Abstract
Purpose
Diffusion of accelerated partial breast irradiation (APBI) into clinical practice is limited by the need for specialized equipment and training. The accessible external beam technique yields unacceptable complication rates, likely due to large post-operative target volumes. We designed a phase I trial evaluating preoperative radiotherapy to the intact tumor utilizing widely available technology.
Methods
Patients received 15, 18, or 21Gy in a single fraction to the breast tumor plus margin. Magnetic resonance imaging (MRI) was used in conjunction with standard computed tomography (CT)-based planning to identify contrast enhancing tumor. Skin markers and an intra-tumor biopsy marker were utilized for verification during treatment.
Results
MRI imaging was critical for target delineation as not all breast tumors were reliably identified on CT scan. Breast shape differences were consistently seen between CT and MRI but did not impede image registration or tumor identification. Target volumes were markedly smaller than historical post-operative volumes and normal tissue constraints were easily met. A biopsy marker within the breast proved sufficient for set up localization.
Conclusions
This single fraction linear-accelerator based ABPI approach can be easily incorporated at most treatment centers. In vivo targeting may improve accuracy and can reduce the dose to normal tissues.
Keywords: radiotherapy, breast cancer, partial breast, preoperative
Introduction
Breast conservation, lumpectomy followed by adjuvant radiotherapy, has been standard of care for treatment of breast cancer since the early 1990’s 1, 2. In the twenty years since, our understanding of the complexity and variability of breast tumor biology has evolved significantly 3. In response, there has been a paradigm shift in utilization of systemic therapy, most notably with development and widespread adoption of the Oncotype DX test for early stage, ER positive tumors4. In contrast, change in breast radiotherapy practice in the US has been limited, with the most standard regimen still approximately 5–6 weeks of whole breast treatment 5.
The most mature research efforts to date focus on reducing duration of radiation treatment. 10 year data from multiple randomized clinical trials now support non-inferiority of a 3 week whole breast radiation course compared to the standard 5 week course 6, 7. Ongoing efforts to further improve convenience and reduce toxicity focus on rapid treatment of the tumor bed plus a small margin of normal tissue, over 1 to 5 days. Accelerated Partial Breast Irradiation (APBI) is the subject of multiple ongoing or recently completed randomized trials 8–10. Early data from these, and prior phase I/II studies, show comparable efficacy to whole breast radiation in low-risk, early-stage patients.
Intracavitary and external beam radiotherapy, the two most common APBI approaches, are widely available and relatively easily applied. However, treatment is still intensive, requiring 10 treatments delivered 6 hours apart over five days. Furthermore, data are accumulating that toxicity outcomes appear to be driven by technique. In particular, soft tissue fibrosis and cosmetic outcomes may be suboptimal with external beam techniques, possibly related to large volumes of tissue treated to high doses in the post-operative setting 11, 12.
Single fraction intra-operative APBI allows women to complete radiotherapy while under anesthesia for breast surgery. This method is more convenient and treats a smaller breast volume than external beam APBI. Results reported to date from randomized Italian and European trials show excellent local control and minimal toxicity 9, 10. However, in addition to potential concerns about dose coverage, many centers do not have the means to incorporate the costly equipment or necessary flexibility in operative scheduling.
In contrast, most radiation facilities can perform stereotactic radiosurgery, high precision, rapid, linear accelerator based external beam irradiation. Highly conformal stereotactic radiosurgery is ideal to minimize volume of uninvolved breast tissue and skin receiving high-dose during APBI, two advantages of intra-operative therapy that likely contribute to acceptable outcomes seen in trials. In fact treatment of the intact tumor pre-operatively allows for an even smaller, and potentially more accurate target volume than IORT. Our prior work evaluating the dosimetric feasibility of this approach, demonstrated a greater than 14% absolute reduction in volume of breast receiving prescription dose and a nearly 40% absolute reduction in volume receiving half prescription dose 13.
We report here implementation aspects of our phase I dose-escalation trial evaluating preoperative single fraction breast radiotherapy for treatment of early stage breast cancer in highly selected women with favorable tumor biology.
Methods
Patient selection
Women ages 55 or older with clinically node negative, unifocal 2.0 cm or less, biopsy proven, low/intermediate grade ductal carcinoma in-situ or invasive carcinoma were eligible for this Institutional Review Board (IRB) approved trial. Lobular cancers meeting size criteria on MRI were eligible but none were enrolled. Patients were breast conservation candidates with ER or PR positive, HER2 negative disease, without lymphovascular invasion. Tumors were deemed T1 clinically by the larger of physical exam or breast imaging (including mammogram, ultrasound, and MRI) measurements. Patients receiving neoadjuvant chemotherapy were excluded as were those with risk factors predictive of poor cosmetic outcomes (e.g. scleroderma, existing breast implant). After informed consent, eligible patients were enrolled and assigned sequentially in groups of 8 to dose cohorts, with an additional 8 enrolled at the final dose.
