Abstract
Previous studies have shown that risks of collection-related pain and symptoms are associated with sex, body mass index (BMI), and age in unrelated donors undergoing collection at National Marrow Donor Program (NMDP) centers. We hypothesized that other important factors (race, socioeconomic status (SES), and number of procedures at the collection center) might affect symptoms in donors. We assessed outcomes in 2,726 bone marrow (BM) and 6,768 peripheral blood stem cell (PBSC) donors collected between 2004 and 2009. Pain/symptoms are reported as maximum levels over mobilization and collection (PBSC) or within 2 days of collection (BM) and at 1 week after collection. For PBSC donors, race and center volumes were not associated with differences in pain/symptoms at any time. PBSC donors with high SES levels reported higher maximum symptom levels 1 week post donation (p=0.017). For BM donors, black males reported significantly higher levels of pain (OR=1.90, CI=1.14-3.19, p=0.015). No differences were noted by SES groups. BM donors from low volume centers reported more toxicity (OR=2.09, CI=1.26-3.46, p=0.006). In conclusion, race and SES have a minimal effect on donation associated symptoms. However, donors from centers performing ≤1 BM collection every 2 months have more symptoms following BM donation. Approaches should be developed by registries and low volume centers to address this issue.
Keywords: Race, socioeconomic status, donor center, unrelated donor, donor toxicities, bone marrow, PBSC
Introduction
The pattern of acute toxicities associated with bone marrow (BM) and peripheral blood stem cell (PBSC) donation in unrelated donors have been well described in several recent studies from the National Marrow Donor Program (NMDP)1-3. Several pre-donation demographic factors from these and other studies have been associated with an increase in acute toxicity; specifically age, gender, body mass index (BMI) (in PBSC, but not BM donors), and anesthetic type1-10. It is important to fully understand factors predictive of increased donor risk as knowledge of their impact on post donation recovery helps us to tailor the pre-donation consent information to the specific donor, more closely follow at risk donors during the recovery period, or institute interventions to prevent symptoms in specific groups of donors.
Race/ethnicity and socioeconomic status (SES) have been linked to pain experience and perception in several studies in other areas of medicine such as orthopedics and chronic pain11-13, but thus far neither have been addressed in the unrelated hematopoietic cell donor population. In addition, the impact on donor outcome of the number of collections performed annually by a center is unknown and recommendations for a minimum number of procedures per year by regulatory bodies are often not based on data. Collection centers vary tremendously in overall numbers of procedures performed and experience of individuals at that center performing BM collection procedures.
The aim of this study was to examine the relationship between donor race/ethnicity, donor SES and collection center volumes on the acute toxicities (up to 1 week) experienced by NMDP donors.
Methods
Study Population
The study population consisted of first time volunteer US donors from the NMDP who underwent Granulocyte Colony Stimulating Factor (G-CSF) (filgastrim, Neupogen, Amgen, Thousand Oaks, CA) mobilized PBSC collection or BM harvest from January 1, 2004 to July 31, 2009. Donors for whom data were available from baseline to the first day of apheresis on the NMDP data collection forms were included. Donors enrolled on BMT CTN protocol 02-0114 and rare donors who donated bone marrow after G-CSF administration were excluded. Donors from centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses (n=534). Donor race/ethnicity was self-reported. Donor race and ethnicity were classified as non-Hispanic white, Hispanic-all races, non-Hispanic black, non-Hispanic Asian/non-Hispanic Pacific Islander and non-Hispanic-other. SES was defined as the median household income in the donor’s census block group. Each donor address was geocoded using the ArcGIS 10.1 Business Analyst US address locater (Esri, Redlands CA, USA). The Esri Business Analyst 2012 dataset was used to extract median household income for each census block group. If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead. Collection center and apheresis center size were based on reaccreditation numbers using the total number of either BM collections for calendar years 2005-2008 or PBSC collections for calendar years 2004-2008 (regardless of whether autologous or allogeneic).
All donors included in the study provided written informed consent for participation in Center for International Blood and Marrow Transplant Research (CIBMTR) research studies approved by the NMDP Institutional Review Board. This study was conducted in accordance with the Declaration of Helsinki. Donors were evaluated for medical suitability, transplantation-transmissible infectious diseases, and contraindications for PBSC or BM donation using standardized NMDP criteria.
Data Collection
Data collection began at the time of the donor's medical evaluation to determine suitability to donate hematopoietic progenitor cells. For PBSC donations, the data collection occurred during each day of G-CSF and on the day of each apheresis procedure. For BM donations, the data collection occurred on the day of BM collection. Both BM and PBSC donors were contacted by the donor center 2 days after donation, 1 week after donation, and weekly thereafter until complete recovery. “Complete recovery” was assessed by the donor center coordinator/medical director and based on reports of return to baseline function with no ongoing symptoms. In view of the fact that this study addressed acute toxicity only day 2 and 1 week forms were analyzed. Detailed questions using the toxicity criteria modeled on Common Terminology Criteria for Adverse Events v4 were used to assess specific symptoms, to measure the donor’s overall health, and to capture any toxicity the donor may have experienced as a result of the hematopoietic progenitor cells donation process. Symptoms assessed included fever, fatigue, rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, syncope, pain, and infections. In addition, a complete blood count and white cell differential were performed at the initial medical evaluation, on the first day of G-CSF, the day(s) of collection, and at annual follow-ups.
PBSC Donation
All PBSC mobilizations were performed according to the NMDP-sponsored and Institutional Review Board-approved research protocol for manufacturing PBSC products, operated under an Investigational New Drug application with the United States Food and Drug Administration. G-CSF dose was approximately 10 μg/kg/day actual body weight rounded to combinations of 300 μg and 480 μg vials, as long as protocol defined targets of 13.3 μg/kg per day were not exceeded. Typically, donors received subcutaneous G-CSF daily for 4 days before and on the first day of apheresis. All donors underwent a maximum of 2 days of apheresis. The volume of whole blood processed was targeted to be between 12 and 24 L per collection. If the PBSC product could not be collected using peripheral veins, a central venous catheter was used.
