Abstract
Decitabine is a hypomethylating agent that irreversibly inhibits DNA methyltransferase I inducing leukemic differentiation and re-expression of epigenetically silenced putative tumor antigens. We assessed safety and efficacy of decitabine maintenance after allogeneic transplantation for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Decitabine maintenance may help eradicate minimal residual disease, decrease the incidence of graft-versus-host disease (GVHD) and facilitate a graft-versus-leukemia effect by enhancing the effect of T-regulatory lymphocytes. Patients with AML/MDS in complete remission (CR) after allotransplant started decitabine between day +50 and +100. We investigated 4 decitabine doses in cohorts of 4 patients: 5, 7.5, 10 and 15 mg/m2/day x 5 days every 6 weeks, for maximum 8 cycles. The Maximum Tolerated Dose (MTD) was defined as the maximum dose at which ≤25% of people experience dose limiting toxicities (DLT) during the 1st cycle of treatment. Twenty-four patients were enrolled and 22 were evaluable. All 4 dose-levels were completed and no MTD was reached. Overall, decitabine maintenance was well tolerated. Grade 3/4 hematological toxicities were experienced by 75% of patients, including all patients treated at the highest dose-level. Nine patients completed all 8 cycles and 8 of them remain in CR. Nine patients have died due to: relapse (n=4), infectious complications (n=3) and GVHD (n=2). Most occurrences of acute GVHD were mild and resolved without interruption of treatment; one patient died of acute gut GVHD. Decitabine maintenance did not clearly impact the rate of chronic GVHD. Although there was a trend of increased FOXP3 expression, results were not statistically significant. In conclusion, decitabine maintenance is associated with acceptable toxicities when given in post-allotransplant setting. Although MTD was not reached, the dose of 10 mg/m2 for 5 days every 6 weeks appeared to be the optimal dose rather than 15 mg/m2, where most hematological toxicities occurred.
Keywords: decitabine, stem cell transplantation, maintenance, myelodysplastic syndrome, acute leukemia
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a potentially curative therapy for patients with high-risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Over the past decade we have witnessed significant advances in therapeutic approaches for alloHSCT including the use of alternative stem cell sources, less toxic conditioning regimens and better supportive care, resulting in improved overall survival.(1–5) However, disease relapse remains the principal cause of treatment failure for these patients.(6–9) The risk of relapse varies from 20–60% depending on the diagnosis and stage of the disease at the time of transplantation, and outcomes of salvage treatments are poor.(10–12) Median time to relapse after nonmyeloablative alloHSCT is 3–6 months and somewhat longer after myeloablative alloHSCT. Therefore, early maintenance therapy, directed at eliminating minimal residual disease and promoting a graft-versus-leukemia (GVL) effect, could be an effective method to improve outcomes after alloHSCT.
The concept of post-transplant maintenance therapy with hypomethylating agents in AML and MDS has been initially studied by investigators at MD Anderson Cancer Center.(13–15) They showed that azacitidine (AZA) can be safely administered to heavily pretreated post-transplant patients and may prolong event-free and overall survival. In addition, recent studies have shown that AZA, followed by DLI, is well tolerated when administered for early AML/MDS relapse after alloHSCT.(16–18) Decitabine (5-aza-2’-deoxycytidine) is a hypomethylating agent that irreversibly inhibits DNA methyltransferase I (DNMT-1) leading to genome-wide global DNA hypomethylation. While AZA incorporates primarily into RNA and to the lesser extent DNA, decitabine is more selective, reducing only DNA methylation. In addition, decitabine is approximately 5-fold more potent inhibitor of DNA methyltransferase I than AZA. Decitabine induces leukemic differentiation and re-expression of tumor-associated genes that had been epigenetically silenced.(19) At high doses, cells die from apoptosis triggered by DNA synthesis arrest, and at low doses, cells survive but change their gene expression profile to favor differentiation, reduced proliferation and increased apoptosis. In addition, maximum effects of DNA hypomethylation have been observed at low doses, and with less side-effects.(20, 21) Decitabine has demonstrated activity in a variety of hematological malignances including AML, MDS and blast phase CML.(22–30) It is generally well tolerated with the primary toxicity being prolonged myelosuppression. Our group has demonstrated that decitabine enhances FOXP3 expression and can convert CD4+CD25−FOXP3− T cells into CD4+CD25+FOXP3+ T regulatory cells (Tregs).(31, 32) Through their immunoregulatory properties Tregs are thought to play an important role in modulating graft-versus-host disease (GVHD) without sacrificing the beneficial GVL effect.(33, 34) In addition, several other groups have demonstrated effects of DNA hypomethylating agents on T cell-mediated anti-tumor activity.(35–39) These include increasing tumor-specific CD8 T cell responses by up-regulating tumor antigen expression on malignant cells and inducing expression of killer-cell immunoglobulin-like receptor in T cells, thereby enhancing cytotoxic effector function of T cells against tumors. In human studies, patients noted to have a higher relative frequency of Tregs after HSCT had lower rate and severity of GVHD, lower rate of non-relapse mortality, and equivalent relapse mortality.(34)
Taken together, these studies provide a rationale for the administration of decitabine after alloHSCT for AML and MDS. We hypothesize that decitabine maintenance may provide direct antileukemic effect both to eradicate minimal residual disease and provide disease control by facilitating a GVL effect. In addition, decitabine may decrease the incidence of GVHD by enhancing the effect of Treg lymphocytes.
Patients and Methods
This was a single-institution, open-label, prospective, dose finding study of low-dose decitabine as a maintenance therapy after alloHSCT. The trial was approved by the Institutional Review Board at the Washington University School of Medicine and informed consent was obtained in accordance with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov (NCT00986804). Eisai Inc. provided decitabine for all enrolled patients.
