Abstract
Objective
Biologically significant amounts of two procoagulant molecules, phosphatidylserine (PS) and tissue factor (TF), are transported by monocyte/macrophage-derived microvesicles (MVs). Because cellular cholesterol accumulation is an important feature of atherosclerotic vascular disease, we now examined effects of cholesterol enrichment on MV release from human monocytes and macrophages.
Methods and Results
Cholesterol enrichment of human THP-1 monocytes, alone or in combination with lipopolysaccharide (LPS), tripled their total MV generation, as quantified by flow cytometry based on particle size and PS exposure. The subset of these MVs that were also TF-positive was likewise increased by cellular cholesterol enrichment, and these TF-positive MVs exhibited a striking 10-fold increase in procoagulant activity. Moreover, cholesterol enrichment of primary human monocyte-derived macrophages also increased their total as well as TF-positive MV release, and these TF-positive MVs exhibited a similar 10-fold increase in procoagulant activity. To explore the mechanisms of enhanced MV release, we found that cholesterol enrichment of monocytes caused PS exposure on the cell surface by as early as 2 hours and genomic DNA fragmentation in a minority of cells by 20 hours. Addition of a caspase inhibitor at the beginning of these incubations blunted both cholesterol-induced apoptosis and MV release.
Conclusions
Cholesterol enrichment of human monocyte/macrophages induces the generation of highly biologically active, PS-positive MVs, at least in part through induction of apoptosis. Cholesterol-induced monocyte/macrophage MVs, both TF-positive and TF-negative, may be novel contributors to atherothrombosis.
Keywords: macrophages, microparticles, tissue factor, apoptosis, atherosclerosis
Microvesicles (MV) are small membranous structures that are released from cells on activation or during apoptosis.1,2 Monocyte/macrophage-derived MVs transport biologically significant amounts of phosphatidylserine (PS), a membrane lipid that enhances clot propagation,3 and tissue factor (TF), a potent initiator of coagulation in vivo.1,4,5 Based on several observations, we hypothesized a link between cellular cholesterol enrichment and monocyte/macrophage MV generation. First, clinical studies have shown an association between hyperlipidemia and elevated numbers of circulating monocyte/macrophage-derived PS-positive MVs,6–9 and monocyte/macrophage-derived MVs have been found in human atherosclerotic lesions as well.4,10 Second, monocyte/macrophages accumulate unesterified cholesterol (UC) in vivo during atherogenesis,11 presumably owing to their uptake of lipoproteins that have been retained and modified within the arterial wall.12–14 Third, cholesterol loading of primary human monocyte-derived macrophages (MDM) in vitro was reported to stimulate the expression of TF,15 and increased TF expression was found in atherosclerotic vessels from cholesterol-fed rabbits,16,17 in human lipid-rich plaque macrophages,18 and in monocytes from hypercholesterolemic patients.19 Moreover, our group recently reported that hyperlipidemia induces a prothrombotic state in vivo in animals,20 although none of these aforementioned studies examined TF-positive MV release. Fourth, plasma lipid lowering by medication,17 diet,21,22 or extracorporeal low-density lipoprotein apheresis23 reduced TF levels in vivo, and extensive cholesterol depletion of monocytes in vitro for the purpose of disrupting normal plasma membrane structure was recently shown to inhibit their calcium ionophore-stimulated release of total MVs.24 Nonetheless, direct effects of cholesterol enrichment on monocyte/macrophage MV release have not been reported. In the current study, we directly loaded human THP-1 monocytic cells and primary human MDMs with exogenous cholesterol and assessed their vesiculation, as well as the biologic activity of the released MVs. LPS, a well-known stimulus for MV generation by THP-1 monocytes,25 served as a positive control.
Methods
Cell Culture and Cholesterol Treatment
THP-1 cells (ATCC, Manassas, Va) were maintained in suspension culture in RPMI-1640 supplemented with 7% FBS, 1% penicillin/streptomycin, and 0.05 mmol 2-mercaptoethanol/L, as recommended by the ATCC. Before each experiment, cells were transferred to serum-free RPMI-1640 containing 0.2% BSA (RPMI/BSA), followed by a 1-hour preincubation at 37°C. Next, the cells were incubated at 37°C without (control) or with 10 µg unesterified cholesterol (UC)/mL, with or without 10 µg LPS/mL, for 0 to 20 hours in serum-free RPMI/BSA. The UC was delivered as a water-soluble complex with methyl-β-cyclodextrin (UC/mcd; 1:6 molar ratio of UC:mcd; Sigma), which has been widely used to modify the cholesterol content of cultured cells without potentially confounding effects from receptor engagement.26–28 We verified that all UC/mcd preparations contained no detectable endotoxin by the Limulus amoebocyte lysate assay (Associates of Cape Cod, Inc).
