Abstract
Purpose
Over the past two decades reduced-intensity conditioning allogeneic hematopoietic cell transplantation (RIC HCT) has increased substantially. Many patients do not have fully HLA-matched donors, and the impact of HLA-mismatch on RIC HCT has not been examined in large cohorts.
Patients and Methods
We analyzed 2,588 recipients of 8/8 HLA-high resolution matched (n=2025) or single-locus mismatched (n=563) unrelated donor (URD) RIC HCT from 1999-2011. Overall survival (OS) was the primary outcome. Secondary endpoints included treatment-related mortality (TRM), relapse, disease-free survival (DFS), and acute/chronic graft-versus-host disease (GVHD).
Results
Adjusted one and three-year OS was better in 8/8 vs. 7/8 matched recipients (54.7% vs. 48.8%, p=0.01 and 37.4% vs. 30.9%, p=0.005, respectively). In multivariate models 7/8 RIC URD HCT recipients had more grade II-IV aGVHD (RR=1.29, p=0.0034), higher TRM (RR=1.52, p<0.0001) and lower DFS (RR=1.12, p=0.0015) and OS (RR=1.25, p=0.0001), with no difference in relapse or chronic GVHD. In subgroup analysis, inferior transplant outcomes were noted regardless of the HLA allele mismatched. Previously reported permissive mismatches at HLA-C (C*03:03/C*03:04) and HLA-DP1 (based on T cell-epitope matching) were not associated with better outcomes.
Conclusion
While feasible, single-locus mismatch in RIC URD HCT is associated with inferior outcomes.
Increasing numbers of patients traditionally considered ineligible for transplantation due to age, performance status or other comorbidities now commonly undergo reduced intensity conditioning followed by unrelated donor hematopoietic cell transplantation (RIC URD HCT). However, only ∼30-75% of patients needing transplantation will have an 8/8 HLA-matched (HLA-A, -B, -C, -DRB1) URD.1, 2 Frequently a 7/8 single HLA-mismatched donor is available for such patients. However, it is currently unknown whether 7/8 URD RIC HCT recipients experience inferior transplantation outcomes. In the context of myeloablative conditioning (MA) HLA 7/8 mismatches increase the incidence of acute graft vs. host disease (aGVHD) and treatment related mortality (TRM), resulting in inferior overall survival (OS).3-9 Considering however, that successful RIC transplant procedure relies more upon graft vs. leukemia (GVL) reactions than after myeloablation and that patients undergoing RIC URD HCT are more likely to be older and/or have more comorbidities, it is important to examine whether outcomes differ between 7/8 mismatched and fully HLA-matched (8/8) URD RIC procedures.
A small number of studies have investigated the impact of HLA-mismatch in URD RIC HCT and have noted the importance of HLA-C locus mismatches. A retrospective report from the Dana-Farber Cancer Institute compared HLA-C antigen- and allele-mismatched donors to fully matched donors and showed that mismatched recipients had significantly increased grade II-IV and III-IV acute GVHD (aGVHD), TRM, and worse 2-year OS.10 In a CIBMTR analysis of HLA-mismatches in URD PB transplantation performed from 1999-2006, a subgroup of 673 URD RIC transplantations was analyzed. Recipients of HLA-C antigen mismatched URD RIC transplants had significantly reduced survival.11 However, HLA-C allele mismatches and other locus mismatches were not identified as relevant, possibly due to limited sample size. A prospective study of T-replete 1-2 locus HLA-mismatched RIC URD utilizing PBSCs also described high rates of grade II-IV and III-IV aGVHD, NRM and a 2-year OS.12
To date, the question of whether HLA mismatching impacts upon the success of URD RIC HCT has not been fully addressed. This information is critical to optimize donor selection and perhaps extend the utility of transplantation. As well, data addressing this topic could constitute a baseline comparator for any novel conditioning or GVHD regimens for RIC URD recipients. Moreover, it is formally possible that HLA mismatching may be beneficial with respect to relapse, especially in RIC. Therefore, a large registry analysis was undertaken using the CIBMTR database to compare RIC URD transplant in recipients of HLA 7/8 antigen or allele matched transplants to recipients of 8/8 fully matched (HLA-A, -B, -C and DRB1) transplants. In subgroup analysis, we determined whether outcomes varied based on individual HLA locus mismatch. Secondary aims were to determine whether there was an interaction between disease risk and HLA mismatch and whether mismatching between specific HLA-C alleles (C*03:03/C*03:04) or HLA-DP (based on T cell-epitope matching) was permissive based on existing models in the MA setting.7, 8, 13, 14
Methods
Study population
This study included patients reported to the National Marrow Donor Program (NMDP)/CIBMTR who received a reduced intensity or non-myeloablative transplant from an URD between 1999 and 2011. Patients receiving MA conditioning were excluded. MA conditioning was defined as the following: single-dose total body irradiation (TBI) greater than 500cGy or more than 800cGy total in fractionated doses, busulfan at least 9mg/kg, melphalan with dose greater than 150mg/m2, or thiotepa dose > 10mg/kg. The study population consisted of recipients who received their first BM or PB URD transplantation for the treatment of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML) or myelodysplastic syndrome (MDS). Early stage disease was defined as AML or ALL in first complete remission, CML in first chronic phase, and MDS subtype refractory anemia. Intermediate stage disease was defined as AML or ALL in second or subsequent complete remission and CML in accelerated phase or second chronic phase. Advanced phase disease was defined as AML in first or higher relapse or primary induction failure, CML in blast phase and MDS subtypes refractory anemia with excess blasts or in transformation.
