Abstract
Background
Previous research indicates that subjects with anterior cruciate ligament reconstruction exhibit abnormal knee joint movement patterns during functional activities like walking. While the sagittal plane mechanics have been studied extensively, less is known about the secondary planes, specifically with regard to more demanding tasks. This study explored the influence of task complexity on functional joint mechanics in the context of graft-specific surgeries.
Methods
In 25 participants (10 hamstring tendon graft, 6 patellar tendon graft, 9 matched controls), three-dimensional joint torques were calculated using a standard inverse dynamics approach during level walking and stair descent. The stair descent task was separated into two functionally different sub-tasks—step-to-floor and step-to-step. The differences in external knee moment profiles were compared between groups; paired differences between the reconstructed and non-reconstructed knees were also assessed.
Findings
The reconstructed knees, irrespective of graft type, typically exhibited significantly lower peak knee flexion moments compared to control knees during stair descent, with the differences more pronounced in the step-to-step task. Frontal plane adduction torque deficits were graft-specific and limited to the hamstring tendon knees during the step-to-step task. Internal rotation torque deficits were also primarily limited to the hamstring tendon graft group during stair descent. Collectively, these results suggest that task complexity was a primary driver of differences in joint mechanics between anterior cruciate ligament reconstructed individuals and controls, and such differences were more pronounced in individuals with hamstring tendon grafts.
Interpretation
The mechanical environment experienced in the cartilage during repetitive, cyclical tasks such as walking and other activities of daily living has been argued to contribute to the development of degenerative changes to the joint and ultimately osteoarthritis. Given the task-specific and graft-specific differences in joint mechanics detected in this study, care should be taken during the rehabilitation process to mitigate these changes.
Keywords: ACL Reconstruction, Knee, Joint Torques, Motion Analysis, Gait, Stair Descent
1. Introduction
The primary goals of anterior cruciate ligament (ACL) reconstruction are to restore knee stability, correct abnormal movement patterns, improve patient outcomes, and return athletes to high-level functional activities (Shelbourne and Rowdon, 1994; Tashman et al., 2007). While ACL reconstruction is successful in returning athletes to competitive sports with a high degree of patient satisfaction, the confluence of evidence indicates that full restoration of normal joint movement patterns during dynamic activities is rarely achieved even years after surgery (Bush-Joseph et al., 2001; Lewek et al., 2002; Papannagari et al., 2006; Ristanis et al., 2006; Stergiou et al., 2007; Tashman et al., 2007; Webster and Feller, 2011; Zabala et al., 2013). Because abnormal movement patterns are a precursor for abnormal joint contact loading, the resulting altered mechanical environment of the articular cartilage (Hosseini et al., 2012), specifically during cyclical repetitive tasks of daily living, has been implicated as a possible explanation for the increased prevalence of early onset degenerative changes in the ACL-reconstructed population (Andriacchi et al., 2004; Chaudhari et al., 2008; Koo et al., 2011; Prodromos et al., 1985).
A number of studies have examined movement patterns during dynamic tasks in individuals with ACL reconstruction. The findings from these studies indicate that subjects often achieve normal sagittal plane kinematic patterns (Bulgheroni et al., 1997; Knoll et al., 2004), especially if the quadriceps muscle has regained most of its pre-injury strength characteristics (Lewek et al., 2002). However, secondary degrees of freedom such as internal/external rotation and abduction/adduction may not be fully restored following ACL reconstruction independent of graft type (Butler et al., 2009; Webster and Feller, 2011). The results of these studies and others also indicate that ACL-reconstructed knees typically exhibit reduced knee adduction moment in comparison to the non-reconstructed contralateral knees and uninjured control knees during level ground and incline walking (Patterson et al., 2014; Varma et al., 2014; Webster et al., 2012; Webster et al., 2011). Furthermore, the complexity of the task has been shown to magnify kinematic and kinetic differences between ACL-reconstructed and healthy knees (Tashman et al., 2007). This may be due in part to the increased magnitude of mechanical loading that the knee must support during more biomechanically challenging activities (Kutzner et al., 2010; Taylor et al., 2004). A recent study examining secondary knee moments during stair navigation and overground gait showed that ACL-reconstructed knees with an Achilles allograft exhibited lower peak adduction moments and lower internal rotation moments compared to contralateral knees (Zabala et al., 2013). While these results are in consensus with evidence from level ground walking studies (Patterson et al., 2014; Varma et al., 2014; Webster et al., 2012; Webster et al., 2011), it remains to be seen if the patterns of differences observed due to complexity of the task will generalize to differences in joint mechanics when the graft is extracted from the patient’s autologous tissue (e.g., hamstring tendon or patellar tendon).
While a number of studies have examined dynamic movement patterns in an ACL-reconstructed population, the majority have considered a pooled population, consisting of multiple graft types and sources (Butler et al., 2009; Gao et al., 2012; Lewek et al., 2002; Scanlan et al., 2010; Tashman et al., 2007); relatively few studies have sought to compare outcomes between subgroups of patients based on graft type. As part of the same research group, Ristanis et al. (2006) and Chouliaras et al. (2007) separately examined similar experimental paradigms of stair descent/pivot on populations of individuals with patellar tendon graft and hamstring tendon graft reconstructions, and anecdotally reported that the amount of excessive tibial rotation was similar for both groups. Recently, a direct comparison between populations of patients with patellar tendon and hamstring tendon autografts found decreased adduction angles during the stance phase of walking in the hamstring group compared to both patellar tendon and control groups (Webster and Feller, 2011). They also found that both patient groups had decreased internal rotation (3 to 5 degrees) compared to the control group and to the contralateral knee during midstance. While small in magnitude, such rotational changes represent a substantial percentage of the overall range of motion in those degrees of freedom and could be indicative of significant changes to the internal mechanics (e.g. contact stress locations) of the joint (Hosseini et al., 2012; Tashman et al., 2007). Whether the magnitude of these demonstrated differences in movement patterns between patient cohorts is amplified during more complex tasks requires further investigation.
