Abstract
Objective
Cholesterol enrichment occurs in vivo when phagocytes ingest retained and aggregated lipoproteins, damaged or senescent cells, and related debris. We previously reported that enrichment of human monocyte/macrophages with unesterified cholesterol (UC) triggers the release of highly procoagulant microvesicles ([MVs], also called microparticles) through induction of apoptosis. We determined whether UC-induced MVs (UCMVs) might transmit endogenous danger signals and, if so, what molecular processes might be responsible for their production, recognition, and detoxification.
Methods and Results
Injection of UCMVs into rats provoked extensive leukocyte rolling and adherence to postcapillary venules in vivo. Likewise, exposure of mouse aortic explants or cultured human endothelial cells to UCMVs augmented the adhesion of human monocytes by several fold and increased endothelial cell intercellular adhesion molecule-1 via nuclear factor-κB activation. To explore molecular mechanisms, we found that UC enrichment of human monocytes, in the absence of other stimuli, induced mitochondrial complex II–dependent accumulation of superoxide and peroxides. A subset of these moieties was exported on UCMVs and mediated endothelial activation. Strikingly, aortic explants from mice lacking lectin–like oxidized low–density lipoprotein receptor-1, a pattern-recognition receptor, were essentially unable to respond to UCMVs, whereas simultaneously treated explants from wild-type mice responded robustly by increasing monocyte recruitment. Moreover, high-density lipoprotein and its associated enzyme paraoxonase-1 exerted unexpected roles in the detoxification of UCMVs.
Conclusion
Overall, our study implicates MVs from cholesterol–loaded human cells as novel carriers of danger signals. By promoting maladaptive immunologic and thrombotic responses, these particles may contribute to atherothrombosis and other conditions in vivo.
Keywords: microvesicles, microparticles, damage–associated molecular patterns, cholesterol, monocytes, endothelium
Damage–associated molecular patterns (DAMPs), also known as endogenous danger signals, provide a major stimulus for helpful or harmful immune responses to sterile tissue insults, such as trauma or ischemia.1-3 Typically, DAMPs contain hydrophobic regions within their structure2 that, when exposed, act as alarm signals to the innate immune system by binding and activating pattern-recognition receptors.2,4,5
In previous work,6 we found that enrichment of human monocyte/macrophages with unesterified cholesterol (UC), a process that occurs within atherosclerotic plaques,7,8 provokes the release of highly procoagulant microvesicles ([MVs], also called microparticles) through induction of apoptosis. For several reasons, we hypothesized that UC-induced MVs (UCMVs) could also serve as novel platforms for the transport of DAMPs. First, apoptotic MVs form by budding from the cell surface,6,9-12 and so they possess a bilayer membrane structure with both hydrophobic and hydrophilic domains that appear uniquely suited to carry partially hydrophobic danger signals.5 Second, MVs have been studied mostly in the context of their transport of tissue factor, a potent procoagulant molecule,6,10,12 but surprisingly, only a minority of MVs carry detectable amounts of tissue factor.6,10,13 This result led us to propose that the MVs in our experimental systems might mediate novel pathogenic effects that are independent from coagulation.6,10 Third, MVs produced under some,12,14-17 but not all,12,15,18 circumstances have been reported to activate endothelial monolayers in vitro. Our initial studies to assess the role of UCMVs in sterile inflammation showed that their injection into rats markedly increased leukocyte rolling and adherence to postcapillary venules, indicative of robust endothelial activation in vivo.6,19 Nevertheless, the molecular mechanisms for the production of MVs that activate endothelial cells and the basis for UCMV-endothelial interactions remain uncharacterized.
In the current study, we focused on the molecular and cellular processes responsible for the production of cholesterol-induced MVs capable of acute endothelial activation; the nature of the danger signals carried by these particles; the pattern-recognition receptors that mediate their recognition by endothelium; and endogenous pathways for detoxification of UCMV-associated DAMPs. Our results implicate UCMVs as novel carriers of DAMPs that may promote maladaptive immune responses in atherosclerosis. Moreover, we now report unexpected functions for 2 molecules previously shown to affect arterial plaque formation in vivo—namely, the lectin–like oxidized low–density lipoprotein receptor-1 (LOX1) receptor20 and paraoxonase-1 (PON1).21,22
Materials and Methods
We prepared UCMVs by enrichment of human THP-1 monocytic cells with UC, in the absence of other manipulations, and then harvested their conditioned media and purified the MVs by sequential ultracentrifugation as previously described.6 We assessed the ability of UCMVs to provoke a classic DAMP–induced, sterile inflammatory response—namely, the recruitment of leukocytes or monocytes to microvascular endothelium in vivo—to aortic explants from wild-type and mutant mice ex vivo and to cultured monolayers of human microvascular endothelial cells (hMVECs) in vitro. To study THP-1 cells during UC enrichment and generation of biologically active UCMVs, we used flow cytometry, confocal fluorescent microscopy, and chemical assay of key mediators. To investigate endogenous pathways for detoxification of UCMV-associated DAMPs, high-density lipoprotein (HDL) was isolated from human plasma and recombinant evolved human PON1 was obtained from Dr Dan S. Tawfik (Weizmann Institute of Science, Rehovot, Israel). Additional experiment details are provided in the online-only Data Supplement.
Results
UCMVs Robustly Enhance Leukocyte Recruitment to Microvascular Endothelium In Vivo, to Aortic Endothelium Ex Vivo, and to Cultured Human Endothelial Monolayers In Vitro
Consistent with our initial preliminary reports,6,19 we found that injection of UCMVs into the tail vein of rats markedly increased leukocyte rolling (Figure 1A and 1C) and adherence (Figure 1B) in vivo to postcapillary mesenteric venules, as assessed by intravital microscopy. The effect was so strong that we also observed leukocyte rolling to precapillary arterioles when they were in the field of view (not shown), despite the higher hemodynamic forces seen by the endothelium of those vessels. To examine direct effects of UCMVs on endothelium, we tested the responses elicited by these particles when added to thoracic aortic explants from wild–type C57BL/6J mice ex vivo (Figure IA–IC in the online-only Data Supplement) and to cultured monolayers of hMVECs in vitro (Figure ID–IJ in the online-only Data Supplement). A 5-hour exposure to UCMVs activated aortic endothelium and human endothelial cells, as indicated by significantly enhanced adherence of monocytes (Figure IA–IJ in the online-only Data Supplement).