Adherent primary human MDMs were prepared from fresh Buffy coats by low-speed Ficoll density gradient centrifugation to purify the mononuclear cell fraction, followed by positive selection of CD14+ monocytes using magnetic beads coated with a monoclonal anti-CD14 antibody (Miltenyi Biotec Inc.). The CD14+ monocytes were differentiated into macrophages by a 7-day incubation at 37°C in DMEM supplemented with 10% FBS, 10% horse serum, 0.5 ng granulocyte-macrophage colony stimulating factor (CSF)/mL, and 0.5 ng macrophage CSF/mL.29,30 Because concentrations of UC/mcd up to 380 µg UC/mL of medium, based on the mass of cholesterol in the UC/mcd complexes, have been used to cholesterol-enrich primary macrophages,26 we chose an intermediate concentration, 100 µg UC/mL, for our studies. Adherent MDMs were incubated at 37°C for 20 hours without (control) or with UC/mcd in RPMI/BSA.
Characterization of MVs and Cells
For flow cytometry, we left the THP-1 cells, which grow in suspension, in their conditioned media. At each time point, samples of cells and media were fixed by addition of paraformaldehyde (PFA) to a final concentration of 2%, followed by storage at −80°C until analysis, as described.31,32 For adherent primary human MDMs, supernatants without cells were fixed in 2% PFA, whereas the cell monolayers were scraped and fixed in suspension in 1% PFA and then 70% ethanol. Consistent with prior literature,31,32 our initial studies indicated that PFA fixation of MVs and cells protects them from changes induced by a single freezing and thawing. Moreover, we specifically verified that the cholesterol-induced increase in MV generation measured after fixation and freezing was the same as that seen using unfixed, unfrozen material (data not shown). The number of MVs in each sample was quantified by flow cytometry, using gating criteria based on particle size, as detected by forward scatter, and surface exposure of PS, as detected by staining with phycoerythrin (PE)-labeled annexin-V (BD Pharmingen).25 The portion of these PS-positive MVs that were also TF-positive was quantified by simultaneous staining with a fluorescein isothiocyanate (FITC)-labeled anti-human TF monoclonal antibody (Cat# 4508CJ, American Diagnostica). For THP-1 cell suspensions, results are reported as MVs per 1000 cells. Because MDMs are adherent, we added 25 000 15-µm latex beads (Molecular Probes) to each 100 µL of their conditioned medium as a reference, and our results are reported as absolute numbers of MVs per ml of conditioned culture medium. Cellular cholesterol content was quantified in isopropanol extracts of unfixed THP-1 cells by the cholesterol oxidase method (Wako Inc).33 In some samples, late-stage apoptosis of THP-1 monocytes or primary human MDMs was assessed by terminal deoxynucleotidyl transferase FITC-dUTP nick end labeling of the cells (TUNEL; APO-DIRECT kit, BD Pharmingen), followed by flow cytometric quantification. For causal studies of the role of apoptosis, a subset of incubations included a caspase-3 inhibitor, Z-DEVD-FMK, at the manufacturer’s highest recommended concentration (100 µmol/L; R&D Systems).
Assessments of Tissue Factor Antigen and Tissue Factor Procoagulant Activity
Cellular release of TF antigen was quantified by ELISA. Culture suspensions (THP-1 monocytes) or conditioned media (primary human MDMs) were centrifuged at low speed (200g for 10 minutes) to remove cells or large debris while leaving MVs in the supernatant. Tissue factor concentrations were then determined (IMUBIND Tissue Factor ELISA kit, American Diagnostica).
Tissue factor procoagulant activity was measured with fresh (unfixed, unfrozen) samples using a chromogenic assay for the activation of clotting factor X (active factor Xa) by MVs captured by platelets, as previously described.24 In brief, MV-containing culture supernatants were obtained by low-speed centrifugation of conditioned medium from THP-1 cells or MDMs after treatment without or with UC. Washed human platelets were activated with 5 µg collagen/mL and then incubated with these MV-containing supernatants for 1 hour at 37°C with continuous rocking. Unbound MVs were then removed by sedimenting the platelets and captured MVs at 1600g for 10 minutes at RT and discarding the supernatants. The pelleted platelets with bound MVs were then resuspended in buffer containing CaCl2, exogenous factor VIIa, and inactive factor X (American Diagnostica) and incubated at 37°C for 30 minutes. To stop the reaction, platelets with bound MVs were removed by filtration (0.2 µm). The filtrate, which contained any activated factor Xa, was portioned into aliquots in a 96-well plate and mixed with a chromogenic Xa substrate, Spectrozyme-Xa (0.5 mmol/L, American Diagnostica). After 60 minutes at 37°C, absorbances at 405 nm were recorded in a microplate reader. As negative controls, TF activity was measured in samples in which factor VIIa, factor X, or Spectrozyme-Xa had each been omitted from the reaction. Lipidated TF (American Diagnostica) was used as standards (0, 10, 20, and 40 pmol/L). We also measured TF procoagulant activity of washed MVs, which were isolated from 1 mL of THP-1 culture supernatant by high-speed centrifugation (100 000g at 4°C for 1 hour), then washed and resuspended in 1 mL RPMI/BSA for analysis of activity, as above.
Statistical Analyses
Values are shown as means±SEM, n=4 to 5. Comparisons among several groups were performed using one-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls test, with P<0.05 considered significant. Comparisons between two groups used Student unpaired t test.