Eligibility Criteria and Enrollment
Adults 18 years of age or greater, with AML or MDS, who achieved a complete remission (CR) after alloHSCT were enrolled in the study between day +50 and +100 after alloHSCT. Exceptions were made for 3 patients who were enrolled shortly after day +100: for 2 patients day +100 fell during the weekend/holiday and one patient, who was already consented, lived far away and had transportation problems. CR was defined as: <5% blasts in the bone marrow with a count of at least 200 nucleated cells, no blasts with Auer rods, no extramedullary disease, absolute neutrophil count (ANC) ≥1,500/µL, platelet count ≥50,000/µL and no blasts in the peripheral blood. The higher threshold for ANC, than that recommended by IWG, was chosen in anticipation of neutropenia secondary to decitabine and lower platelet threshold was allowed to facilitate enrollment. No platelet transfusion within 7 days of enrollment or growth factor support was allowed to meet those criteria. Other major eligibility criteria included Eastern Cooperative Oncology Group performance status ≤2, no grade 3–4 acute GVHD, no uncontrolled infection, creatinine <1.5 × upper limit of normal (ULN), bilirubin ≤1.5 × ULN, and hepatic enzymes ≤2.5 × ULN. Both myeloablative and non-myeloablative conditioning regimens were allowed, and both related and unrelated donors were permitted using either peripheral blood or bone marrow as a source of graft. Donors could be mismatched at a single antigen at HLA-A, -B or -DR locus, plus a single antigen mismatch at HLA-C; two-antigen mismatch at a single locus was not allowed. Acute GVHD prophylaxis was according to the treating physician.
Treatment Plan
Decitabine was administered as an intravenous infusion for 5 consecutive days every 6 weeks, for up to 8 cycles. The study consisted of 5 escalating dose-levels only one of which was open for accrual at a given time. The first cohort of 4 patients started decitabine at 5 mg/m2/day. In subsequent cohorts the dose was escalated to 7.5, 10 and 15 mg/m2/day according to the dose limiting toxicity (DLT) experienced at the previous dose level. Decitabine dose could be deescalated to 2.5 mg/m2/day if DLT was observed in the first cohort. There was an observation period of 42 days between enrolling each subsequent cohort.
The primary goal was to determine the maximum tolerated dose (MTD) of decitabine, defined as the maximum dose at which ≤25% of patients experience DLT during the first cycle of treatment. DLT was defined as: (1) ANC <500/µL and/or platelet count <30,000/µL sustained for two consecutive weeks without platelet transfusions, (2) inability to achieve ANC ≥1000/µL and platelet count ≥50,000/µl after a delay of the second cycle by a maximum 2 weeks, and (3) grade 3–4 non-hematological toxicities related to decitabine. Patients who met criteria for DLT started second cycle at one level dose reduction. Patients with inadequate counts 6 weeks after a previous cycle had their next cycle delayed by maximum of 2 weeks. Each cohort could contain 4 or 8 evaluable patients until MTD or a dose of 15 mg/m2/day were reached. Patients with documented progressive disease were removed from the study.
Dose adjustments:
0/4 DLT observed: current dose is acceptable, proceed with dose escalation.
1/4 DLT observed: enroll additional 4 patients at the same dose-level. If there is 1/8 DLT observed then the current dose is acceptable and escalated; if there are ≥ 2/8 DLTs then the current dose is deemed over-toxicity and the previous dose is considered MTD.
≥2/4 DLT observed: current dose is deemed over-toxicity and the previous dose level is considered MTD.
Evaluation of Response
All patients completing the first cycle of decitabine were included in the safety and efficacy assessment. History, physical exam, complete blood count and complete metabolic panel were performed at baseline and every 2 weeks thereafter. Bone marrow biopsy was performed at baseline, at cycle 3 day 1 and cycle 8 day 42 (or at end of study for any reason). Acute and chronic GVHD evaluation was performed every 2 cycles or sooner if clinically indicated. Chronic GVHD was diagnosed and graded according to the NIH Criteria.(40) Determination of relapse was based on the peripheral blood, bone marrow biopsy, or evidence of new extramedullary disease. Patients are being followed for survival and relapse for 5 years.
Toxicity Assessment
All patients receiving at least one dose of decitabine were included in the toxicity assessments. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTC), Version 3.0. Serious Adverse Event (SAE) was defined as any toxicity that met any of the following conditions: resulted in death, was life-threatening, required hospitalization, resulted in significant disability or incapacity. Toxicity assessment was performed at the beginning of each cycle.
Correlative Studies
Peripheral blood samples to examine levels of lymphocyte populations were obtained at baseline, immediately prior to administration of decitabine on Cycle 1 Day 1 (C1D1) and Cycle 3 Day 1 (C3D1), immediately following administration of decitabine on Cycle 1 Day 5 (C1D5) and Cycle 3 Day 5 (C3D5), and at the end of study (EOS). Flow cytometry of peripheral blood was performed to examine lymphocyte populations including CD3+CD4+ T cells, CD3+CD8+ T cells, CD4+FOXP3+ Tregs, CD3-CD19+ B cells, and CD3-CD56+ NK cells. The following antibodies were used: CD3 PerCP-Cy5.5 (OKT3, eBioscience, San Diego, CA), CD4 PE (RPA-T4, BD Biosciences, Franklin Lakes, NJ), CD4 APC-eFluor780 (RPA-T4, eBiosciences), CD8 APC (RPA-T8, BD Biosciences), CD19 APC (HIB19, BD Biosciences), CD56 PE (B159, BD Biosciences), CD45 FITC (HI30, BD Biosciences), and FOXP3 PE (236A/E7, eBiosciences). To determine absolute numbers of these cells, Sphero Accucount Fluorescent Particles (Spherotech, Inc., Lake Forest, IL) were used. To measure DNA methylation in the FOXP3 locus, CD4+ T cells were isolated from the cohort treated with the highest dose of decitabine (15mg/m2/day) using a Sony SY3200 cell sorter (Sony Biotechnology, San Jose, CA) operated by the Siteman Cancer Center Flow Cytometry shared resource. The purities of the cells were 90% or higher. Direct bisulfite modification from cells and pyrosequencing analysis were performed by EpigenDx, Inc (Hopkinton, MA). A total of 16 CpGs were analyzed: 5 CpGs in 5’ UTR region (−6278 to −6216 from ATG, Ensembl Transcript ID ENST00000376207), 2 CpGs in the human Treg specific demethylated region (TSDR) (−2376 to −2371 from ATG), and 9 CpGs in the TSDR (−2330 to −2263 from ATG).(41–43)
Statistical Analysis
Demographic and clinical characteristics of patients, as well as outcomes and length of follow-up, were listed for each patient. Preliminary efficacy was also assessed from pooled data of all cohorts. The endpoints for efficacy included relapse of the hematological malignancy, incidence and severity of GVHD, as well as overall survival (OS) and disease-free survival (DFS). OS was defined as the time from transplantation to death due to any cause, and survivors were censored at the date of last contact. DFS was defined as time from transplantation to relapse or death, whichever occurred first. Those patients alive and relapse-free were censored at date of last contact. Probabilities of OS and DFS were estimated using the Kaplan-Meier product-limit method. The cumulative incidence of relapse was estimated using Gray’s sub-distribution method to account for the presence of competing risk due to non-relapse mortality (NRM).(44) Statistical analyses were performed using statistical packages cmprsk (http://biowww.dfci.harvard.edu/~gray) for competing risk analysis and SAS 9.2 (SAS Institutes, Cary, NC) for all other analyses.