Simulation
Prior to radiation treatment planning, a clip marking tumor location (placed during biopsy) was confirmed, and served as a fiducial marker for radiotherapy localization. Surface markers were placed on the four quadrants of the ipsilateral breast to identify breast rotation or shape change at treatment and facilitate initial set-up. These were initially placed during both MRI and CT, but after low utility in MRI for a few patients, were only placed during CT for the remainder of the trial. Patients underwent non-contrast computed tomography (CT) in the prone position on a CDR prone breast board (CDR systems Inc, Calgary, Alberta, Canada). A contrast-enhanced treatment planning MRI was also required and patients were placed in the prone position on a dedicated breast surface coil with arms raised overhead. The MRI protocol included T1- and T2-weighted imaging, diffusion-weighted (DW) MRI and dynamic contrast enhanced (DCE) MRI. For DCE-MRI, gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany/ Bayer, Whippany, NJ) was intravenously administered by power injection at 0.1 mmol/kg bodyweight at 2 ml/s flow rate. Every effort was made to conserve the same breast shape for MRI, CT, and radiation treatment. For two patients, prone positioning yielded suboptimal results and MRI was acquired supine using a 4-channel Torso array coil. A planning CT scan was acquired in the same position for fusion.
Treatment Planning
MR and CT images were imported into Brain Lab version 4.1 (Brainlab, Feldkirch, Germany) for manual rigid registration in three dimensions. Priority was given to alignment of the biopsy marker and soft tissue near the tumor. Gross tumor volume (GTV) was contoured by the treating radiation oncologist based on enhancing tumor on MRI. Tumor was identified on the CT as well in most patients, but priority was given to the MR volume. GTVs were reviewed with a diagnostic radiology colleague (typically JB) to ensure accurate tumor delineation. The GTV was uniformly expanded by 1.5cm to create the clinical target volume (CTV). If greater than 1cm from the GTV, the first 5 mm of subcutaneous tissue and chest wall (pectoralis muscle and deeper) were excluded from this volume. An additional 0.3 cm margin excluding the first 5 mm of subcutaneous tissue was used to generate the planning target volume (PTV) to account for set up uncertainty. Initially, a separation of at least 1.5cm was required between skin and tumor edge, to allow a minimum 1cm CTV margin and decrease the dose to the skin. This constraint was relaxed as the trial progressed and limited acute toxicity was observed.
To assess the value of CT in delineating target volumes, one of two radiation oncologists (RB or JH) segmented tumors on the initial treatment planning CT scans more than one year after trial completion. Clinical information (e.g. expected tumor size, location) was utilized but not the MRI images so as to avoid bias. CT and MRI volumes were then compared.
The remainder of contouring and treatment planning was performed in Eclipse version 10 (Varian Medical Systems, Palo Alto, CA). The skin surface, spinal cord, heart and lungs were contoured in standard fashion. The ipsilateral and contralateral breast tissue was segmented per NSABP B39/RTOG 0413 protocol guidelines. Skin was defined as a 3 mm layer from the external body surface based on other available data in the literature in similar clinical scenarios14, 15
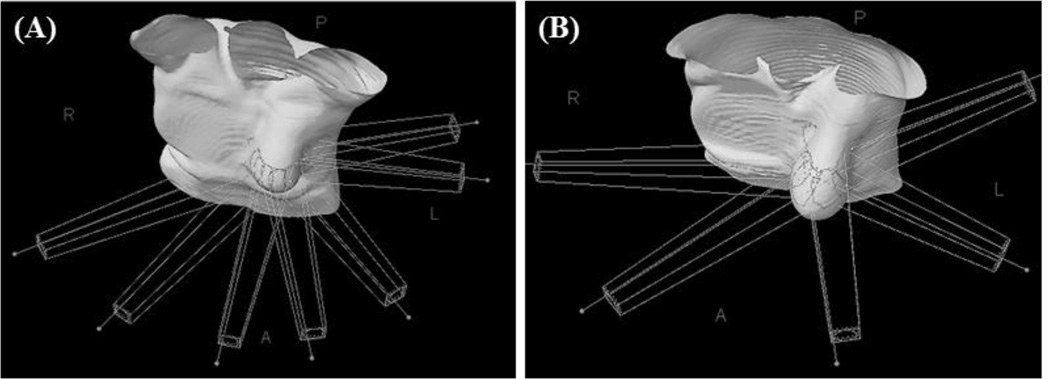