Results and Discussion
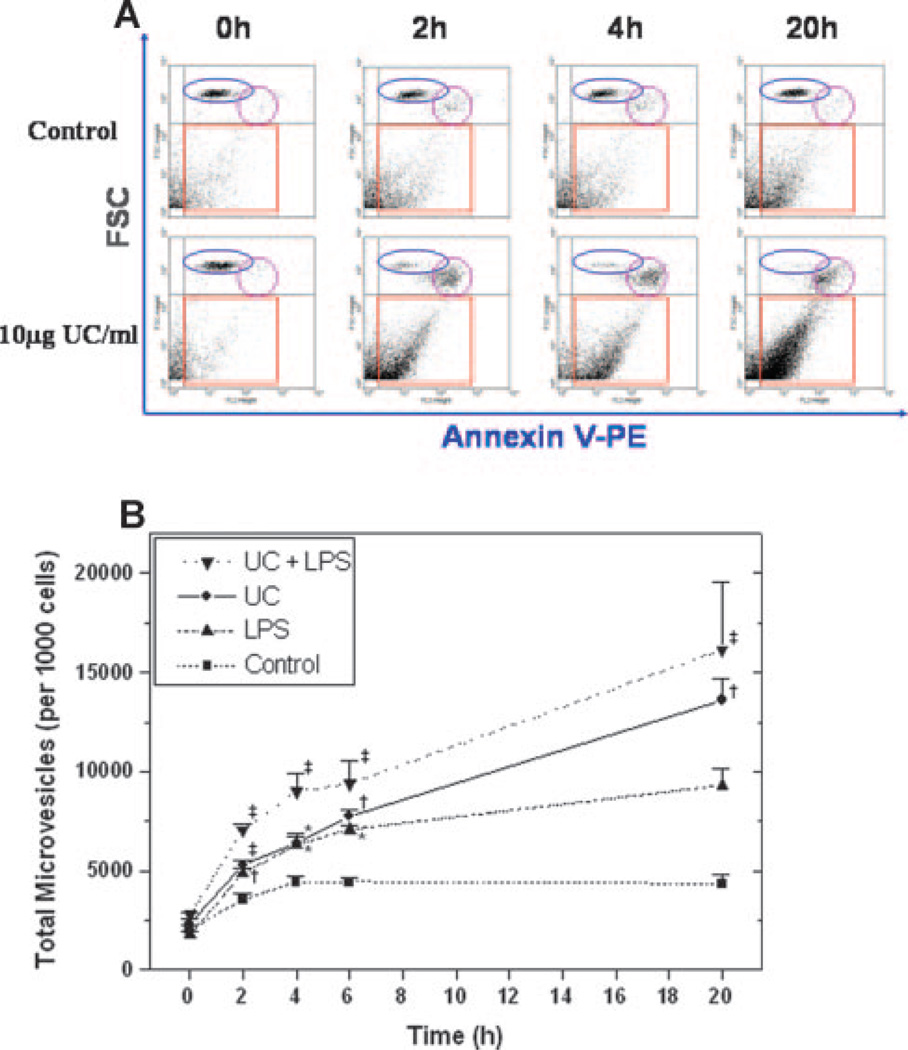
We found that cholesterol enrichment of human THP-1 monocytes provoked a significant increase in the release of PS-positive MVs (Figure 1A and 1B). The effect was evident as early as 2 hours and persisted for the entire 20-hour period we examined (Figure 1A and 1B). By 20 hours, the total MV count from cholesterol-enriched THP-1 cells was nearly 3 times the value from untreated control cells. As a positive control,25 LPS also stimulated THP-1 monocyte MV release, but we now found that cholesterol given simultaneously with LPS increased microvesiculation at each time point as compared with either agent alone (Figure 1B). To establish that cholesterol was responsible for these effects, we verified that incubation with UC/mcd caused significant cholesterol enrichment of THP-1 monocytes throughout the 20-hour time-course (51.1±4.0 at 0 hours, 86.3±12.7 at 2 hours, and 123.0±13.2 µg cholesterol/mg cell protein at 20 hours, n=4, P<0.01 for 2 hours and for 20 hours versus 0 hours). Similar cholesterol enrichment was seen in cells incubated with the combination of UC and LPS, whereas no changes in cellular cholesterol content were observed in the control or LPS-only groups (data not shown). Furthermore, media supplemented with the same low concentration of methyl-β-cyclodextrin (0.15 mmol/L), but not complexed to cholesterol, failed to measurably affect THP-1 MV generation over time (eg, the MV count after 20 hours in the presence of methyl-β-cyclodextrin without UC was 93%±3% of the control value in unsupplemented medium, NS).
Figure 1.
Cholesterol enrichment of monocytic cells increases total MV generation. Suspensions of THP-1 monocytes were treated for 0 to 20 hours at 37°C with UC, LPS, both, or neither (Control), as indicated, and then samples were analyzed by flow cytometry. A, Representative dot-plots of forward scatter (FSC, indicating size) vs annexin V-PE staining (indicating PS exposure) for suspensions of control and UC-enriched cells. The squares enclose the population of PS-positive MVs. The ovals indicate cellular populations with the same size range and PE intensity as control THP-1 cells at t=0 hours, whereas the circles identify a population of cells that appeared over time, particularly with cholesterol enrichment. B, Quantitative counts of PS-positive MVs generated over time under the four experimental conditions. P<0.01 by ANOVA. *P<0.05, †P<0.01, and ‡P<0.001 vs the control group by the Student-Newman-Keuls test.