All surviving recipients included in this analysis provided informed consent for participation in the NMDP research program. Research was approved and conducted under the supervision of the NMDP Institutional Review Board. A modeling process was used as previously described to adjust for any bias introduced by exclusion of non-consenting survivors.5, 15 This adjustment is standard for all studies using NMDP data.
HLA Typing and Permissive Mismatch Analysis
High-resolution typing was performed as previously described for HLA-A, -B, -C, -DRB1, -DQB1 and -DPB1.16 Low-resolution (serologic or antigen-level) disparities were derived through conversion of DNA-based typing to serologic equivalent according to the 2010 WHO nomenclature for factors of the HLA system.17 Mismatch at HLA-DQ (and –DP) included only –DQB1 (and –DPB1), as there is strong linkage disequilibrium between the alpha and beta subunits (> 98%), and –DQA1 and –DPA1 typing data was limited (not available for the majority of cases in the dataset). DQB1 and DPB1 data was available for 97% and 53% of the population, respectively. As previously described,18 the directionality of HLA mismatch was considered for the analysis of GVHD and engraftment. Mismatches at homozygous alleles were considered single mismatches. Donor-recipient high-resolution HLA matching at HLA-A, -B, -C, and –DRB1 defined an 8/8 matched pair. Allele- or antigen-level mismatch at one (7/8) of these loci defined the mismatch group of interest in the main analysis. Secondary analyses examined the following: Mismatch at HLA-DPB1 or –DQB1, HLA-C*03:03/03:04 vs. other -C allele or antigen mismatch,19 and -DPB1 permissive vs. non-permissive mismatches according to T-cell epitope grouping, as previously reported.20 The –DPB1 permissive mismatch analysis was performed separately in 8/8 and 7/8 cases. These analyses did not consider –DQB1, as –DQB1 mismatch was infrequent (allele matched in 89% of cases).
Outcome definitions
OS was defined as time from HCT to death from any cause. Engraftment was defined as achieving an absolute neutrophil count of 500/μl for three consecutive days. Treatment-related mortality (TRM) was death in continuous remission from the primary malignancy. Disease free survival (DFS) and relapse were defined per Center for International Blood and Marrow Transplant Research (CIBMTR) criteria.21 Grades II-IV and III-IV aGVHD were defined by the Glucksberg scale,22 and chronic GVHD was defined as limited or extensive chronic GVHD according to the Seattle criteria.23
Statistical analysis
For univariate analysis, probabilities for OS and DFS were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood's formula. Values for other outcomes were calculated according to cumulative incidence. Death was considered a competing risk for all endpoints except OS and DFS. Relapse and TRM were considered competing risks for each other.
The association between number and type of HLA mismatches were evaluated by Cox proportional hazards models adjusting for significant clinical covariates. Stepwise selection procedures were fit to determine which risk factors were related to a given outcome. All variables were tested for the affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted for using time-varying covariates. Engraftment was reported as the cumulative incidence by day 100, with death as a competing risk. Interactions were checked between each selected variable and the main effect. Covariates tested include disease, disease status (early vs. intermediate vs. advanced), performance status, patient age, donor age, donor/recipient sex match (F/M vs. other), donor/recipient CMV match, graft source (PB vs. BM), T-cell depletion (anti-thymocyte globulin/alemtuzumab vs. none), GVHD regimen (calcineurin inhibitor-based (CNI) vs. other), and year of transplantation. Variables were categorized after examination of the data or based on current evidence for relevant breakpoints. The main analysis compares subgroups of HLA-mismatched pairs with 8/8 HLA-matched pairs. Given the multiple comparisons, p values <0.01 were be considered significant.
Results
Patient Characteristics
From 1999-2011, 2,588 patients underwent RIC URD HCT from 144 centers and 12 countries and were reported to the CIBMTR. 2,025 patients received transplantation from an HLA-matched unrelated donor (8/8 URD; HLA-A, -B, -C, -DRB1) and 563 patients were transplanted with a 7/8 allele or antigen matched unrelated donor (7/8 URD). Patient and transplant characteristics are shown in table 1. Briefly, the 8/8 URD recipients were older (p<0.003), more likely Caucasian (p<0.001), and were transplanted with younger donors (34 vs. 36 years, p=0.04). The two groups did not differ in disease status (p=0.09), conditioning (p=0.92), graft type (p=0.42), donor parity (p=0.07), use of TBI (p=0.33), in vivo T-cell depletion (p=0.10), or GVHD prophylaxis (p=0.74). Considering the whole group (both 8/8 and 7/8 URD patients), the conditioning intensity was more likely RIC compared to NMA (79% vs. 21%), and GVHD prophylaxis was mainly CNI-based, with either tacrolimus (70%) or cyclosporine A (27%). The median follow-up for the 8/8 URD group was 38 months and for 7/8 URD recipients it was 48 months, thus 7/8 transplants were performed earlier (p<0.001).