Figure 1.
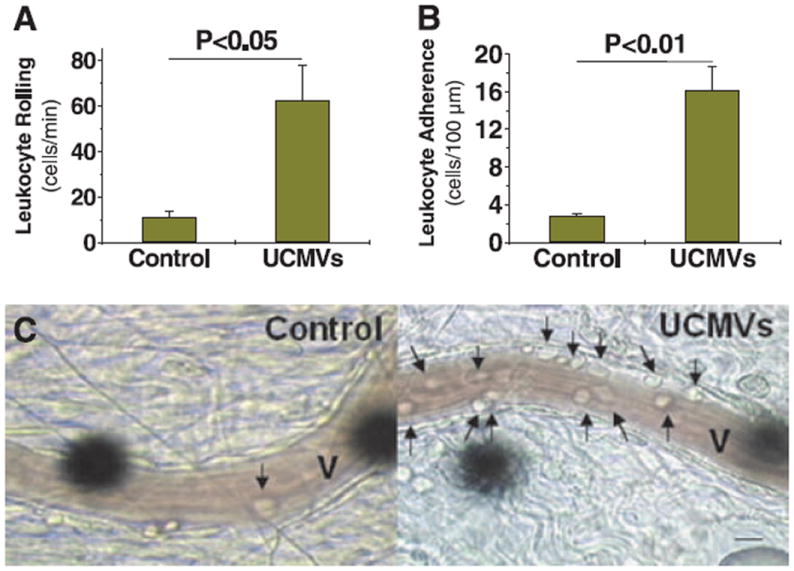
Unesterified cholesterol–induced microvescicles (UCMVs) robustly enhance leukocyte recruitment to microvascular endothelium in vivo. Rolling (A) and adherence (B) of leukocytes to the endothelium of postcapillary mesenteric venules of live rats were quantitatively assessed from video recordings of intravital microscopic images taken 5 hours after tail-vein injections of saline (Control) or UCMVs. Displayed are mean±SEM, n=4; P values were computed using Student unpaired, 2-tailed t test. C, Representative still images captured from these video recordings. Each venule lumen is indicated as V. Arrows indicate adherent leukocytes. The round black objects are projections of the optical Doppler device used to measure blood velocity. Scale bar, 10 μm.
Next, we examined candidate molecules that could mediate the recruitment of monocytes to endothelial cells exposed to UCMVs. A 5-hour exposure of cultured hMVECs to UCMVs specifically increased cellular levels of intercellular adhesion molecule-1 (ICAM1), without affecting vascular cell adhesion molecule-1 or E-selectin in this system (Figure IIA in the online-only Data Supplement). Activation of a fourth major cell adhesion molecule, P-selectin, involves translocation from intracellular stores to the cell surface, not regulated expression.23 To test functional roles for ICAM1 and P-selectin in UCMV–induced monocyte adherence to endothelium, we found that anti-ICAM1 blocking antibodies, but not nonimmune isotype controls, essentially blocked the ability of UCMVs to induce monocyte adherence to thoracic aortic explants from wild-type mice (Figure IIB in the online-only Data Supplement). However, anti-P-selectin blocking antibodies did not inhibit the ability of UCMVs to induce monocyte adherence to mouse thoracic aortic explants (Figure IIB in the online-only Data Supplement). These results, along with our earlier work,6 indicate that UCMVs provide a damage-associated signal that provokes endothelial recruitment of leukocytes12 through the display of functional ICAM1.
Enrichment of Human THP-1 Monocytes With UC in the Absence of Other Stimuli Disrupts Mitochondrial Function and, Thereby, Induces Complex II–Dependent Accumulation of Superoxide, H2O2, and Byproducts of Lipid Peroxidation
Because endogenous danger signals can originate from mitochondria,3,24,25 we examined the effect of UC enrichment alone, without scavenger receptor engagement or esterification inhibitors,26 on mitochondrial integrity in human THP-1 monocytes, the cells of origin for UCMVs. We found that enrichment of these cells with UC, in the absence of other manipulations, induced severe mitochondrial dysfunction, as shown by markedly impaired mitochondrial membrane potential (Figure 2A). To identify possible biologically active molecules, we found that UC enrichment induced time-dependent accumulation of the superoxide anion (O2•−) in mitochondria, assessed by MitoSOX staining (Figure 2B). The superoxide byproduct H2O2 also accumulated intracellularly, assessed by staining with 2’,7’-dichlorofluorescein (Figure 2C). In addition, by 20 hours, UC enrichment more than doubled the cellular content of lipid peroxides, assessed by the thiobarbituric acid reactive substances assay (Figure 2D). Next, we used chemical inhibitors to determine whether damaged mitochondria are the source of these new superoxide and peroxide molecules. Mitochondrial complexes I and III are commonly thought to be sites for superoxide anion generation27 because of premature electron leakage to oxygen, but some experimental evidence suggests that complex II may also serve as a source of mitochondrial superoxide during exposure of cells to artificially peroxidized low-density lipoprotein.28 We found that pretreatment with 2-thenoyltrifluoroacetone, a mitochondrial complex II inhibitor, essentially blocked UC-induced accumulation of superoxide and H2O2 in human THP-1 cells, detected by mitoSOX and by 2’,7’-dichlorofluorescein (Figure 2E and 2F). Inhibitors of the other mitochondrial complexes (rotenone for complex I, myxothiazol for complex III, NaCN for complex IV, and the mitochondria uncoupler carbonyl cyanide m-chlorophenylhydrazone) and inhibitors of NAD(P)H oxidases (apocynin and diphenyleneiodonium) each failed to affect UC-induced accumulation of peroxide or H2O2 (not shown). Thus, mitochondria, and specifically complex II, are the major source of intracellular superoxide and peroxides generated during UC enrichment of human THP-1 cells.