Results
Patient Characteristics
Between May 2010 and April 2014, 24 patients were enrolled and treated on this study. Two patients failed to complete the first cycle of treatment due to early relapse; they were replaced and excluded from all analyses other than toxicity. The characteristics of 22 evaluable patients are shown in Table 1. There were 15 males. Median age was 59 years (range, 21–68). Seventeen patients had AML and 5 MDS. Twenty patients received myeloablative conditioning (2 TBI-based) and 2 patients non-myeloablative conditioning. Six patients received alloHSCT from 10/10 HLA-matched siblings, 1 from 8/10 mismatched sibling, 11 from 10/10 matched unrelated donors and 4 from mismatched unrelated donors. All patients received mobilized peripheral blood stem cells as the graft source, and methotrexate and tacrolimus as GVHD prophylaxis. All patients were in CR at the time of enrollment. The median time from alloHSCT to start of decitabine was 95 days (range 62–115).
Table 1.
Patents Characteristics
| Cohort (Dose-Level) |
Pt # |
Sex/ Age, y |
Diagnosis-status at alloHSCT |
Disease Risk | Donor type |
Conditioning Regimen |
Days from alloHSCT to Dec |
Chimerism at enrollment, % |
|---|---|---|---|---|---|---|---|---|
| 1 (5 mg/m2) |
1 | M/62 | AML-PD | Ph+ AML | mMUD | BU/CY | 95 | 100 |
| 2 | F/59 | AML-PD | Prior MF, trisomy 8, FLT3-ITD + | MRD | BU/CY | 62 | Mixed | |
| 3 | M/37 | AML-CR1 | Monosomy 7 | MUD | BU/CY | 75 | 100 | |
| 4 | M/62 | AML-PD | Prior MDS, trisomy 19 | MUD | TBI/CY | 95 | Mixed | |
| 5 | M/57 | MDS | Trisomy 8, 5q- | MUD | BU/CY | 76 | 100 | |
| 6 | M/59 | AML-CR2 | t (8,21), CR2 | MUD | BU/CY | 77 | 100 | |
| 7 | M/42 | AML-CR1 | Del 12/ETV6 | mMUD | BU/CY | 115 | 100 | |
| 8 | M/52 | MDS | Del 5, Del 6 | MUD | BU/CY | 90 | 100 | |
| 9 | M/58 | AML-CR1 | Complex cytogenetics | MRD | BU/CY | 98 | 100 | |
| 2 (7.5 mg/m2) |
10 | F/68 | AML-CR1 | Complex cytogenetics | MRD | FLU/BU | 96 | 100 |
| 11 | F/65 | AML-CR1 | FLT 3-ITD + | MUD | TBI/CY | 96 | 100 | |
| 12 | F/45 | AML-CR1 | Therapy related AML | mMRD | BU/CY | 98 | 100 | |
| 13 | M/63 | AML-CR1 | Prior MDS | mMUD | BU/CY | 103 | 100 | |
| 3 (10 mg/m2) |
14 | M/21 | AML-CR1 | FLT3-ITD + | MUD | BU/CY | 97 | 100 |
| 15 | F/56 | AML-CR1 | Complex cytogenetics | MUD | BU/CY | 87 | 100 | |
| 16 | M/62 | MDS | High-grade MDS | MUD | BU/CY | 95 | 100 | |
| 17 | M/63 | AML-CR1 | Prior MDS | MRD | BU/CY | 102 | 100 | |
| 4 (15 mg/m2) |
18 | F/54 | AML-CR2 | CR2 | MRD | BU/CY | 95 | 100 |
| 19 | F/51 | AML-CR1 | MLL rearrangement | MUD | BU/CY | 82 | 100 | |
| 20 | M/57 | MDS | Del 7 | mMUD | BU/CY | 86 | 100 | |
| 21 | M/67 | AML-CR1 | Del 17p (p53) | MUD | FLU/BU | 74 | 100 | |
| 22 | M/66 | MDS | High-grade MDS | MRD | BU/CY | 100 | 100 |
AlloHSCT indicates allogeneic hematopoietic stem cell transplantation; AML, acute myelogenous leukemia; BU, busulfan; CR, complete remission; CY, cyclophosphamide; Dec, decitabine; Del, deletion; Flu, fludarabine; GVHD, graft-versus-host disease; MDS, melodysplastic syndrome; MF, myelofibrosis; mMRD, mismatched related donor; mMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; PD, persistent disease; Ph, Philadelphia chromosome; Pt, patient; TBI, total body irradiation.
Dosing of Decitabine
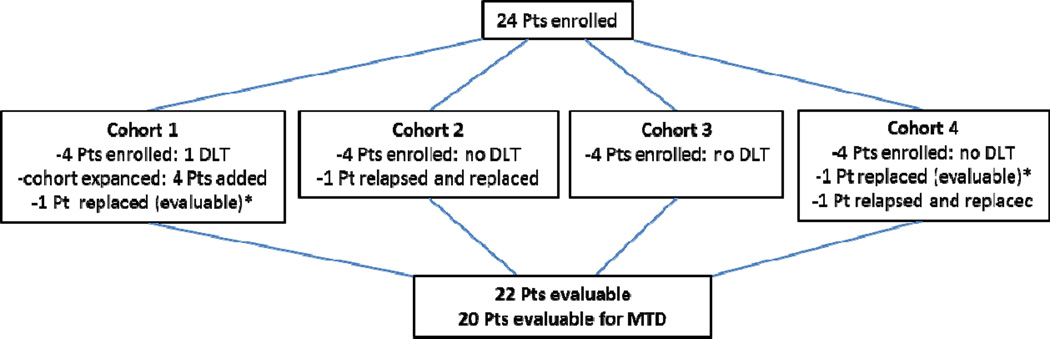
Four patients were initially enrolled in cohort 1 (5 mg/m2/day), Figure 1. One patient experienced a hematological DLT and therefore 4 additional patients were added to expand that cohort. None of the additional patients experienced a DLT. One patient was replaced as he failed to receive all cycle 1 doses due to non-compliance, however, this patient received subsequent cycles and remains evaluable for all assessments besides MTD. Four patients were enrolled in cohort 2 (7.5 mg/m2/day). One patient relapsed before completing cycle 1 and was replaced. No DLTs were observed in that cohort, therefore 4 patients were enrolled in cohort 3 (10 mg/m2/day). None of those patients experienced a DLT and 4 patients were enrolled in cohort 4 (15 mg/m2/day). There were no DLTs at this dose-level, however 2 patients were replaced. One patient was replaced do to the early relapse, before completing cycle 1. The other replaced patient was deemed non-evaluable for DLT because he missed several laboratory data points and received growth-factors for neutropenia during the first cycle; his decitabine dose was reduced to 10 mg/m2/day and he completed the remaining cycles at that dose. In summary, as only 1 patient met the criteria for DLT, MTD was not established.