Table 1. Patient Characteristics.
| Variable | 8/8 | 7/8 | p-value |
|---|---|---|---|
| Number of patients | 2025 | 563 | |
| Conditioning intensity | 0.92 | ||
| RIC | 1601 (79) | 444 (79) | |
| NMA | 424 (21) | 119 (21) | |
| Recipient age | 0.03 | ||
| Median (range) | 59 (18-81) | 58 (19-76) | 0.003 |
| 18-39 | 215 (11) | 67 (12) | |
| 40-54 | 441 (22) | 154 (27) | |
| 55-59 | 397 (20) | 107 (19) | |
| 60-64 | 512 (25) | 129 (23) | |
| >=65 | 460 (23) | 106 (19) | |
| Recipient race | <0.001 | ||
| Caucasian | 1925 (95) | 508 (90) | |
| African-American | 44 (2) | 30 (5) | |
| Asian | 23 (1) | 8 (1) | |
| Pacific islander | 1 (<1) | 0 | |
| Native American | 2 (<1) | 3 (<1) | |
| Other | 2 (<1) | 0 | |
| Unknown | 28 (1) | 14 (2) | |
| Recipient gender | 0.20 | ||
| Male | 1162 (57) | 306 (54) | |
| Female | 863 (43) | 257 (46) | |
| Karnofsky score | 0.06 | ||
| <90% | 728 (36) | 233 (41) | |
| >=90% | 1190 (59) | 303 (54) | |
| Missing | 107 (5) | 27 (5) | |
| Disease at HCT | 0.11 | ||
| AML | 1318 (65) | 372 (66) | |
| ALL | 151 (7) | 44 (8) | |
| CML | 138 (7) | 51 (9) | |
| MDS | 418 (21) | 96 (17) | |
| Disease status prior to HCT | 0.09 | ||
| Early | 1038 (51) | 268 (48) | |
| Intermediate | 365 (18) | 124 (22) | |
| Advanced | 622 (31) | 171 (30) | |
| Graft type | 0.42 | ||
| Bone marrow | 296 (15) | 90 (16) | |
| Peripheral blood | 1729 (85) | 473 (84) | |
| Total body irradiation | 0.33 | ||
| No | 1575 (78) | 427 (76) | |
| Yes | 450 (22) | 136 (24) | |
| GVHD prophylaxis | 0.74 | ||
| FK506 +- others | 1431 (71) | 392 (70) | |
| CSA +- others | 540 (27) | 158 (28) | |
| Others | 54 (3) | 13 (2) | |
| In vivo T-cell depletion | 0.10 | ||
| No | 1194 (59) | 310 (55) | |
| Yes | 831 (41) | 253 (45) | |
| Donor age | <0.001 | ||
| Median (range) | 34 (<1-221) | 36 (19-221) | 0.04 |
| 19-32 | 1017 (50) | 231 (41) | |
| 32-49 | 890 (44) | 288 (51) | |
| >=50 | 114 (6) | 40 (7) | |
| Not available | 4 (<1) | 4 (<1) | |
| Donor Parity | p=0.07 | ||
| Male or not parous | 1674 (83) | 444 (79) | |
| Parous | 307 (15) | 100 (18) | |
| Not available | 44 (2) | 19 (3) | |
| Year of HSCT | <0.001 | ||
| 1999-2002 | 104 (5) | 48 (9) | |
| 2003-2006 | 578 (29) | 190 (34) | |
| 2007-2011 | 1343 (66) | 325 (58) | |
| Median follow-up of survivors (range), months | 38 (3-149) | 48 (3-145) |
Outcomes of URD 7/8 vs. 8/8 URD RIC Transplants
Table 2 shows the univariate analysis comparing outcomes between HLA 7/8 and HLA 8/8 RIC URD transplants. As shown, 7/8 RIC URD recipients had more frequent grade II-IV (43% vs. 35%, p=0.0018) and III-IV aGVHD (21% vs. 13%, p<0.001) and TRM (29% vs. 20%, p<0.001). There was no association with relapse at 1, 3 or 5 years (p=0.73, p=0.78, p=0.84) or chronic GVHD at 1, 3 or 5 years (p=0.3, p=0.06, p=0.06, respectively). OS was worse in 7/8 matched recipients at 1 year (48% vs. 55, p=0.003), 3 years (30% vs. 38%, p<0.001) and 5 years (22% vs. 30%, p=0.004).