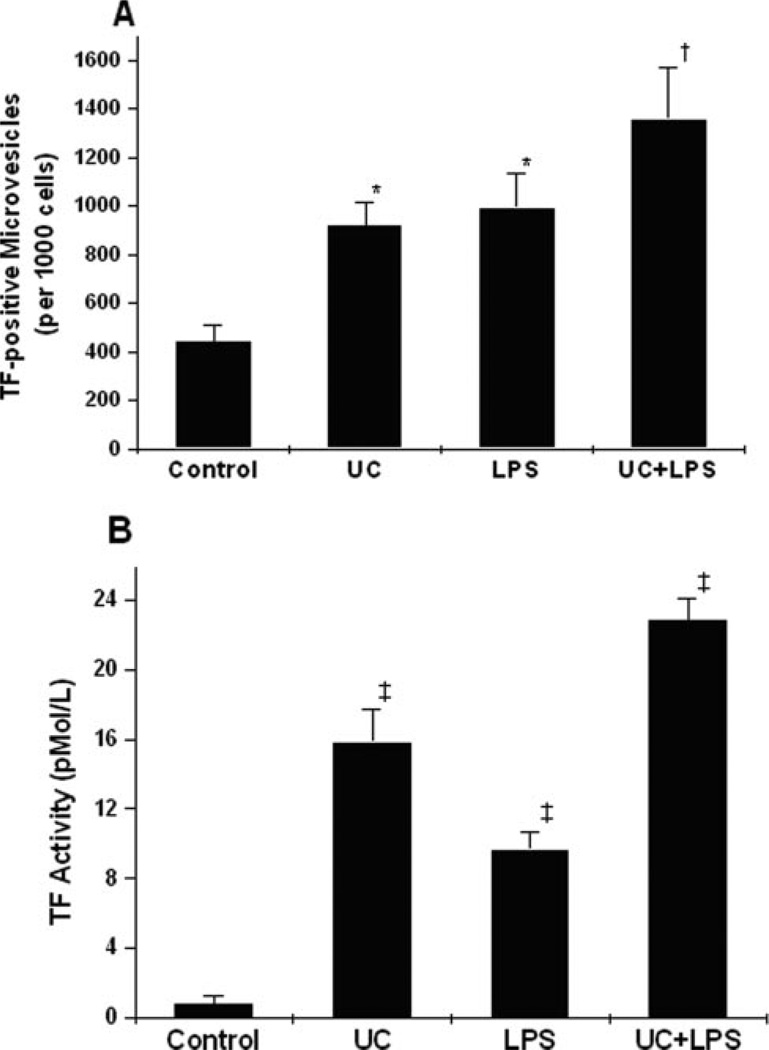
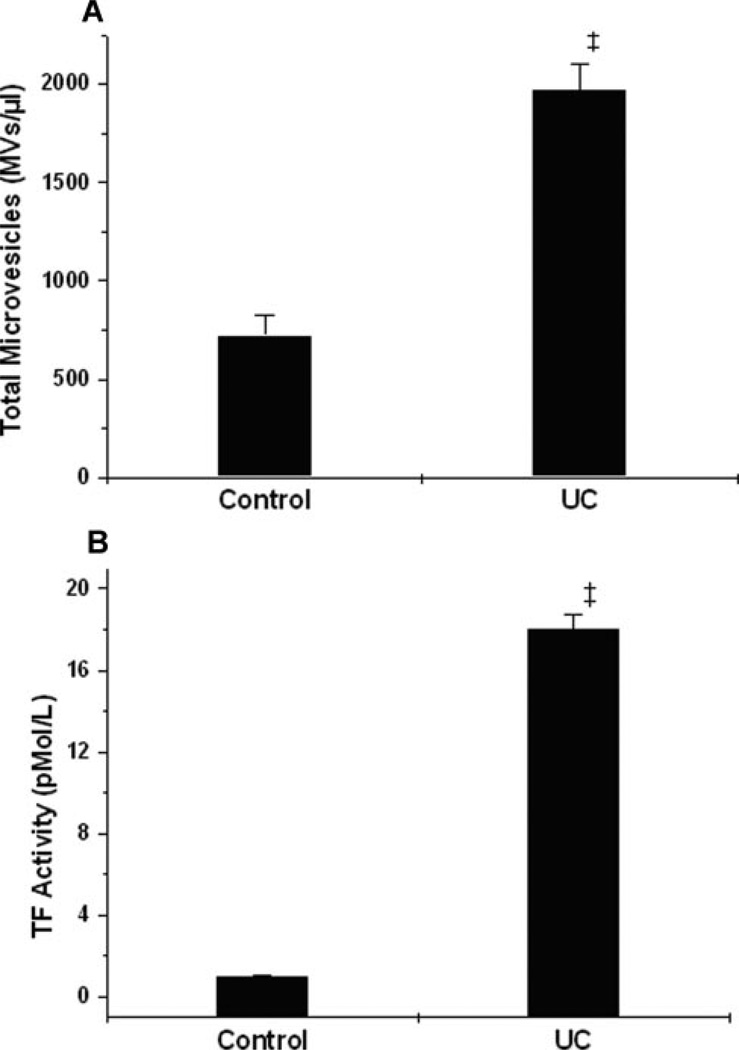
In addition to increasing total cellular output of MVs, cholesterol loading of THP-1 cells in the absence or presence of LPS caused large increases in the cellular release of TF-positive MVs, as measured either by flow cytometry of the cell culture suspension (Figure 2A), or by TF ELISA of cell-culture supernatants (supplemental Figure I, available online at http://atvb.ahajournals.org). Importantly, cellular cholesterol loading of THP-1 cells caused a remarkable 10-fold increase in the TF procoagulant activity of MV-containing cell culture supernatants (Figure 2B) and of washed THP-1 MVs (not shown), consistent with the increase in TF mass (supplemental Figure I) and possibly TF de-encryption (see references34–36). To examine these effects in a primary cell, we found that cholesterol-enrichment of human MDMs caused large, statistically significant increases in total (Figure 3A) and TF-positive (supplemental Figure II) MV release compared with control. Moreover, MV-containing cell culture supernatants from cholesterol-enriched primary human MDMs also showed a striking 10-fold increase in TF procoagulant activity over control (Figure 3B). These results suggest that enhanced release of procoagulant MVs from cholesterol-enriched monocyte/macrophages could contribute to the general prothrombotic state in hypercholesterolemia,19,20,23,37 and to the prothrombotic interior of lipid-rich, vulnerable plaques.4,5,10,14
Figure 2.
Cholesterol enrichment of monocytic cells increases the generation of biologically active TF-positive MVs. Suspensions of THP-1 monocytes were treated as in Figure 1. A, Quantitative counts at 4 hours of PS-positive MVs that were also TF-positive. B, TF procoagulant activity of MV-containing supernatants in an assay using exogenous factor VIIa and collagen-activated platelets. P<0.01 by ANOVA. *P<0.05, †P<0.01, and ‡P<0.001 vs the control group by the Student-Newman-Keuls test.