Figure 2.
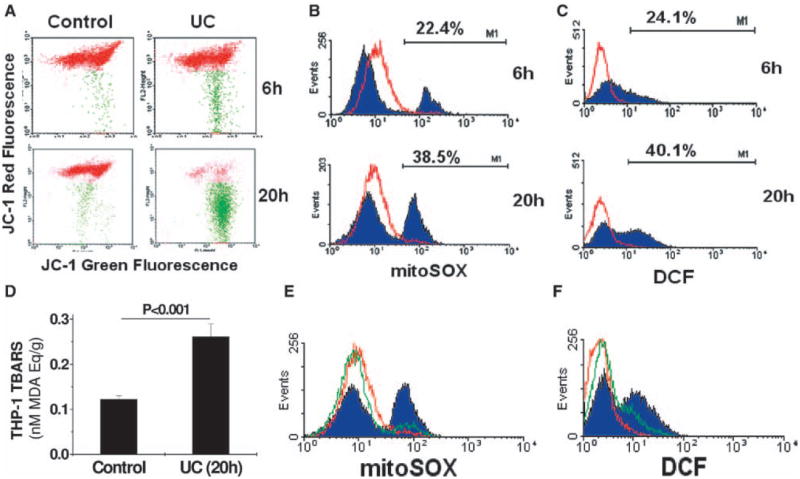
Enrichment of human THP-1 monocytes with unesterified cholesterol (UC) in the absence of other stimuli disrupts mitochondrial function and, thereby, induces complex II–dependent accumulation of superoxide, H2O2, and byproducts of lipid peroxidation. Suspensions of human THP-1 monocytes were incubated at 37°C for 6 hours or 20 hours, as indicated, in control medium or in a medium supplemented with 10 μg UC per mL. A, Representative dot plots from flow cytometry after staining living cells with JC-1, a dye that undergoes a potential-dependent accumulation within healthy mitochondria, indicated by a shift in its fluorescence from green (≈529 nm, x axis) to red (≈590 nm, y axis). Each cell with a normal mitochondrial potential is indicated by a red dot, whereas cells with abnormally low potentials are labeled green. B and C, Suspensions of human THP-1 monocytes were incubated at 37°C for 6 hours or 20 hours, as indicated, in control medium (red histogram curves) or in a medium supplemented with 10 μg UC per mL (filled purple histogram curves). B, Representative histograms of cellular fluorescence after staining living cells with mitoSOX, a dye that detects mitochondrial superoxide. Percentages indicate the proportion of UC-enriched cells that had abnormally high levels of mitoSOX staining. For comparison, the percentages for control cells were 0.5% (6 hours) and 1.6% (20 hours). C, Representative histograms of 2’,7’-dichlorofluorescein (DCF) fluorescence, as an indicator of total intracellular H2O2, a major downstream product of superoxide. Percentages indicate the proportion of UC-enriched cells that had abnormally high levels of DCF staining. The percentages for control cells were 0.2% (6 hours) and 0.9% (20 hours). D, Quantitative assays of THP-1 cells for thiobarbituric acid reactive substances (TBARS), an indicator of peroxidized lipids, expressed as malondialdehyde (MDA) equivalents (mean±SEM, n=5). The P value was computed using Student t test. E and F, Histograms of mitoSOX and DCF fluorescence in control THP-1 cells (red curves), cells enriched with UC for 20h (filled purple curves), and cells pretreated with 10 μmol/L 2-thenoyltrifluoroacetone (TTFA), a mitochondrial complex II inhibitor, before UC loading (green curves).
UCMVs Carry Malondialdehyde–Like Peroxidized Epitopes on Their Surface
We next sought to determine whether peroxidized molecules are present on UCMVs, and if so, whether these molecules might be biologically active. UC enrichment of human THP-1 monocytes for 20 hours caused the production of UCMVs with abundant surface epitopes recognized by the EO14 monoclonal antibody (dots within the orange ovals, Figure 3A–3C). This antibody is known primarily for its binding to low-density lipoprotein that has been modified by malondialdehyde (MDA),29 a reactive compound generated during autoxidation of polyunsaturated fatty acids.30 More recent studies indicate that EO14 also recognizes MDA–like peroxidized epitopes on apoptotic cells.29 Consistent with our previous report that UC enrichment induces apoptosis of human monocyte/macrophages6 and our current finding of thiobarbituric acid reactive substances in UC-enriched cells (Figure 2D), we now found that the EO14 antibody also recognizes the surface of UC–enriched, but not control, THP-1 cells (dots within the green ovals in Figure 3A–3C and green fluorescent regions in Figure 3D and 3G).
Figure 3.