BM Donation
One or two autologous blood units were potentially collected from the donor prior to donation, based on individual assessment. BM was collected from the donor's posterior iliac crests in an operating room under either general or regional (spinal or epidural) anesthesia. The NMDP guidelines recommend a duration of anesthesia of less than 150 minutes, and a maximum collection volume of 20ml/kg.
Endpoints and Statistical Analysis
The following end points were analyzed: incidence of grade 2 to 4 and grade 3 to 4 skeletal pain and highest toxicity level (Modified Toxicity Criteria, MTC) across selected body symptoms frequently associated with collection (fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope). Skeletal pain was defined as pain in at least 1 of the following sites: back, bone, headache, hip, limb, joint, or neck. The severity of skeletal pain was defined as the maximum grade among these pain sites. Pain/symptoms are reported and analyzed as maximum levels over mobilization and collection (PBSC) or within 2 days of collection (BM), and at 1 week after collection. Donor and collection characteristics were described using frequencies/percentages or median/range as appropriate, separately by groups based on center volume. Variables were compared using the Pearson chi-squared test for categorical variables and the Kruskal-Wallis test for continuous variables. Incidence rates for pain and symptoms were described using frequencies and percentages. We examined the impact of the main effects of race, SES, and center volume in multivariate models on 4 outcomes for each donor type: grade 2-4 maximum skeletal pain at day 2 (BM) or from mobilization through collection (PBSC), grade 2-4 maximum skeletal pain at 1 week, grade 2-4 maximum MTC score at day 2 (BM) or from mobilization through collection (PBSC), and grade 2-4 maximum MTC score at 1 week. Other toxicity outcomes were generally too low in frequency for multivariate modeling. Generalized linear mixed models were used to fit logistic regression models to each outcome with random effects for collection center (BM) or apheresis center (PBSC). In each case, the 3 main effects were forced into the model, while other donor characteristics were added in a stepwise manner. The optimal cut point based on maximum likelihood was investigated for the number of BM performed and found to be ≤1 BM collection every 2 months. All model results use this optimal cut point. All statistical analyses were performed using SAS EG 4.3 and SAS 9.3 (Cary, North Carolina).
Results
Donor Demographics
The characteristics of 2,726 BM and 6,768 PBSC donors are shown in Tables 1 and 2 respectively, displayed in quartiles reflecting the total activity of all centers (as defined in the methods).
Table 1.
Characteristics of First-Time NMDP Donors Who Donated BM Between January 1, 2004 and July 31, 2009 by Collection Center Sizea
| Variable | 0-18 collections N (%) |
19-43 collections N (%) |
44-76 collections N(%) |
77-573 collections N(%) |
p-value |
|---|---|---|---|---|---|
| Number of donors | 248 | 548 | 635 | 1295 | |
| Number of centers | 19 | 27 | 20 | 17 | |
| Median household income in the donor's census block group, 2012b,c |
0.059 | ||||
| 0-25,000 | 4 (2) | 16 (3) | 25 (4) | 33 (4) | |
| 25,001-50,000 | 74 (30) | 179 (35) | 231 (37) | 241 (30) | |
| 50,001-100,000 | 118 (49) | 245 (48) | 274 (44) | 415 (51) | |
| >100,000 | 40 (16) | 63 (12) | 72 (12) | 108 (13) | |
| Unknown | 7 (3) | 11 (2) | 20 (3) | 16 (2) | |
| Donor race | <0.001 | ||||
| Non-Hispanic white | 213 (86) | 370 (68) | 440 (69) | 912 (70) | |
| Hispanic, all races | 10 (4) | 88 (16) | 78 (12) | 127 (10) | |
| Non-Hispanic black | 11 (4) | 40 (7) | 32 (5) | 82 (6) | |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
7 (3) | 23 (4) | 46 (7) | 68 (5) | |
| Non-Hispanic other, unknown | 7 (3) | 27 (5) | 39 (6) | 106 (8) | |
| Donor-related | |||||
| Donor age at donation | <0.001 | ||||
| 18 to 29 | 72 (29) | 143 (26) | 206 (32) | 466 (36) | |
| 30 to 39 | 78 (31) | 209 (38) | 186 (29) | 407 (31) | |
| 40 to 49 | 68 (27) | 151 (28) | 171 (27) | 329 (25) | |
| 50+ | 30 (12) | 45 (8) | 72 (11) | 93 (7) | |
| Median (Range) | 37 (19-60) | 36 (19-61) | 36 (19-61) | 34 (19-61) | 0.007 |
| Donor sex | <0.001 | ||||
| Female | 105 (42) | 243 (44) | 299 (47) | 441 (34) | |
| Male | 143 (58) | 305 (56) | 336 (53) | 854 (66) | |
| Donor BMI (kg/m2) | 0.166 | ||||
| Underweight (<18.5) | 1 (<1) | 4 (1) | 5 (1) | 4 (<1) | |