Figure 3.
Cholesterol enrichment of primary human monocyte-derived macrophages increases the generation of total as well as biologically-active TF-positive MV. Adherent primary human MDMs in 6-well plates were treated without (Control) or with UC for 20 hours. A, Total concentrations of PS-positive MVs in the conditioned medium, as assessed by flow cytometry. B, TF procoagulant activity of MV-containing culture supernatants. ‡P<0.001 vs the control group by Student unpaired t test.
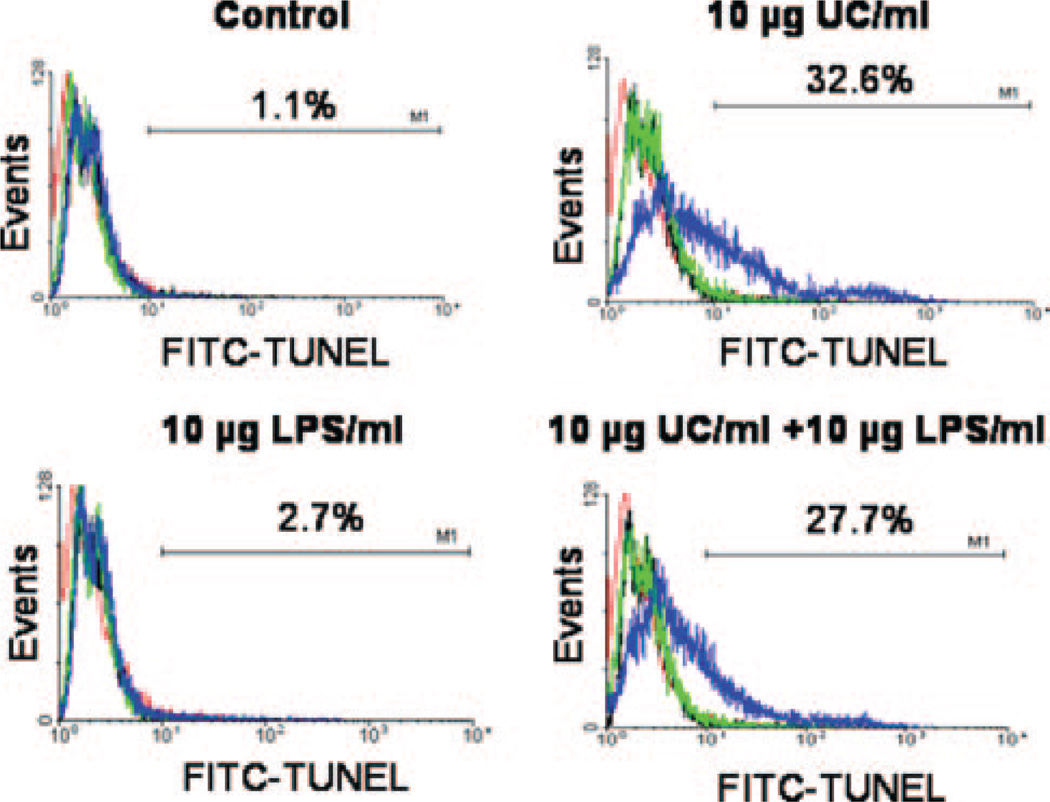
The flow cytometry protocol for THP-1 monocytes allowed us to simultaneously examine MVs and the cells that generated them. Our data showed no significant changes in either cell-surface exposure of PS or cellular size when THP-1 cells were incubated in unsupplemented serum-free medium (Figure 1A and supplemental Figure III) or treated with LPS (supplemental Figure III; size data not shown). Under these conditions, a low level of cellular PS exposure was seen in the current (Figure 1A) and previous studies25 which could play a role in generation of PS-positive MVs by these cells.38,39 In contrast, cholesterol enrichment caused a majority of the THP-1 monocytes to promptly display strong staining for PS on their surface by 2 hours (Figure 1A and supplemental Figure III), and to gradually shrink over time, as shown by a downward shift in forward scatter (Figure 1A). Because cell-surface exposure of PS not only induces procoagulant responses5 but may also indicate early-stage apoptosis,5,40 we examined a definitive marker of late-stage apoptosis, namely DNA fragmentation by TUNEL. At the 20-hour time point, the proportions of TUNEL-positive cells were only 1% to 2% in serum-free medium, and only 2% to 3% with LPS stimulation, but rose to approximately 30% with cholesterol enrichment of THP-1 cells (Figure 4). Likewise, the proportions of primary human MDMs that were TUNEL-positive were only 1% in serum-free medium but rose to