Figure 1.
Cohorts characteristics
*Evaluable for all the assessments except MTD
Abbreviations: DLT-dose limmiting toxicity; MTD- maximal tolerated dose; Pts- patients
Response and Mortality
The median number of cycles completed was 5 (range 1–8). The causes for early discontinuation were: toxicity (n=5), relapse (n=3), new-onset severe gut GVHD (n=1), physician discretion (n =1), non-compliance (n=2) and consent withdrawal (n=1), Table 2. Nine of 22 patients (41%) completed 8 cycles of decitabine and all of them are alive: 8 patients are in CR and 1 patient developed central nervous system (CNS) relapse and leukemia cutis one year after completing decitabine maintenance and underwent 2nd alloHSCT. The remaining 13 patients discontinued decitabine before cycle 8 and only 4 are alive: 3 patients are in CR and 1 patient developed CNS relapse and underwent 2nd alloHSCT. Overall, 11 of 22 evaluable patients are alive in CR with full donor chimerism (50%) and additional 2 patients are alive after 2nd alloHSCT for CNS/extramedulary relapse.
Table 2.
Summary of Outcomes
| Cohort (Dose-Level) |
Pt # |
No. of cycles |
DLT | Reason for Discontinuation |
Relapse | DLI | PFS (mo) |
OS (mo) |
Acute GVHD |
Chronic GVHD, grade |
Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (5 mg/m2) |
1 | 4 | No | Relapse | After 4 cycles | No | 5.9 | 28.6 | No | Yes, severe | Relapse |
| 2 | 2 | Yes | Relapse | After 2 cycles | 1 mo after last dose | 2.6 | 4.4 | No | N/A | Relapse | |
| 3 | 6 | No | Non-Compliance | No | No | 23.0 | 23.0 | Yes | Yes, severe | Infection | |
| 4 | 1 | No | Relapse | After 1 cycle | 3 mo after last dose | 1.5 | 6.8 | No | N/A | Relapse | |
| 5 | 8 | No | Completed | No | No | 49.1+ | 49.1+ | No | No | Alive | |
| 6 | 8 | No | Completed | No | No | 48.8+ | 48.8+ | No | No | Alive | |
| 7 | 2 | N/A | Non-Compliance | 1 yr after 2nd cycle | 19 mo after last dose | 12.3 | 24.7 | No | Yes, severe | Relapse | |
| 8 | 8 | No | Completed | No | No | 47.7+ | 47.7+ | No | Yes, moderate | Alive | |
| 9 | 1 | No | Patient withdrew | No | No | 44.9+ | 44.9+ | No | Yes, severe | Alive | |
| 2 (7.5 mg/m2) |
10 | 3 | No | Toxicity: Infection | No | No | 7.7 | 7.7 | No | No | Infection |
| 11 | 8 | No | Completed | 1 yr after 8th cycle | No, 2nd AlloHSCT | 23.1+ | 40.4+ | Yes | No | Alive | |
| 12 | 2 | No | PI Decision | No | No | 35.2+ | 35.2+ | No | No | Alive | |
| 13 | 8 | No | Completed | No | No | 35.5+ | 35.5+ | No | Yes, severe | Alive | |
| 3 (10 mg/m2) |
14 | 8 | No | Completed | No | No | 34.1+ | 34.1+ | Yes | Yes, moderate | Alive |
| 15 | 8 | No | Completed | No | No | 33.9+ | 33.9+ | Yes | Yes, severe | Alive | |
| 16 | 8 | No | Completed | No | No | 31.6+ | 31.6+ | Yes | Yes, moderate | Alive | |
| 17 | 2 | No | Toxicity: Ileus, Infection | No | No | 18.8 | 18.8 | Yes | Yes, severe | Lung GVHD | |
| 4 (15 mg/m2) |
18 | 2 | No | Toxicity: Infection | No | No | 4.7 | 4.7 | Yes | No | Infection |
| 19 | 4 | No | Toxicity: Neutropenia | 1 yr after 4th cycle | No, 2nd AlloHSCT | 15.2+ | 21.2+ | No | Yes, moderate | Alive | |
| 20 | 6 | No | Toxicity: Fatigue | No | No | 19.7+ | 19.7+ | No | Yes, moderate | Alive | |
| 21 | 8 | N/A | Completed | No | No | 13.1+ | 13.1+ | Yes | No | Alive | |
| 22 | 1 | No | Acute gut GVHD | No | No | 3.4 | 3.4 | Yes | N/A | Acute gut GVHD |
AlloHSCT indicates allogeneic hematopoietic stem cell transplantation; DLT, dose limiting toxicity; GVHD, graft-versus-host disease; PFS, progression free survival; OS, overall survival; PI, principal investigator; Pt, patient.