Accordingly, the purpose of this study was to calculate three-dimensional (3D) knee joint kinematics and kinetics during overground walking and stair descent tasks in two groups of ACL-reconstructed subjects based on graft type (hamstring tendon and patellar tendon autografts) as well as healthy control subjects. Our overarching hypothesis was that task complexity would be the primary driver of differences in the aggregate joint mechanics between groups. In this context, we argue that complexity is influenced not only by the biomechanical demand on the ipsilateral leg, but also by the demand placed on the contralateral leg (Kozanek et al., 2008). To that end, we analyzed the joint mechanics between tasks with increasing contralateral demand in the form of change in vertical height—walking, step-to-floor, and step-to-step. Additionally, we sought to explore the compensatory/adaptive mechanisms of the non-reconstructed knees across the three tasks. In an attempt to delineate the potential effect of surgery-specific alterations in the musculoskeletal structure on joint mechanics, both the reconstructed and non-reconstructed knees were compared to healthy control knees.
2. Methods
2.1. Subject Population
The subject population consisted of three groups—two ACL reconstructed groups (10 hamstring tendon and 6 patellar tendon autograft; reconstructed and contralateral knees) and an uninjured control group (n = 9; dominant leg knees) that was matched for age, height, weight, and activity level (Table 1; detailed demographics are provided in the Supplementary Material, Table S1). ACL-reconstructed subjects were included if they had an isolated ACL rupture and a subsequent surgical reconstruction using either a hamstring tendon (HT) or patellar tendon (PT) autograft at least 6 months and up to 15 months prior to the study sessions. Subjects were expected to be able to perform daily functional activities without the use of a knee brace. Subjects were excluded if they were obese (BMI ≥ 30.0), had a history of significant knee pain prior to the injury and/or at time of testing, contralateral knee injury/surgery, or prior injury/surgery to the reconstructed knee. Most ACL-reconstructed subjects were identified and recruited using the Northwestern Medical Enterprise Data Warehouse (EDW). All experimental procedures were approved by the Institutional Review Board of Northwestern University and complied with the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects prior to participation in the research study.
Table 1.
Group summary demographics; data reported as mean (standard deviation).
| ACLR (HT) | ACLR (PT) | Control | |
|---|---|---|---|
| Count | N = 10 | N = 6 | N = 9 |
| Age (years) | 32.6 (8.0) | 30.8 (5.5) | 27.2 (3.7) |
| Height (cm) | 176.1 (8.8) | 179.1 (14.1) | 170.6 (12.0) |
| Weight (kg) | 74.6 (7.4) | 74.8 (18.0) | 67.1 (13.4) |
| BMI | 24.1 (1.9) | 23.0 (2.9) | 22.8 (1.9) |
| Months Post Surgery | 9.4 (3.7) | 9.7 (4.3) | N/A |
| Tegner Activity Scale | 6.4 (1.9) | 7.0 (1.3) | 6.0 (2.3) |
| Marx Activity Scale | 10.1 (5.5) | 11.8 (1.9) | 10.6 (5.2) |
No significant differences (p > 0.05) were observed between groups for any of the demographic variables. (ACLR: Anterior Cruciate Ligament Reconstruction; HT: Hamstring Tendon; PT: Patellar Tendon).
2.2. Experimental Protocol
All subjects were asked to fill out a series of questionnaires to characterize their current activity level, the incidence and severity of their anterior knee pain, and their knee health-related quality of life. The activity level was evaluated using the Tegner Activity Scale (Tegner and Lysholm, 1985) and the Marx Activity Rating Scale (Marx et al., 2001). The Knee Injury and Osteoarthritis Outcome Score (KOOS) survey (Roos et al., 1998) was used to obtain the subject’s opinion about their knee and associated problems. The Anterior Knee Pain Scale (Shelbourne and Trumper, 1997) was used to determine the frequency and severity of pain in the kneecap or anterior knee region during sporting and daily activities, prolonged sitting, stair climbing, and kneeling.
Prior to functional testing, knee ligamentous laxity of the ACL-reconstructed subjects was measured on both legs using a KT1000 arthrometer (MEDmetric® Corporation, San Diego, CA). Three measurements were obtained using the manual maximum tests for each leg, and the average value rounded to the nearest millimeter was recorded.
Functional motion analysis was performed on all subjects using an eight camera passive motion capture system (Motion Analysis Corporation, Santa Rosa, CA). The 3D motion of 32 retroreflective markers affixed to the torso, pelvis, thighs, shanks, and feet was tracked by the system at a sampling frequency of 120 Hz. Please refer to the Supplementary Material for more detailed information.
Following the initial setup period, subjects were instructed to perform various functional tasks in the laboratory. The primary tasks of interest for this particular study included overground walking and stair descent, with the complexity of the task driven by the change in vertical height of both legs. For the stance leg, the walking task required no change in vertical height from the previous step; however, in both stair descent tasks, the change from the prior step was equivalent to two stair riser units (15 inches). Additionally, the stair descent to step task enforced an additional constraint over the stair descent to floor task based on the tread depth of the stairs. For the contralateral leg, the three tasks presented a linear increase in the number of stair riser units (1 unit = 7.5 in) traversed during the cycle: walking (0), stair descent to floor (1), and stair descent to step (2).
Tasks were repeated a number of times to ensure that sufficient information about both legs could be obtained (at least 6 trials per leg per task). Three force plates in the ground (AMTI, Watertown, MA) were used to record 3D ground reaction forces and moments, and two force plates on the steps of the stairs (Kistler, Winterthur, Switzerland) recorded 3D ground reaction forces. Marker trajectories and ground reaction forces were simultaneously recorded during all trials; all analog data were acquired at 20 times the camera frame rate, or 2400 Hz.
2.3. Data Analysis
Marker trajectories were identified and tracked in 3D space using Cortex software (v4.0, Motion Analysis Corporation). Prior to further calculations, marker coordinates were smoothed using a fourth order zero lag 6 Hz low-pass Butterworth filter. The rigid body segments representing the thigh (hip joint center to knee joint center), shank (knee joint center to ankle joint center), and foot (ankle joint center to second metatarsal head) were defined using custom Matlab (vR2014a, The Mathworks Inc., Natick, MA) algorithms. Knee joint angles were calculated using the joint coordinate system method described by Grood and Suntay (1983).
Ground reaction forces and moments measured from the force plates were smoothed using a fourth order zero lag low-pass Butterworth filter with a cutoff frequency of 15 Hz (Besier et al., 2009). Forces and moments were then converted to an equivalent system (forces and free torque) acting on the surface of the plate at the center of pressure (COP) of the foot.