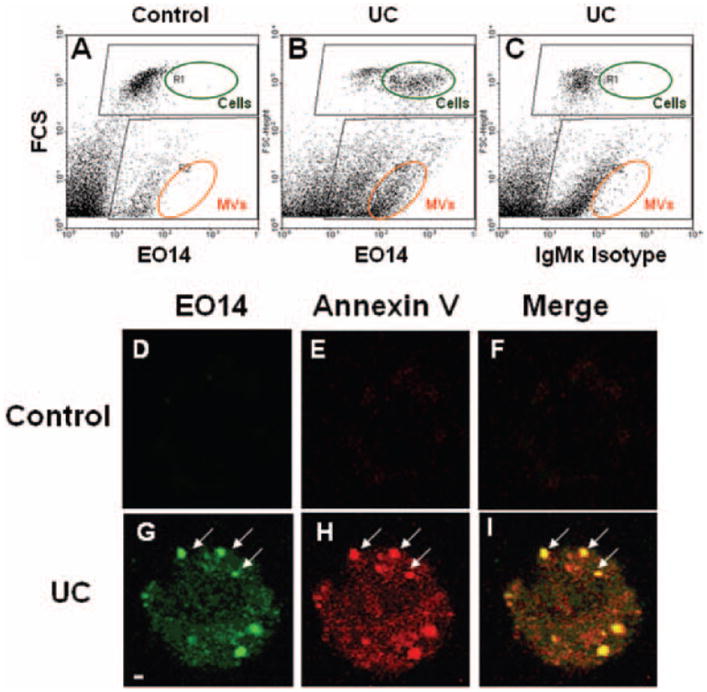
Unesterified cholesterol–induced microvescicles (UCMVs) carry malondialdehyde (MDA)–like peroxidized molecules on their surface. Suspensions of human THP-1 monocytes were incubated at 37°C for 20 hours in control medium (Control) or in a medium supplemented with 10 μg unesterified cholesterol (UC)/mL, followed by fixation of cells and conditioned medium together, as described. A–C, Representative dot plots from flow cytometry after staining samples with EO14, an IgMk antibody that recognizes MDA-like epitopes, or with a nonimmune isotype control antibody, as indicated. Plots show forward scatter on the vertical axis (FSC, indicating size) vs intensity of antibody staining on the horizontal axis. Because we analyzed whole culture suspensions here, positively stained cells (R1, dark green ovals) and microvesicles (MVs) (R2, orange ovals) appear on these plots. D-I, Confocal fluorescent micrographs of representative THP-1 cells that were stained simultaneously with EO14 (green) and annexin V (red), as indicated. The confocal slices were taken to visualize the upper surface of the cells. The yellow color in the merged images (Merge) demonstrates colocalization of the 2 labels. Arrows indicate several of the small, circumscribed, cell-surface domains that stain intensely with EO14 and annexin V and exhibit the same size as UCMVs, consistent with MV blebbing. Scale bar, 1 μm.
Surface-exposed phosphatidylserine is characteristic of both MVs and apoptotic cells.6 Exteriorization of phosphatidylserine from the inner to the outer leaflet of the plasma membrane during apoptosis may be facilitated by its peroxidation by specific reactive molecules that originate from dysfunctional mitochondria.31 To determine whether there is a relationship between UC–induced MDA-like epitopes and cell-surface exposure of phosphatidylserine, we performed colocalization studies. By confocal fluorescence microscopy, both EO14 (green fluorescence in Figure 3G and 3I) and annexin V (a marker of exteriorized phosphatidylserine, indicated by red fluorescence in Figure 3H and 3I) bound to the surface of UC–enriched THP-1 monocytes but not detectably to control cells (Figure 3D–3F). Importantly, EO14 and annexin V colocalized on the surface of UC–treated THP-1 cells, concentrated in small, circumscribed domains (arrows in Figure 3G–3I). These domains exhibit approximately the same size as UCMVs (≈1 μm) and resemble published images of microvesicle release.15 These results indicate that UC enrichment of the human THP-1 monocytes induces the generation of molecules that are detected by the thiobarbituric acid reactive substances assay and recognized on the cell surface by the EO14 antibody. Moreover, these molecules appear to be directly released on MVs.
MDA-Like Peroxidized Molecules Formed Through Mitochondrial Dysfunction Are a Major Class of UCMV-Associated Danger Signals Required to Activate Endothelium
To determine whether peroxidized molecules on UCMVs contribute to the ability of these particles to activate endothelium to recruit leukocytes/monocytes, we isolated UCMVs and then treated them for 1 hour with 0 (control) or 70 μmol/L NaBH4, a chemical reducing agent. The UCMVs were then washed and reisolated by ultracentrifugation. Flow cytometry of the UCMVs indicated that NaBH4 treatment produced a substantial loss of MDA-like epitopes recognized by the EO14 antibody (Figure 4A). More importantly, we found that the NaBH4 treatment significantly attenuated UCMV-induced recruitment of monocytes to mouse thoracic aorta explants ex vivo (Figure 4B) and essentially attenuated UCMV–induced monocyte recruitment to cultured hMVECs (Figure III in the online-only Data Supplement).
Figure 4.