A treatment plan utilizing intensity modulated radiotherapy (IMRT) was designed. Coplanar or non-coplanar beam arrangements were allowed. Most commonly a 5–7 beam arrangement was utilized (Figure 1). A dose escalation format was utilized with sequential dose levels of 15Gy, 18Gy, and 21Gy. Acceptable dose coverage was defined as greater than 95% of the CTV receiving prescription dose. A maximum dose of 120% was selected based on the NSABP/RTOG randomized partial breast protocol Normal tissue dose constraints were also adopted from the NSABP/RTOG partial breast trial, with modifications for the single dose approach. Less than 60% of the ipsilateral whole breast could receive 50% of prescription dose, and less than 35% could receive prescription dose. The contralateral whole breast was limited to less than 15% of prescribed dose at any point. Lung constraints (<1000mL should receive 7Gy or greater) were adopted from radiosurgery approaches felt to be more clinically relevant. Heart dose was limited to less than 5mL receiving a dose of 3Gy or higher and spinal cord dose was restricted to a maximum dose of 3Gy to 1 cc. Skin dose was constrained to minimize dose as much as possible and evaluated case by case.
Figure 1.
Representative beam arrangements for breast radiosurgery plans. (A) Coplanar beams. (B) Non-coplanar beams.
Radiation treatment
Patients were positioned using the four skin surface markings placed during the planning CT scan. Target localization was performed using orthogonal kilovoltage (KV) images to align the biopsy marker and skin surface markers with treatment planning images. Cone-beam CT (CBCT) was acquired to confirm and improve initial alignment. The biopsy marker, tumor and neighboring soft tissue were utilized to verify positioning. Treatment was delivered after the treating radiation oncologist confirmed positioning. Post treatment KV imaging was performed to assess intrafraction patient movement. For purposes of comparison, the shifts along each axis were utilized to generate a composite vector.
Post-radiation Treatment
Surgical tumor resection was completed within 10 days after radiotherapy. Patients underwent intraoperative lymphatic mapping and sentinel lymphadenectomy per standard at our institution. Standard pathologic assessment followed. Close or positive margins (<2mm) required re-excision. If pathology revealed tumor characteristics outside entry criteria (e.g. large tumor size, nodal involvement), whole breast and regional nodal (as indicated) radiotherapy was delivered. Systemic therapy was at the discretion of the treating Medical Oncologist.
Statistical Analysis
Descriptive statistics were used to analyze frequencies and percentages of responses in each category for discrete variables. For continuous variables, medians, ranges, and frequencies as well as percentages were calculated within delineated categories.
Results
Forty-two women were screened, consented, and underwent treatment planning CT and MRI scans. In 10 women, MRI imaging revealed findings making them ineligible for the trial. These included lesion size (n=5), proximity to skin (n=3), additional ipsilateral lesion (n=1), or additional contralateral lesion (n=1). These women received standard of care radiotherapy and 32 women were enrolled for final analysis. Eight women received 15Gy, eight 18Gy, and sixteen 21Gy. Table 1 lists selected patient characteristics.
Table I.
Patient Characteristics
| Mean (n=32) |
Range | |
|---|---|---|
| Age at enrollment | 66.2 | 55–78 |
| BMI | 30 | 21–47 |
| Breast Volume (cc) | 1520 | 267–3604 |
| n (%) | ||
| Clinical Tumor Stage | ||
| T0 | 7 (22%) | |
| T1a | 4 (13%) | |
| T1b | 14 (44%) | |
| T1c | 7 (22%) | |
| GTV (cc) | 1 | 0.1–4 |
| CTV (cc) | 42 | 20–66 |
| PTV (cc) | 63 | 31–97 |
| Ipsilateral breast V100 (%) | 4 | 1–9 |
| Ipsilateral breast V50 (%) | 14 | 6–22 |
| Pathological Tumor Stage | ||
| T0 | 6 (19%) | |
| T1a | 4 (13%) | |
| T1b | 10 (31%) | |
| T1c | 12 (38%) |
All patients completed treatment planning with CT and MRI per protocol. Twenty-two patients underwent treatment planning MRI in the radiation oncology department. Ten underwent diagnostic planning MRI due to decreased GFR (n=2), body habitus requiring open MRI (n=4), or existing or planned diagnostic MRI at enrollment (n=4). Diagnostic MRI images were incorporated into treatment planning without issue. Two patients had tumors too close to the skin on prone CT scan, were re-imaged supine resulting in more favorable tumor to skin position, and continued on trial.