approximately 11% after 20 hours of cholesterol enrichment. Thus, similar to prior literature,26 we saw significant cholesterol- induced apoptosis, which raises the possibility that at least some of the cholesterol-induced MV generation in our system might be from apoptotic blebbing. Interestingly, our human monocyte/macrophages underwent UC-induced apoptosis even in the absence of other manipulations (see reference26), indicating an unexpectedly robust response in these cells.
Figure 4.
Apoptosis during cholesterol-induced MV generation. THP-1 monocytes were incubated at 37°C with or without UC, LPS, or combinations, as indicated, then analyzed by flow cytometry. Displayed are representative histograms of TUNEL staining after 0 hours (red), 2 hours (black), 4 hours (green), and 20 hours (blue). Each histogram includes approximately 1000 cells. Percentages indicate the proportion of cells that were TUNEL positive at 20 hours.
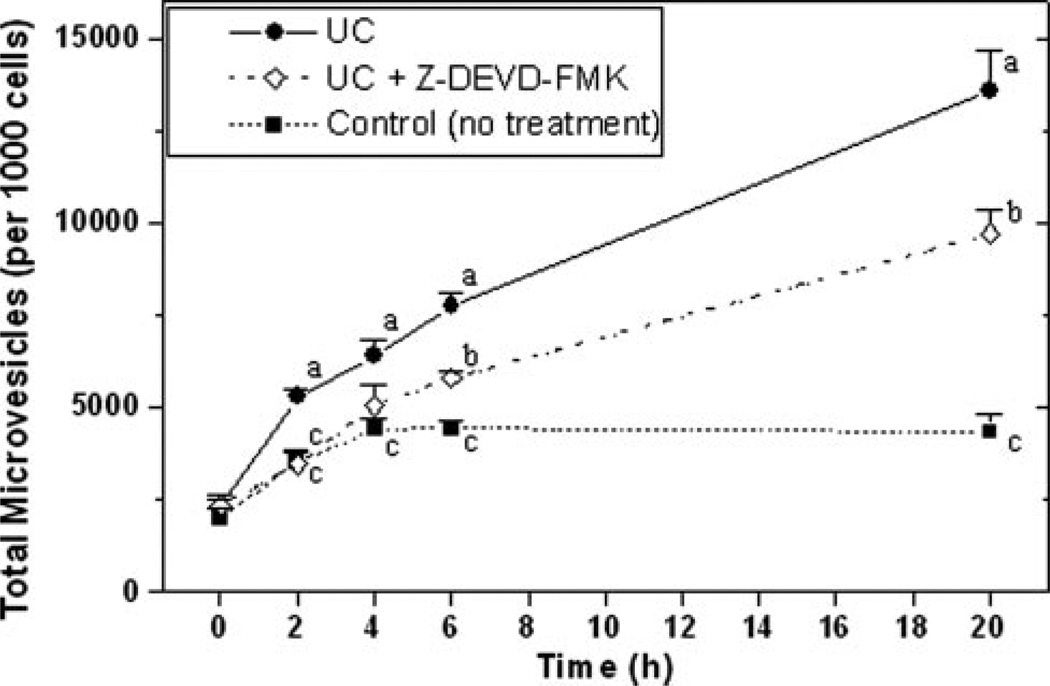
To assess a causal role for apoptosis, we used the caspase-3 inhibitor Z-DEVD-FMK. Addition of this inhibitor during cholesterol enrichment of THP-1 monocytes delayed cell-surface exposure of PS by 2 to 4 hours, but did not affect the final percentage of PS-positive cells at 20 hours (Supplemental Figure IVA). The inhibitor significantly decreased the percentage of TUNEL-positive cells at 20 hours from 28±2.8% to 12.5±2.2% (supplemental Figure IVB, P<0.001). Importantly, the caspase-3 inhibitor significantly decreased the number of PS-positive MVs released during UC-enrichment over time, although not entirely to the level of unenriched control cells at 6 hours and 20 hours (Figure 5 and supplemental Figure IVA). In contrast, we did not see any effect of the caspase-3 inhibitor on the number of TF-positive MVs released after UC-enrichment (data not shown), suggesting that TF synthesis and TF-positive MV release may come from a subset of these cells that are not in late-stage apoptosis (see reference41).