Joint torques were calculated using a standard Newton-Euler inverse dynamics approach (Winter, 2009) with segment inertial properties obtained from de Leva (1996). Joint forces and torques were solved segment-by-segment starting at the foot and moving proximally up the leg. Using the 3D positions of both actual and virtual markers, an orthogonal local coordinate system was established for each segment with the z-axis pointed proximally along the direction of the bone, the x-axis pointed to the right (laterally for the right leg and medially for the left leg), and the y-axis pointed anteriorly. Ground reaction forces and moments acting at the center of pressure of the foot were used as inputs to the system. Joint torques at the knee were expressed as external moments with respect to the joint coordinate system (Grood and Suntay, 1983) described above, and were normalized to percentage of body weight times height (% BW × H).
A combination of ground reaction force thresholds and kinematic gait event detection (Zeni et al., 2008) was used to detect the beginning and end of the stance phase for all tasks analyzed (overground walking, stair descent to the floor, and stair descent to a step). Each stance cycle was normalized to 101 data points (t = 0% – 100%). For further analysis, the peaks (positive or negative) in the torque profiles during the first and second halves of the stance cycle were identified for all tasks and all degrees of freedom (Zabala et al., 2013); the timing of these torque peaks were also used for the examination of differences in kinematics.
2.4. Statistical Analysis
All statistical analyses were performed using R (v3.1.3). The differences in knee joint moments (and kinematics) between groups were tested separately for the reconstructed (3 groups: controls, PT reconstructed, HT reconstructed) and the non-reconstructed (3 groups: controls, PT contralateral, HT contralateral) legs using the one-way analysis of variance (ANOVA) test. A significant omnibus test was followed by post-hoc analysis using Tukey’s honest significance test for multiple comparisons. Paired t-tests were used to compare the joint torque profiles (and kinematics) of the reconstructed and non-reconstructed contralateral legs in the two ACL-reconstructed cohorts. A significance level of α = 0.05 was set for all statistical analyses. Estimates of effect size were reported using partial η2.
3. Results
3.1. Demographics and Clinical Characteristics
There were no significant differences in demographics between groups (p > 0.05), indicating that the groups were adequately matched (Table 1). Significant differences were not detected in ligament laxity (p = 0.63), KOOS (range p = [0.23, 0.81]), or the anterior knee pain scores (p = 0.5) between the hamstring and the patellar tendon groups; however, significant differences in KOOS and anterior knee pain scores were observed between the ACL-reconstructed groups and the healthy control group (p < 0.05; Table 2).
Table 2.
Knee-related outcome measures; data reported as mean (standard deviation).
| ACLR (HT) | ACLR (PT) | Control | |
|---|---|---|---|
| KT1000 Difference (mm) | +2.5 (2.8) | +1.8 (1.7) | N/A |
| KOOS Pain | 86.1 (11.2) | 88.8 (12.5) | 95.6 (5.5) |
| KOOS Symptoms | 78.2 (14.1) * | 83.8 (14.0) | 97.1 (3.0) |
| KOOS Activities of Daily Living | 93.8 (7.4) * | 97.8 (3.4) | 99.7 (1.0) |
| KOOS Sports | 76.5 (22.2) * | 80.0 (15.8) | 96.7 (6.6) |
| KOOS Quality of Life | 60.0 (16.7) * | 62.5 (23.8) * | 89.6 (15.7) |
| Anterior Knee Pain Scale | 85.9 (15.1) | 80.7 (13.8) * | 96.1 (5.5) |
Significant (p < 0.05) differences from controls are indicated with an asterisk (*). KOOS and pain scale survey results were out of a maximum of 100 (no deficiencies). (KOOS: Knee Injury and Osteoarthritis Outcome Score; ACLR: Anterior Cruciate Ligament Reconstruction; HT: Hamstring Tendon; PT: Patellar Tendon).
3.2. Kinematics
There were several instances of significant kinematic differences between groups. Please refer to the Supplementary Material for details.
3.3. Sagittal Plane Kinetics
3.3.1. Walk
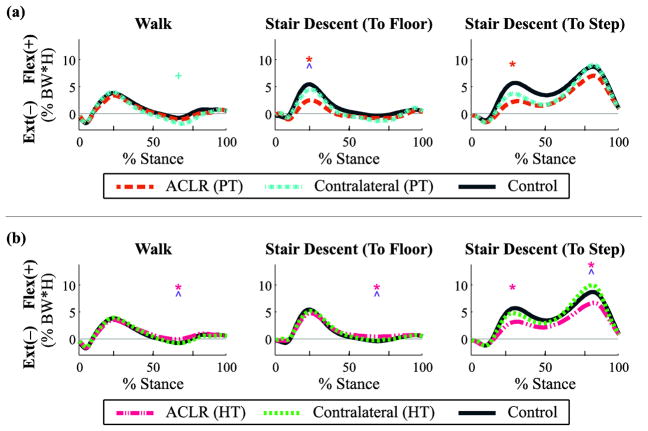
There were significant main effects of group on kinetics only during the second peak for reconstructed (p = 0.033, partial η2 = 0.2, observed power = 0.5) and non-reconstructed knees (p = 0.049, partial η2 = 0.2, observed power = 0.6). Post-hoc analysis revealed a significant increase in the extension moment in the PT graft non-reconstructed knees compared to control knees (p = 0.039; Fig. 1a; Table 3) and a significant decrease in the extension moment in the HT graft reconstructed knees compared to control knees (p = 0.043; Fig. 1b; Table 4). Paired t-tests revealed a significant decrease in the second peak extension moment in the HT graft reconstructed knees compared to the non-reconstructed knees (p < 0.001; Fig. 1b; Table 4).
Fig. 1.
Sagittal plane joint torques (flexion +/extension −) throughout the stance phase for walking and stair descent tasks by graft type: (a) patellar tendon (PT) and (b) hamstring tendon (HT). Mean data reported as a percentage of body weight × height for ACL reconstructed (ACLR) knees, non-reconstructed contralateral knees, and healthy control knees, with regions of statistical significance (p < 0.05) indicated: ACLR vs. control [*], contralateral vs. control [+], ACLR vs. contralateral [^].
Table 3.