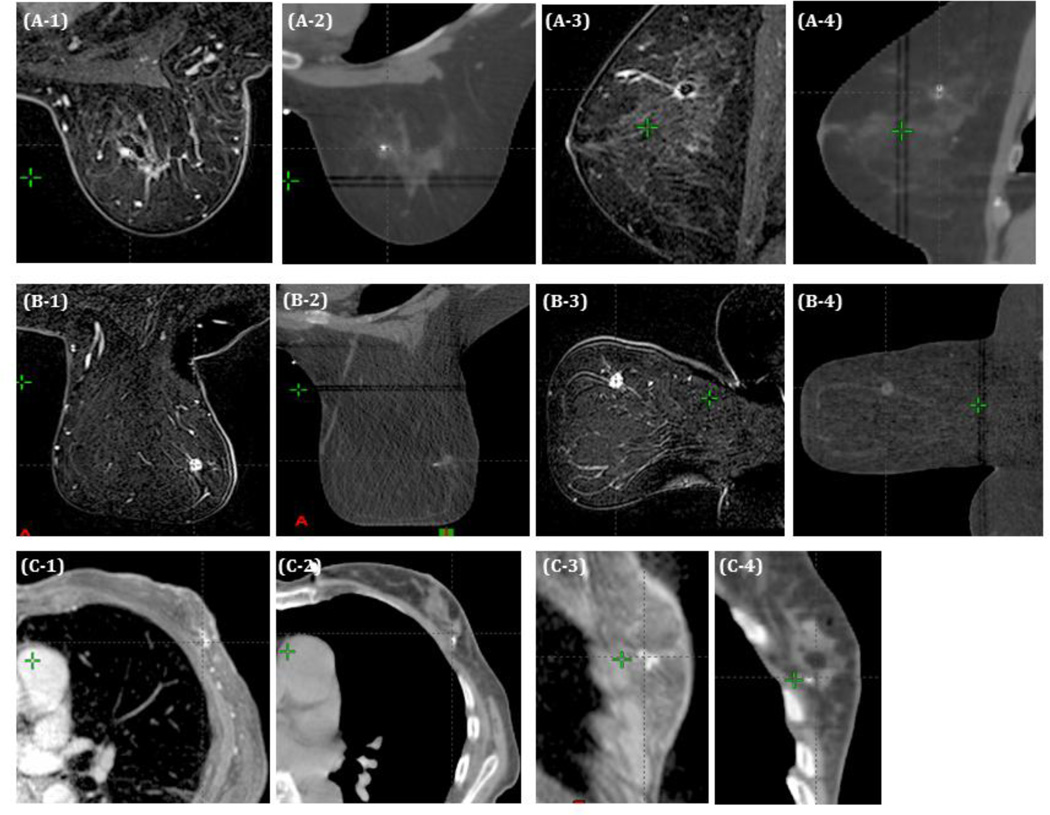
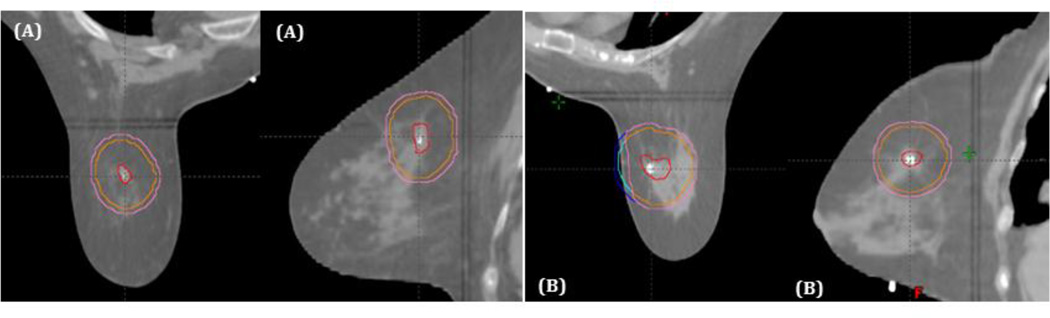
MRI images were used for confirmation of tumor volume and in-breast location on CT. While registering MRI and CT scans for planning, breast shape differences were noted, potentially impacting image fusion and tumor localization. This effect was variable, and consisted primarily of superior breast tissue constriction by the MRI coil. Figure 2A shows a patient with little shape difference between MRI (2A1, 2A3) and CT scan (2A2, 2A4) in axial and sagittal planes, whereas 2B shows a patient with greater breast shape variability between MRI (Figure 2B1, 2B3) and CT scan (Figure 2B2, 2B4). Despite this, the small region of interest allowed image registration for all patients. No difficulties were noted during image registration for supine patients (Figure 2C).