Table 2. Univariate Analysis Comparing Transplant Outcomes Between Fully Matched (HLA 8/8) Donors to Single Locus (HLA 7/8) Mismatched Donors.
| HLA 8/8 Donor | HLA 7/8 Donor | ||||
|---|---|---|---|---|---|
| Outcomes | n | Prob (95% CI) | n | Prob (95% CI) | p value |
| Acute GVHD, grade II-IV | 1398 | 385 | |||
| 100-day | 35 (33-38)% | 43 (38-48)% | 0.0018 | ||
| Acute GVHD, grade III-IV | 1403 | 387 | |||
| 100-day | 13 (11-15)% | 21 (17-25)% | <0.001 | ||
| Chronic GVHD | 1989 | 516 | |||
| 6 months | 27 (25-29)% | 30 (26-34)% | 0.018 | ||
| 1-year | 44 (42-47)% | 44 (39-48)% | 0.30 | ||
| 3-year | 50 (48-52)% | 50 (45-54)% | 0.064 | ||
| 5-year | 50 (48-53)% | 50 (46-55)% | 0.057 | ||
| Extensive chronic GVHD | 2000 | 518 | |||
| 6 months | 20 (18-22)% | 24 (20-27)% | 0.017 | ||
| 1-year | 33 (31-35)% | 33 (29-37)% | 0.27 | ||
| 3-year | 37 (35-39)% | 38 (34-43)% | 0.056 | ||
| 5-year | 37 (35-40)% | 38 (34-43)% | 0.056 | ||
| Engraftment | 2061 | 510 | |||
| 100-day | 97 (96-98)% | 94 (92-96)% | 0.22 | ||
| Relapse | 2004 | 555 | |||
| 1-year | 36 (33-38)% | 33 (29-37)% | 0.78 | ||
| 3-year | 42 (40-44)% | 39 (35-43)% | 0.73 | ||
| 5-year | 44 (41-46)% | 40 (36-45)% | 0.84 | ||
| TRM | 2004 | 555 | |||
| 1-year | 20 (18-22)% | 29 (25-33)% | <0.001 | ||
| 3-year | 27 (25-29)% | 36 (31-40)% | 0.0011 | ||
| 5-year | 31 (29-34)% | 40 (35-44)% | 0.0088 | ||
| Disease free survival | 2004 | 555 | |||
| 1-year | 44 (42-47)% | 39 (35-43)% | 0.02 | ||
| 3-year | 31 (29-33)% | 25 (21-29)% | 0.01 | ||
| 5-year | 25 (23-27)% | 20 (16-24)% | 0.03 | ||
| Overall survival | 2025 | 563 | |||
| 1-year | 55 (53-57)% | 48 (44-52)% | 0.003 | ||
| 3-year | 38 (35-40)% | 30 (26-34)% | <0.001 | ||
| 5-year | 30 (27-32)% | 22 (19-27)% | 0.004 | ||
The consequences of a 7/8 URD RIC transplant were also considered relative to an 8/8 URD RIC transplants in multivariable analysis (Table 3). When adjusting for other significant covariates, the 7/8 URD RIC recipients were more likely to develop grade II-IV aGVHD (RR=1.29; p=0.003) (Figure 1A). Grade III-IV aGVHD (RR=1.69; p=0.05) did not meet the criteria for significance. Higher TRM (RR=1.52; p<0.0001) (Figure 1B), inferior DFS (RR=1.20; p=0.0015), and lower OS (RR=1.25; p=0.0001, Figure 1C) were seen in 7/8 compared to 8/8 URD RIC recipients. The impact of allele vs. antigen level mismatches in aggregate was also assessed. Comparing single allele mismatches to single antigen mismatches showed no differences in TRM (RR=1.37; p=0.27) or OS (RR=0.74; p=0.08). Whether ATG mitigated the negative impact of 7/8 donors was also investigated. However, there was no interaction between ATG and HLA matching, thus, while ATG may be improving outcomes in recipients 7/8 matched donors (compared to no ATG), it is not abrogating the negative effect of mismatch.
Table 3. Multivariable Analysis Comparing outcomes between HLA 8/8 to HLA 7/8 Donors.
| Variable | RR | 95% CI Lower Limit | 95% CI Upper Limit | p value | |
|---|---|---|---|---|---|
| aGVHD II-IV | |||||
| 8/8 allele match | 1 | 0.003 | |||
| 7/8 allele match | 1.29 | 1.09 | 1.53 | ||
| aGVHD III-IV | |||||
| 8/8 allele match | 1 | 0.05 | |||
| 7/8 allele match | 1.69 | 1.00 | 3.36 | ||
| CGVHD | |||||
| 8/8 allele match | 1 | 0.15 | |||
| 7/8 allele match | 1.11 | 0.96 | 1.28 | ||
| TRM | |||||
| 8/8 allele match | 1 | <.0001 | |||
| 7/8 allele match | 1.52 | 1.29 | 1.79 | ||
| Relapse | |||||
| 8/8 allele match | 1 | 0.92 | |||
| 7/8 allele match | 1.007 | 0.87 | 1.17 | ||
| DFS | |||||
| 8/8 allele match | 1 | 0.0015 | |||
| 7/8 allele match | 1.20 | 1.07 | 1.34 | ||
| OS | |||||
| 8/8 allele match | 1 | 0.0001 | |||
| 7/8 allele match | 1.25 | 1.12 | 1.40 | ||
Multivariable mo dels also included the following c ovariates: aGV HD II-IV: G VHD prophylaxis, graft type, in vivo T cell depletion, DPB1 matching; aGVHD III-IV: GVHD prophylaxis, TBI, in vivo T cell depletion; cGVHD: year of transplant, in vivo T cell depletion; TRM: graft type, recipient age, Karnofsky performance score, disease, GVHD prophylaxis; Relapse: Karnofsky performance score, disease status, conditioning intensity, in vivo T cell depletion; DFS: Karnofsky performance score, disease status, conditioning intensity; OS: graft type, recipient age, Karnofsky performance score, donor age, and disease status.
