Abstract
To determine whether receiving melanoma genetic test results undermines perceived control over melanoma prevention, control-related beliefs were examined among 60 adults from melanoma-prone families receiving CDKN2A/p16 test results (27 unaffected noncarriers, 15 unaffected carriers, 18 affected carriers; response rate at 2 years=64.9% of eligible respondents). Multilevel modeling of perceived control ratings over a 2-year period revealed significant variation in individual trajectories: most participants showed increases (45%) or no change (38.3%), while 16.7% showed decreases. At the group level, noncarriers reported sustained increases through the 2-year follow-up (ps<.05); unaffected carriers reported significant short-term increases (ps<.05); and affected carriers reported no change. Participants in all groups continued to rate photoprotection as highly effective in reducing melanoma risk and reported decreased belief that carrying the p16 mutation would inevitably lead to the development of melanoma. Qualitative responses immediately following counseling and test reporting corroborated these findings, as 93% indicated it was possible to either prevent (64.9%) or decrease the likelihood (28.1%) of future melanomas. Thus, genetic test reporting does not generally undermine perceived control over melanoma prevention, though variability in response to positive results warrants future study.
Keywords: CDKN2A/p16, familial melanoma, genetic counseling, prevention, genetic determinism, perceived control
Predictive genetic testing for hereditary cancer can alert people to elevated disease risk prior to disease onset, when early detection and prevention may still be possible. Determining whether genetic and genomic information improves prevention behaviors is a major priority for communications and social sciences research (McBride et al., 2010; Marteau et al., 2010; Collins et al., 2003; Khoury et al., 2007). As researchers identify environmental and behavioral contributors to genetically linked diseases, it will become increasingly important to understand whether members of high-risk families believe their genetic risk is at least partially modifiable through their own actions.
Of particular concern, research to date suggests that providing information about genetic causes of disease may induce a sense of fatalism about disease onset and risk management, depressing both uptake of genetic testing and interest in prevention information (Marteau & Weinman, 2006). Beliefs such as genetic essentialism (the bias toward perceiving genetic makeup as determining characteristics and behaviors, Dar-Nimrod & Heine, 2011) and genetic determinism (e.g., “Genes are the most important contributor to human health,” Parrott et al., 2004) may influence important perceptions and decisions (Nelkin & Lindee, 2004). For example, an experimental study showed that people automatically associate genes with fate rather than choice (Gould & Heine, 2012). Further, experiments that manipulate information about the causes of disease find that conditions said to have genetic origins are seen as less under the personal control of the affected person (Aspinwall et al., 2012; Claassen et al., 2010; Frosch et al., 2005; Senior et al., 2000).
Diminished perceived control may not be an inevitable consequence of receiving information about genetic risk for disease, however. The effects of biological or genetic attributions for medical conditions can be reversed by emphasizing the malleability of biological contributors to illness (Cameron et al., 2012; Lebowitz et al., 2013). Research that determines whether, when, and in what ways genetic risk information may reduce (or enhance) perceived control is critical because low control perceptions may interfere with performing recommended preventive behaviors (Armitage & Conner, 2001).
At present, there are relatively few data concerning the impact of genetic counseling and test reporting on perceived control over cancer prevention. For most hereditary cancer syndromes, patients are recommended to reduce cancer risk through accelerated screening and/or prophylactic surgery, and there are few preventive actions that can be performed in daily life (Aspinwall, Taber, Kohlmann, & Leachman, 2013). An important exception is familial melanoma. The CDKN2A/p16 (or simply, p16) mutation confers an extremely elevated risk (30–70x population risk, 76% lifetime risk for US residents), yet genetic factors may interact with environmental exposure to ultraviolet radiation (UVR), determined largely by personal behavior, to determine risk (Bishop et al., 2002). Thus, one of the primary goals of melanoma genetic testing and counseling is to educate patients about how they can manage their risk through prevention and early detection. It is, therefore, important to study whether providing positive p16 test results undermines control-related beliefs, including beliefs about the effectiveness of recommended sun-protection behaviors, and increases deterministic beliefs about melanoma development.
Overview and Predictions
In the present study, we prospectively examined the impact of melanoma genetic test reporting and counseling, particularly the impact of a positive p16 test result, on perceived control over melanoma prevention. Changes in control perceptions were examined over a 2-year period in three groups of high-risk family members undergoing melanoma genetic test reporting: unaffected carriers, affected carriers, and unaffected noncarriers. Because the genetic counseling sessions emphasized prevention and early detection measures that can be taken to manage melanoma risk, we predicted that unaffected carriers’ control perceptions would not necessarily decrease following genetic test reporting and counseling. For affected carriers, a positive test result would provide a genetic cause for prior melanoma diagnoses and confer risk for new melanomas, which could potentially undermine perceived control. Finally, we predicted that unaffected noncarriers would report sustained increases in perceived control over melanoma development.
Method
Study Population and Procedures
Companion test-reporting and follow-up studies were approved by the Institutional Review Board (IRB#s 7916 and 13816). Participants were recruited from two large melanoma pedigrees who had contributed DNA samples for prior melanoma genetic research, beginning with an IRB-approved study in the late 1980s that utilized the Utah Population Database to identify pedigrees with a hereditary pattern of melanoma (Cannon-Albright et al., 1999; Kamb et al., 1994). In this research, two kindreds were found to have deleterious p16 mutations (V126D and 5′UTR34G>T). In the early 2000s, every living participant in the gene-identification studies was invited to participate in a phenotyping study which included mutation testing, but no participants learned that the p16 mutation was present in their family or of their personal mutation status (Florell et al., 2005).