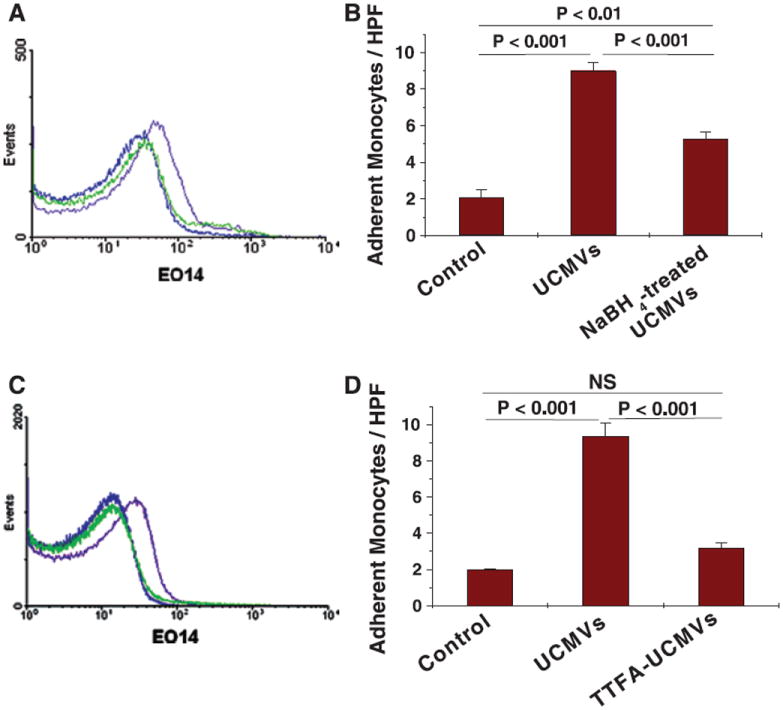
MDA–like peroxidized molecules formed through mitochondrial dysfunction are a major class of unesterified cholesterol–induced microvescicles (UCMV)–associated danger signals that activate endothelium. A and B, UCMVs were isolated from culture supernatants of human THP-1 monocytes and then treated for 1 hour without or with a chemical reducing agent (70 μmol/L NaBH4). Particles were repurified from vehicle or NaBH4 before our analyses. A, Representative histograms from flow cytometry of UCMVs stained with an IgMk isotype control antibody (blue curves), UCMVs stained with the EO14 antibody (filled purple curves), and NaBH4-treated UCMVs stained with the EO14 antibody (green curves). B, Wild–type murine aortic explants were incubated for 5 hours ex vivo with 0 (Control) or 3×104 per μL of UCMVs or NaBH4-treated UCMVs. The explants were rinsed and then endothelial activation was assessed by the subsequent adherence of fluorescent-labeled monocytes. C and D, Human THP-1 monocytes were pretreated without or with the mitochondrial complex II inhibitor 2-thenoyltrifluoroacetone (TTFA) (10 μmol/L) for 1 hour before loading the cells with UC for 20 hours, after which microvesicles were isolated from culture supernatants. C, Representative histograms from flow cytometry of UCMVs stained with an IgMk isotype control anti-body (blue curves), UCMVs stained with the EO14 antibody (filled purple curves), and UCMVs produced in the presence of TTFA and then isolated and stained with the EO14 antibody (green curves). D, Wild–type murine aortic explants were incubated for 5 hours ex vivo with 0 (Control) or 3×104 per μL of UCMVs or UCMVs produced in the presence of TTFA (TTFA-UCMVs). Explants were rinsed and then endothelial activation was assessed by the adherence of labeled monocytes. B and D, Display means±SEM, n=3–5. In each of these panels, P<0.001 by ANOVA, displayed are significance levels for individual pairwise comparisons by the Student-Newman-Keuls test. NS indicates not significant; HPF, high-power field.
To determine whether these biologically active, MDA-like molecules carried by UCMVs originate from cholesterol–induced mitochondrial dysfunction, we pretreated human THP-1 monocytes with a complex II inhibitor (10 μmol/L 2-thenoyltrifluoroacetone) for 1 hour before UC enrichment. Complex II inhibition blocked cholesterol–induced cellsurface display of MDA–like peroxidized epitopes, as shown by decreased EO14 binding by flow cytometry (Figure IVA in the online-only Data Supplement). This effect is presumably a consequence of the strong attenuation by 2-thenoyltrifluoroacetone of UC-induced accumulation of superoxide and H2O2 in human THP-1 cells (Figure 2E and 2F). Surprisingly, 2-thenoyltrifluoroacetone pretreatment did not protect THP-1 monocytes at all from UC-induced apoptosis (Figure IVB in the online-only Data Supplement). Consistent with its effects on cell-surface display of EO14-positive epitopes, complex II inhibition markedly attenuated the appearance of MDA-like molecules on the UCMVs produced by these cells (Figure 4C). Moreover, complex II inhibition during UCMV generation blocked the ability of these particles to induce monocyte adherence to mouse thoracic aorta explants (Figure 4D). Thus, the danger signals carried by UCMVs originate from mitochondrial dysfunction, specifically requiring complex II.
The LOX1 Mediates Endothelial Recognition of Danger Signals on UCMVs
Recent studies have demonstrated that the LOX1 (HGMW-approved symbol OLR1), a scavenger receptor expressed on endothelium,20,32 strongly affects atherogenesis in murine models in vivo.20,32 Moreover, human LOX1 gene variants have been linked to myocardial infarction.33 Because LOX1 is also involved in innate immunity,34,35 including recognition of at least 2 DAMPs, heat shock protein-60 and -70,35 we explored whether this receptor might also recognize danger signals on UCMVs. We found that treatment of cultured hMVECs with UCMVs induced endothelial LOX1 expression, assessed by immunoblots (Figure 5A). We then used 2 approaches to test a functional role for LOX1: antibody inhibition and use of aortic explants from Lox1 knockout mice (Figure 5B–5C). Inhibitory anti-LOX1 monoclonal antibodies, but not an IgG2b isotype control, significantly inhibited the ability of UCMVs to induce monocyte adherence to cultured hMVECs (Figure 5B). Most importantly, we found that aortic explants from Lox1 knockout mice were essentially unable to respond to UCMVs, whereas simultaneously treated explants from wild-type mice responded robustly by increasing monocyte recruitment (Figure 5C). These findings indicate that LOX1 is required for UCMV–induced endothelial activation. Moreover, microvesicle–associated danger signals may be the key endogenous ligands for LOX1.
Figure 5.