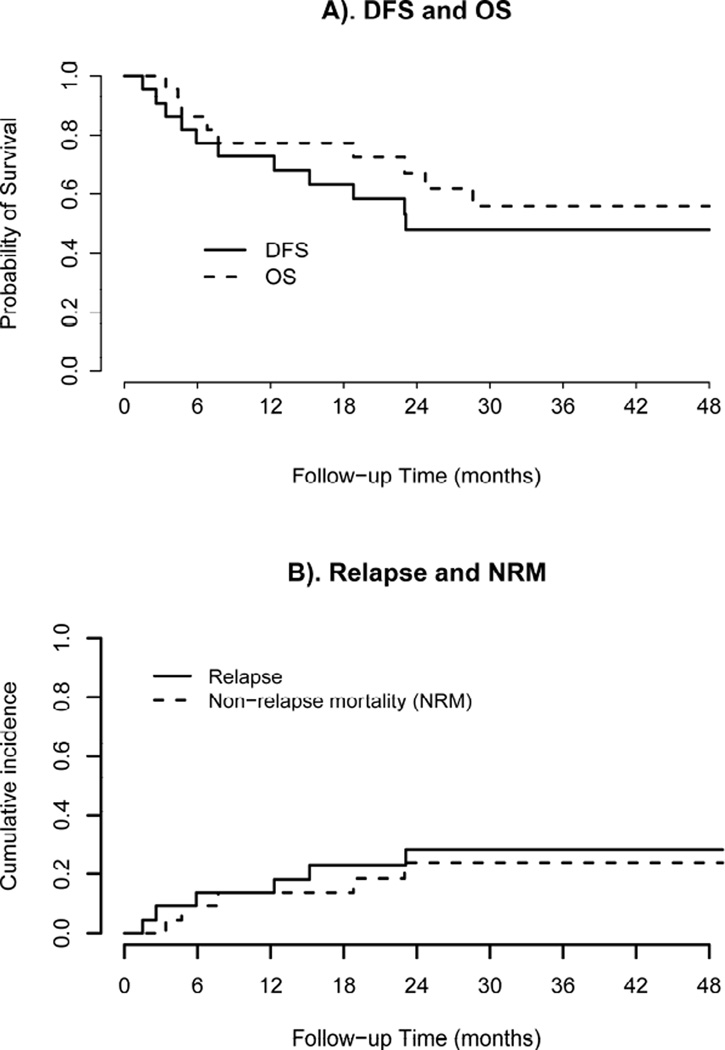
After a median follow up of 26.7 months (range 3.4–49.1) 6 patients have relapsed and 9 have died. All of the 6 patients that relapsed had high-risk disease (3 were transplanted with persistent disease, 1 had MLL rearrangement, 1 had FLT3 mutation, and one had ETV6 gene amplification with persistent dysplasia after induction chemotherapy). Only one of the relapsed patients had evidence of mild acute GVHD. The 2-year OS and DFS were 56% (95% CI 38–83%) and 48% (95% CI 30–75%), respectively, Figure 2. After adjusting for competing risk of non-relapse mortality, the 2-year cumulative incidence of relapse was 28% (95% CI 8–48%). Causes of death included: relapse (n=4), infectious complications (n=3) and GVHD (n=2; one from acute gut GVHD, one from chronic lung GVHD). Among 6 patients who relapsed: 2 patients are alive after second alloHSCT and 4 died from their disease. Only one of the relapsed patients completed all 8 cycles of decitabine, others received 1–4 cycles. Pre-transplant conditioning chemotherapy did not appear to impact the results.
Figure 2.
Acute and Chronic GVHD
Five patients had resolving grade I-II classic acute GVHD at the time of starting on the study, and all of those completely resolved while on decitabine, with taper of steroids. One patient developed stage I gut GVHD during the second cycle of decitabine that completely resolved after a brief course of high-dose steroids with rapid taper, and another patient developed stage IV gut GVHD coinciding with the first cycle of decitabine, requiring high dose steroids, that also completely resolved while on decitabine. One patient developed stage IV gut GVHD immediately after the first cycle, was taken off the study and eventually died from progressive acute gut GVHD. One patient had late-onset acute GI GVHD that resolved.
Among 9 patients who completed all cycles of decitabine, 5 have developed chronic GVHD: 2 have severe and 3 patients have moderate chronic GVHD, Table 3. Among 13 patients that discontinued decitabine earlier, 10 are at risk for chronic GVHD (2 patients died of early relapse and 1 patient died from acute gut GVHD). Seven of those 10 patients have developed chronic GVHD: 5 have severe chronic GVHD (1 with features of overlap syndrome) and 2 have moderate.
Table 3.
GVHD Rates
| GVHD type | No. of Pts with GVHD/ No. of Pts at risk |
|---|---|
| Classic acute GVHD | |
| Grade I-II | 6/22 |
| Grade III-IV GI | 2/22 |
| Late-onset acute GVHD | 1/22 |
| Chronic GVHD | |
| Moderate | |
| Pt completed 8 cycles | 3/9 |
| Pt completed < 8 cycles | 2/10 |
| Severe | |
| Pt completed 8 cycles | 2/9 |
| Pt completed < 8 cycles | 5/10* |
One of these 5 patients had overlap features of GVHD
GVHD indicates graft-versus-host disease; GI, gastrointestinal; Pt, patient.
Toxicity Assessment
All 24 patients enrolled on the study were assessed for toxicity. Generally, study treatments were well tolerated, Table 4. Grade 3/4 hematological toxicities were experienced by 18 of 24 patients (75%), including all 6 patients treated at the 15 mg/m2/day dose level. However, typically hematological toxicities resolved quickly between treatment cycles and accounted for very few treatment delays. The only frequently occurring grade 3/4 non-hematological toxicities were infections which occurred in 8 of 24 patients (33%). Five patients discontinued study due to the toxicities: two of them for infection, one for neutropenia, one for bowel obstruction and one for fatigue. Among 3 patients who discontinued decitabine because of infections, only one was neutropenic when infection developed.
Table 4.