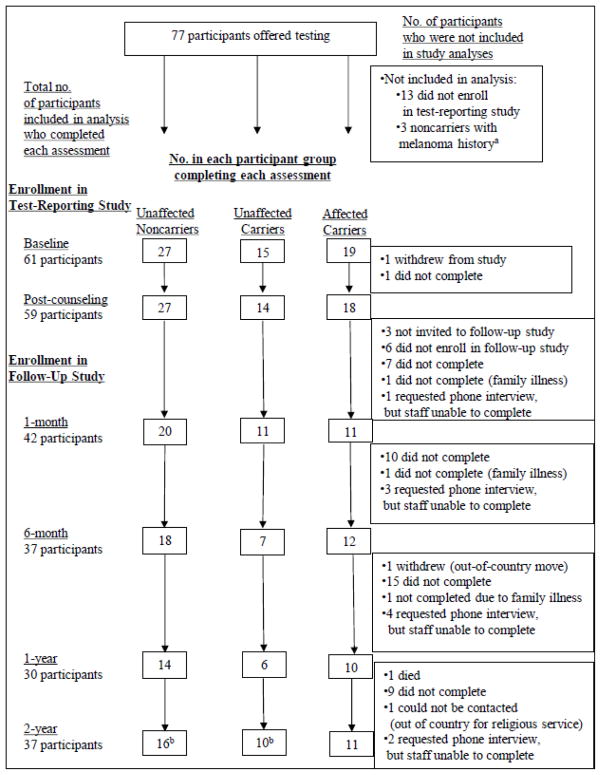
For the present study, every participant in the phenotyping study who was a member of a p16-positive kindred (n=77) was invited to participate (see Aspinwall et al., 2008). Recruitment and retention are summarized in Figure 1. From May through November 2005, 61 participants (82.4% of the participants from the three eligible groups) completed a written baseline questionnaire immediately prior to undergoing pre-disclosure genetic counseling followed by test reporting. All 61 participants elected to receive results, but one withdrew after counseling due to fatigue. After completing a post-counseling questionnaire, 57 participants were then invited to enroll in the companion follow-up study of long-term psychological and behavioral responses to p16 test reporting (Aspinwall et al., 2008, 2009, 2013a, 2013b; 2014a, 2014b); of these, 51 (89.5% of eligible participants) enrolled. As shown in Figure 1, response rates for follow-up assessments were 82.4% of enrolled participants at 1 month, 72.6% at 6 months, 58.8% at 1 year and 72.6% at 2 years. Participants received modest non-monetary incentives (e.g., water bottles and tote bags) for completing follow-up questionnaires.
Figure 1.
Recruitment, retention, and attrition of unaffected noncarriers, unaffected carriers, and affected carriers in the genetic test reporting and follow-up studies.
a3 affected noncarriers who received genetic counseling and test results and who completed multiple assessments were excluded from analysis because there were too few participants to permit statistical analyses from which meaningful inferences or comparisons to other participant groups could be made.
b3 unaffected noncarriers and 2 unaffected carriers completed an abbreviated version of the survey by telephone that did not include the perceived control ratings. Thus, the effective sample size for perceived control ratings at 2 years is 32 (56.1% of eligible participants).
Pre- and Post-Disclosure Genetic Education and Counseling
Genetic testing was performed in a Clinical Laboratory Improvement Amendments certified laboratory. Melanoma genetics education and test-reporting (Aspinwall et al., 2008) were conducted in a single session. Melanoma risk associated with p16 mutations was presented as a 35- to 70-fold increase from general population risk (approximately 1%) and illustrated with a bar graph indicating a risk of 50% by age 50 and 76% by age 80. Those testing negative were informed that they still may have up to a 1.7-fold residual risk due to other co-inherited familial risks such as melanoma-prone phenotype. All participants were informed they could minimize their risk for developing an advanced melanoma through screening (annual total body skin exams and monthly self-exams) to detect and remove abnormal lesions. Participants were educated about the role of UVR in melanoma development and presented with several strategies to minimize exposure including protective clothing and use of sunblock.
Measures
Demographic information and medical history
Participants completed standard demographic questions. Melanoma history was confirmed through medical records (Aspinwall et al., 2008).
Perceived control over the development of future melanomas
Control beliefs were assessed both qualitatively and quantitatively. First, immediately following genetic counseling and test reporting, participants were asked, “In general, do you believe that it is possible for you to prevent future melanomas? Why or why not?” Second, at baseline and all follow-up assessments, participants completed the item, “Overall, how much personal control do you feel you have over whether you develop melanoma in the future?” (1=no control, 5=a lot of control).
Perceived effectiveness of specific sun-protection behaviors in reducing personal melanoma risk
At baseline, post-counseling, and 1 year, participants indicated agreement with the following statement: “For me, using sunscreen is (or would be) effective in reducing my risk of developing melanoma” (1=not at all, 5=very much). Parallel items assessed the effectiveness of “wearing protective clothing (long sleeves, long pants, and hats)” and “avoiding UV exposure during peak hours (10am–4pm).”
Belief that p16 mutation carriers will inevitably develop melanoma
At baseline, post-counseling, and 1 month participants indicated agreement with the following statement, “A person who tests positive for a gene change that increases his or her risk of developing melanoma [the p16 gene change] will definitely get melanoma during his or her lifetime” (1=strongly disagree, 5=strongly agree; an “I don’t know” option was provided and coded as 3, “neither agree nor disagree”). At baseline, we used the more general wording regarding “a gene change” because this assessment preceded specific education regarding p16 mutations.
Overview of Data Analysis
Multilevel modeling in Hierarchical Linear and Nonlinear Modeling (HLM, Raudenbush & Bryk, 2002) was conducted to examine changes up to 2 years following test reporting and counseling in perceived control over melanoma prevention in each participant group. Multilevel modeling was used because it allowed modeling of within-person trajectories over time in addition to testing for group differences and because it allowed us to retain all participants for analysis for whom we had at least one perceived control rating following test reporting (n=60; 199 included level-1 data units). Of the 60 participants included in the perceived control analyses, 24 (40%) had complete data for all six assessments, 8 (13.3%) had complete data for five assessments, 5 (8.3%) had complete data for four assessments, 9 (15%) had complete data for three assessments, and 14 (23.3%) had complete data for two assessments. Multilevel modeling accommodates missing data using a maximum likelihood approach (which weights cases that contribute less to overall variance, usually cases with more complete data, more heavily in the estimation of fixed effects) and is preferable to listwise deletion (Graham, 2009).