Patellar tendon (PT) group differences in joint kinetics for walking and stair descent tasks; significant (p < 0.05) differences in bold (*).
| Plane | Task | Phase | Mean Difference, [95% Confidence Interval] (% BW*H)
|
||
|---|---|---|---|---|---|
| Control–ACLR (PT) | Control–Contralateral (PT) | Contralateral (PT)–ACLR (PT) | |||
| Sagittal | Walk | Peak 1 | 0.43, [−1.25, 2.12] | −0.18, [−1.87, 1.5] | 0.62, [−0.82, 2.06] |
| Peak 2 | 0.3, [−0.74, 1.33] | 1.08, [0.05, 2.12] * | −0.79, [−1.69, 0.11] | ||
| Stair Descent (Step To Floor) | Peak 1 | 2.95, [0.6, 5.3] * | 0.91, [−1.44, 3.25] | 2.04, [0.95, 3.13] * | |
| Peak 2 | 0.43, [−0.53, 1.4] | 0.92, [−0.04, 1.88] | −0.49, [−1.35, 0.38] | ||
| Stair Descent (Step To Step) | Peak 1 | 3.35, [1.29, 5.41] * | 1.98, [−0.08, 4.04] | 1.37, [−0.49, 3.22] | |
| Peak 2 | 1.64, [−0.79, 4.07] | −0.4, [−2.83, 2.03] | 2.04, [−0.9, 4.98] | ||
| Frontal | Walk | Peak 1 | −0.15, [−1.13, 0.83] | −0.27, [−1.25, 0.71] | 0.12, [−0.32, 0.56] |
| Peak 2 | 0.07, [−0.64, 0.78] | −0.52, [−1.23, 0.2] | 0.59, [−0.22, 1.39] | ||
| Stair Descent (Step To Floor) | Peak 1 | 0.23, [−1.26, 1.71] | −0.45, [−1.94, 1.03] | 0.68, [−0.66, 2.02] | |
| Peak 2 | −0.2, [−1.11, 0.71] | −0.85, [−1.68, −0.02] * | 0.62, [−0.15, 1.38] | ||
| Stair Descent (Step To Step) | Peak 1 | 0.38, [−1.14, 1.9] | 0.1, [−1.41, 1.62] | 0.28, [−1.21, 1.77] | |
| Peak 2 | 0.17, [−1.95, 2.29] | −0.08, [−2.2, 2.04] | 0.25, [−1.34, 1.84] | ||
| Transverse | Walk | Peak 1 | 0.03, [−0.34, 0.41] | 0.14, [−0.24, 0.52] | −0.11, [−0.4, 0.19] |
| Peak 2 | −0.24, [−0.48, −0.01] * | 0.02, [−0.22, 0.26] | −0.27, [−0.51, −0.03] * | ||
| Stair Descent (Step To Floor) | Peak 1 | −0.35, [−1.04, 0.34] | 0.17, [−0.52, 0.86] | −0.52, [−1.5, 0.45] | |
| Peak 2 | −0.06, [−0.36, 0.24] | 0.2, [−0.1, 0.5] | −0.27, [−0.53, −0.01] * | ||
| Stair Descent (Step To Step) | Peak 1 | −0.5, [−1.24, 0.24] | −0.14, [−0.88, 0.61] | −0.36, [−1.19, 0.46] | |
| Peak 2 | −0.27, [−2.41, 1.86] | 0.43, [−1.7, 2.57] | −0.71, [−2.56, 1.14] | ||
Table 4.
Hamstring tendon (HT) group differences in joint kinetics for walking and stair descent tasks; significant (p < 0.05) differences in bold (*).
| Plane | Task | Phase | Mean Difference, [95% Confidence Interval] (% BW*H)
|
||
|---|---|---|---|---|---|
| Control–ACLR (HT) | Control–Contralateral (HT) | Contralateral (HT)–ACLR (HT) | |||
| Sagittal | Walk | Peak 1 | 0.28, [−0.97, 1.54] | −0.11, [−1.37, 1.14] | 0.4, [−0.51, 1.31] |
| Peak 2 | −0.73, [−1.43, −0.02] * | 0.04, [−0.67, 0.75] | −0.77, [−1.07, −0.46] * | ||
| Stair Descent (Step To Floor) | Peak 1 | 0.66, [−1.89, 3.21] | 0.24, [−2.31, 2.79] | 0.42, [−1.46, 2.29] | |
| Peak 2 | −0.86, [−1.7, −0.01] * | −0.15, [−1, 0.69] | −0.7, [−1.09, −0.31] * | ||
| Stair Descent (Step To Step) | Peak 1 | 2.48, [0.23, 4.73] * | 0.9, [−1.35, 3.15] | 1.58, [−0.12, 3.27] | |
| Peak 2 | 2.02, [0.17, 3.86] * | −1.29, [−3.14, 0.55] | 3.31, [2.13, 4.49] * | ||
| Frontal | Walk | Peak 1 | 0.64, [−0.34, 1.62] | 0.62, [−0.36, 1.6] | 0.01, [−0.45, 0.48] |
| Peak 2 | 0.21, [−0.5, 0.92] | 0.37, [−0.34, 1.08] | −0.16, [−0.97, 0.64] | ||
| Stair Descent (Step To Floor) | Peak 1 | 0.99, [−0.43, 2.41] | 0.88, [−0.55, 2.3] | 0.11, [−0.54, 0.77] | |
| Peak 2 | 0.04, [−0.76, 0.84] | 0.29, [−0.57, 1.15] | −0.15, [−1.26, 0.95] | ||
| Stair Descent (Step To Step) | Peak 1 | 1.49, [0.42, 2.55] * | 1.19, [0.12, 2.25] * | 0.3, [−0.25, 0.85] | |
| Peak 2 | 0.9, [−0.73, 2.53] | 0.26, [−1.36, 1.89] | 0.64, [−0.37, 1.65] | ||
| Transverse | Walk | Peak 1 | −0.09, [−0.4, 0.22] | −0.08, [−0.39, 0.23] | −0.01, [−0.14, 0.12] |
| Peak 2 | −0.16, [−0.36, 0.04] | −0.09, [−0.28, 0.11] | −0.07, [−0.29, 0.15] | ||
| Stair Descent (Step To Floor) | Peak 1 | −0.47, [−1.19, 0.24] | −0.07, [−0.78, 0.65] | −0.41, [−0.76, −0.06] * | |
| Peak 2 | −0.16, [−0.41, 0.1] | −0.09, [−0.36, 0.18] | −0.06, [−0.41, 0.28] | ||
| Stair Descent (Step To Step) | Peak 1 | −0.91, [−1.68, 0.14] * | −0.46, [−1.23, 0.31] | −0.45, [−0.99, 0.09] | |
| Peak 2 | −1.3, [−3, 0.4] | −0.23, [−1.93, 1.47] | −1.08, [−2.4, 0.25] | ||
3.3.2. Stair Descent (Step-to-Floor)
In the step-to-floor task, there was a significant main effect of group on sagittal plane kinetics for the reconstructed knees during both peaks (peak 1: p = 0.016, partial η2 = 0.3, observed power = 0.7, peak 2: p = 0.049, partial η2 = 0.2, observed power = 0.5). Post-hoc analysis revealed a significant decrease in the first peak flexion torque in the PT graft reconstructed knees compared to controls (p = 0.012; Fig. 1a; Table 3) and a significant decrease in the second peak extension moment in the HT graft reconstructed knees compared to controls (p = 0.046; Fig. 1b; Table 4). Paired t-tests also revealed a significant decrease in the first peak flexion torque in the PT graft reconstructed knees compared to non-reconstructed knees (p = 0.005; Fig. 1a; Table 3) and a significant decrease in the second peak extension moment in the HT graft reconstructed knees compared to non-reconstructed knees (p = 0.003; Fig. 1b; Table 4).