Figure 1. Impact of Single Allele Mismatch on (A) aGVHD II-IV, (B) cumulative incidence of TRM and (C) OS.
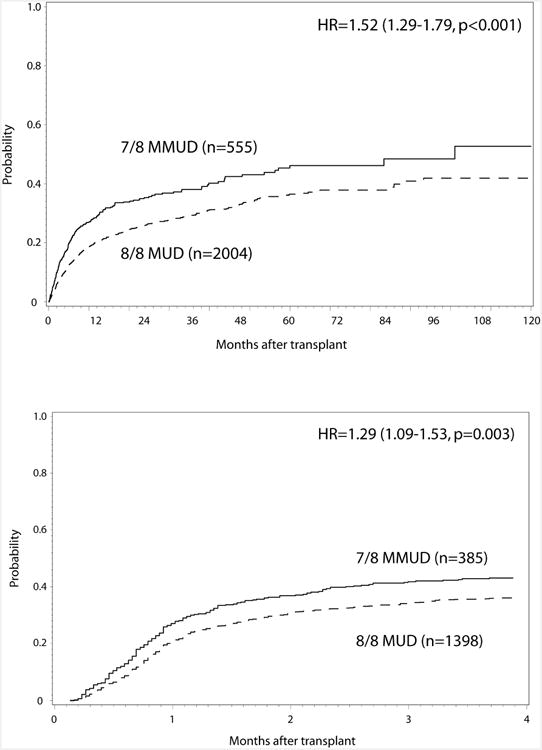
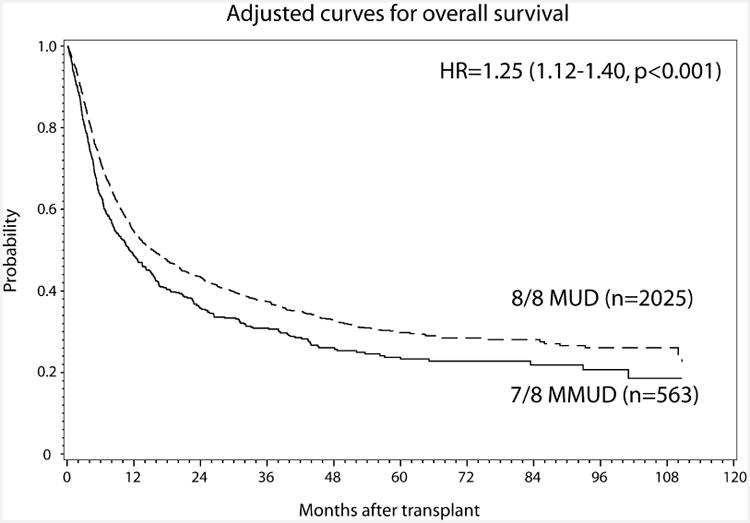
Potential differences in the association of HLA mismatching with disease status prior to transplantation were also considered, as described in myeloablative HLA-mismatched transplantation.5 There was no interaction between disease status and HLA matching in the OS analysis (p=0.42). Survival differences at 3 years based on HLA mismatching were not statistically different among early stage disease (43% HLA-matched vs. 38% HLA-mismatched), intermediate stage disease (40% vs. 31%), and advanced stage disease (27% vs. 19%).
Individual Locus Mismatch and Patient Outcomes
We investigated whether mismatching at specific HLA loci impacted RIC URD transplant outcomes. Mismatching at HLA-DQ and –DP were not associated with TRM, aGVHD, relapse, DFS and OS (not shown). Recently, certain HLA-DP mismatches have been identified as being permissive (or non-permissive) on the basis of T cell-epitope matching.20 Patients with available HLA-DP typing (n=1,056) were categorized as either matched (n=160), mismatched permissive (n=498) or mismatched non-permissive (n=398). In multivariable analysis, non-permissive mismatches were not associated with greater aGVHD II-IV (p=0.26) III-IV (p=0.57), chronic GVHD (p=0.79), TRM (p=0.3), relapse (p=0.47), DFS (p=0.8) or OS (p=0.45). Similarly, HLA C*03:03/C*03:04 mismatches have been reported to be permissive in MA transplantation.19 Only a small numbers (n=50-59, depending on the outcome measure) were available to assess whether the HLA-C*03:03/C*03:04 mismatch was better tolerated than other HLA C mismatches or other allele mismatches (HLA-A, -B or –DRB1). No differences were seen in TRM, GVHD, DFS or OS (not shown).