| Normal (18.5-24.9) | 71 (29) | 187 (34) | 193 (30) | 374 (29) | |
| Overweight (25-29.9) | 102 (41) | 197 (36) | 223 (35) | 522 (40) | |
| Obese (30+) | 74 (30) | 160 (29) | 214 (34) | 395 (31) | |
| Median (Range) | 27.5 (18.2-42.1) | 27.0 (17.6-45.6) | 27.7 (16.1-50.9) | 27.4 (17.8-48.5) | 0.314 |
| Baseline WBC (×109/L) | |||||
| N Eval | 248 | 548 | 635 | 1295 | |
| Median (Range) | 6.3 (2.9-12.3) | 6.3 (2.3-14.2) | 6.5 (3.1-12.9) | 6.4 (3.0-14.2) | 0.077 |
| Baseline platelets (×109/L) | |||||
| N Eval | 248 | 548 | 634 | 1295 | |
| Median (Range) | 259 (138-419) | 254 (139-490) | 256 (130-465) | 252 (104-534) | 0.374 |
| Baseline hemoglobin (g/dL) | |||||
| N Eval | 248 | 548 | 635 | 1295 | |
| Median (Range) | 14.6 (10.7-18.3) | 14.6 (8.6-17.9) | 14.7 (10.4-17.6) | 14.7 (9.2-19.0) | 0.129 |
| Baseline neutrophils (×109/L) | |||||
| N Eval | 247 | 548 | 635 | 1295 | |
| Median (Range) | 4.1 (1.5-9.9) | 4.0 (1.0-12.5) | 4.1 (1.6-9.9) | 4.0 (1.2-10.3) | 0.052 |
| Baseline mononuclear cells (×109/L) |
|||||
| N Eval | 248 | 548 | 632 | 1289 | |
| Median (Range) | 2.2 (1.2-5.3) | 2.3 (0.9-5.2) | 2.3 (0.9-5.0) | 2.3 (0.9-7.2) | 0.473 |
| Donor CMV | 0.065 | ||||
| Negative | 161 (65) | 305 (56) | 348 (55) | 738 (57) | |
| Positive | 86 (35) | 243 (44) | 287 (45) | 555 (43) | |
| Unknown/Inconclusive | 1 (<1) | 0 | 0 | 2 (<1) | |
| Collection-related | |||||
| Year of donation | <0.001 | ||||
| 2004 | 88 (35) | 100 (18) | 112 (18) | 191 (15) | |
| 2005 | 61 (25) | 93 (17) | 93 (15) | 202 (16) | |
| 2006 | 36 (15) | 97 (18) | 124 (20) | 222 (17) | |
| 2007 | 24 (10) | 95 (17) | 114 (18) | 238 (18) | |
| 2008 | 21 (8) | 106 (19) | 113 (18) | 297 (23) | |
| 2009 | 18 (7) | 57 (10) | 79 (12) | 145 (11) | |
| Type of anesthesia | <0.001 | ||||
| Epidural | 2 (1) | 3 (1) | 4 (1) | 22 (2) | |
| General | 229 (92) | 533 (97) | 608 (96) | 1249 (97) | |
| Local | 0 | 0 | 1 (<1) | 0 | |
| Spinal | 17 (7) | 12 (2) | 22 (3) | 23 (2) | |
| Unknown | 0 N/A | 0 N/A | 0N/A | 1N/A |
Collection center size based on NMDP reaccreditation numbers: uses total number of bone marrow collections, regardless of donor source, for calendar years 2005-2008.
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: NMDP – National Marrow Donor Program; BM – bone marrow; BMI – body mass index; WBC – white blood cells; CMV – Cytomegalovirus.
Table 2.
Characteristics of First-Time NMDP Donors Who Donated PBSC Between January 1, 2004 and July 31, 2009 by Collection Center Sizea
| Variable | 0-204 collections N (%) |
205-520 collections N (%) |
521-1135 collections N (%) |
1136-5953 collections N (%) |
Unknown N(%) |
p-value |
|---|---|---|---|---|---|---|
| Number of donors | 543 | 1674 | 2845 | 1686 | 20 | |
| Number of centers | 22 | 24 | 24 | 22 | 6 | |
| Median household income in the donor's census block group, 2012b,c |
<0.001 | |||||
| 0-25,000 | 27 (5) | 37 (2) | 75 (4) | 43 (3) | 0 | |
| 25,001-50,000 | 184 (34) | 475 (30) | 512 (30) | 473 (31) | 4 (57) | |
| 50,001-100,000 | 251 (47) | 813 (51) | 827 (49) | 713 (46) | 2 (29) | |
| >100,000 | 56 (10) | 205 (13) | 252 (15) | 276 (18) | 0 | |
| Unknown | 19 (4) | 55 (3) | 34 (2) | 32 (2) | 1 (14) | |
| Donor race | <0.001 | |||||
| Non-Hispanic white | 458 (84) | 1168 (70) | 2183 (77) | 1258 (75) | 16 (80) | |
| Hispanic, all races | 26 (5) | 202 (12) | 241 (8) | 139 (8) | 1 (5) | |
| Non-Hispanic black | 13 (2) | 72 (4) | 148 (5) | 87 (5) | 0 | |
| Non-Hispanic Asian/non-Hispanic Pacific Islander |
17 (3) | 117 (7) | 58 (2) | 102 (6) | 0 | |
| Non-Hispanic other, unknown |
29 (5) | 115 (7) | 215 (8) | 100 (6) | 3 (15) | |
| Donor-related | ||||||
| Donor age at donation | <0.001 | |||||
| 18 to 29 | 141 (26) | 509 (30) | 995 (35) | 521 (31) | 7 (35) | |
| 30 to 39 | 185 (34) | 531 (32) | 935 (33) | 550 (33) | 6 (30) | |
| 40 to 49 | 158 (29) | 431 (26) | 672 (24) | 430 (26) | 3 (15) | |
| 50+ | 59 (11) | 203 (12) | 243 (9) | 185 (11) | 4 (20) | |
| Median (Range) | 37 (19-60) | 36 (18-60) | 35 (19-61) | 36 (19-61) | 35 (20-61) | <0.001 |
| Donor sex | <0.001 | |||||
| Female | 230 (42) | 746 (45) | 908 (32) | 706 (42) | 8 (40) | |
| Male | 313 (58) | 928 (55) | 1937 (68) | 980 (58) | 12 (60) | |
| Donor BMI (kg/m2) | 0.085 | |||||
| Underweight (<18.5) | 2 (<1) | 9 (1) | 17 (1) | 13 (1) | 0 | |
| Normal (18.5-24.9) | 146 (27) | 504 (30) | 795 (28) | 521 (31) | 4 (20) | |
| Overweight (25-29.9) | 202 (37) | 641 (38) | 1183 (42) | 625 (37) | 8 (40) | |
| Obese (30+) | 193 (36) | 520 (31) | 849 (30) | 527 (31) | 8 (40) | |