Next, we examined participants’ qualitative accounts of whether and how it was possible to prevent new melanomas. Participants’ responses concerning whether and how it was possible to prevent new melanomas were content coded using a coding scheme developed for this study by the authors (see Table 1). Responses concerning whether it was possible to prevent new melanomas were coded into four response categories (yes, can reduce but not eliminate risk, maybe, no) with accompanying reasons coded into four broad categories: 1) the use of precautionary behaviors to protect skin from sun exposure; 2) the practice of regular skin screening; 3) any explicit mention of genetic factors related to melanoma risk and prevention; and 4) other factors not related to prevention, screening, or genetics. Two independent raters coded all responses and obtained 86.6% agreement. Disagreements were resolved in conference.
Table 1.
Qualitative explanations obtained immediately following genetic counseling and test reporting of whether and how participants in each group believed it was possible for them to prevent a future melanoma.
| Unaffected Noncarriers (n=26) | Unaffected carriers (n=13) | Affected carriers (n=18) | Total (n=57) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| Possible for you to prevent future melanomas? | ||||||||
| Yes | 18 | 69.2 | 8 | 61.5 | 11 | 61.1 | 37 | 64.9 |
| Can reduce but not eliminate risk | 8 | 30.8 | 5 | 38.5 | 3 | 16.7 | 16 | 28.1 |
| Maybe | 0 | 0.0 | 0 | 0.0 | 2 | 11.1 | 2 | 3.5 |
| No | 0 | 0.0 | 0 | 0.0 | 2 | 11.1 | 2 | 3.5 |
| Reasons why it is or is not possible to prevent a future melanoma | ||||||||
| Use Precautionary Behaviors to Protect Skin | 22 | 84.6a | 11 | 84.6a | 6 | 33.3b | 39 | 68.4 |
| Limit sun exposure | 14 | 53.8 | 7 | 53.8 | 1 | 5.6 | 22 | 38.6 |
| Unspecified (Can take precautions to reduce the chance of developing melanoma) | 5 | 19.2 | 5 | 38.5 | 5 | 27.8 | 15 | 26.3 |
| Use proper sunscreen | 5 | 19.2 | 2 | 15.4 | 0 | 0.0 | 7 | 12.3 |
| Wear protective clothing or hats | 3 | 11.5 | 0 | 0.0 | 0 | 0.0 | 3 | 5.3 |
| Avoid tanning beds | 1 | 3.8 | 0 | 0.0 | 0 | 0.0 | 1 | 1.8 |
| Practice Regular Screening | 13 | 50.0a | 3 | 23.1b | 10 | 55.6a | 26 | 45.6 |
| Early detection and removal of questionable moles or dysplastic nevi | 6 | 23.1 | 2 | 15.4 | 7 | 38.9 | 15 | 26.3 |
| Skin self-exams | 6 | 23.1 | 1 | 7.7 | 6 | 33.3 | 13 | 22.8 |
| Annual total body skin exams | 4 | 15.4 | 0 | 0.0 | 2 | 11.1 | 6 | 10.5 |
| Unspecified mention of screening | 4 | 15.4 | 1 | 7.7 | 0 | 0.0 | 5 | 8.8 |
| Genetic Factors | 4 | 15.4 | 1 | 7.7 | 1 | 5.6 | 6 | 11.0 |
| p16 gene can lead to cancer | 1 | 3.8 | 1 | 7.7 | 1 | 5.6 | 3 | 5.3 |
| Negative status reduces risk, but the presence of other risk factors means must still be careful | 2 | 7.7 | 0 | 0.0 | 0 | 0.0 | 2 | 3.5 |
| Negative status means not at risk | 1 | 3.8 | 0 | 0.0 | 0 | 0.0 | 1 | 1.8 |
| Additional Factors | 0 | 0.0 | 2 | 15.4 | 1 | 5.6 | 3 | 5.3 |
| Can reduce risk by diet | 0 | 0.0 | 2 | 15.4 | 0 | 0.0 | 2 | 3.5 |
| Can take care of ourselves | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 1 | 1.8 |
| There is always new research | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 1 | 1.8 |
Notes. Totals for each bolded major category (e.g., Use Precautionary Behaviors to Protect Skin) consist of the number of participants who mentioned one or more of the subcategories. Major category percentages with differing superscripts differed between groups according to Fisher’s exact tests (p<.05; if italicized, p<.10)
Finally, repeated-measures analyses of variance (ANOVAs) tested prospective changes in the perceived effectiveness of recommended sun-protection behaviors for reducing one’s own melanoma risk and the belief that mutation carriers would inevitably develop melanoma.
Results
Participant Characteristics
Participants were on average 45.2 years old (SD=14.65). All were White, 54.1% were male, 86.5% were married, 91.9% had a high school education or higher, and median total household income assessed in annual increments of $10,000 was $60–$69,000. Nearly all participants (95%) were members of one of two large kindreds (Kindred A, n = 26; Kindred B, n = 31). Participants with a melanoma history (n=18) had an average of 2.44 (SD=2.12) past melanomas. There were no significant differences in age, sex, education, household income, or baseline control beliefs among the three patient groups. Participants who did not complete the 2-year assessment did not differ from others in group membership, age, sex, education, household income, or baseline control beliefs.