Figure 2.
Representative axial and sagittal images from MRI and CT treatment planning scans. (A) Patient without significant breast shape change between MRI and CT. (B) Patient with significant compression of the superior breast from the MRI coil. (C) Patient treated supine.
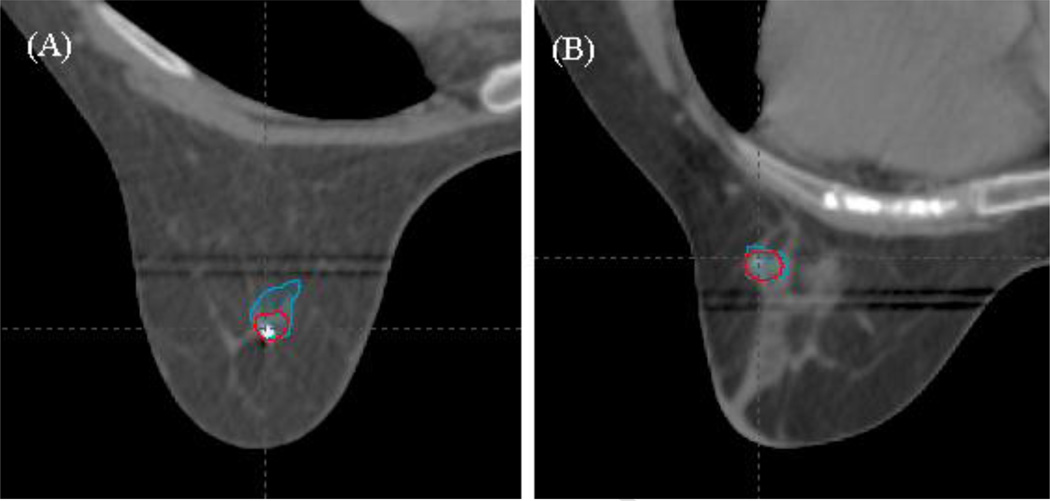
We could delineate the GTV on MRI for all patients. However, not all tumors were easily visualized on CT. CT-based target volumes differed from MR-delineated volumes in 29/32 patients on post-treatment analysis. The mean difference in volume for MR defined targets (0.9cc3) compared to CT defined targets (0.7cc3) was only 0.2cc3. However, the mean of the absolute volume differences between MRI and CT for each individual patient was 0.5cc3 suggesting significant variability in MR/CT agreement. Figure 3 illustrates one patient with limited overlap of the two volumes, despite similar absolute volumes (Figure 3A), and one with similar CT and MRI delineated target volumes (Figure 3B).
Figure 3.
Representative axial images of MRI and CT delineated GTVs. (A) Patient in whom the two volumes did not align well despite having similar total volumes on MRI (blue) and CT (red). (B) Patient in whom the two volumes aligned well and were very similar between MRI (blue) and CT (red).