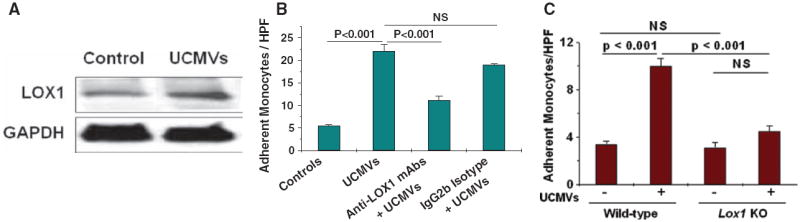
The lectin–like oxidized low–density lipoprotein receptor-1 (LOX1) mediates endothelial recognition of danger signals on unesterified cholesterol–induced microvescicles (UCMVs). A, Cultured monolayers of human heart microvascular endothelial cells were incubated for 5 hours at 37°C with 0 (Control) or 3.0×104 UCMVs per μL, followed by rinsing and then detergent extraction. Displayed are immunoblots for LOX1 and, as a loading control, GAPDH. B, Human endothelial monolayers were preincubated for 1 hour in unsupplemented medium (2 leftmost columns) or in a medium supplemented with a monoclonal blocking antibody against LOX1 (anti-LOX1 mAb) or a non-immune isotype control antibody (IgG2b Isotype). Additional medium without (Control) or with UCMVs was then added, followed by 5 more hours of incubation and then rinsing. Endothelial activation was assessed by the subsequent adherence of fluorescent-labeled monocytes. C, Thoracic aortic explants from wild-type or Lox1 knockout mice (Lox1 KO) were incubated ex vivo for 5 hours at 37°C without or with UCMVs, as indicated, followed by rinsing and then the adherence of fluorescently labeled monocytes was assessed. B and C, Counts of adherent, fluorescent monocytes per high-power field ([HPF]; mean±SEM, n=3–5). In these panels, P<0.001 by ANOVA, displayed are significance levels for individual pairwise comparisons by the Student-Newman-Keuls test.
Earlier work by us36 and others32 indicated that LOX1 can induce endothelial expression of cell adhesion molecules via activation of nuclear factor-κB.32,36 Here, we found that inhibition of nuclear factor-κB signaling in hMVECs by the nuclear factor-κB inhibitor MG-132 significantly attenuated UCMV–induced ICAM1 expression (Figure VA in the online-only Data Supplement). The effects were even more striking when we exposed hMVECs to the UCMVs that we had generated from primary human peripheral blood mononuclear cells. Addition of the UCMVs produced by cholesterol enriched human peripheral blood mononuclear cells to hMVECs substantially induced ICAM1 expression, and this effect was essentially blocked by pretreating hMVECs with the nuclear factor-κB inhibitor (Figure VB in the online-only Data Supplement).
HDL and Its Associated Enzyme PON1 Detoxify Danger Signals on UCMVs
Mechanisms to limit maladaptive responses to DAMPs released during sterile tissue damage have been documented in several circumstances,4,37 although not in the context of atherosclerosis or cholesterol enrichment.25 Studies in animal models have shown direct antiatherogenic roles for HDL38 and the HDL-associated enzyme PON1 in vivo.21,22 Moreover, HDL has been reported to suppress certain immune reactions,39 but its effects on DAMPs have not been previously described. Here, we determined whether HDL or PON1 can act on UCMVs and their associated danger signals. We first treated isolated UCMVs with a subphysiological concentration of HDL, equivalent to 4 mg HDL cholesterol per dL, followed by reisolation. This treatment substantially lowered the content of MDA-like epitopes on UCMVs, as assessed by EO14 antibody binding (Figure 6A) and significantly blunted the ability of the treated UCMVs to induce monocyte adherence to the aortic endothelium (Figure 6B) or to the cultured hMVECs (Figure VI in the online-only Data Supplement). Although paraoxonases were named for their ability to act on a man-made insecticide from the 1940s,21 endogenous natural substrates have not been definitively identified. Here, we isolated UCMVs and then incubated them for 1 hour at 37°C without or with 100 μg of recombinant human PON1 per mL, a concentration that falls within the range of PON1 levels we previously found in human plasma.22 These UCMVs were then reisolated by ultracentrifugation and analyzed. We found that the enzyme decreased MDA-like epitopes on UCMVs (Figure 6C) and significantly attenuated the ability of these particles to activate the endothelium of mouse thoracic aorta explants, as assessed by the subsequent adherence of monocytes (Figure 6D). Thus, detoxification of endogenous danger signals on persistent apoptotic MVs may be a normal function of HDL and PON1. Moreover, our results suggest that DAMPs on UCMVs and other apoptotic membranes might be natural substrates for PON1.
Figure 6.
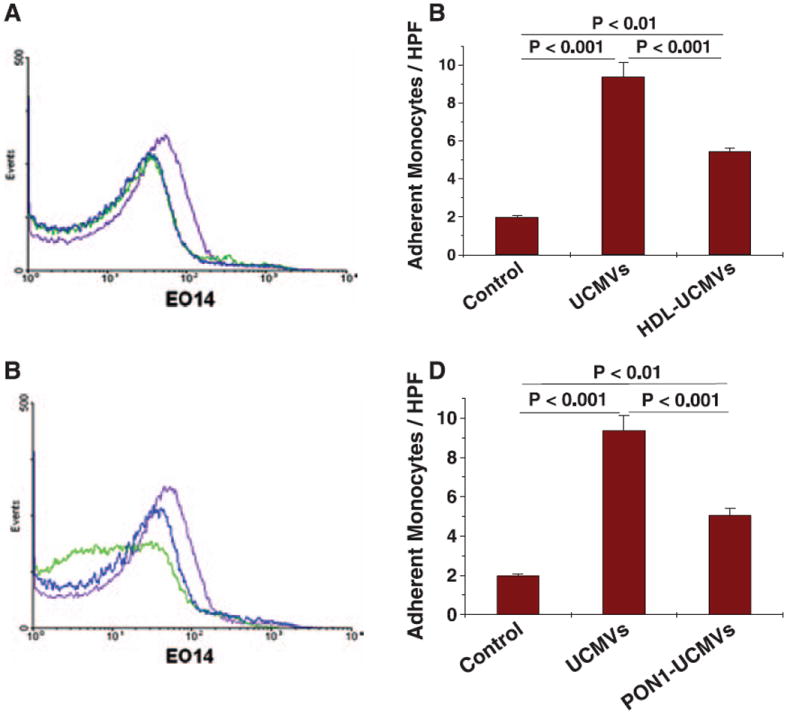
High-density lipoprotein (HDL) and its associated enzyme paraox-onase-1 (PON1) detoxify danger signals on UCMVs. A and B, Unesterified cholesterol–induced microvescicles (UCMVs) were isolated from culture supernatants of human THP-1 monocytes and then incubated for 1 hour at 37°C without or with a subphysiological concentration of human HDL (100 μg HDL protein/mL, equivalent to 4 mg HDL cholesterol per 100 mL). Microvesicles were repurified from vehicle or HDL before our analyses. A, Representative histograms from flow cytometry of UCMVs stained with an IgMk isotype control antibody (blue curves), UCMVs stained with the EO14 antibody (filled purple curves), and HDL-treated UCMVs stained with the EO14 antibody (green curves). B, Wild–type murine aortic explants were incubated for 5 hours ex vivo with 0 (Control) or 3×104 per μL of UCMVs or HDL-treated UCMVs. Explants were rinsed and then endothelial activation was assessed by the adherence of labeled monocytes. C and D, UCMVs were isolated from culture supernatants of human THP-1 monocytes and then incubated for 1 hour at 37°C without or with recombinant evolved PON1 (100 μg/mL). Microvesicles were repurified from vehicle or PON1 before our analyses. C, Representative histograms from flow cytometry of UCMVs stained with an IgMk isotype control antibody (blue curves), UCMVs stained with the EO14 antibody (filled purple curves), and PON1-treated UCMVs stained with the EO14 antibody (green curves). D, Wild–type murine aortic explants were incubated for 5 hours ex vivo with 0 (Control) or 3×104 per μL of UCMVs or PON1-treated UCMVs. Explants were rinsed and then endothelial activation was assessed by the adherence of labeled monocytes. B and D, Mean±SEM, n=3–4. In each of these panels, P<0.001 by ANOVA, displayed are significance levels for individual pairwise comparisons by the Student-Newman-Keuls test.