Impact of Melanoma Genetic Test Reporting on Perceived Control over Melanoma Development
We used multilevel modeling to examine the effect of genetic counseling and test reporting on perceived control over prevention in the three participant groups.1 Values for perceived control over melanoma development at post-counseling, 1 month, 6 months, 1 year, and 2 years served as the outcome variable and were centered at each participant’s baseline value of perceived control over prevention; therefore, the values graphed below represent change from baseline, with positive values signifying increases above baseline values. Time was scaled in months, with both the linear and quadratic terms included in the model to test for curvilinear effects. Dummy codes accounted for participant group. For example, the following model tested whether unaffected carriers reported elevated perceived control 1 month following counseling and test reporting:
- Level 1:
- Level 2:
In the initial model reported below, the level-2 variable melanoma history was coded as 0 for unaffected participants and 1 for affected participants, and the level-2 variable carrier was coded as 0 for p16 mutation carriers and 1 for noncarriers. Time was centered at the 1-month assessment. Thus, unaffected carriers are the reference (all zero) group in this model. In subsequent models, coding of these variables was varied to test whether there was a significant difference from baseline values at each assessment within each group.
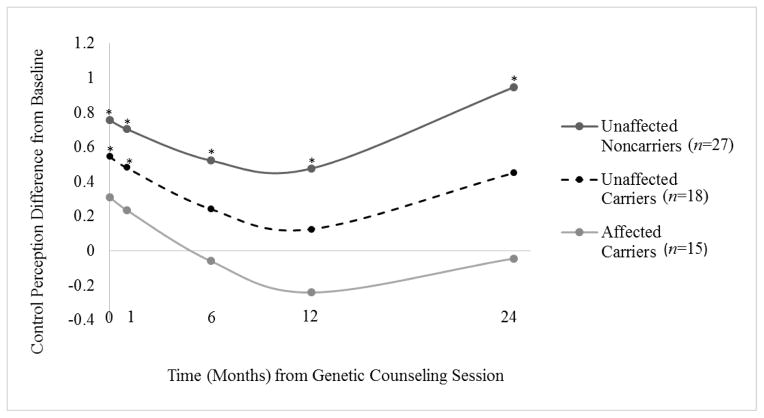
Trajectories of change tended to be curvilinear, γ20 =.003, t(57)=1.84, p=.07. As shown in Figure 2, at both post-counseling and 1 month, unaffected carriers’ reports of perceived control were significantly elevated above baseline values, but did not differ from baseline values at subsequent assessments. For noncarriers, perceived control estimates remained significantly elevated above baseline throughout the 2-year follow-up. Affected participants’ perceived control did not change significantly from baseline at any follow-up assessment. Finally, there was significant variation between participants in both the slope for the quadratic term for time, u2 = .00004, χ2 (36) = 59.21, p=.009, as well as in the linear slopes in each analysis, u1s = .01 – .02, p < .01. Evidence of this individual variability in trajectories led us to more closely examine individual trajectories of change in each participant group.
Figure 2.
Impact of melanoma genetic test reporting and counseling on changes in perceived control over the development of a new melanoma in the three participant groups over the 2-year period following test reporting.
*Indicates a significant difference from baseline, p<.05
Note. On the x-axis, 0 months indicates the assessment completed immediately after genetic counseling and test reporting. On the y-axis, positive values indicate increases above baseline values, and negative values indicate decreases below baseline.
Net trajectories of change in the three patient groups
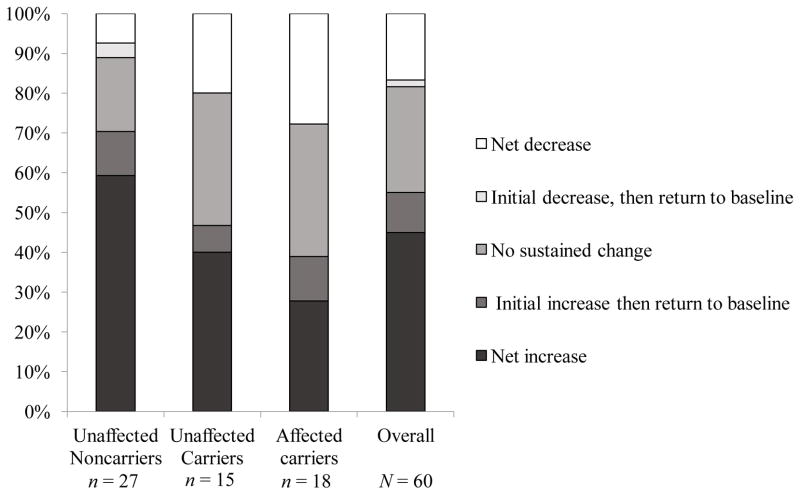
We evaluated individual short-term and net trajectories of change in perceived control up to 2 years following test reporting and counseling, using the latest available follow-up assessment for each of the 60 participants who provided at least one post-counseling assessment. As shown in Figure 3, increases (45%) or no change (38.33%) in perceived control over melanoma prevention were observed in the majority of the sample. Reported decreases in perceived control over melanoma prevention were less frequent (16.67%), but were concentrated in the two carrier groups (20% of unaffected carriers, 27.78% of affected carriers vs. 7.4% of noncarriers, p = .08). These decreases were relatively small -- an average of 1.1 points (SD = 0.3) on a 5-point scale. Additionally, among the eight carriers who reported decreases, all but one indicated that they perceived at least some control over melanoma prevention by responding at or above the midpoint of the scale at all assessments.
Figure 3.
Trajectories of changes from baseline in perceived control over melanoma development in the three participant groups up to 2 years following melanoma genetic test reporting and counseling
Qualitative Analysis of Participants’ Accounts of Whether and How They Can Prevent a New Melanoma
As shown in Table 1, 57 out of 59 (96.6%) respondents completing the post-counseling assessment listed one or more reasons why they could or could not prevent melanoma. Of these, 53 (93%) indicated that it was possible to prevent melanoma. Some participants (28.1%) qualified their answers by explaining that they believed it was possible to decrease but not eliminate the likelihood of developing melanoma. For example, one affected carrier wrote, “Prevent? No. Decrease likelihood? Yes.”