3.3.3. Stair Descent (Step-to-Step)
There were significant main effects of group in kinetics for reconstructed knees during both peaks (peak 1: p = 0.038, partial η2 = 0.3, observed power = 0.8; peak 2: p = 0.003, partial η2 = 0.4, observed power = 0.9). In the first peak, post-hoc analysis revealed a significant decrease in the flexion torque in the PT graft reconstructed knees compared to controls (p = 0.002; Fig. 1a; Table 3), as well as a significant decrease in the flexion torque in the HT graft reconstructed knees compared to controls (p = 0.029; Fig. 1b; Table 4). In the second peak, post-hoc analysis revealed a significant decrease in the flexion torque in the HT graft reconstructed knees compared to controls (p = 0.03; Fig. 1b; Table 4). Paired t-tests revealed a significant decrease in the flexion torque during the second peak in the HT graft reconstructed knees compared to non-reconstructed knees (p = 0.001; Fig. 1b; Table 4).
3.4. Frontal Plane Kinetics
3.4.1. Walk
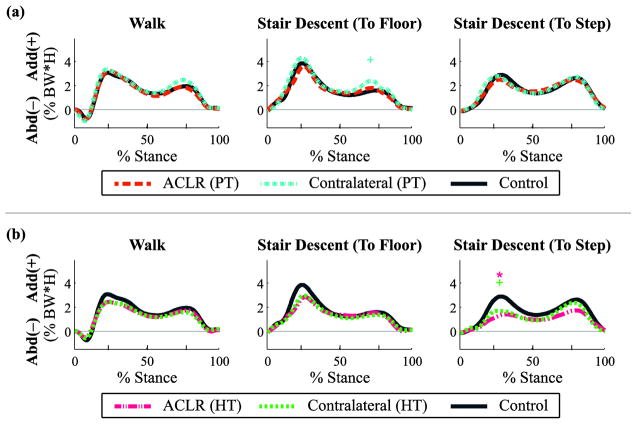
There were no significant main effects of group on frontal plane kinetics for the reconstructed or the non-reconstructed knees (p > 0.05, range p = [0.17, 0.78]; Fig. 2; Tables 3/4). Paired t-tests also indicated no differences between the reconstructed and non-reconstructed contralateral knees (p > 0.05, range p = [0.12, 0.95]).
Fig. 2.
Frontal plane joint torques (adduction +/abduction −) throughout the stance phase for walking and stair descent tasks by graft type: (a) patellar tendon (PT) and (b) hamstring tendon (HT). Mean data reported as a percentage of body weight × height for ACL reconstructed (ACLR) knees, non-reconstructed contralateral knees, and healthy control knees, with regions of statistical significance (p < 0.05) indicated: ACLR vs. control [*], contralateral vs. control [+], ACLR vs. contralateral [^].
3.4.2. Stair Descent (Step-to-Floor)
There was a significant main effect of group on frontal plane kinetics in the non-reconstructed knees during the second peak of the step-to-floor task (p = 0.049, partial η2 = 0.2, observed power = 0.5). Post-hoc analysis revealed a significant increase in peak knee adduction moment in the PT graft non-reconstructed knees in comparison to the control knees (p = 0.044; Fig. 2a; Table 3). There were no significant effects of group in the reconstructed knees (p > 0.05, range p = [0.16, 0.70], as well as no significant difference between reconstructed and non-reconstructed knees (p > 0.05, range p = [0.09, 0.75]).
3.4.3. Stair Descent (Step-to-Step)
There was a significant main effect of group on frontal plane kinetics during the first peak of the step-to-step task in both reconstructed (p = 0.003, partial η2 = 0.4, observed power = 0.9) and non-reconstructed knees (p = 0.005, partial η2 = 0.4, observed power = 0.8). Post-hoc analysis revealed a reduced adduction moment in the HT graft reconstructed knees compared to the control knees (p = 0.006; Fig. 2b; Table 4), as well as a reduction in the HT graft non-reconstructed knees compared to control knees (p = 0.028; Fig. 2b; Table 4).