Adjusted multivariable outcomes for single allele mismatched recipients are shown in table 5. The number of patients in each HLA locus mismatch group became relatively small, ranging from 52-219 patients depending on the group and the transplant outcome analyzed. Mismatches at HLA-A and –C were more common than HLA-B or –DRB1 (33% vs. 39% vs. 14% vs. 13%, respectively, table 4). No differential impact of mismatching at a particular locus (HLA-A, -B, -C or –DRB1) was detected for either relapse or chronic GVHD. Mismatching at HLA-A was not associated with grade II-IV aGVHD, but did significantly increase grade III-IV aGVHD (RR=1.75; p=0.005) and TRM (RR=1.62; p=0.0002), and lower DFS (RR=1.26; p=0.009) and OS (RR=1.43; p<0.0001). Single allele mismatches at HLA-B showed no association with aGVHD grades II-IV or III-IV, but were strongly associated with higher TRM (RR=2.26; p<0.0001), and lower DFS (RR=1.51, p=0.001) and OS (RR=1.57; p=0.0005). HLA-C mismatched pairs had increased grade II-IV aGVHD (RR=1.4; p=0.005), but not grade III-IV aGVHD, TRM, DFS or OS. Lastly, HLA-DRB1 mismatches were associated with increased grade III-IV aGVHD (RR=2.49; p=0.001), but not grade II-IV aGVHD, TRM, DFS or OS. Collectively, these results show that mismatching at any loci (HLA-A, -B, and –C, or -DRB1) was associated with at least one inferior transplant outcome following RIC URD.
Table 5. Transplant Outcomes Based On Allele Locus Mismatches.
| Matching | n | RR | p-value | 95% CI Lower Limit | 95% CI Upper Limit | Overall p-value | |
|---|---|---|---|---|---|---|---|
| aGVHD II-IV | 0.025 | ||||||
| 8/8 allele match | 1366 | 1 | |||||
| Single MM at -A | 127 | 1.1 | 0.52 | 0.82 | 1.47 | ||
| Single MM at -B | 63 | 1.27 | 0.24 | 0.85 | 1.90 | ||
| Single MM at -C | 175 | 1.40 | 0.005 | 1.11 | 1.77 | ||
| Single MM at -DRB1 | 52 | 1.46 | 0.08 | 0.96 | 2.22 | ||
| aGVHD III-IV | 0.0004 | ||||||
| 8/8 allele match | 1371 | 1 | |||||
| Single MM at -A | 128 | 1.75 | 0.0 05 | 1.1 8 | 2.5 8 | ||
| Single MM at -B | 63 | 1.72 | 0.06 | 0.98 | 3.01 | ||
| Single MM at -C | 176 | 1.43 | 0.07 | 0.98 | 2.09 | ||
| Single MM at -DRB1 | 52 | 2.49 | 0.001 | 1.45 | 4.30 | ||
| TRM | <.00 01 | ||||||
| 8/8 allele match | 2004 | 1 | |||||
| Single MM at -A | 186 | 1.62 | 0.0002 | 1.25 | 2.09 | ||
| Single MM at -B | 80 | 2.26 | <.0001 | 1.63 | 3.14 | ||
| Single MM at -C | 216 | 1.32 | 0.03 | 1.03 | 1.70 | ||
| Single MM at -DRB1 | 73 | 1.18 | 0.45 | 0.77 | 1.79 | ||
| DFS | 0.0004 | ||||||
| 8/8 allele match | 2004 | 1 | |||||
| Single MM at -A | 186 | 1.26 | 0.0 09 | 1.0 6 | 1.5 1 | ||
| Single MM at -B | 80 | 1.51 | 0.001 | 1.18 | 1.93 | ||
| Single MM at -C | 216 | 1.18 | 0.049 | 1.00 | 1.38 | ||
| Single MM at -DRB1 | 73 | 0.85 | 0.29 | 0.63 | 1.15 | ||
| OS | <.00 01 | ||||||
| 8/8 allele match | 2025 | 1 | |||||
| Single MM at -A | 188 | 1.43 | <.00 01 | 1.2 0 | 1.7 1 | ||
| Single MM at -B | 81 | 1.57 | 0.0005 | 1.22 | 2.02 | ||
| Single MM at -C | 219 | 1.13 | 0.16 | 0.96 | 1.34 | ||
| Single MM at -DRB1 | 75 | 0.97 | 0.82 | 0.71 | 1.31 |
Multivariable models also included the following covariates: aGVHD II-IV: GVHD prophylaxis, graft type, in vivo T cell depletion; aGVHD III-IV: GVHD prophylaxis, TBI, in vivo T cell depletion; cGVHD: year of transplant, in vivo T cell depletion; TRM: graft type, recipient age, Karnofsky performance score, disease, GVHD prophylaxis; Relapse: Karnofsky performance score, disease status, conditioning intensity, in vivo T cell depletion; DFS: Karnofsky performance score, disease status, conditioning intensity; OS: graft type, recipient age, Karnofsky performance score, donor age, and disease status.