Table 1 lists the four major categories of reasons provided, specific subcategories for each major category, and the number of participants in each group who listed one or more reasons in each category and subcategory. Many respondents (64.8%) reported that melanoma could be prevented by protecting one’s skin from sun exposure. Unaffected participants were significantly more likely to mention preventive measures than affected participants (84.6% versus 33.3%, p<.01). For example, one unaffected carrier noted, “Yes, for sun exposure is a great risk and that is something I, as a person and in command of my own life, can control.” Nearly half of respondents (45.6%) listed screening as a method to prevent melanoma, including routine skin self-exams, total body skin exams by medical professionals, and early detection and removal of questionable moles. Screening and early detection were cited by about half of the affected carriers and unaffected noncarriers, but marginally fewer unaffected carriers (23.1%, p=.079). A few participants in this category specifically stated that melanoma cannot be prevented, but that screening can be used to detect melanoma early. For example, one affected carrier noted, “No - however you can catch them early and avoid them growing into a major problem.”
A small subset of patients (11%) listed genetic factors as a determinant of whether melanoma could be prevented. Only three participants (5.3%) mentioned that the p16 mutation can or will lead to cancer. Two of these three participants mentioned that behaviors could still offset risk. One unaffected carrier observed, “Sure, the gene does not pre-determine cancer, just that the cells have less changes to make before turning malignant. Reducing exposure still lessens the chance for mutations.” Conversely, only one participant, an unaffected noncarrier, indicated that he or she did not have the p16 mutation without acknowledging additional risk factors (“I do not have the gene.”). Two unaffected noncarriers specifically mentioned elevated melanoma risk, despite testing negative for p16 (“Yes, because although I tested negative for p16, I still have other risk factors, so I should prevent it by precautionary behavior and early screening.”). Finally, a small percentage (5.3%), all of whom were mutation carriers, listed additional prevention strategies not included in the genetic counseling protocol, such as improved diet and taking care of oneself. Importantly, these participants also cited reduced sun exposure.
Impact of Melanoma Genetic Testing on Beliefs regarding the Effectiveness of Sun-Protection Behaviors for Reducing Melanoma Risk
For all three recommended sun-protection behaviors, perceived effectiveness was high at baseline with no differences among participant groups (sunscreen, M=4.50, SD=.95; protective clothing, M=4.38, SD=.85, avoiding peak UVR exposure, M=4.33, SD=.93). For sunscreen and protective clothing, ratings remained high and did not change immediately following test reporting and counseling (sunscreen, n=56, M=4.41, SD=.95; protective clothing, n=58, M=4.26, SD=1.04) or 1 year later (sunscreen, n=25, M=4.28, SD=.74; protective clothing, n=29, M=4.48, SD=.83). For avoiding peak UVR exposure, perceived effectiveness decreased following testing among unaffected carriers (M at baseline=4.64, SD=.63, M at post-counseling=3.57, SD=1.34, p=.001) with no significant changes for the other patient groups, but returned to high baseline levels at 1 year with no differences among participant groups (n=29, M=4.41, SD=.87).
Impact of Melanoma Test Reporting on Deterministic Beliefs about Melanoma Development
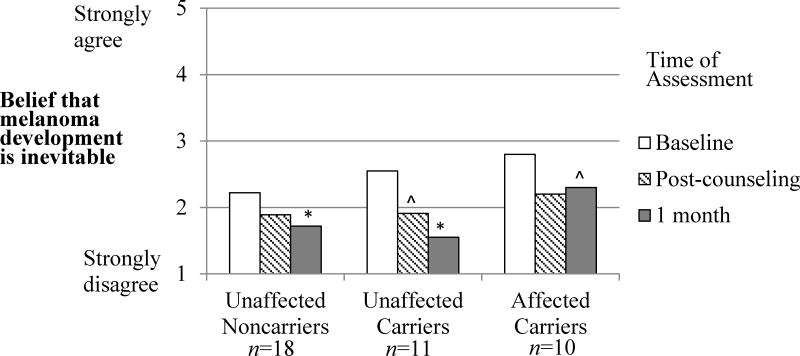
Last, we examined the impact of counseling and test reporting on beliefs about whether mutation carriers will inevitably develop melanoma. A repeated-measures ANOVA indicated that agreement significantly decreased from 2.46 (SD=.79) at baseline to 1.82 (SD=.82) at 1 month following test reporting (main effect of Time, F(2,35)=11.67, η2=.25, p<.001). As shown in Figure 4, this decrease was significant for noncarriers (p=.024) and unaffected carriers (p=.001), and marginally significant for affected carriers (p=.087), with no differences among groups.
Figure 4.
The prospective impact of melanoma genetic test reporting on beliefs that melanoma development is inevitable among p16 mutation carriers in the three participant groups.
*Indicates a significant difference from baseline, p<.05
^Indicates a marginally significant difference from baseline, p<.10
Exploratory Analyses Comparing Decliners to Other Participants
To explore whether participants who reported net decreases in perceived control differed from other participants in other post-counseling control-related beliefs, we conducted a series of independent-samples t-tests. Participants who reported a net decrease in perceived control tended to more strongly endorse the belief that melanoma is inevitable (Mdecline=2.40, SD=1.17; Mothers=1.86, SD=.82; t(57)=1.77, d=.60, p=.08), but did not differ from other participants in beliefs regarding the effectiveness of sun protection (Msdecline=4.30–4.60, SDs=.70–1.06, Msothers=4.06–4.35, SDs=1.03–1.18; ps>.10). Additionally, those reporting net decreases had higher baseline perceived control beliefs (Mdecline=4.20, SD=.63; Mothers=3.16, SD=.89; t(58) = 3.52, d=1.13, p<.01).