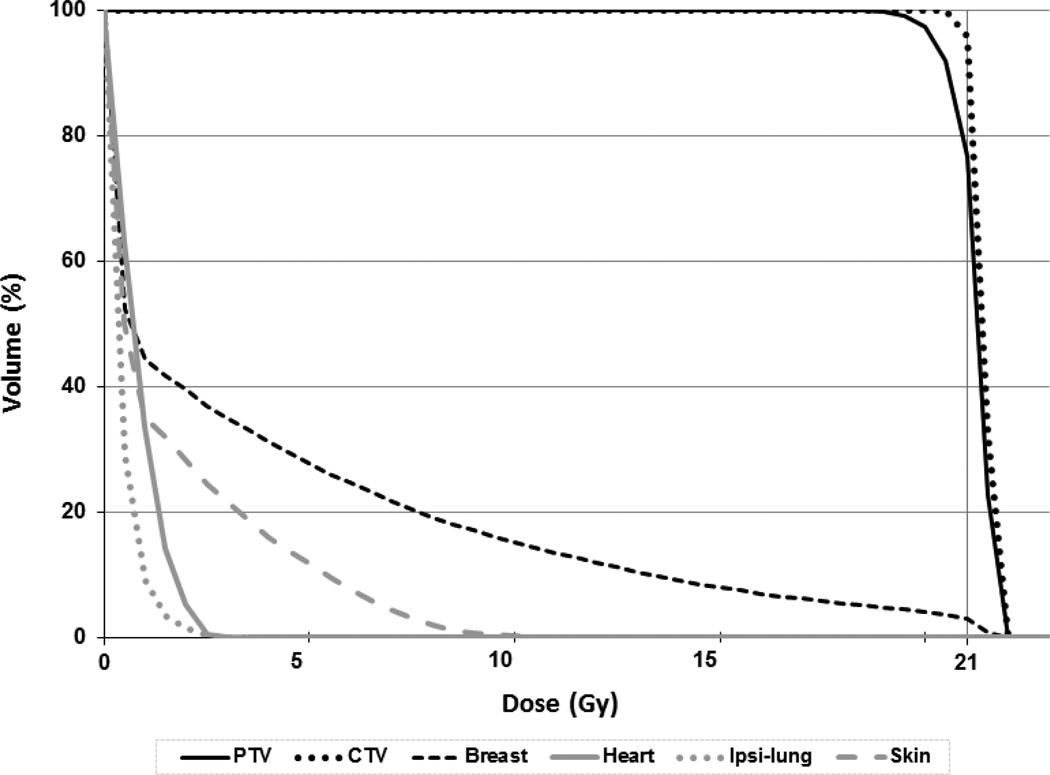
All required dosimetric parameters were easily met in all dose cohorts, as were normal tissue constraints. The mean breast volume receiving prescription dose (V100) was 4% [Table 1]. This agrees with our prior analysis 13 and improves on reported historical V100 values of 14–24% 16 for post-operative APBI patients. Similarly, the mean breast volume receiving half prescription dose (V50) in our cohort was only 14%, despite heterogeneous breast sizes. In fact, the patient with the greatest volume had a V50 of only 20%, improving on previously reported mean V50 values of 38–49% [14]. The remaining normal tissues received negligible doses though non-coplanar beam arrangements were sometimes required to minimize exit dose through critical organs. Spinal cord dose was minimal and no patient required contouring of thyroid or brachial plexus, as these structures were clearly distant from treatment fields and not within the low dose region for all cases (Figure 4).
Figure 4.
Representative dose volume histogram. Dose volume histogram for a representative patient treated pre-operatively with 21Gy showing target volume and normal tissue doses.
Skin dose constraints were not set, as skin tolerance was a primary trial objective. We achieved a mean skin Dmax of 14 +/− 3 Gy. The dose to 1cc and 10cc of skin was 11 +/− 3 Gy and 7 +/− 2 Gy respectively. The volume of skin receiving 8, 10, and 12 Gy was 4 +/− 4 cc, 2 +/− 3 cc, and 1 +/− 1 cc respectively. CTV and PTV expansions required modification to meet trial-mandated avoidance of the first 5 mm of subcutaneous tissue for 16/32 patients. However, the resulting mean percent volume change was only 4% (0–43%) for CTV and 6% (0–44%) for PTV. Figure 5 shows a tumor volume that did not require modification (A) and one that required modification in the axial dimension (B).
Figure 5.
Impact of tumor location on expansion volumes. (A) GTV (red) expanded 1.5cm to generate CTV (orange) plus 3mm to delineate PTV (pink), with no adjustment required in any dimension. (B) Target volumes extend beyond skin in the axial plan, requiring modification to exclude the first 5mm of subcutaneous tissue.