Table 4. Graft Matching Characteristics.
| Variable | 8/8 | 7/8 | P-value |
|---|---|---|---|
| High resolution matching for HLA-A,-B,-C and -DRB1 | |||
| Fully matched | 2025 | 0 | |
| Single MM at –A | 0 | 188 (33) | |
| Single MM at –B | 0 | 81 (14) | |
| Single MM at –C | 0 | 219 (39) | |
| Single MM at -DRB1 | 0 | 75 (13) | |
| Low resolution matching for HLA-A,-B,-C and -DRB1 | |||
| Fully matched | 2025 | 197 (35) | |
| Single MM at –A | 0 | 143 (25) | |
| Single MM at –B | 0 | 36 (6) | |
| Single MM at –C | 0 | 170 (30) | |
| Single MM at -DRB1 | 0 | 17 (3) | |
| DQB1 match | <0.001 | ||
| Allele matched | 1804 (89) | 488 (87) | |
| Single allele mismatch | 70 (3) | 36 (6) | |
| Double allele mismatch | 0 | 1 (<1) | |
| Single antigen mismatch | 71 (4) | 27 (5) | |
| One allele and one antigen mismatch | 2 (<1) | 1 (<1) | |
| Double antigen mismatch | 1 (<1) | 1 (<1) | |
| Missing | 77 (4) | 9 (2) | |
| DPB1 match | 0.06 | ||
| Allele matched | 160 (8) | 41 (7) | |
| Single allele mismatch | 559 (28) | 175 (31) | |
| Double allele mismatch | 337 (17) | 110 (20) | |
| Missing | 969 (48) | 237 (42) |
Discussion
A significant proportion of patients lack a fully HLA-matched URD for transplantation and one option is to use HLA partially-matched URDs. Over the past 10-15 years a number of large studies have shown that MA conditioning and HLA mismatched URD RIC transplantation results in inferior transplant outcomes.3-9 However, over the past two decades utilization of RIC conditioning has steadily increased worldwide. With this change, it is important to identify the risks and benefits of partially HLA-matched URD transplantation. There are a number of fundamental differences between RIC and MA transplants including the degree of tissue injury induced by the conditioning regimen, the kinetics of donor engraftment, and immunological reconstitution. As well, patients undergoing RIC conditioning tend to be older, have worse performance status and are more likely to have chronic or indolent malignancies relative to MA treated patients. In this large international registry study we demonstrate that single allele mismatched (7/8) RIC URD transplantation resulted in significantly inferior outcomes when compared to a cohort of HLA 8/8 matched donors. In particular, 7/8 mismatched RIC URD recipients experienced greater rates of aGVHD (grade II-IV) and TRM and inferior DFS and OS. RIC URD 7/8 recipients had an approximately 6.5 % reduction in OS at 3 years compared to RIC URD 8/8 recipients. These decrements are similar to, but perhaps lower than those observed in the MA setting where mismatched donors yielded a 9-14% reduction in OS.5, 6, 11 Thus, despite inherent differences between RIC and MA conditioning the overall impact of single locus mismatch appears to be similar.
The other goal of this analysis was to determine the impact of mismatching at individual HLA loci on RIC transplant outcomes to help identify better mismatched donors. Despite being a registry study of >2,500 patients, when the groups were analyzed based on individual locus mismatch (at HLA-A, -B, -C and –DRB1), patient numbers were reduced and thus, caution should be used in interpreting negative results since power may be limited. Importantly, all mismatch groups had at least one transplant outcome which was inferior to fully matched recipients. These findings contrast with Woolfrey who in a CIBMTR RIC URD subgroup analysis (n=673) reported that only HLA-C antigen mismatches had worse outcomes.11 In the current study, we had greater power to address this question and found that mismatching at any locus resulted in inferior transplant outcomes compared to fully matched donors. Of note, there was only 19% overlap between these two CIBMTR studies.
A number of interesting findings differed when compared to prior MA studies. First, we were surprised to observe that mismatches in HLA-DRB1 (n=75) were as well tolerated as HLA class I mismatches. In fact, in multivariable analysis HLA-DRB1 mismatches were not associated with reductions in DFS or OS, contrasting with prior data in the MA data.6, 7 Also interesting was that HLA-B mismatches were not associated with aGVHD grade II-IV or III-IV, but were strongly associated with TRM. In contrast, we found that mismatches at HLA-C were associated with aGVHD grade II-IV and TRM, but not severe grade III-IV aGVHD. Acknowledging the caveat of small patient numbers, the current results suggest the use of HLA-C or –DRB1 mismatched donors may be preferable over mismatches at HLA-A and or –B for RIC URD transplantation.