Supplementary Analyses Examining Emotional and Behavioral Correlates of Changes in Perceived Control Following Test Reporting
Last, improvements (or declines) in perceived control may have important relations to emotional and behavioral outcomes of genetic testing. We have previously described both psychological and behavioral outcomes of melanoma genetic testing in this sample, finding low reported distress overall (Aspinwall et al., 2013a) and sustained improvements in sun-protection behavior among unaffected carriers and noncarriers at the 2-year follow-up (Aspinwall et al., 2014b). To examine the association of changes in perceived control with these outcomes, we conducted exploratory analyses of the relationships between changes in perceived control and changes in sun-protection behavior and psychological distress following test reporting and counseling.2
First, among the 37 participants who provided complete data concerning their frequency of practice of specific photoprotection behaviors at the 2-year follow-up (see Aspinwall et al., 2014b, for details), we used repeated-measures ANOVA to examine changes in sun-protection behavior from baseline to 2 years among participants who reported increased, decreased, or unchanged estimates of perceived control over the course of the study. In the case of protective clothing use only, this analysis yielded a significant Participant Group x Change in Perceived Control x Time interaction (F(4,28) =2.902, p <.040), such that both unaffected carriers and unaffected noncarriers who reported increases in perceived control reported significant increases in protective clothing use at 2 years. Specifically, unaffected carriers (n=5) increased from 36% (SD=44.64%) of the time at baseline to 88% (SD=6.71%) at 2 years, p<.001, and unaffected noncarriers (n=10) increased from 43% (SD=30.39%) of the time to 66.5% (SD=33.92%), p<.029. These increases were not observed among participants who reported no net change in perceived control (unaffected carriers, n=4, 71.25% [SD=17.02%] to 53.75% [SD=29.55%], p<.288; unaffected noncarriers, n=5, 58% [SD=38.99%] to 70% [SD=33.17], p<.413). Among affected carriers, participants who reported decreases in perceived control (n=4) reported marginally decreased use of photoprotective clothing (from 96.25% [SD=7.5%] to 67.50% [SD=23.63%] of the time, p<.086). Consistent with these findings, increases in perceived control from baseline to 2 years tended to be positively correlated with increases in reported use of photoprotective clothing over the same interval in the total sample, r=.34, p<.06.
We next conducted parallel analyses to examine the relation of reported changes in perceived control to changes in anxiety, depression, and cancer worry. These analyses were conducted among the 60 participants who provided one or more post-counseling ratings of anxiety, depression, and cancer worry (see Aspinwall et al., 2013a, for details). Although perceived control was correlated with psychological distress at several assessments, these analyses yielded no significant relation between changes in perceived control and changes in distress, likely because distress was consistently low (Aspinwall et al., 2013a).
Discussion
A persistent concern in the translation of genetic and genomic knowledge to patient care is that providing information about the genetic causes of disease, particularly for highly penetrant mutations such as p16 or BRCA1/2, will undermine participants’ beliefs that their own actions may effectively mitigate disease risk (Marteau & Weinman, 2006). Contrary to these concerns, we did not find that receiving a genetic test result generally undermined control perceptions related to melanoma development. Instead, nearly all participants who received melanoma genetic test results and counseling, including those who tested positive, subsequently reported that they could prevent the development of a new melanoma through sun-protection behaviors and/or early detection and removal of suspicious skin changes. Additionally, both groups of unaffected participants reported increased perceived control at the post-counseling and 1-month follow-ups, with sustained gains throughout the 2-year follow-up reported by unaffected noncarriers. Beliefs that recommended sun-protection behaviors would be effective in reducing personal melanoma risk were uniformly high among the three participant groups. Finally, counter to the concern that information about genetic causes of melanoma would induce deterministic beliefs about vulnerability to disease, participants reported significant decreases in the belief that p16 mutation carriers would inevitably develop melanoma.
Both quantitative and qualitative results indicated potentially important differences in perceived control between family members with and without a personal history of melanoma. In contrast to unaffected participants, participants with a prior melanoma diagnosis did not report increases in perceived control over melanoma development at any assessment. Further, the qualitative accounts obtained immediately following test reporting and counseling indicated that compared to unaffected carriers, affected carriers were somewhat more likely to cite screening and significantly less likely to cite reductions in UVR exposure as reasons they believed they could prevent the development of a new melanoma. As patients with a history of melanoma report that they are highly likely to develop a new melanoma (Aspinwall et al., 2014a), they may be particularly receptive to more detailed counseling regarding early detection strategies. Future research might examine how specific experiences with melanoma development, detection, and treatment influence the impact of melanoma genetic test results on control-related beliefs.
Despite the general finding of sustained increases in perceived control among unaffected noncarriers and short-term increases among unaffected carriers, our multilevel analyses revealed individual variability in responses to genetic test reporting. These types of individual trajectories are often overlooked when analyzing group means. For instance, although there was no net change in perceived control among affected carriers, 27.8% of affected carriers reported net increases and 27.8% reported net decreases. Further, although unaffected carriers did not report a long-term increase in perceived control over melanoma development, examining individual trajectories revealed that 40% of participants in this group reported a net increase in perceived control and 20% reported a net decrease. For these reasons, we recommend both formal and descriptive analyses of trajectories of change in response to genetic test reporting, as group averages are likely to obscure this potentially meaningful variation.
Overall, a minority of participants receiving positive test results reported a net decrease in control perceptions. To effectively support such participants, it will be important to understand these responses in conjunction with other beliefs about melanoma risk and prevention. For example, decreased control perceptions may not necessarily indicate that a patient holds beliefs that interfere with prevention efforts; instead, decreases may reflect an increased appreciation of the multifactorial nature of melanoma risk following detailed education about genetic, phenotypic, and environmental contributors to risk. Importantly, those who reported decreases in perceived control nevertheless reported beliefs that sun-protection behaviors would be highly effective.
Finally, it is important to note that the provision of negative test results resulted in increased perceived control over the 2-year follow-up period. Importantly, these noncarrier family members overwhelmingly listed reduced UVR exposure and, to a lesser extent, screening as the methods they would use to manage their risk. Thus, we find little evidence that receipt of negative results reduces interest in or reported practice of sun-protection behaviors.