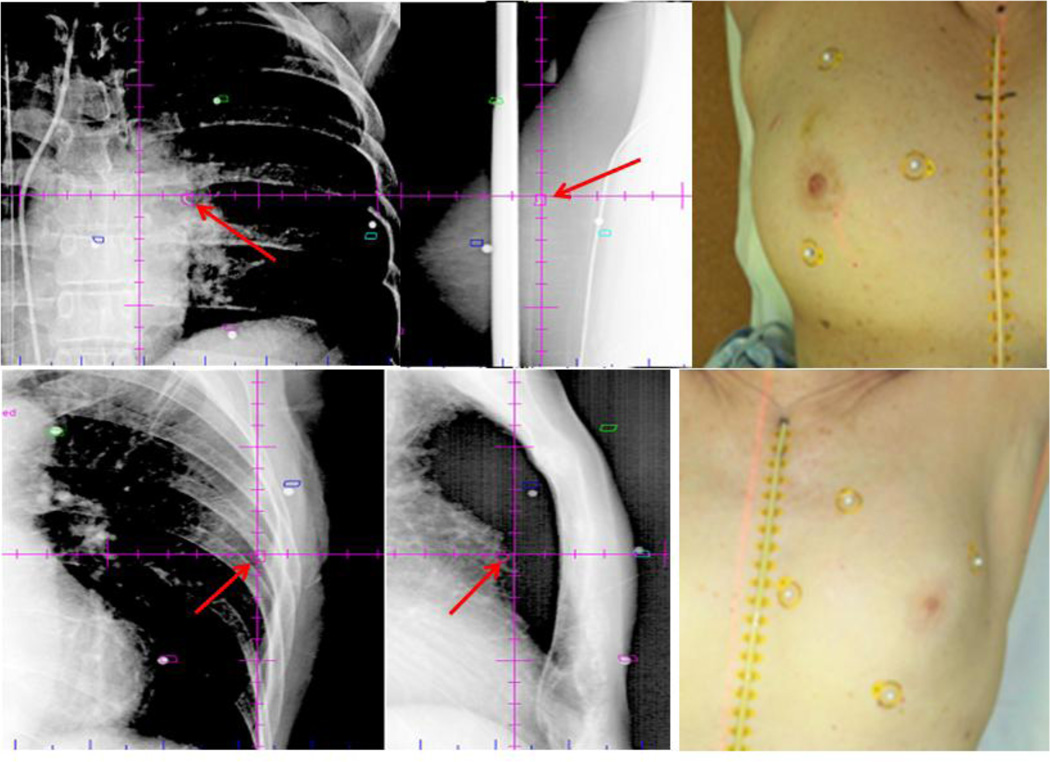
Treatment positioning via skin surface markers was more variable than using the biopsy marker due to breast shape differences. Therefore shifts were primarily performed to align the biopsy marker. All patients required a shift from initial set up prior to treatment. Subsequent shifts between kV set-up images and CBCT, as well as pre- to post-treatment images, were significantly less (one patient had CBCT without KV imaging during initial set up and one patient had no post-treatment imaging) [Figure 6, Table 2]. Of note, only 3/32 patients had any intra-fraction shift larger than 3mm in a single direction.
Figure 6.
Representative orthogonal kv images (left) and corresponding patient (right) illustrating surface marker orientation. Primary alignment was based on biopsy fiducial marker (red arrow) with surface markings providing supplemental guidance.
Table II.
Patient set-up and dose delivery parameters.
| Mean | Range | |
|---|---|---|
| Shift (cm) | ||
| Initial shift | 1.00 | 0.20–4.25 |
| Residual shift | 0.28 | 0.00–2.11 |
| Intrafraction motion | 0.21 | 0.00–0.86 |
| Treatment Time (mins) | ||
| First image to last image | 42.10 | 24.28–69.47 |
| Coplanar | 36.17 | 24.28–68.21 |
| Non-coplanar | 49.44 | 29.82–69.47 |
| First beam to last beam | 8.74 | 4.77–13.99* |
| Coplanar | 7.08 | 4.77–11.77 |
| Non-coplanar | 10.75 | 6.63–13.99* |
For one early patient a collision issue arose during treatment. This was easily resolved, and thereafter we tested permissible beam angles on the treatment machine at time of simulation, with no further collision issues. Mean treatment time and total time on the table were shorter for coplanar beam arrangements, although the range overlapped for coplanar and non-coplanar plans [Table 2]. We therefore prioritized coplanar plans to reduce treatment time, improve patient comfort, and reduce risk of intra-fraction movement.
Discussion
As breast cancer treatment moves toward individualized risk assessment, it is important that alternative treatment regimens are considered, whether shorter schedules, partial breast methods, or both. We successfully implemented a single fraction preoperative radiotherapy program for early stage favorable breast cancers within our department.
Implementation did have challenges. We found MRI imaging essential for target delineation, as not all tumors were visualized on CT. We noted patients for whom volumes on MRI and CT were similar in size but with minimal overlap, with potential amplification of divergence with CTV and PTV expansions. Tumors were more accurately defined on MRI, making this the preferred target delineation method. However, MRI imaging also caused screen failure of almost a quarter of clinically eligible patients; slowing patient accrual and adding an additional logistical consideration. Nonetheless, given the critical contribution to target delineation and elimination of inappropriate patients, we feel MRI treatment planning (departmental or diagnostic) is necessary for preoperative breast radiotherapy. Given routine use of contrasted MRI for other radiotherapy treatments 17–20, many programs likely already have experience incorporating this into treatment planning.