Figure 5.
Role for apoptosis in cholesterol-induced MV generation. THP-1 monocytic cells were incubated at 37°C without or with UC, Z-DEVD-FMK (a caspase-3 inhibitor), or combinations, as indicated, then analyzed by flow cytometry. Displayed are quantitative counts of PS-positive MVs over time after the indicated treatments. P<0.01 by ANOVA. Symbols labeled with different lowercase letters are statistically different by the Student- Newman-Keuls test (P<0.05).
Taken together, our results directly link cellular cholesterol enrichment, a known consequence of hyperlipidemia, arterial retention of lipoproteins, and atherogenesis,12,14 with the enhanced generation of biologically active monocyte/macrophage-derived MVs, at least in part through induction of apoptosis. Related effects may occur with other cell types, though independent of apoptosis.42 The effects of TF-positive monocyte/macrophage-derived MVs and their potential contributions to atherothrombosis have been investigated in the present as well as in previous studies.4,43 Nevertheless, more than 80% of the MVs in our study (Figures 1B and 2A; Figure 3A and supplemental Figure II) and in prior literature44 were PS-positive but TF-negative. We speculate that these particles may be biologically active as well, for example, by enhancing clot propagation,3,5 causing apoptosis of nearby cells,45,46 blocking PS receptors that could otherwise promote phagocytosis of apoptotic bodies,47 and altering gene expression via their display of PS48 and perhaps other biologically active molecules.49,50 Of interest, we recently found that injection of cholesterol-induced monocyte MVs into rats provoked substantial leukocyte rolling and adherence to post-capillary venules in vivo, indicative of endothelial activation (Liu M-L, Scalia R, Williams KJ, unpublished data, 2006). Thus, cholesterol-induced PS-positive monocyte/macrophage MVs, both TF-positive and TF-negative, may be novel contributors to atherothrombosis. Because cholesterol is an exchangeable molecule that can be removed by acceptor particles, such as high-density lipoprotein, cellular production of these vesicles could be amenable to therapeutic interventions.14,51
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health (USA) grants HL56984 and HL73898 (to K.J.W.) and HL69471 and HL081503 (to M.P.R.).
Footnotes
A preliminary report of this work was presented at 6th Annual Conference on Arteriosclerosis, Thrombosis and Vascular Biology, Washington, DC, 30 April 2005.
Disclosures
None.
References
- 1.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 3.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 4.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 5.Mallat Z, Tedgui A. Current perspective on the role of apoptosis in atherothrombotic disease. Circ Res. 2001;88:998–1003. doi: 10.1161/hh1001.090571. [DOI] [PubMed] [Google Scholar]
- 6.Nomura S, Hattori N, Sakakibara I, Fukuhara S. Effects of Saikoka-ryukotsu-borei-to in patients with hyperlipidemia. Phytomedicine. 2001;8:165–173. doi: 10.1078/0944-7113-00035. [DOI] [PubMed] [Google Scholar]
- 7.Nomura S, Kanazawa S, Fukuhara S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with Type 2 diabetes mellitus. J Diabetes Complications. 2003;17:153–159. doi: 10.1016/s1056-8727(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 8.Nomura S, Takahashi N, Inami N, Kajiura T, Yamada K, Nakamori H, Tsuda N. Probucol and ticlopidine: effect on platelet and monocyte activation markers in hyperlipidemic patients with and without type 2 diabetes. Atherosclerosis. 2004;174:329–335. doi: 10.1016/j.atherosclerosis.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles. disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 10.Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 11.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 12.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 14.Williams KJ, Tabas I. Lipoprotein retention-and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 15.Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1992;89:10370–10374. doi: 10.1073/pnas.89.21.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato K, Elsayed YA, Namoto M, Nakagawa K, Sueishi K. Enhanced expression of tissue factor activity in the atherosclerotic aortas of cholesterol-fed rabbits. Thromb Res. 1996;82:335–347. doi: 10.1016/0049-3848(96)00083-7. [DOI] [PubMed] [Google Scholar]
- 17.Camera M, Toschi V, Comparato C, Baetta R, Rossi F, Fuortes M, Ezekowitz MD, Paoletti R, Tremoli E. Cholesterol-induced thrombogenicity of the vessel wall: inhibitory effect of fluvastatin. Thromb Haemost. 2002;87:748–755. [PubMed] [Google Scholar]
- 18.Hutter R, Valdiviezo C, Sauter BV, Savontaus M, Chereshnev I, Carrick FE, Bauriedel G, Luderitz B, Fallon JT, Fuster V, Badimon JJ. Caspase-3 and tissue factor expression in lipid-rich plaque macrophages: evidence for apoptosis as link between inflammation and atherothrombosis. Circulation. 2004;109:2001–2008. doi: 10.1161/01.CIR.0000125526.91945.AE. [DOI] [PubMed] [Google Scholar]