Limitations
This study included a relatively small sample of high-risk participants who had previously received counseling regarding elevated familial risk and the necessity for sun-protection and screening as part of prior research protocols. Nearly all participants were drawn from two extended families. Thus, these findings await confirmation in a larger sample drawn from a greater number of extended families, ideally those with less prior research participation. A larger sample would allow the examination of whether specific beliefs about the mutability of genetic risks may moderate responses to melanoma genetic test reporting, as well as analyses of the reciprocal relations between control perceptions and the practice of sun-protection and screening behaviors. Also, this 2-year prospective longitudinal study had considerable attrition across multiple assessments, beginning with non-invitation to (5%) or non-enrollment in (10%) the follow-up study following participation in the test-reporting study. Additionally, of those enrolled in the follow-up study, noncompletion of specific assessments ranged from 17.65% to 41.2%, with an additional 5.3% lost to follow-up through mortality, moving out of the country, or religious service. This degree of attrition, while not unusual for studies of this length and complexity (four lengthy mailed follow-up surveys over a 2-year period), decreases our confidence in the findings at later assessments. Importantly, we were able to test curvilinear patterns of change using multi-level modeling (which allows for missing within-subjects data) because 82.5% of participants eligible for the follow-up study provided at least one perceived control rating in the 2 years following counseling and test reporting. We believe that this method also produces results more representative of the larger initial sample by retaining for analysis those participants who either declined to enroll in the follow-up study or who passively withdrew by not completing questionnaires. Finally, there was no evidence of differential attrition in the three participant groups.
While the quantitative analysis of within-participant trajectories of perceived control indicated considerable variability over time, qualitative accounts of participants’ reasoning about whether and how they could prevent a future melanoma were obtained only at a single assessment immediately following counseling and test reporting. It would strengthen further investigations to collect qualitative accounts in parallel with quantitative ones to examine potential changes over time, as participants adjust to the information provided and attempt to implement consistent sun-protection and screening behaviors. This information could also be useful to genetic counselors in understanding the kinds of information about prevention and early detection that could be bolstered in booster sessions for members of high-risk families to support sustained increases in perceived control over melanoma risk.
An additional limitation of this study is that objective measures of sun exposure (e.g., reflectance spectroscopy) were not included. Including such measures would have allowed us to examine more precisely the relationship between changes in perceived control over melanoma development and changes in UVR exposure. Such analyses are a priority for future research, as we obtained exploratory evidence in the present study that increases in perceived control over the development of melanoma among unaffected family members (both carriers and noncarriers) were associated with sustained increases in the reported use of photoprotective clothing two years after test reporting and counseling. Conversely, decreases in perceived control were associated with trends toward decreased protective clothing use among affected carriers, although reported frequency remained high. These findings should be considered exploratory because of the small sample sizes created by stratifying by both participant group and net changes in perceived control. Additionally, changes in single sun-protection behaviors should be interpreted with caution, as multiple other strategies (e.g., sunscreen, avoidance of peak UVR exposure) may also be used. Therefore, it will be important in future research to corroborate these findings with objective measures of sun exposure, such as UVR dosimetry and reflectance spectroscopy. It will also be important to model the reciprocal relations between increased control perceptions and improved adherence over time.
Finally, it should be acknowledged that the effects of test reporting cannot be separated from the effects of counseling regarding photoprotection and screening, as counseling was always provided with test results. A formal test of the effectiveness of providing behavioral information (i.e., randomly assigning participants to receive or not to receive behavioral information in combination with a test result) would be unethical. Nevertheless, our finding that genetic test reporting does not generally decrease control beliefs from baseline levels is consistent with findings from scenario studies that included specific behavioral information. In scenario studies that describe both behavior and genetics as impacting disease risk, those who were asked to imagine that they received a positive test result remained confident in the effectiveness of risk-reducing behaviors (Cameron et al., 2012; Ugalde et al., 2008).
Conclusion and Future Directions
Providing melanoma genetic test results does not generally undermine perceived control over melanoma prevention. Further, participation in genetic counseling and test reporting appeared to reduce perceptions that disease is inevitable, even among people at highly elevated risk. Importantly, responses to test reporting were variable, with a subset of participants reporting declines in perceived control following positive test results. Future research should elucidate the reasons for this variability, and the ways in which increased or decreased control perceptions may either promote or impede prevention and screening behavior. Researchers should also develop ways to communicate test results and behavioral recommendations to family members who may be at risk for decreased control perceptions.
Acknowledgments
This work was supported by a Funding Incentive Seed Grant, Office of the Vice President for Research, University of Utah, and a Huntsman Cancer Institute (HCI) CCPS Pilot Project Award to Drs. Aspinwall and Leachman. Additional support was received from the Huntsman Cancer Foundation (HCF), the Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment, HCI’s Pedigree and Population Resource, the Utah Population Database, and the Utah Cancer Registry, which is funded by contract N01-PC-35141 from the National Cancer Institute (NCI) SEER Program with additional support from the Utah State Department of Health and the University of Utah. The authors used core facilities supported by the National Institutes of Health (NIH) through NCI Cancer Center Support Grant 5P30CA420-14 awarded to HCI, the genetic counseling core facility supported by HCF, and National Center for Research Resources grant 1KL2RR025763-01 awarded to the University of Utah by the NIH Office of the Director. The authors acknowledge NCI R01 CA158322-01 for partial support during article preparation. Content is the authors’ responsibility and does not necessarily represent views of NCI or NIH. The authors gratefully acknowledge study participants; and Marybeth Hart, Erin Dola, Lisa Wadge, Amber Kostial, Emily Bullough, Michelle Welch, Candace Larson, and Taylor Haskell for contributions to the conduct or management of the study.
Footnotes
Because 95% of participants came from two large extended families or kindreds, we examined whether responses depended on family membership. Because immediate family groups were not mutually exclusive (i.e., a participant could be a brother in one group, but a father in another group), we did not include family unit as a level of nesting. Instead, we accounted for dependencies among members of the same extended family by adding kindred as a level-2 variable in the model testing change in perceived control at 1 month. Of note, the interactions between kindred and participant group were not significant predictors of the intercept, slope for time, or the quadratic effect. Likewise, in a separate model, we found that average changes in perceived control reported by one’s siblings did not predict one’s own changes and did not interact with participant group. As these analyses did not suggest that outcomes depended on family unit, we did not retain either kindred or sibling group as factors in the model.