Grade 3–4 Adverse Events
| Event | Grade 3–4 |
|---|---|
| Anemia | 3 |
| Lymphopenia | 2 |
| Neutropenia | 11 |
| Thrombocytopenia | 13 |
| Hypotension | 1 |
| Pain, not specified | 2 |
| Diarrhea | 1 |
| SGOT (AST) | 1 |
| SGPT (ALT) | 2 |
| Infection/Sepsis | 8 |
| Hypokalemia | 1 |
| Hyponatremia | 1 |
| Neuropathy | 2 |
| Dyspnea | 1 |
| Renal Failure | 1 |
Correlative Studies
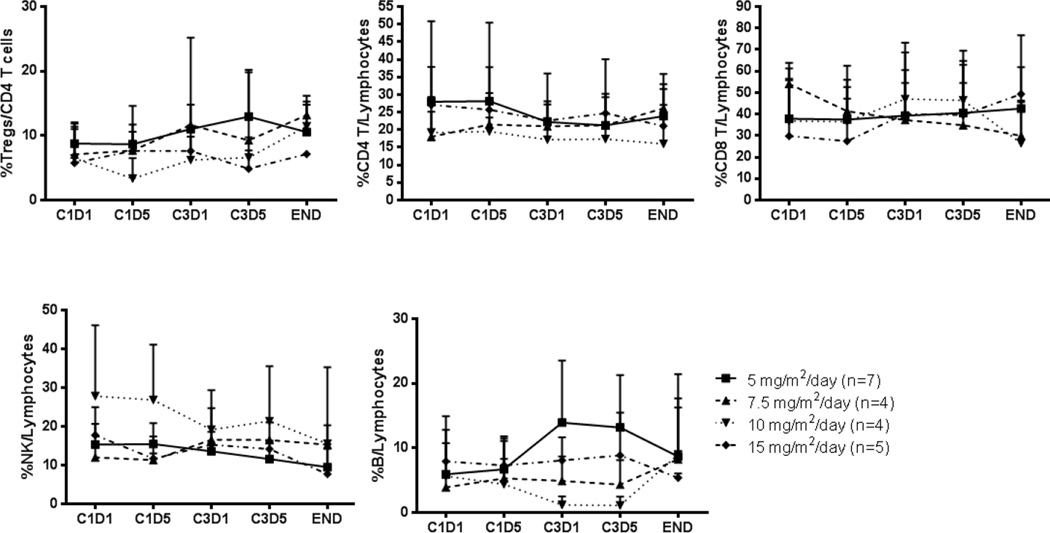
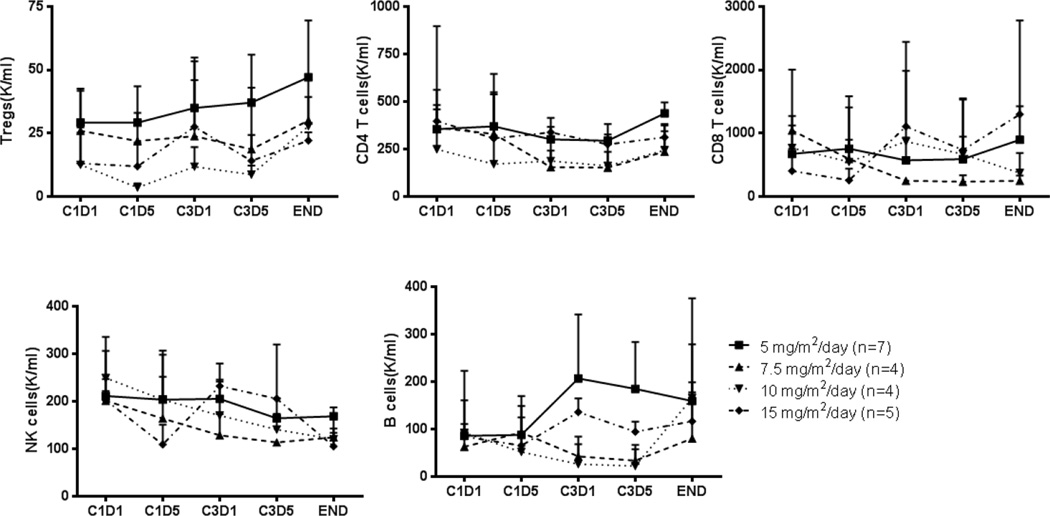
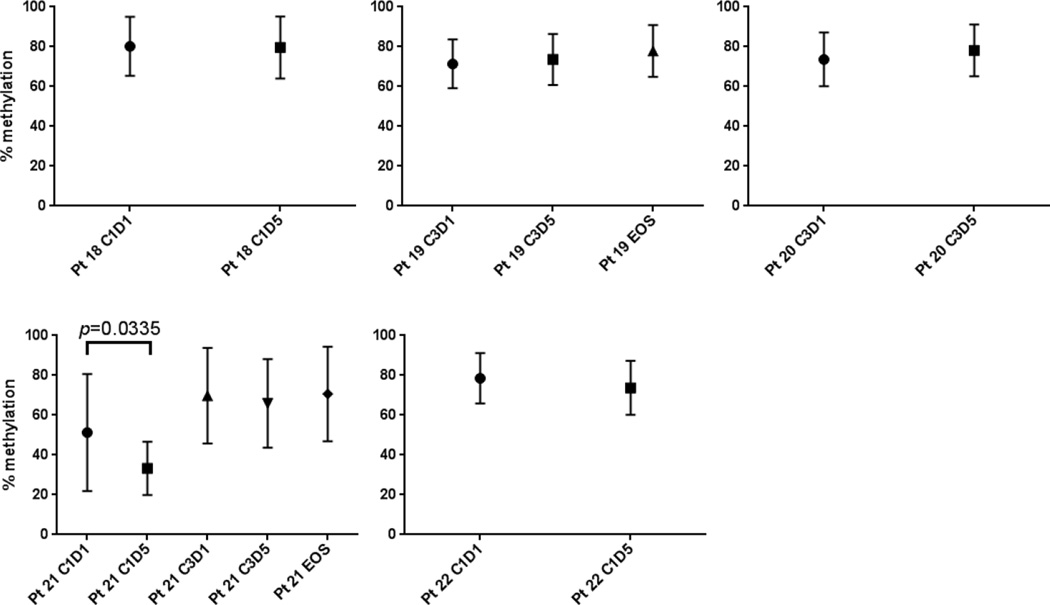
We examined the effect of decitabine on lymphocyte subpopulations, such as T cells, B cells, NK cells, and Tregs, in all the patient samples, using flow cytometry (Figures 3 and 4). Although we observed a trend toward increase of Tregs after decitabine treatment, the difference was not statistically significant. In addition, a total of 16 CpG sites of the FOXP3 locus in CD4+ T cells isolated from the cohort treated with the highest dose of decitabine (15mg/m2/day) were analyzed, as described in Methods. The gene structure of the FOXP3 locus is presented in Supplemental Figure 1. As shown in Figure 5 we found that there is no statistically significant difference in the degree of DNA methylation in the FOXP3 locus before (C1D1 and C3D1) and after (C1D5 and C3D5) treatment and end-of-study (EOS) even though there was a trend toward decrease in DNA methylation in Cycle 1. C1D5 sample of patient S101 shows decrease in DNA methylation when compared with C1D1 sample of the same patient. Accordingly, its FOXP3 expression is higher than that of C1D1 sample (15.7% vs 11.8% FOXP3+ Tregs among CD4+ T cells). All of these data indicate that our DNA methylation data is consistent with the FOXP3 protein expression data. Interestingly, we found that NK cells and CD8 T cells are more sensitive to decitabine treatment in vivo with a consistent quantitative decrease in these subsets after treatment while CD4 T cells were relatively resistant to decitabine treatment (Figures 3 and 4). The CD4 T cell number at EOS in the 5 mg/m2/day cohort is higher than at C3D1. The explanation for this observation is unknown and may simply represent a more rapid recovery of CD4 T cells compared to other T and NK cells after treatment.