| Unknown | 0 N/A | 0N/A | 1N/A | 0N/A | 0N/A | |
| Median (Range) | 28.0 (18.0-49.7) | 27.4 (16.2-49.1) | 27.4 (16.7-51.8) | 27.4 (16.9-56.2) | 27.7 (19.5-39.6) | 0.082 |
| Baseline WBC (×109/L) | ||||||
| N Eval | 543 | 1674 | 2845 | 1686 | 20 | |
| Median (Range) | 6.4 (3.2-14.0) | 6.3 (2.9-15.6) | 6.3 (2.4-16.0) | 6.4 (2.3-14.5) | 6.6 (4.5-11.0) | 0.683 |
| Baseline platelets (×109/L) | ||||||
| N Eval | 543 | 1673 | 2844 | 1686 | 20 | |
| Median (Range) | 252 (122-474) | 257 (127-474) | 251 (100-548) | 257 (112-494) | 242 (170-397) | 0.002 |
| Baseline hemoglobin (g/dL) | ||||||
| N Eval | 543 | 1674 | 2845 | 1686 | 20 | |
| Median (Range) | 14.7 (10.5-17.9) | 14.5 (9.6-18.3) | 14.9 (9.4-18.6) | 14.6 (9.6-19.0) | 14.6 (12.5-16.5) | <0.001 |
| Baseline neutrophils (×109/L) |
||||||
| N Eval | 543 | 1673 | 2844 | 1686 | 20 | |
| Median (Range) | 4.1 (1.3-10.9) | 3.9 (1.2-12.9) | 3.9 (1.0-12.8) | 4.0 (1.3-11.8) | 3.8 (2.4-6.1) | 0.152 |
| Baseline mononuclear cells (×109/L) |
||||||
| N Eval | 538 | 1672 | 2836 | 1681 | 20 | |
| Median (Range) | 2.3 (1.1-4.9) | 2.3 (0.7-7.3) | 2.3 (0.8-5.9) | 2.3 (0.3-5.0) | 2.5 (1.4-5.0) | 0.100 |
| Donor CMV | <0.001 | |||||
| Unknown/Inconclusive | 0 | 4 (<1) | 3 (<1) | 3 (<1) | 0 | |
| Negative | 329 (61) | 937 (56) | 1790 (63) | 1046 (62) | 15 (75) | |
| Positive | 214 (39) | 733 (44) | 1052 (37) | 637 (38) | 5 (25) | |
| Year of donation | <0.001 | |||||
| 2004 | 107 (20) | 169 (10) | 356 (13) | 176 (10) | 6 (30) | |
| 2005 | 111 (20) | 258 (15) | 426 (15) | 303 (18) | 4 (20) | |
| 2006 | 90 (17) | 294 (18) | 504 (18) | 299 (18) | 1 (5) | |
| 2007 | 79 (15) | 331 (20) | 574 (20) | 307 (18) | 6 (30) | |
| 2008 | 88 (16) | 393 (23) | 648 (23) | 367 (22) | 1 (5) | |
| 2009 | 68 (13) | 229 (14) | 337 (12) | 234 (14) | 2 (10) | |
| Two-day collection | <0.001 | |||||
| No | 290 (53) | 1288 (77) | 2293 (81) | 1058 (63) | 15 (75) | |
| Yes | 253 (47) | 386 (23) | 552 (19) | 628 (37) | 5 (25) | |
| Pre-collection WBC (×109/L) | ||||||
| N Eval | 543 | 1674 | 2845 | 1685 | 20 | |
| Median (Range) | 37.5 (9.6-98.9) | 38.3 (11.3-93.4) | 38.9 (11.2-117) | 38.9 (10.6-89.7) | 37.6 (18.9-64.3) | 0.431 |
| CD34+ at collection (×106) | ||||||
| N Eval | 409 | 1508 | 2430 | 1480 | 18 | |
| Median (Range) | 636.0 (7.1-3486.2) | 611.4 (0.9-4013.6) | 743.6 (2.8-5967.0) | 657.2 (0.7-13428.1) | 592.2 (77.8-1262.7) | <0.001 |
| Day 1 G-CSF dose per donor weight (μg/kg) |
||||||
| N Eval | 543 | 1674 | 2836 | 1685 | 20 | |
| Median (Range) | 10.5 (5.3-14.6) | 10.6 (7.1-14.5) | 10.6 (2.5-15.7) | 10.6 (4.5-17.7) | 10.5 (9.0-12.8) | 0.021 |
| Total G-CSF dose per donor weight (μg/kg) |
||||||
| N Eval | 543 | 1674 | 2842 | 1686 | 20 | |
| Median (Range) | 52.3 (35.0-73.2) | 52.9 (31.2-70.6) | 52.7 (5.2-74.4) | 52.7 (21.2-71.4) | 52.3 (35.9-63.9) | <0.001 |
Apheresis center size based on reaccreditation numbers; uses total number of autologous, related and unrelated allogeneic PBSC collections for calendar years 2004-2008.
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: NMDP – National Marrow Donor Program; PBSC – peripheral blood stem cell; BMI – body mass index; WBC – white blood cells; CMV – Cytomegalovirus.
BM donations were facilitated by 81 donor centers and 83 collection centers. The median number of BM collections per center in this study population was 43 (range 0-573). The median volume harvested was 12.70 ml/kg of donor weight. In 4.12% of donors this exceeded the recommended maximum collection volume. There were several significant differences between the donors based on center volume (Table 1); however this variation was not distributed in a linear fashion. For example while the lowest and highest volume centers had the largest proportion of non-Hispanic whites and highest median income, the low volume centers had older donors and more female donors compared to the highest volume centers. In addition, more collections occurred in low volume centers in the early years of the study.
PBSC donations were facilitated by 76 donor centers and 98 apheresis centers. The median number of apheresis procedures per center in this study population was 520 (range 0-5,953). We found that the lowest volume centers had more non-Hispanic whites and the lowest median household income. The lowest volume centers had the highest percentage of second day collections. Several differences existed between baseline blood counts (Table 2). As in BM, more collections occurred in low volume centers in the early years of the study.