We considered using latent change score models to evaluate the relationships among difference scores over time (for example, changes in perceived control and changes in protective clothing use); however, these models assume linear relationships and equal time intervals across the assessments, assumptions that are not met by our study design (with assessments at post-counseling, 1 month, 6 months, 12 months, and 24 months) and findings (the curvilinear pattern of increases in perceived control over these time points).
Ms. Stump, Dr. Taber, Dr. Leaf, and Ms. Kohlmann declare no conflict of interest. Dr. Aspinwall’s work is funded by the NIH. Dr. Leachman’s work is funded by the NIH. Dr. Leachman serves on a Medical and Scientific Advisory Board for Myriad Genetics Laboratory, for which she has received an honorarium. She has collaborated with Myriad on a project to validate an assay that is unrelated to the research reported here.
Contributor Information
Lisa G. Aspinwall, University of Utah
Tammy K. Stump, University of Utah
Jennifer M. Taber, University of Utah
Wendy Kohlmann, Huntsman Cancer Institute.
Samantha L. Leaf, University of Utah
Sancy A. Leachman, Huntsman Cancer Institute
References
- Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: A meta-analytic review. British Journal of Social Psychology. 2001;40:471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Brown TR, Tabery J. The double-edged sword: Does biomechanism increase or decrease judges’ sentencing of psychopaths? Science. 2012;337:846–849. doi: 10.1126/science.1219569. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Leaf SL, Dola ER, Kohlmann W, Leachman SA. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiology Biomarkers & Prevention. 2008;17:1510–1519. doi: 10.1158/1055-9965.EPI-08-0010. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Leaf SL, Kohlmann W, Dola ER, Leachman SA. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. Journal of the American Academy of Dermatology. 2009;60:745–757. doi: 10.1016/j.jaad.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leachman SA. Psychological aspects of hereditary cancer risk counseling and genetic testing. In: Carr BI, Steel J, editors. Psychological aspects of cancer: A guide to emotional and psychological consequences of cancer, their causes and their management. New York: Springer; 2013. pp. 31–64. [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Perceived risk following melanoma genetic testing: A 2-year prospective study distinguishing subjective estimates from recall. Journal of Genetic Counseling. 2014a;23:421–437. doi: 10.1007/s10897-013-9676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genetics in Medicine. 2014b doi: 10.1038/gim.2014.37. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Genetic testing for hereditary melanoma and pancreatic cancer: A longitudinal study of psychological outcome. Psycho-Oncology. 2013a;22:276–289. doi: 10.1002/pon.2080. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiology Biomarkers & Prevention. 2013b;22:1687–1697. doi: 10.1158/1055-9965.EPI-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, Tucker MA. Geographical variation in the penetrance of CDKN2A mutations for melanoma. Journal of the National Cancer Institute. 2002;94:894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- Cameron LD, Marteau TM, Brown PM, Klein WM, Sherman KA. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: the role of coherence. Journal of Behavioral Medicine. 2012;35:286–298. doi: 10.1007/s10865-011-9361-5. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright LA, Goldgar DE, Meyer LJ, Lewis CM, Anderson DE, Fountain JW, Bale AE. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13–p22. Science. 1992;258:1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Claassen L, Henneman L, De Vet R, Knol D, Marteau T, Timmermans D. Fatalistic responses to different types of genetic risk information: exploring the role of self-malleability. Psychology and Health. 2010;25:183–196. doi: 10.1080/08870440802460434. [DOI] [PubMed] [Google Scholar]
- Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422(6934):835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Heine SJ. Genetic essentialism: on the deceptive determinism of DNA. Psychological Bulletin. 2011;137:800–818. doi: 10.1037/a0021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florell SR, Boucher KM, Garibotti G, Astle J, Kerber R, Mineau G, Leachman SA. Population-based analysis of prognostic factors and survival in familial melanoma. Journal of Clinical Oncology. 2005;23:7168–7177. doi: 10.1200/JCO.2005.11.999. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1485–1489. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Gould WA, Heine SJ. Implicit essentialism: genetic concepts are implicitly associated with fate concepts. PloS One. 2012;7:e38176. doi: 10.1371/journal.pone.0038176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A, Shattuck-Eidens D, Eeles R, Liu Q, Gruis NA, Ding W, Cannon-Albright LA. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nature Genetics. 1994;8:22–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: How can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- Lebowitz MS, Ahn WK, Nolen-Hoeksema S. Fixable or fate? Perceptions of the biology of depression. Journal of Consulting and Clinical Psychology. 2013;81:518–527. doi: 10.1037/a0031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, Hollands GJ. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Systematic Review. 2010;10 doi: 10.1002/14651858.CD007275.pub2. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Social Science & Medicine. 2006;62:1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McBride CM, Bowen D, Brody LC, Condit CM, Croyle RT, Gwinn M, Valente TW. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. American Journal of Preventive Medicine. 2010;38:556–565. doi: 10.1016/j.amepre.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin D, Lindee S. The DNA mystique. WH Freeman & Company; 1995. [Google Scholar]
- Parrott R, Silk K, Weiner J, Condit C, Harris T, Bernhardt J. Deriving lay models of uncertainty about genes’ role in illness causation to guide communication about human genetics. Journal of Communication. 2004;54:105–122. doi: 10.1111/j.1460-2466.2004.tb02616.x. [DOI] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Senior V, Marteau TM, Weinman J. Impact of genetic testing on causal models of heart disease and arthritis: an analogue study. Psychology & Health. 2000;14:1077–1088. doi: 10.1080/08870440008407368. [DOI] [PubMed] [Google Scholar]
- Ugalde A, Martin P, Rees G. Psychological impact of receiving genetic risk information for breast cancer, with and without lifestyle information. Australian Journal of Psychology. 2008;60:1–9. doi: 10.1080/00049530701449497. [DOI] [Google Scholar]