Discussion
In the current study, we found that upon cholesterol enrichment, human monocytic cells release MVs that contain biologically active danger signals. Cholesterol enrichment occurs in vivo when monocyte/macrophages ingest retained and aggregated lipoproteins in the vessel wall,8,38 damaged or senescent cells, and related debris. Our results suggest that the release of UCMVs may function to recruit additional phagocytes when the local cholesterol load becomes too great. Because macrophages cannot catabolize the steroid nucleus, they must use other processes to accommodate exogenous UC. These processes include transport of UC into mitochondria for processing by CYP27A1, also known as sterol 27-hydroxylase.40 In our experimental system, similar to atherosclerotic plaques in vivo,41,42 the pathways to accommodate UC become overwhelmed, leading to mitochondrial dysfunction (Figure 2), apoptosis,6 and the release of MVs with biologically active danger signals that activate endothelium (Figures 1, 3, and 4).12 Consistent with this model, our previous work showed that removal of UC in vivo from the tissues of genetically hypercholesterolemic animals can normalize leukocyte adherence to microvascular endothelium.43
Endothelial activation by UCMVs was manifested by increased cellular levels of LOX1 and ICAM1 and robust adherence of monocytes (see Results). The effect of UCMVs on endothelial ICAM1 was highly specific (Figure IIA and IIB in the online-only Data Supplement), thereby, accurately modeling the effects of MVs isolated from human atherosclerotic plaques.17 In our study, the mechanism by which UCMVs increased endothelial ICAM1 required engagement of LOX1 (Figure 5B and 5C) and active nuclear factor-κB, a known inducer of ICAM1 gene transcription32 (Figure VA and VB in the online-only Data Supplement). These results are in line with our previous report, in which we showed that other ligands for LOX1 induce endothelial expression of ICAM1.44 An additional possibility was suggested by a recent report that plaque MVs can donate ICAM1 protein to endothelial cells. The ICAM1 content of plaque MVs in that study was low,17 and putative transfer of ICAM1 from UCMVs to endothelial cells in our experimental systems should presumably remain unaffected by inhibition of nuclear factor-κB, an intracellular factor. Thus, our data support a model in which the dominant mechanism for UCMV-induced expression of ICAM1 by endothelial cells occurs via LOX1 engagement and nuclear factor-κB activation.
Mechanisms for noninflammatory removal of apoptotic bodies have been extensively studied,45 as have the consequences of persistent apoptotic bodies and blebs,12,45,46 but less information is available regarding the safe disposal of biologically active MVs. Here, we found a novel role for HDL and its associated enzyme, PON1, in the detoxification of danger signals on UCMVs. In healthy human adults, over 1 million cells die per second,37 requiring mechanisms for the disposal and detoxification of potentially immunostimulatory material, which we now presume to include MVs and their associated DAMPs. In related work, Perkmann et al47 preliminarily reported that a portion of circulating MVs from healthy human subjects display oxidized epitopes, which emphasizes the potential importance of our current finding that HDL and PON1 can neutralize such particles. Consistent with this premise, genetic deficiency of HDL48 and human variants of PON149 have been linked to certain forms of autoimmunity. In the context of atherosclerosis, human coronary plaques have been shown to contain oxidation-specific epitopes on macrophages, including foam cells.50,51 Such cell-associated material would be a likely source of biologically active plaque MVs that should also be targets for HDL and PON1.
As additional triggers for sterile inflammation become identified, the concept of endogenous danger signals continues to broaden. The first few DAMPs to be recognized were intracellular molecules, such as heat shock proteins and high–mobility group box 1 protein, that were released from damaged cells.2,4,45 Damaged cells may also release mitochondria, which can activate innate immunity through persistent generation of ATP, a known DAMP,3,45 as well as through their misidentification as foreign by the innate immune system, because of the organelle’s similarities with its bacterial ancestors.24 In all of these circumstances, DAMPs originate from intracellular components that are inadvertently disgorged as a consequence of cellular damage in an apparently passive, unregulated fashion.