Multivariate Analysis
Pain and Toxicity in PBSC Donors
As has been previously shown female (pain at day 2: OR=1.62, p<0.001; and 1 week: OR=1.53, p=0.048; MTC at day 2: OR=1.96, p<0.001; and 1 week: OR=1.67, p=0.014) and obese (pain at day 2: OR=1.31, p<0.001; MTC at day 2: OR=1.47, p<0.001) donors experienced more symptoms with donation. There was no impact of race/ethnicity or apheresis center volume on any pain or toxicity outcome of PBSC donors at any time. Of interest, donors in a higher income census block reported higher maximum toxicity levels 1 week post donation (p=0.025). We also found a differential effect of age, with donors over the age of 40 years having lower pain with donation compared to younger donors (p<0.001), but all donors over 30 years having greater pain at 1 week (p=0.003). Donors aged 30-39 had a higher MTC through donation compared to both younger and older donors (p=0.021). Finally, we found a white blood cell count (WBC) of >7.6 ×109/L at baseline to be associated with higher MTC through donation (OR=1.21, p=0.004) and at 1 week post (OR=1.86, p=0.003) and a mononuclear cell count of >2.7 ×109/L at baseline to be associated with higher pain levels through donation (OR=1.2, p=0.002).
Pain and Toxicity in BM Donors
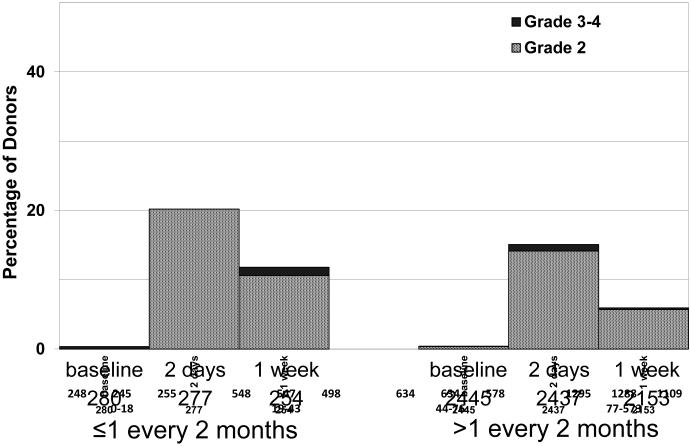
All statistically significant outcomes related to pain and maximum toxicity on day 2 and 1 week post donation are displayed in Tables 3-6. Female donors experienced more symptoms with donation (pain at 1 week: OR=2.07, p<0.001; MTC at day 2: OR=2.08, p<0.001; and 1 week OR=2.28, p<0.001) and donors older than 30 years had significantly higher MTC at 1 week (p=0.020).There was no impact of SES on any pain or toxicity outcome at any time. The only significant impact associated with race/ethnicity was a higher incidence of grade 2-4 skeletal pain on day 2 post BM collection in black males (OR=1.91, p=0.014). The collection center volume had a significant impact on the MTC on day 2 (p=0.068) and 1 week (p=0.004) post donation (Tables 3 and 4, Figure 1). At 1 week, donors from any center performing fewer than 24 collections reported more toxicity (OR=2.09, CI=1.26-3.46, p=0.004) (Table 4). Finally, we found that donors with neutrophils >3.2 ×109/L at baseline had more pain on day 2 (p=0.002); those who were cytomegalovirus positive had more pain at 1 week (p=0.009) and normal/underweight donors had higher MTC at day 2 (p=0.029).
Table 3.
Multivariate Analysis of Grade 2-4 Maximum MTC Grade at Day 2 After BM Donation
| Variable | n | OR | Lower | Upper | p-value |
|---|---|---|---|---|---|
| Median household income in the donor's census block group, 2012a,b |
0.996 | ||||
| 0-25,000 | 78 | 1.00 | |||
| 25,001-50,000 | 721 | 1.01 | 0.51 | 2.00 | 0.985 |
| 50,001-100,000 | 1050 | 0.97 | 0.49 | 1.92 | 0.927 |
| >100,000 | 281 | 0.93 | 0.44 | 1.95 | 0.839 |
| Donor race | 0.821 | ||||
| Non-Hispanic white | 1920 | 1.00 | |||
| Hispanic, all races | 302 | 0.81 | 0.54 | 1.20 | 0.294 |
| Non-Hispanic black | 165 | 0.98 | 0.62 | 1.56 | 0.937 |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
144 | 1.14 | 0.69 | 1.88 | 0.599 |
| Non-Hispanic other, unknown | 178 | 0.99 | 0.63 | 1.56 | 0.974 |
| Collection center size | 0.068 | ||||
| ≥24 (≥1 every 2 months) | 2138 | 1.00 | |||
| <24 (<1 every 2 months) | 265 | 1.55 | 0.97 | 2.47 | 0.068 |
| Sex | <0.001 | ||||
| Male | 1627 | 1.00 | |||
| Female | 1082 | 2.08 | 1.66 | 2.62 | <0.001 |
| Donor BMI (kg/m2) | 0.029 | ||||
| Normal/underweight (<24.9) | 835 | 1.00 | |||
| Overweight (25-29.9) | 1036 | 0.76 | 0.59 | 0.99 | 0.041 |
| Obese (30+) | 838 | 0.70 | 0.53 | 0.93 | 0.013 |
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: MTC – modified toxicity criteria; BM – bone marrow; OR – odds ratio; BMI – body mass index.
Table 6.
Multivariate Analysis of Grade 2-4 Maximum Skeletal Pain at 1 Week After BM Donation
| Variable | n | OR | Lower | Upper | p-value |
|---|---|---|---|---|---|
| Median household income in the donor's census block group, 2012a,b |
0.114 | ||||
| 0-25,000 | 70 | 1.00 | |||
| 25,001-50,000 | 648 | 0.83 | 0.38 | 1.82 | 0.649 |
| 50,001-100,000 | 958 | 1.11 | 0.51 | 2.39 | 0.790 |
| >100,000 | 254 | 0.59 | 0.24 | 1.44 | 0.248 |
| Donor race | 0.479 | ||||
| Non-Hispanic white | 1720 | 1.00 | |||
| Hispanic, all races | 273 | 0.78 | 0.49 | 1.23 | 0.280 |
| Non-Hispanic black | 137 | 1.25 | 0.76 | 2.07 | 0.383 |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
121 | 0.72 | 0.36 | 1.43 | 0.352 |
| Non-Hispanic other, unknown | 153 | 0.82 | 0.47 | 1.42 | 0.479 |
| Collection center size | 0.164 | ||||
| ≥24 (≥1 every 2 months) | 2138 | 1.00 | |||
| <24 (<1 every 2 months) | 265 | 1.41 | 0.87 | 2.28 | 0.164 |
| Sex | <0.001 | ||||
| Male | 1432 | 1.00 | |||
| Female | 972 | 2.07 | 1.59 | 2.70 | <0.001 |
| Donor CMV | 0.009 | ||||
| Negative | 1363 | 1.00 | |||
| Positive | 1041 | 1.43 | 1.09 | 1.87 | 0.009 |
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: BM – bone marrow; OR – odds ratio; CMV – Cytomegalovirus.