3.5. Transverse Plane Kinetics
3.5.1. Walk
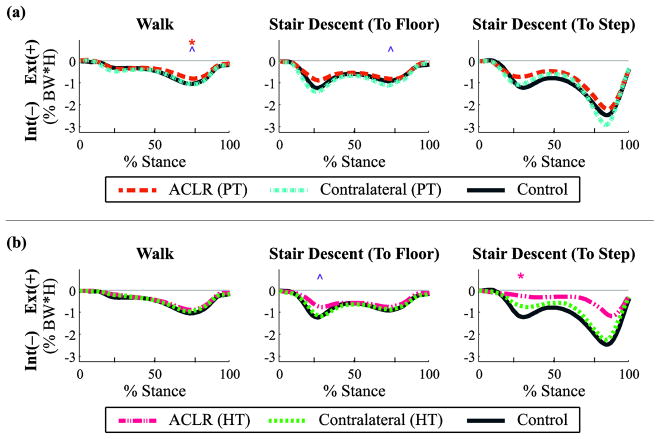
There was a significant main effect of group on kinetics for the reconstructed knees during the second peak (p = 0.04, partial η2 = 0.2, observed power = 0.6). Post-hoc analysis revealed a significant decrease in the peak internal rotation moment in the PT graft reconstructed knees compared to control knees (p = 0.044; Fig. 3a; Table 3). Paired t-tests also revealed a significant decrease in the second peak internal rotation moment in the PT graft reconstructed knees compared to non-reconstructed knees (p = 0.036; Fig. 3a; Table 3).
Fig. 3.
Transverse plane joint torques (external +/internal −) throughout the stance phase for walking and stair descent tasks by graft type: (a) patellar tendon (PT) and (b) hamstring tendon (HT). Mean data reported as a percentage of body weight × height for ACL reconstructed (ACLR) knees, non-reconstructed contralateral knees, and healthy control knees, with regions of statistical significance (p < 0.05) indicated: ACLR vs. control [*], contralateral vs. control [+], ACLR vs. contralateral [^].
3.5.2. Stair Descent (Step-to-Floor)
There were no significant main effects of group on transverse plane kinetics for the reconstructed and non-reconstructed knees during the step-to-floor task (p > 0.05, range p = [0.18, 0.33]). Paired t-tests revealed a significant decrease in the first peak internal rotation moment in the HT graft reconstructed knees compared to non-reconstructed knees (p = 0.027; Fig. 3b; Table 4). In the second peak there was a significant decrease in the PT graft reconstructed knees compared to non-reconstructed knees (p = 0.045; Fig. 3a; Table 3).
3.5.3. Stair Descent (Step-to-Step)
There was a significant main effect of group on kinetics for the reconstructed knees during the first peak of the step-to-step task (p = 0.022, partial η2 = 0.3, observed power = 0.8). Post-hoc analysis revealed a significant decrease in the first peak internal rotation moment in the HT graft reconstructed knees compared to healthy control knees (p = 0.018; Fig. 3b; Table 4).
4. Discussion
In this study we sought to examine the effect of task complexity on 3D joint mechanics in ACL-reconstructed individuals and healthy control subjects. The primary tasks examined were overground walking and stair descent, with the stair descent task divided into step-to-floor and step-to-step sub-tasks. The outcomes of these examinations were tested for the potential effect of graft type used in the ACLR procedure. The results support our overarching hypothesis that task complexity is the primary driver of differences in the aggregate joint mechanics between ACL-reconstructed individuals and controls. In general, differences in kinematics and torques were more pronounced, both in magnitude and duration, in the stair descent tasks compared to the walking task. The addition of the tread-depth constraint and the increase in contralateral leg demand during the step-to-step task compared to the step-to-floor task further highlighted such differences. Our results also provide new evidence supporting the notion of graft-specific (patellar tendon and hamstring tendon) kinematic/kinetic adaptations. While certain adaptations seemed to be consistent regardless of graft type (e.g., flexion angle and flexion torque deficits during stair descent), other changes were primarily limited to one particular graft (e.g., frontal plane adaptations and internal torque deficits in the hamstring tendon group). Furthermore, our results suggest that such adaptations may not be limited to the reconstructed limb, though future work is needed in this area to elucidate the magnitude and impact of these changes. Because our clinical population was tested approximately 9 months post-operatively, we did not assess degenerative changes to the knee. However, the differences in global knee joint movement patterns and mechanics during activities of daily living could potentially be a precursor to the long-term increased prevalence of degenerative joint diseases in the ACL-reconstructed population (Andriacchi et al., 2004; Butler et al., 2009; Lohmander et al., 2004; Papannagari et al., 2006; Scanlan et al., 2010).
Irrespective of the graft type, there was a reduction in the knee flexion angle of the reconstructed limbs compared to controls during midstance of the stair descent to step task, which corresponded to a reduced first peak knee flexion moment. This reduced flexion strategy has also been observed in a recent examination of the joint mechanics in Achilles allograft ACLR subjects (Zabala et al., 2013). While both groups exhibited a reduced flexion strategy compared to healthy controls during the step-to-step task, only the PT graft group exhibited this strategy during the less complex step-to-floor task. Our results are consistent with prior examinations under similar testing paradigms (Bush-Joseph et al., 2001; Webster et al., 2005). These findings suggest that task complexity plays a significant role in the relative effect of graft type and other surgical variables on the observed changes in sagittal plane joint mechanics. Functionally, it has been proposed that the consequences of a reduced flexion strategy over time may interfere with the normal shock-absorbing ability of the knee joint and lead to potential degenerative changes (Cook et al., 1997). Biomechanically, the reduction in the external flexion torque corresponds to a reduction in the internal extension torque generated by the quadriceps muscles (Kowalk et al., 1997). The underlying mechanism associated with this reduction in extension torque in an ACL-reconstructed population has been studied and debated, implicating muscle weakness and/or impaired neuromuscular control as the primary causes of reduced knee flexion after ACL surgery (Bush-Joseph et al., 2001; Gokeler et al., 2003; Krishnan and Williams, 2011; Lewek et al., 2002; Patel et al., 2003). While the magnitude of quadriceps weakness reduces over time (Roewer et al., 2011), it is well-established that profound deficits are observed during the initial year of the surgery. In this context, and given that our study participants were tested on an average of 9 months post-surgery, quadriceps muscle weakness is the most likely reason for the observed “stiff-knee” gait.
