. [DOI] [PubMed] [Google Scholar]
- 22.Lichy MP, Anastasiadis AG, Aschoff P, et al. Morphologic, functional, and metabolic magnetic resonance imaging-guided prostate biopsy in a patient with prior negative transrectal ultrasound-guided biopsies and persistently elevated prostate-specific antigen levels. Urology. 2007 Jun;69:1208, e5–8. doi: 10.1016/j.urology.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006 Jun;16:1237–43. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 24.Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012 Apr;30:213–8. doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 25.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology. 2011 Apr;259:162–72. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 26.Zangos S, Melzer A, Eichler K, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011 Jun;259:903–10. doi: 10.1148/radiol.11101559. [DOI] [PubMed] [Google Scholar]
- 27.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012 Jan;61:177–84. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011 Oct;108:E171–8. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 29.Park BK, Park JW, Park SY, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011 Nov;197:W876–81. doi: 10.2214/AJR.11.6829. [DOI] [PubMed] [Google Scholar]
- 30.Sciarra A, Panebianco V, Ciccariello M, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010 Mar;16:1875–83. doi: 10.1158/1078-0432.CCR-09-2195. [DOI] [PubMed] [Google Scholar]
- 31.Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010 Mar;13:65–70. doi: 10.1038/pcan.2009.41. [DOI] [PubMed] [Google Scholar]
- 32.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013 Mar;189:860–6. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Puech P, Rouvière O, Renard-Penna R, et al. Prostate Cancer Diagnosis: Multiparametric MR-targeted Biopsy with Cognitive and Transrectal US-MR Fusion Guidance versus Systematic Biopsy--Prospective Multicenter Study. Radiology. 2013 Aug;268:461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 34.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013 Feb;189:493–9. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan I, Oldenburg NE, Meskell P, Blake M, Church P, Holupka EJ. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging. 2002 Apr;20:295–9. doi: 10.1016/s0730-725x(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 36.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013 Jul; doi: 10.1002/cncr.28216. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011 Oct;186:1281–5. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Usefulness of Multiparametric Magnetic Resonance Imaging Suspicion Levels in Detecting Prostate Cancer. J Urol. 2013 May; doi: 10.1016/j.juro.2013.05.052. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy. Eur Urol. 2013 Jun; doi: 10.1016/j.eururo.2013.05.059. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011 May-Jun;29:334–42. doi: 10.1016/j.urolonc.2011.02.014. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013 Jan;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ukimura O, Desai MM, Palmer S, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol. 2012 Mar;187:1080–6. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 43.Rud E, Baco E, Eggesbø HB. MRI and ultrasound-guided prostate biopsy using soft image fusion. Anticancer Res. 2012 Aug;32:3383–9. [PubMed] [Google Scholar]
- 44.Fiard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, Long JA. Targeted MRI-guided prostate biopsies for the detection of prostate cancer: initial clinical experience with real-time 3-dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology. 2013 Jun;81:1372–8. doi: 10.1016/j.urology.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of MRI-targeted TRUS-guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013 Apr; doi: 10.1016/j.juro.2013.04.043. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 46.Miyagawa T, Ishikawa S, Kimura T, et al. Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol. 2010 Oct;17:855–60. doi: 10.1111/j.1442-2042.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 47.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013 Jan;63:125–40. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011 Feb;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of Reporting for MRI-targeted Biopsy Studies (START) of the Prostate: Recommendations from an International Working Group. Eur Urol. 2013 Mar; doi: 10.1016/j.eururo.2013.03.030. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]