- 19.Sanguigni V, Ferro D, Pignatelli P, Del Ben M, Nadia T, Saliola M, Sorge R, Violi F. CD40 ligand enhances monocyte tissue factor expression and thrombin generation via oxidative stress in patients with hypercholesterolemia. J Am Coll Cardiol. 2005;45:35–42. doi: 10.1016/j.jacc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Reilly MP, Taylor SM, Franklin C, Sachais BS, Cines DB, Williams KJ, McKenzie SE. Prothrombotic factors enhance heparin-induced thrombocytopenia and thrombosis in vivo in a mouse model. J Thromb Haemost. 2006;4:2687–2694. doi: 10.1111/j.1538-7836.2006.02201.x. [DOI] [PubMed] [Google Scholar]
- 21.Aikawa M, Voglic SJ, Sugiyama S, Rabkin E, Taubman MB, Fallon JT, Libby P. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–1222. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
- 22.Jeanpierre E, Le Tourneau T, Six I, Zawadzki C, Van Belle E, Ezekowitz MD, Bordet R, Susen S, Jude B, Corseaux D. Dietary lipid lowering modifies plaque phenotype in rabbit atheroma after angioplasty: a potential role of tissue factor. Circulation. 2003;108:1740–1745. doi: 10.1161/01.CIR.0000089370.84709.51. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Blessing F, Walli AK, Uberfuhr P, Fraunberger P, Seidel D. Effects of heparin-mediated extracorporeal low-density lipoprotein precipitation beyond lowering proatherogenic lipoproteins–reduction of circulating proinflammatory and procoagulatory markers. Atherosclerosis. 2004;175:145–150. doi: 10.1016/j.atherosclerosis.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 25.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 26.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 27.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 29.Akagawa KS, Komuro I, Kanazawa H, Yamazaki T, Mochida K, Kishi F. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Respirology. 2006;11:S32–S36. doi: 10.1111/j.1440-1843.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 30.Dave RS, Pomerantz RJ. Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains. J Virol. 2004;78:13687–13696. doi: 10.1128/JVI.78.24.13687-13696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;26:26. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 32.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 33.Fuki IV, Meyer ME, Williams KJ. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem J. 2000;351:607–612. [PMC free article] [PubMed] [Google Scholar]
- 34.Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest. 1996;75:281–289. [PubMed] [Google Scholar]
- 35.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 36.Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10:201–210. [PubMed] [Google Scholar]
- 37.Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, Falco A, Cuccurullo F, Davi G. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation. 2002;106:399–402. doi: 10.1161/01.cir.0000025419.95769.f0. [DOI] [PubMed] [Google Scholar]
- 38.Tepper AD, Ruurs P, Wiedmer T, Sims PJ, Borst J, van Blitterswijk WJ. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol. 2000;150:155–164. doi: 10.1083/jcb.150.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadeel B, Xue D. PS externalization: from corpse clearance to drug delivery. Cell Death Differ. 2006;13:360–362. doi: 10.1038/sj.cdd.4401836. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- 41.Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 2000;7:603–615. doi: 10.1038/sj.cdd.4400695. [DOI] [PubMed] [Google Scholar]
- 42.Llorente-Cortes V, Otero-Vinas M, Camino-Lopez S, Llampayas O, Badimon L. Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation. 2004;110:452–459. doi: 10.1161/01.CIR.0000136032.40666.3D. [DOI] [PubMed] [Google Scholar]
- 43.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 44.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 45.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zadelaar AS, von der Thusen JH, Boesten LS, Hoeben RC, Kockx MM, Versnel MA, van Berkel TJ, Havekes LM, Biessen EA, van Vlijmen BJ. Increased vulnerability of pre-existing atherosclerosis in ApoE-deficient mice following adenovirus-mediated Fas ligand gene transfer. Atherosclerosis. 2005;183:244–250. doi: 10.1016/j.atherosclerosis.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zachlederova M, Jarolim P. Gene expression profiles of microvascular endothelial cells after stimuli implicated in the pathogenesis of vasoocclusion. Blood Cells Mol Dis. 2003;30:70–81. doi: 10.1016/s1079-9796(03)00011-1. [DOI] [PubMed] [Google Scholar]
- 49.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadl A, Bochkov VN, Huber J, Leitinger N. Apoptotic cells as sources for biologically active oxidized phospholipids. Antioxid Redox Signal. 2004;6:311–320. doi: 10.1089/152308604322899378. [DOI] [PubMed] [Google Scholar]
- 51.Williams KJ, Werth VP, Wolff JA. Intravenously administered lecithin liposomes: a synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984;27:417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.