Abnormal motions and loadings in the secondary planes at the human knee have been implicated in the development and progression of degenerative diseases (Andriacchi and Mundermann, 2006; Butler et al., 2009; Tashman et al., 2007; Zabala et al., 2013). Unlike sagittal plane motions, which are largely controlled by active muscle contractions, frontal plane motions are primarily influenced by the stiffness of the musculotendon units in addition to the material properties of other connective tissues such as the collateral and cruciate ligaments (Buchanan et al., 1996; Cammarata and Dhaher, 2010; Dhaher et al., 2005; Olmstead et al., 1986). Interestingly, the results of this study revealed that frontal plane mechanics are primarily affected in individuals with HT graft as compared to those with PT graft. Relative to the healthy knees, subjects with hamstring tendon grafts had significant deficiencies in the adduction angle during the end of loading response and early midstance of both stair descent tasks, corresponding to a significant reduction in the first peak adduction torque in the reconstructed limbs. While the exact mechanism for this observation is not clear, alterations in musculotendinous stiffness properties of the lateral hamstring compartment due to morphological changes following surgery could partly account for the reduced adduction in the HT group (Williams et al., 2004). Given the potential link between increased loading and osteoarthritis development, the observation of reduced frontal plane loading is somewhat contrary to what one might expect, but are in agreement with recent work examining ACL-reconstructed subjects with Achilles tendon allografts (Zabala et al., 2013). It is important to note, though, that unloading of the joint may be equally detrimental to joint health as overloading, especially when combined with poor muscular control and weakness (Herzog et al., 2004).
Because the secondary role of the ACL is to resist internal rotation of the tibia with respect to the femur, internal/external rotation angles following ACLR have been examined in a number of studies (Chouliaras et al., 2007; Papannagari et al., 2006; Ristanis et al., 2006; Scanlan et al., 2010; Stergiou et al., 2007; Webster and Feller, 2011). While we did not observe significant differences in tibial rotation kinematics between ACL-reconstructed individuals of either graft type and healthy controls, there were significant differences between the reconstructed and the non-reconstructed legs of both the PT and HT group. Further, the kinematic differences between legs were more profound during stair descent tasks than overground level walking. In general, the ACL-reconstructed knees exhibited lower internal rotation in comparison to the non-reconstructed legs and this phenomenon was more striking in the PT group. Interestingly, while the kinematic differences were more profound in the PT group, the differences in transverse plane torque profiles were more profound in the HT group. The altered torque profiles in the HT group are understandable considering the anatomical role of the hamstring muscle group on tibial rotations. However, further research incorporating modeling or simulations is required to fully understand the repercussions of graft-specific kinematic and kinetic changes on knee joint cartilage health.
The results of this study should be interpreted with consideration of its limitations. Graft type is only one of many surgical variables that may concurrently contribute to functional outcomes following ACLR; geometric factors such as graft tunnel position and orientation as well as mechanical factors such as graft pre-tensioning and fixation methods may also play a large role. In this study, several different surgeons performed the reconstructions, so we expect significant variability in these surgical factors which may have affected the results. Moreover, the limited sample size, specifically with respect to the patellar tendon cohort, could have reduced the power to detect certain kinematic or kinetic differences between legs or groups. Further, similar to others (Krishnan and Williams, 2011; Zabala et al., 2013), we assumed that there are minimal differences between legs in the control subjects. However, the validity of this assumption needs to be verified experimentally, as it would assist in better interpretation of between-leg differences in ACL-reconstructed individuals. Additionally, motion analysis studies using skin-based reflective markers such as this one are susceptible to picking up changes in overlying skin movement in addition to bone movement. While more advanced techniques such as biplanar fluoroscopy are available (Li et al., 2005; Tashman et al., 2007), they were not a feasible option for the types of tasks analyzed in this study. As a result, we recommend caution when interpreting the results of transverse plane mechanics, as they lack sufficient reliability and are particularly susceptible to large errors (McGinley et al., 2009). We also suggest that readers use the established minimal detectable change scores (Wilken et al., 2012) when making inferences about mean differences observed between legs and groups in this study.
5. Conclusions
This study offers a comprehensive look at the influence of task complexity and graft type on 3D joint kinetics following ACL reconstruction. The results of this study indicate that the differences in kinematics and torques were typically more pronounced in the stair descent tasks compared to the walking task. These findings emphasize the importance of task complexity in elucidating kinematic and kinetic differences after ACL reconstruction. The results also indicate graft-specific differences in kinematic and torque profiles after ACL reconstruction. Specifically, frontal plane adaptations and internal rotation torque deficits were primarily limited to the hamstring tendon graft cohort. While the precise mechanisms for the observed differences are not clear, these results highlight the need for further research in this area, particularly considering the increased risk of post-traumatic knee osteoarthritis in this population.
Supplementary Material
Highlights.
We examine knee joint kinetics following anterior cruciate ligament reconstruction.
Task complexity (walking vs. stair descent) influences functional mechanics.
Graft type (hamstring tendon and patellar tendon) influences patient outcomes.
Bilateral adaptations occur post anterior cruciate ligament reconstruction.