Table 4.
Multivariate Analysis of Grade 2-4 Maximum MTC Grade at 1 Week After BM Donation
| Variable | n | OR | Lower | Upper | p-value |
|---|---|---|---|---|---|
| Median household income in the donor's census block group, 2012a,b |
0.599 | ||||
| 0-25,000 | 70 | 1.00 | |||
| 25,001-50,000 | 648 | 0.51 | 0.20 | 1.30 | 0.159 |
| 50,001-100,000 | 956 | 0.66 | 0.26 | 1.66 | 0.380 |
| >100,000 | 254 | 0.60 | 0.22 | 1.68 | 0.331 |
| Donor race | 0.813 | ||||
| Non-Hispanic white | 1720 | 1.00 | |||
| Hispanic, all races | 272 | 0.87 | 0.49 | 1.55 | 0.634 |
| Non-Hispanic black | 137 | 0.71 | 0.33 | 1.53 | 0.383 |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
121 | 0.68 | 0.28 | 1.66 | 0.395 |
| Non-Hispanic other, unknown | 153 | 0.86 | 0.41 | 1.77 | 0.675 |
| Collection center size | 0.004 | ||||
| ≥24 (≥1 every 2 months) | 2138 | 1.00 | |||
| <24 (<1 every 2 months) | 265 | 2.09 | 1.26 | 3.46 | 0.004 |
| Sex | <0.001 | ||||
| Male | 1432 | 1.00 | |||
| Female | 971 | 2.28 | 1.62 | 3.22 | <0.001 |
| Age at donation | 0.020 | ||||
| 18 to 29 | 767 | 1.00 | |||
| 30 to 39 | 783 | 1.62 | 1.02 | 2.56 | 0.040 |
| 40 to 49 | 633 | 2.05 | 1.29 | 3.25 | 0.002 |
| 50+ | 220 | 1.91 | 1.03 | 3.54 | 0.040 |
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: MTC – modified toxicity criteria; BM – bone marrow; OR – odds ratio.
Figure 1. Highest Toxicity Level Across All Body Symptoms in BM Donors Depending on the Number of BM Collections in the Center.
Highest toxicity level of key symptoms (fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope) for BM donors by collection center volume: at baseline, during peri-collection period and post-donation.
Discussion
The results of this study show that center volume was an important factor associated with acute toxicity for BM donors. In contrast, we did not find an impact of center volumes on recovery of PBSC donors, and race and SES had only a minor effect on acute toxicity symptoms associated with either PBSC or BM donation.
We were reassured to see little consistent impact of the additional demographic factors of race and SES on donor toxicities. Previous studies have demonstrated increased pain in African Americans13,15 which we noted here only for a single outcome where black males had a significantly higher skeletal pain score on day 2 post BM donation (which resolved by 1 week). The reasons for this disparity are not clear. Likewise the finding of a higher MTC in donors with a higher SES at 1 week after PBSC donation was not found at other time points. Although SES has been shown to impact symptoms in some chronic conditions such as fibromyalgia16 and arthritis17, it is generally those in lower SES groups who have more troublesome symptoms. Interestingly, one study has shown that analgesia use is lower in those with a higher SES following a medical procedure18, an outcome we did not examine in our study. Since SES assignment was based only on the census block group that the donor lived in at the time of donation, it is possible that we did not have enough information to properly understand this outcome. Education level and occupation were not considered in this study.
To date, a comprehensive study investigating the toxicity outcomes of donors has not looked at the variable of center size and experience, and our findings with regards to BM donors are of great interest and require further investigation. While a few studies have investigated a center effect or donor demographic factors on the quality of BM harvested19-21, none of these have reported on the donor’s outcome. This issue is of critical importance to the NMDP and other donor registries not only to ensure the best donor experience, but also to assist in accreditation of centers. This finding may represent a predictable result of less experience at a given center, warranting special attention and intervention to ensure appropriate outcomes for BM donors harvested at small centers.
The number of BM harvests performed in unrelated donors annually has reduced dramatically over the last decade, although there is some evidence of a plateau in recent years. Currently, 20% of unrelated donor transplants reported to the CIBMTR are performed using BM22. The percentage of donors undergoing BM harvest vs. PBSC is even lower in (adult) related donors, with the overall effect being that of a lack of exposure to this procedure for many hematologists/BMT specialists and other BM harvest team members. A recently published study by Remberger, et al,23 reported a significant reduction in the number of CD34 cells harvested from BM in a more contemporary time period (2010-2011) compared to an earlier time period (1995-1997). In addition, a single center study has shown a marked downward trend in total nucleated cell numbers harvested from BM over time (Nicole L. Prokopishyn, personal communication, April 27, 2015). Authors of both of these studies speculate that these effects are due to a reduction in operator expertise. Indeed, one study which assessed the impact of a new BM needle on harvest quality24, reported a “learning effect” for the same operators performing more harvest over time, as larger collection volumes were consistently obtained in the later time period.
These diminishing numbers of BM collections have led the Foundation for the Accreditation of Cellular Therapy (FACT) standards committee to lower the minimum number of BM harvests required by a facility to be accredited such that: A minimum of one marrow collection procedure shall have been performed in the twelve month period immediately preceding facility accreditation, and a minimum average of one marrow collection procedure per year shall be performed within the accreditation cycle25. In this study we defined center size by the total number of collections performed (related and unrelated) and found that in those centers performing ≤1 BM collection every 2 months over a 3-4 year period donors had a longer time to recovery. This is well above the current minimum standard required by FACT for center accreditation, and suggests that the standard should be revised or that other measures should be taken to address this issue. Given the results of this study and the trends in BM harvesting volumes, we may not only see an increase in donor acute toxicities, but also a reduction in the quality of the harvest if practice is not changed. We were not able to accurately assess the number of BM harvests performed by individual practitioners at collection centers. However, we believe that the harvesting process is a composite one requiring expertise not only of the harvesting practitioner, but also the ancillary operating room staff and anesthesiologists.