Figure 3.
Effect of decitabine on the frequency of Tregs, CD4 and CD8 T cells, B cells and NK cells in the peripheral blood at stated time points (C1D1: cycle 1 day 1; C1D5: cycle 1 day 5; C3D1: cycle 3 day 1; C3D5: cycle 3 day 5; END: end of study)
Figure 4.
Effect of decitabine on the absolute number of Tregs, CD4 and CD8 T cells, B cells and NK cells in the peripheral blood and stated time points (C1D1: cycle 1 day 1; C1D5: cycle 1 day 5; C3D1: cycle 3 day 1; C3D5: cycle 3 day 5; END: end of study)
Figure 5.
DNA methylation status at the FOXP3 locus of cohort 4 (15 mg/m2/days)
Legend: Shown are means and standard deviations of DNA methylation status of 16 CpGs of each patient in cohort 4 (patients 18–22). Pt indicates patient; C, cycle; D, day.
Discussion
Disease recurrence after alloHSCT is the major cause of treatment failure for patients with AML and MDS. Treatment options for those patients are limited, responses poor and prognosis dismal. Since most of relapses occur in the first year after alloHSCT, preventive maintenance therapy should be considered early in the post-transplant course. De Lima et al. first described that AZA maintenance after reduced-intensity alloHSCT for high-risk AML and MDS is well tolerated and may prolong event-free and OS.(13)
In this study we examined the toxicity and responses to low-dose decitabine as a maintenance therapy after alloHSCT for patients with AML and MDS. To our knowledge, this is the first report of decitabine use in this setting. We have demonstrated that decitabine can be given safely in the out-patient setting to this group of post-alloHSCT patients. Approximately 67% of our patients were able to receive at least 4 cycles of decitabine and 41% received all 8 cycles. Main dose-limiting toxicity associated with decitabine use is myelosuppression.(45) The interval between cycles of 6 weeks rather than 4 weeks, as commonly used for treatment of AML and MDS, was chosen to facilitate count recovery. Several patients experienced neutropenia and one of these qualified as DLT. Interestingly, that patient relapsed a month later and it is possible that the neutropenia in this patient was due to the early relapse rather than a direct effect of decitabine. All patients treated at the highest dose-level (15 mg/m2/day) experienced grade 3/4 hematological toxicities, none qualifying for DLT. However, one patient from the last cohort was discontinued after 4 cycles due to neutropenia and the other required growth factor support. Therefore, although no formal MTD was reached, 10 mg/m2/day may be a more optimal and better tolerated dose for decitabine maintenance in post-alloHSCT setting.
Three patients were discontinued from the study due to the relapse. All of them had high-risk disease and 2, in retrospect, already had early signs of relapse at the time of enrollment (manifested as mixed chimerism and evidence of multilineage dysplasia). Three additional patients, also with high risk disease, relapsed later, after being off decitabine. Interestingly, only one patient who completed all 8 cycles of decitabine relapsed. Longer administration of decitabine was associated with prolonged OS and DFS.
Although decitabine could have been started between day +50 and +100 after transplantation, majority of patients started close to day +100. Study required bone marrow biopsy within 2 weeks of starting decitabine. In some instances treating physicians were waiting for the “standard-of-care” 3-month post-alloHSCT biopsy, rather than obtaining “study” biopsy earlier. Many of those patients had blood counts adequate to start decitabine closer to day +50 and, in retrospect, should have been biopsied and, if eligible, enrolled.
Since most of our patients started decitabine around day +100 after alloHSCT it is difficult to determine the effect of decitabine on incidence of acute GVHD. Several patients had grade I-II acute GVHD at the time of starting decitabine which completely resolved and one patient had biopsy proven grade IV gut GVHD that started during the first week of decitabine therapy and completely resolved. However, one additional patient died from grade IV gut GVHD developing after the first cycle of decitabine. Decitabine maintenance did not clearly impact the rate of chronic GVHD, however, lack of comparison in this early phase study precludes any further conclusion. Although the severity of chronic GVHD appears to be lower in the group that completed all 8 cycles of decitabine, this is merely the observation; there were too few patients to suggest correlation between decitabine and chronic GVHD severity. Earlier post-transplant initiation of decitabine might have greater impact on the incidence of GVHD. This is another reason to support, for future studies, the use of lower decitabine dose of 10 mg/m2 that should be better tolerated and likely result in less hematological toxicities and infectious complications.
Goodyear et al. used AZA maintenance after alemtuzumab-containing reduced-intensity alloHSCT confirming the tolerability of AZA in post-transplant setting.(35) They observed increased number of Tregs within first 3 months after transplantation and relatively low incidence of acute and chronic GVHD. However, important differences between their study and ours are that they started AZA earlier in post-transplant course and their patients all received alemtuzumab-containing reduced-intensity conditioning. Although we observed a trend of increased FOXP3 expression, results were not statistically significant. There are several possible explanations why we have not observed an increase in FOXP3 expression. First, we only examined peripheral blood. Considering that most alloreactive T cells traffic to GVHD-target organs, it might be possible that those alloreactive T cells converted into FOXP3 expressing T cells might be differentially localized in the GVHD-target organs instead of the peripheral blood. Thus, it might have been more informative to examine Tregs and T cell subsets from GVHD target organs (skin, liver and GI tract) and not the peripheral blood. Second, decitabine needs to incorporate into replicating DNA to block DNMT-1. Therefore, unless alloreactive T cells are actively proliferating, the degree of incorporation of decitabine into DNA would be limited, thereby minimizing conversion of alloreactive T cells into suppressive FOXP3 expressing Tregs. Since decitabine maintenance was not performed immediately after T cell infusion (stem cell transplantation) and was performed in the context of standard GVHD prophylaxis (calcineurin inhibitors) that also limit T cell proliferation and viability, it is possible that the optimal effect of decitabine on GVHD and FOXP3 expression was not realized in this study. Finally, it is also possible that the doses of decitabine tested in this study are not optimal to convert alloreactive T cells in vivo into Tregs although they might be optimal for reduction of GVHD and maintenance of GVL. Decitabine does appear to reduce the numbers of NK cells and CD8 T cells while maintaining a relatively low rate of leukemia relapse suggesting possible direct anti-leukemia effect of decitabine in vivo and increase of tumor-specific CD8 T cells responses.