Acknowledgments
The authors gratefully acknowledge the financial support of the Arthritis Foundation (Doctoral Dissertation Award), National Institutes of Health (NIAMS R01 AR049837, R01 EB019834, U01 EB015410), National Science Foundation (#0966535/0966742), and the United States Department of Defense (#DR080326); subject identification was aided by the use of the Northwestern Medical Enterprise Data Warehouse (NUCATS grant UL1RR025741). The authors would also like to thank Brian Chilelli, Jay Kalawadia, John Paul Manalo, and Despina Kotsapouikis for assistance with patient recruitment, as well as Winston Ku and Jordan Wong for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Besier TF, Fredericson M, Gold GE, Beaupre GS, Delp SL. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J Biomech. 2009;42:898–905. doi: 10.1016/j.jbiomech.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TS, Kim AW, Lloyd DG. Selective muscle activation following rapid varus/valgus perturbations at the knee. Med Sci Sports Exerc. 1996;28:870–876. doi: 10.1097/00005768-199607000-00014. [DOI] [PubMed] [Google Scholar]
- Bulgheroni P, Bulgheroni MV, Andrini L, Guffanti P, Giughello A. Gait patterns after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1997;5:14–21. doi: 10.1007/s001670050018. [DOI] [PubMed] [Google Scholar]
- Bush-Joseph CA, Hurwitz DE, Patel RR, Bahrani Y, Garretson R, Bach BR, Jr, Andriacchi TP. Dynamic function after anterior cruciate ligament reconstruction with autologous patellar tendon. Am J Sports Med. 2001;29:36–41. doi: 10.1177/03635465010290011101. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- Cammarata ML, Dhaher YY. Evidence of gender-specific motor templates to resist valgus loading at the knee. Muscle Nerve. 2010;41:614–623. doi: 10.1002/mus.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone-patellar tendon-bone autografts: an in vivo study comparing tibial internal-external rotation. Am J Sports Med. 2007;35:189–196. doi: 10.1177/0363546506296040. [DOI] [PubMed] [Google Scholar]
- Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE. Effects of restricted knee flexion and walking speed on the vertical ground reaction force during gait. J Orthop Sports Phys Ther. 1997;25:236–244. doi: 10.2519/jospt.1997.25.4.236. [DOI] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Dhaher YY, Tsoumanis AD, Houle TT, Rymer WZ. Neuromuscular reflexes contribute to knee stiffness during valgus loading. J Neurophysiol. 2005;93:2698–2709. doi: 10.1152/jn.00921.2004. [DOI] [PubMed] [Google Scholar]
- Gao B, Cordova ML, Zheng NN. Three-dimensional joint kinematics of ACL-deficient and ACL-reconstructed knees during stair ascent and descent. Hum Mov Sci. 2012;31:222–235. doi: 10.1016/j.humov.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Gokeler A, Schmalz T, Knopf E, Freiwald J, Blumentritt S. The relationship between isokinetic quadriceps strength and laxity on gait analysis parameters in anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2003;11:372–378. doi: 10.1007/s00167-003-0432-1. [DOI] [PubMed] [Google Scholar]
- Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- Herzog W, Clark A, Longino D. Joint mechanics in osteoarthritis. Novartis Found Symp. 2004;260:79–95. discussion 95–79, 100–104, 277–109. [PubMed] [Google Scholar]
- Hosseini A, Van de Velde S, Gill TJ, Li G. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J Orthop Res. 2012;30:1781–1788. doi: 10.1002/jor.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll Z, Kocsis L, Kiss RM. Gait patterns before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12:7–14. doi: 10.1007/s00167-003-0440-1. [DOI] [PubMed] [Google Scholar]
- Koo S, Rylander JH, Andriacchi TP. Knee joint kinematics during walking influences the spatial cartilage thickness distribution in the knee. J Biomech. 2011;44:1405–1409. doi: 10.1016/j.jbiomech.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalk DL, Duncan JA, McCue FC, 3rd, Vaughan CL. Anterior cruciate ligament reconstruction and joint dynamics during stair climbing. Med Sci Sports Exerc. 1997;29:1406–1413. doi: 10.1097/00005768-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Kozanek M, Van de Velde SK, Gill TJ, Li G. The contralateral knee joint in cruciate ligament deficiency. Am J Sports Med. 2008;36:2151–2157. doi: 10.1177/0363546508319051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011 doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43:2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Li G, Defrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23:340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- McGinley JL, Baker R, Wolfe R, Morris ME. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture. 2009;29:360–369. doi: 10.1016/j.gaitpost.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Olmstead TG, Wevers HW, Bryant JT, Gouw GJ. Effect of muscular activity on valgus/varus laxity and stiffness of the knee. J Biomech. 1986;19:565–577. doi: 10.1016/0021-9290(86)90162-4. [DOI] [PubMed] [Google Scholar]
- Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006–2012. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- Patel RR, Hurwitz DE, Bush-Joseph CA, Bach BR, Jr, Andriacchi TP. Comparison of clinical and dynamic knee function in patients with anterior cruciate ligament deficiency. Am J Sports Med. 2003;31:68–74. doi: 10.1177/03635465030310012301. [DOI] [PubMed] [Google Scholar]
- Patterson MR, Delahunt E, Caulfield B. Peak knee adduction moment during gait in anterior cruciate ligament reconstructed females. Clin Biomech. 2014;29:138–142. doi: 10.1016/j.clinbiomech.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188–1194. [PubMed] [Google Scholar]
- Ristanis S, Stergiou N, Patras K, Tsepis E, Moraiti C, Georgoulis AD. Follow-up evaluation 2 years after ACL reconstruction with bone-patellar tendon-bone graft shows that excessive tibial rotation persists. Clin J Sport Med. 2006;16:111–116. doi: 10.1097/00042752-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44:1948–1953. doi: 10.1016/j.jbiomech.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43:1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelbourne KD, Rowdon GA. Anterior cruciate ligament injury. The competitive athlete. Sports Med. 1994;17:132–140. doi: 10.2165/00007256-199417020-00005. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Trumper RV. Preventing anterior knee pain after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:41–47. doi: 10.1177/036354659702500108. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37:601–613. doi: 10.2165/00007256-200737070-00004. [DOI] [PubMed] [Google Scholar]
- Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. J Orthop Res. 2004;22:625–632. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985:43–49. [PubMed] [Google Scholar]
- Varma RK, Duffell LD, Nathwani D, McGregor AH. Knee moments of anterior cruciate ligament reconstructed and control participants during normal and inclined walking. BMJ Open. 2014;4:e004753. doi: 10.1136/bmjopen-2013-004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Feller JA. Alterations in joint kinematics during walking following hamstring and patellar tendon anterior cruciate ligament reconstruction surgery. Clin Biomech. 2011;26:175–180. doi: 10.1016/j.clinbiomech.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Webster KE, Feller JA, Wittwer JE. Longitudinal changes in knee joint biomechanics during level walking following anterior cruciate ligament reconstruction surgery. Gait Posture. 2012;36:167–171. doi: 10.1016/j.gaitpost.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2011 doi: 10.1136/bjsm.2010.080770. [DOI] [PubMed] [Google Scholar]
- Webster KE, Wittwer JE, O’Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33:247–254. doi: 10.1177/0363546504266483. [DOI] [PubMed] [Google Scholar]
- Wilken JM, Rodriguez KM, Brawner M, Darter BJ. Reliability and Minimal Detectible Change values for gait kinematics and kinetics in healthy adults. Gait Posture. 2012;35:301–307. doi: 10.1016/j.gaitpost.2011.09.105. [DOI] [PubMed] [Google Scholar]
- Williams GN, Snyder-Mackler L, Barrance PJ, Axe MJ, Buchanan TS. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. J Bone Joint Surg Am. 2004;86-A:1936–1946. doi: 10.2106/00004623-200409000-00012. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. 4. John Wiley & Sons, Inc; 2009. [Google Scholar]
- Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013;46:515–520. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni JA, Jr, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27:710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.