Interestingly, the effects on BM donors were most marked at 1 week post donation for both MTC, with little or no effect for skeletal pain. It is thus possible that the increased symptoms are not only related to collection variables, but also to other factors. This may include factors not directly addressed in this study, such as hospitalization, advice on activities, use of and prescriptions for analgesia and iron supplementation and infusion of autologous blood units26, 27. Practice at NMDP centers is generally to collect one or more autologous unit prior to donation, but in many cases these units are not returned. It is unclear how this variable may impact the donor experience, but this may warrant further analysis. We also did not consider aspects of anesthesia (duration/method) in this study as this is extremely standardized at NMDP centers, following earlier studies showing the important impact of this variable on toxicities3. This study was only focused on short term toxicities and pain and was not designed to examine long term donor outcomes.
Several possible strategies to address the problems associated with collecting fewer BM could be proposed. First, knowledge of this issue by centers might lead to training and standardization of practices within centers to address this concern. If such interventions did not lead to improvements in low volume centers, a possible solution would be to consolidate collections into fewer facilities. This would be relatively easy to achieve for registries looking after unrelated donors, however, it would be a challenge for centers performing related donor collections. In some countries, registries have taken on the collection of BM from related donors for the transplant centers. Concerning the issue of lower total nucleated cell numbers over time, CIBMTR is undertaking a large study to explore this issue in more detail28.
In conclusion, despite a reassuring lack of major impact of race and SES on acute toxicities in unrelated donors, we found an increase in toxicities in BM donors who donated at small volume BM harvesting centers. We speculate that this is part of a worrying trend towards reduction in the experience of the BM operators, which will impact not only on the donor (as we have shown), but also on the quality of the harvest, with obvious detriments to the patient. A global effort is required to address these issues.
Highlights.
We investigate short term toxicities in donors by race, SES and donor center volume
Race and SES have only a minor effect on toxicities
Center volume was an important factor associated with acute toxicity for BM donors
Table 5.
Multivariate Analysis of Grade 2-4 Maximum Skeletal Pain on Day 2 After BM Donation
| Variable | n | OR | Lower | Upper | p-value |
|---|---|---|---|---|---|
| Donor race | 0.001 | ||||
| Male | 0.008 | ||||
| Non-Hispanic white | 1220 | 1.00 | |||
| Hispanic, all races | 146 | 0.75 | 0.48 | 1.18 | 0.213 |
| Non-Hispanic black | 71 | 1.91 | 1.14 | 3.20 | 0.014 |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
86 | 0.61 | 0.34 | 1.10 | 0.100 |
| Non-Hispanic other, unknown | 106 | 0.63 | 0.37 | 1.08 | 0.094 |
| Female | 0.136 | ||||
| Non-Hispanic white | 702 | 1.00 | |||
| Hispanic, all races | 156 | 0.81 | 0.54 | 1.23 | 0.323 |
| Non-Hispanic black | 94 | 0.57 | 0.34 | 0.95 | 0.030 |
| Non-Hispanic Asian/non- Hispanic Pacific Islander |
58 | 1.04 | 0.57 | 1.92 | 0.892 |
| Non-Hispanic other, unknown | 72 | 1.29 | 0.77 | 2.18 | 0.333 |
| Median household income in the donor's census block group, 2012a,b |
0.689 | ||||
| 0-25,000 | 78 | 1.00 | |||
| 25,001-50,000 | 722 | 0.68 | 0.40 | 1.16 | 0.160 |
| 50,001-100,000 | 1051 | 0.69 | 0.41 | 1.16 | 0.160 |
| >100,000 | 281 | 0.66 | 0.37 | 1.17 | 0.154 |
| Collection center size | 0.338 | ||||
| ≥24 (≥1 every 2 months) | 2138 | 1.00 | |||
| <24 (<1 every 2 months) | 265 | 1.25 | 0.79 | 1.95 | 0.338 |
| Neutrophils at baseline (×109/L) | |||||
| <3.2 | 650 | 1.00 | |||
| >3.2 | 2061 | 1.38 | 1.13 | 1.70 | 0.002 |
Donors from the centers who provided only non-residential zip codes (e.g. work, university or donor center zip codes) were excluded from the SES analyses.
If the census block group could not be located from reported street address, median household income from donor’s zip code was used instead.
Abbreviations: BM – bone marrow; OR – odds ratio.
Acknowledgement
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; *Gilead Sciences, Inc.; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; *Mesoblast; *Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; *Strakan, Inc.; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Dr. M. A. Pulsipher’s contribution to this work is supported by R01 HL085707 from the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions:
Acquisition and interpretation of SES data: Kelsey Besse and EPW. MAP, BES, DLC, BRL, DMK, PC where involved with the design, analysis and interpretation of the data. All authors were involved in critical review of the protocol and results. The final manuscript was reviewed and approved by all authors.
Disclosure of Conflicts of Interest: The authors have no relevant conflicts to declare.