In conclusion, low-dose decitabine maintenance is associated with acceptable toxicities when given in post-alloHSCT setting. Although MTD was not reached the dose of 10 mg/m2 for 5 days every 6 weeks appears to be the optimal dose rather than 15 mg/m2. Starting decitabine closer to the stem cell and T cell infusion, when T cells are rapidly expanding in allogeneic stem cell transplant recipients, could potentially be more efficacious in limiting GVHD. This study provides essential data for subsequent studies of decitabine maintenance. The availability of oral hypomethylating agents might make this approach of post-transplant maintenance even more attractive.
Supplementary Material
Highlights.
Decitabine maintenance after allogeneic transplantation is associated with acceptable toxicities.
Although the Maximum Tolerated Dose was not reached, the dose of 10 mg/m2 for 5 days every 6 weeks appears to be a more optimal and better tolerated dose than 15 mg/m2.
Decitabine maintenance didn’t clearly impact the rate of chronic GVHD.
Acknowledgments
· This publication was supported by the following grants: Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 (PI: Evanoff) from the National Center for Advancing Translational Sciences; NIH/NCI P01 CA101937 (PI: J. DiPersio); NIH P50 Mol Imaging Ctr Grant P50 CA094056 (PI: Achilefu); NIH P50 CA171963-01 (PI: Link). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
· The Siteman Flow Cytometry Core provided cell sorting operation. The Siteman Cancer Center is supported in part by a NCI Cancer Center Support Grant #P30 CA91842
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None
REFERENCES
- 1.Solomon SR, Sizemore CA, Sanacore M, et al. Total Body Irradiation-Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Malard F, Milpied N, Blaise D, et al. Effect of Graft Source on Unrelated Donor Hemopoietic Stem Cell Transplantation in Adults with Acute Myeloid Leukemia after Reduced-Intensity or Nonmyeloablative Conditioning: A Study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Chitphakdithai P, Logan B, et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant. 2015;21:142–150. doi: 10.1016/j.bbmt.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: A review of ten years experience with new transplant concepts and new therapeutic agents. Leukemia. 2006;20:1661–1672. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 5.Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2015;21:151–158. doi: 10.1016/j.bbmt.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oran B, Giralt S, Couriel D, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia. 2007;21:2540–2544. doi: 10.1038/sj.leu.2404828. [DOI] [PubMed] [Google Scholar]
- 7.Bishop MR, Alyea EP, 3rd, Cairo MS, et al. National Cancer Institute’s First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2011;17:443–454. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant. 2014;20:4–13. doi: 10.1016/j.bbmt.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454–459. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter DL, Collins RH, Jr, Hardy C, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214–1221. [PubMed] [Google Scholar]
- 11.Mielcarek M, Storer BE, Flowers MED, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2007;13:1160–1168. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. 2007;40:1027–1032. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 13.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hourigan CS, McCarthy P, de Lima M. Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:154–163. doi: 10.1016/j.bbmt.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant. 2015;21:653–660. doi: 10.1016/j.bbmt.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27:1229–1235. doi: 10.1038/leu.2013.7. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder T, Frobel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. 2013;27:1910–1913. doi: 10.1038/leu.2013.64. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 21.Strathdee G, Brown R. Epigenetic cancer therapies: DNA methyltransferase inhibitors. Expert Opin Investig Drugs. 2002;11:747–754. doi: 10.1517/13543784.11.6.747. [DOI] [PubMed] [Google Scholar]
- 22.Sacchi S, Kantarjian HM, O’Brien S, et al. Chronic myelogenous leukemia in nonlymphoid blastic phase: analysis of the results of first salvage therapy with three different treatment approaches for 162 patients. Cancer. 1999;86:2632–2641. doi: 10.1002/(sici)1097-0142(19991215)86:12<2632::aid-cncr7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2007;109:1133–1137. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 26.Lubbert M, Wijermans P, Kunzmann R, et al. Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2’-deoxycytidine. Br J Haematol. 2001;114:349–357. doi: 10.1046/j.1365-2141.2001.02933.x. [DOI] [PubMed] [Google Scholar]
- 27.Wijermans P, Lubbert M, Verhoef G, et al. Low-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18:956–962. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 28.Wijermans PW, Lubbert M, Verhoef G, Klimek V, Bosly A. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2’-deoxycytidine (decitabine) in 177 patients. Ann Hematol. 2005;84(Suppl 1):9–17. doi: 10.1007/s00277-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 29.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi J, Ritchey J, DiPersio J. Generation of Treg-Like Cells from CD4+CD25- T Cells Via Epigenetic Modification Using a Demethylating Agent Decitabine. ASH Annual Meeting Abstracts. 2007;110:62. [Google Scholar]
- 32.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Ziga ED, Ritchey J, et al. IFNgammaR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120:4093–4103. doi: 10.1182/blood-2012-01-403196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magenau JM, Qin X, Tawara I, et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant. 2010;16:907–914. doi: 10.1016/j.bbmt.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119:3361–3369. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 36.Coral S, Sigalotti L, Gasparollo A, et al. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2’-deoxycytidine (5-AZA-CdR) J Immunother. 1999;22:16–24. doi: 10.1097/00002371-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Hambach L, Ling KW, Pool J, et al. Hypomethylating drugs convert HA-1-negative solid tumors into targets for stem cell-based immunotherapy. Blood. 2009;113:2715–2722. doi: 10.1182/blood-2008-05-158956. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Kuick R, Hanash S, Richardson B. DNA methylation inhibition increases T cell KIR expression through effects on both promoter methylation and transcription factors. Clin Immunol. 2009;130:213–224. doi: 10.1016/j.clim.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stubig T, Badbaran A, Luetkens T, et al. 5-azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity. Mediators of inflammation. 2014;2014:418292. doi: 10.1155/2014/418292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YC, Bhairavabhotla R, Yoon J, et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–2818. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:330–338. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 45.Momparler RL, Rivard GE, Gyger M. Clinical trial on 5-aza-2’-deoxycytidine in patients with acute leukemia. Pharmacology & therapeutics. 1985;30:277–286. doi: 10.1016/0163-7258(85)90052-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.