Danger signals on MVs differ from these canonical DAMPs in at least 3 ways. First, the generation of MVs requires active cellular processes, including plasma membrane blebbing via actomyosin-based abscission.9,11,52 Second, the process of plasma membrane blebbing preserves the boundary between intracellular and extracellular compartments and, thereby, avoids indiscriminant release of intracellular contents. Thus, danger signals have to be transported or created on the external surface. Some MVs do not seem to contain danger signals,12,15,18 suggesting selectivity in these processes. Third, our characterization of the biologically active ligands for LOX1, HDL, and PON1 implicates MDA-like moieties that are susceptible to chemical reduction. In contrast, several previously described DAMPs, such as high–mobility group box 1 protein, persist in reducing environments and become inactivated by oxidation.4 Thus, these different DAMPs may be suited for different circumstances.
Overall, our study has implicated UCMVs as platforms for the transport of danger signals, LOX1 as a key pattern- recognition receptor that recognizes these signals, and HDL and PON1 as detoxifiers. Danger signals on MVs may participate in healthy inflammation. Nevertheless, by promoting maladaptive immunologic and thrombotic responses, MVs from cholesterol–loaded human cells may be novel contributors to atherothrombosis and other disorders in vivo.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the American Heart Association (AHA)-Great Rivers Affiliate Beginning Grant-In-Aid (Dr Liu), the Temple University Department of Medicine Career Development Award (Dr Liu), and grants from the National Institutes of Health, HL73898 (Dr Williams) and DK064344 (Dr Scalia).
Footnotes
Disclosures
None.
Portions of this work were presented at the ATVB Annual Conference, 2008, in Atlanta, GA.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.255471/-/DC1.
References
- 1.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 2.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 3.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tör M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 6.Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430–435. doi: 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985;56:93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- 8.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol. 2010;30:1818–1824. doi: 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 12.Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:121–127. doi: 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 14.Barry OP, Praticò D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 16.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1ß-dependent manner. Blood. 2011;118:2366–2374. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 18.Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–2741. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 19.Liu ML, Scalia R, Williams KJ. Novel cholesterol-induced membrane microvesicles stimulate monocyte-endothelial interactions through engagement of the LOX-1 receptor. Arterioscler Thromb Vasc Biol. 2008;28:E12. [Google Scholar]
- 20.Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 21.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 22.Liu ML, James RW, Ylitalo K, Taskinen MR. Associations between HDL oxidation and paraoxonase-1 and paraoxonase-1 gene polymorphisms in families affected by familial combined hyperlipidemia. Nutr Metab Cardiovasc Dis. 2004;14:81–87. doi: 10.1016/s0939-4753(04)80014-0. [DOI] [PubMed] [Google Scholar]
- 23.Denis CV, André P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De vries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JS, Lee T, Chow SE. Role of exercise intensities in oxidized low-density lipoprotein-mediated redox status of monocyte in men. J Appl Physiol. 2006;101:740–744. doi: 10.1152/japplphysiol.00144.2006. [DOI] [PubMed] [Google Scholar]
- 29.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 31.Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, Dekosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta. 2006;31:31. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- 33.Mango R, Biocca S, del Vecchio F, Clementi F, Sangiuolo F, Amati F, Filareto A, Grelli S, Spitalieri P, Filesi I, Favalli C, Lauro R, Mehta JL, Romeo F, Novelli G. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circ Res. 2005;97:152–158. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka S, Zhang XY, Miura K, Kim S, Iwao H. The human gene encoding the lectin-type oxidized LDL receptor (OLR1) is a novel member of the natural killer gene complex with a unique expression profile. Genomics. 1998;54:191–199. doi: 10.1006/geno.1998.5561. [DOI] [PubMed] [Google Scholar]
- 35.Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185:2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- 36.Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–1435. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- 37.Kroemer G, Zitvogel L. Death, danger, and immunity: an infernal trio. Immunol Rev. 2007;220:5–7. doi: 10.1111/j.1600-065X.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 38.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 39.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 40.Björkhem I, Diczfalusy U, Lütjohann D. Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr Opin Lipidol. 1999;10:161–165. doi: 10.1097/00041433-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 42.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 43.Williams KJ, Scalia R, Mazany KD, Rodrigueza WV, Lefer AM. Rapid restoration of normal endothelial functions in genetically hyperlipidemic mice by a synthetic mediator of reverse lipid transport. Arterioscler Thromb Vasc Biol. 2000;20:1033–1039. doi: 10.1161/01.atv.20.4.1033. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Li D, Saldeen T, Mehta JL. Transforming growth factor-beta(1) modulates oxidatively modified LDL-induced expression of adhesion molecules: role of LOX-1. Circ Res. 2001;89:1155–1160. doi: 10.1161/hh2401.100598. [DOI] [PubMed] [Google Scholar]
- 45.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr, Walsh H. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkmann T, Hörkkö S, Wagner O, Witztum J, Binder C. Circulating microparticles express oxidation-specific epitopes that are recognized by natural IgM antibodies: the 53rd Annual Meeting of the Society of Thrombosis and Haemostasis Research; Vienna: Gesellschaft für Thrombose- und Hämostaseforschung; 2009. [Google Scholar]
- 48.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, Batliwalla F, Li W, et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol Med. 2008;14:575–581. doi: 10.2119/2008-00056.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien KD, Alpers CE, Hokanson JE, Wang S, Chait A. Oxidation-specific epitopes in human coronary atherosclerosis are not limited to oxidized low-density lipoprotein. Circulation. 1996;94:1216–1225. doi: 10.1161/01.cir.94.6.1216. [DOI] [PubMed] [Google Scholar]
- 51.Maor I, Kaplan M, Hayek T, Vaya J, Hoffman A, Aviram M. Oxidized monocyte-derived macrophages in aortic atherosclerotic lesion from apolipoprotein E-deficient mice and from human carotid artery contain lipid peroxides and oxysterols. Biochem Biophys Res Commun. 2000;269:775–780. doi: 10.1006/bbrc.2000.2359. [DOI] [PubMed] [Google Scholar]
- 52.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schore C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