References
- 1.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121:197–206. doi: 10.1182/blood-2012-03-417667. doi:10.1182/blood-2012-03-417667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113:3604–3611. doi: 10.1182/blood-2008-08-175323. doi:10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JP, Perry EH, Price TH, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:29–36. doi: 10.1016/j.bbmt.2008.05.018. doi:10.1016/j.bbmt.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Martino M, Console G, Dattola A, et al. Short and long-term safety of lenograstim administration in healthy peripheral haematopoietic progenitor cell donors: a single centre experience. Bone Marrow Transplant. 2009;44:163–168. doi: 10.1038/bmt.2008.440. doi:10.1038/bmt.2008.440. [DOI] [PubMed] [Google Scholar]
- 5.Murata M, Harada M, Kato S, et al. Peripheral blood stem cell mobilization and apheresis: analysis of adverse events in 94 normal donors. Bone Marrow Transplant. 1999;24:1065–1071. doi: 10.1038/sj.bmt.1702038. [DOI] [PubMed] [Google Scholar]
- 6.Nishimori M, Yamada Y, Hoshi K, et al. Health-related quality of life of unrelated bone marrow donors in Japan. Blood. 2002;99:1995–2001. doi: 10.1182/blood.v99.6.1995. [DOI] [PubMed] [Google Scholar]
- 7.Machaczka M, Kalaitzakis E, Eleborg L, et al. Comparison of general vs regional anaesthesia for BM harvesting: a retrospective study of anaesthesia-related complications. Bone Marrow Transplant. 2010;45:53–61. doi: 10.1038/bmt.2009.109. doi:10.1038/bmt.2009.109. [DOI] [PubMed] [Google Scholar]
- 8.Chen SH, Yang SH, Chu SC, et al. The role of donor characteristics and post-granulocyte colony-stimulating factor white blood cell counts in predicting the adverse events and yields of stem cell mobilization. Int J Hematol. 2011;93:652–659. doi: 10.1007/s12185-011-0844-5. doi:10.1007/s12185-011-0844-5. [DOI] [PubMed] [Google Scholar]
- 9.Stroncek DF, Clay ME, Petzoldt ML, et al. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601–610. doi: 10.1046/j.1537-2995.1996.36796323059.x. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S, Ziman A, Smeltzer B, et al. Moderate and severe adverse events associated with apheresis donations: incidences and risk factors. Transfusion. 2010;50:478–486. doi: 10.1111/j.1537-2995.2009.02443.x. doi:10.1111/j.1537-2995.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane GJ, Norrie G, Atherton K, et al. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis. 2009;68:1591–1595. doi: 10.1136/ard.2008.093088. doi:10.1136/ard.2008.093088. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Moric M, Husfeldt B, et al. Ethnic similarities and differences in the chronic pain experience: a comparison of african american, Hispanic, and white patients. Pain Med. 2005;6:88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 13.Rahim-Williams FB, Riley JL, Herrera D, et al. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129:177–184. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. doi:10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh M, Davidovitch RI, Egol KA. Ethnic disparities in recovery following distal radial fracture. J Bone Joint Surg Am. 2010;92:1082–1087. doi: 10.2106/JBJS.H.01329. doi:10.2106/JBJS.H.01329. [DOI] [PubMed] [Google Scholar]
- 16.Fitzcharles MA, Rampakakis E, Ste-Marie PA, et al. The association of socioeconomic status and symptom severity in persons with fibromyalgia. J Rheumatol. 2014;41:1398–1404. doi: 10.3899/jrheum.131515. doi:10.3899/jrheum.131515. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Bykerk VP, Boire G, et al. Does socioeconomic status affect outcomes in early inflammatory arthritis? Data from a canadian multisite suspected rheumatoid arthritis inception cohort. J Rheumatol. 2015;42:46–54. doi: 10.3899/jrheum.131382. [DOI] [PubMed] [Google Scholar]
- 18.Daugbjerg SB, Brandsborg B, Ottesen B, et al. The impact of socioeconomic and clinical factors on purchase of prescribed analgesics before and after hysterectomy on benign indication. Clin J Pain. 2014;30:46–54. doi: 10.1097/AJP.0b013e318285d26f. doi:10.1097/AJP.0b013e318285d26f. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer TR, Areman EM, Cirenza E, et al. The impact of harvest center on quality of marrows collected from unrelated donors. J Hematother. 1994;3(1):65–70. doi: 10.1089/scd.1.1994.3.65. Spring. [DOI] [PubMed] [Google Scholar]
- 20.Fagioli F, Quarello P, Pollichieni S, et al. Quality of harvest and role of cell dose in unrelated bone marrow transplantation: an Italian Bone Marrow Donor Registry-Gruppo Italiano Trapianto di Midollo Osseo Study. Hematology. 2014 Jan;19(1):1–9. doi: 10.1179/1607845413Y.0000000086. doi:10.1179/1607845413Y.0000000086. [DOI] [PubMed] [Google Scholar]
- 21.Kao RH, Li CC, Shaw CK, et al. Correlation between characteristics of unrelated bone marrow donor and cell density of total nucleated cell in bone marrow harvest. Int J Hematol. 2009 Mar;89(2):227–30. doi: 10.1007/s12185-008-0235-8. doi:10.1007/s12185-008-0235-8. [DOI] [PubMed] [Google Scholar]
- 22.Cell sources 2014 Nov 17; Retrieved November 17, 2014, from https://bethematchclinical.org/Transplant-Therapy-and-Donor-Matching/Cell-Sources/
- 23.Remberger M, Ringden O, Mattsson J. Bone marrow aspiration technique has deteriorated in recent years. Bone Marrow Transplantation. 2015 doi: 10.1038/bmt.2015.75. Vol in Press. [DOI] [PubMed] [Google Scholar]
- 24.Lannert H, Able T, Becker S, et al. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplant. 2008;42:443–447. doi: 10.1038/bmt.2008.196. doi:10.1038/bmt.2008.196. [DOI] [PubMed] [Google Scholar]
- 25.Fact news 2015 Mar 3; Retrieved from http://www.factwebsite.org/News.aspx#news-id1082.
- 26.Mijovic A, Britten C, Regan F, et al. Preoperative autologous blood donation for bone marrow harvests: are we wasting donors' time and blood? Transfus Med. 2006;16:57–62. doi: 10.1111/j.1365-3148.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 27.Parkkali T, Juvonen E, Volin L, et al. Collection of autologous blood for bone marrow donation: how useful is it? Bone Marrow Transplant. 2005;35:1035–1039. doi: 10.1038/sj.bmt.1704967. [DOI] [PubMed] [Google Scholar]
- 28.Retrospective examination of the role quantity of bone marrow harvests performed by a harvest center has on the overall quality of the harvested product: assessment of the potential impacts bone marrow product quality has on utilization of bone marrow as a cell source for transplant. 2014 Retrieved from http://www.cibmtr.org/Studies/Observational/StudyLists/pages/ObservationalStudy.aspx?OSID=a0JE000000DANyXMAX.