While MHC-disparity may drive allogeneic reactions that may protect against relapse, there was no reduction in relapse for the HLA-mismatched RIC cohort. Prior studies showed that disease status modified outcomes of HLA mismatched MA transplants, with advanced disease patients not experiencing as large an absolute decrement in survival with HLA mismatching as low risk patients.24 In this analysis there was no interaction between disease status and HLA-matching status. While on the one hand, relapse rates are generally higher in the RIC setting and this might have allowed us to more readily detect an association of HLA mismatching with relapse, this study was not specifically designed to address this question. Thus, the disease groups were heterogeneous, making it difficult to identify any protective effect of HLA mismatching. As well, HLA mismatching was strongly associated with the development of aGVHD. Therefore any benefit of HLA mismatching (i.e., increased GVL) might have been blunted by GVHD therapy. These findings are consistent with other studies that fail to show differences in relapse when comparing sibling to URD stem cell sources, where the latter might be expected to have more alloreactivity, but also increased aGVHD.25, 26
In summary, in the largest study to examine the association of HLA mismatching with RIC URD transplant outcomes, we found that compared to HLA 8/8 matched recipients, a single locus mismatch (i.e., 7/8 match) was associated with a considerable reduction in survival, mainly due to increased rates of aGVHD and TRM, with no change in relapse rates. We also found that mismatching at individual HLA loci had differential negative impacts on transplant outcomes, but caution is needed in interpreting these findings since the sample size of each subgroup is relatively small. Nonetheless, recipients of mismatched RIC URD donors showed inferior outcomes compared to fully matched donor transplants. Overall these findings show that while mismatched RIC URD transplantation is feasible, it comes at a significant cost in the form of toxicity and reduction in survival. Novel regimens to improve 7/8 URD RIC HCT outcomes are needed.
Manuscript Highlights.
Large registry cohort of RIC 8/8 vs. 7/8 matched URD donor transplantation
HLA 7/8 mismatched donors were associated with significantly higher grade II-IV aGVHD (RR=1.29, p=0.0034), higher TRM (RR=1.52, p<0.0001) and lower DFS (RR=1.12, p=0.0015) and OS (RR=1.25, p=0.0001)
HLA 7/8 mismatched donors were not associated with relapse protection
HLA 7/8 mismatched donors were not associated with higher cGVHD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foeken LM, Green A, Hurley CK, et al. Monitoring the international use of unrelated donors for transplantation: the WMDA annual reports. Bone Marrow Transplant. 2010;45:811–818. doi: 10.1038/bmt.2010.9. [DOI] [PubMed] [Google Scholar]
- 2.Petz LD SS, Gragert L. Cord Blood: Biology, Transplantation, Banking, and Regulation. Vol. 1. AABB Press; 2011. The underutilization of cord blood transplantation: extent of the problem, causes, and methods improvement; pp. 557–584. [Google Scholar]
- 3.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99:4200–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 4.Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 6.Loiseau P, Busson M, Balere ML, et al. HLA Association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transplant. 2007;13:965–974. doi: 10.1016/j.bbmt.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Gooley T, Malkki M, Horowitz M. Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69(Suppl 1):25–30. doi: 10.1111/j.1399-0039.2006.759_2.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaw BE, Gooley TA, Malkki M, et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110:4560–4566. doi: 10.1182/blood-2007-06-095265. [DOI] [PubMed] [Google Scholar]
- 9.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho VT, Kim HT, Liney D, et al. HLA-C mismatch is associated with inferior survival after unrelated donor non-myeloablative hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:845–850. doi: 10.1038/sj.bmt.1705315. [DOI] [PubMed] [Google Scholar]
- 11.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamae H, Storer BE, Storb R, et al. Low-dose total body irradiation and fludarabine conditioning for HLA class I-mismatched donor stem cell transplantation and immunologic recovery in patients with hematologic malignancies: a multicenter trial. Biol Blood Marrow Transplant. 2010;16:384–394. doi: 10.1016/j.bbmt.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauterbach N, Crivello P, Wieten L, et al. Allorecognition of HLA-DP by CD4+ T cells is affected by polymorphism in its alpha chain. Mol Immunol. 2014;59:19–29. doi: 10.1016/j.molimm.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Fleischhauer K, Fernandez-Vina MA, Wang T, et al. Risk associations between HLA-DPB1 T-cell epitope matching and outcome of unrelated hematopoietic cell transplantation are independent of HLA-DPA1. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14:37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Marsh SG, Albert ED, Bodmer WF, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley CK, Woolfrey A, Wang T, et al. The impact of HLA unidirectional mismatches on the outcome of myeloablative hematopoietic stem cell transplantation with unrelated donors. Blood. 2013;121:4800–4806. doi: 10.1182/blood-2013-01-480343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Vina MA, Wang T, Lee SJ, et al. Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood. 2014;123:1270–1278. doi: 10.1182/blood-2013-10-532671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13:366–374. doi: 10.1016/S1470-2045(12)70004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 24.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124:2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisdorf DJ, Nelson G, Lee SJ, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009;15:1475–1478. doi: 10.1016/j.bbmt.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]