We observed breast shape differences between planning CT and MRI scans due to the MRI coil, but these did not prevent registration of the images or interfere with target delineation. This was again noted between treatment planning and treatment imaging. We planned to utilize skin surface markers to correct breast shape differences. However, we found significant skin marker variability at treatment that could not be fully corrected. The two skin markers closest to the biopsy marker, and the marker itself, were reliable for set up. Fortunately, given small target volumes 13, 21 shape variability did not significantly impact the treatment volume. Therefore, we do not feel this impedes our approach or that more stringent immobilization is required.
A CTV expansion of 1.5cm was utilized based on correlative studies of MRI imaging and pathologic findings22. This same numerical expansion is typically used postoperatively. One could argue that this could be reduced in the post-operative setting to decrease normal tissue coverage. However, given that the location of the tumor relative to the edge of the seroma cannot be known, this might result in target underdosing. Additionally, given that the post-operative seroma is generally orders of magnitude larger than the intact tumor, it is highly unlikely that the small normal breast treatment volumes we observed with in situ targeting in the pre-operative setting could be achieved.
For the PTV expansion post-operative APBI techniques generally utilize a larger margin primarily due to interfraction variability over ten treatments. Given our single fraction approach with onboard imaging, as well as our finding that only 3/32 patients had a shift in any one direction larger than 3mm, we feel a PTV expansion in the range of 3–5mm maintains the value of reduced normal tissue dose while ensuring target coverage. For future trials, we feel it is not necessary to trim the PTV near the skin surface given the lact of toxicity observed in out study.
We easily met normal tissue constraints from partial breast and lung SBRT protocols for all patients, likely due to smaller target volumes than for post-operative APBI where tumor bed plus margin can represent a significant volume. Given the ease with which parameters were met, we may consider stricter normal tissue constraints moving forward to minimize potential acute and chronic toxicity. In contrast we reduced the required tumor to skin surface distance during the trial given excellent treatment tolerance. In the future, we suggest tumor to skin allowance be based on coverage of the target rather than a specific physical distance.
Patient positioning and optimal beam angle considerations were generally straightforward, and not outside the complexity of other disease sites. Most patients were treated prone, and we feel this is optimal. However, for two patients we were able to delineate tumors and achieve dosimetric parameters in the supine position. Optimizing supine positioning during future trials may make this approach more accessible for a larger subset of patients and treatment centers.
Small sample size limits our study. A larger patient cohort is required to determine suitability of wider implementation. Though whole breast radiotherapy will appropriately remain standard of care for many patients, APBI approaches may be increasingly utilized for selected low risk patients. It is important that radiotherapy research keeps pace with advances in estimating recurrence risk by tumor biology to maximize efficiency of treatment. Our pre-operative single fraction SBRT approach represents an excellent potential alternative to less easily performed intra-operative radiotherapy. Furthermore, it addresses normal tissue dose-volume issues thought to contribute to negative cosmetic outcomes in external beam APBI trials. We anticipate most radiation oncology facilities would find this approach technically feasible. A phase II trial to further evaluate long-term effects of this approach is in development.
Acknowledgements
JH has research funding relevant to this project from Varian Medical Systems. Salary support for Dr. Horton and research support for the preclinical portions of this project (not reported here) was provided by K12HD043446 – Building Interdisciplinary Research Careers in Women’s Health and Susan G. Komen for the Cure, CCR12225923. The study sponsors (noted above) had no role in the design, collection, analysis, or interpretation of the data. Furthermore, they had no involvement in the writing of the manuscript or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification:
The authors have no conflicts of interest to declare.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England journal of medicine. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. The New England journal of medicine. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, nodenegative breast cancer. The New England journal of medicine. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30(14):1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haviland J, Agrawal R, Aird E, et al. The UK START (Standardisation of Breast Radiotherapy) Trials: 10-year follow-up results. Cancer research. 2012;72(Supplement 3) (abs)(24). [Google Scholar]
- 7.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. The New England journal of medicine. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 8.Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(32):4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. The lancet oncology. 2013;14(13):1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383(9917):603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 11.Hepel JT, Tokita M, Macausland SG, et al. Toxicity of Three-Dimensional Conformal Radiotherapy for Accelerated Partial Breast Irradiation. International journal of radiation oncology, biology, physics. 2009 doi: 10.1016/j.ijrobp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Jagsi R, Ben-David M, Moran J, et al. Adverse Cosmesis in a Protocol Investigating IMRT with Active Breathing Control for Accelerated Partial Breast Irradiation (APBI) International Journal of Radiation Oncology, Biology, Physicis. 2008;72(1):S153. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palta M, Yoo S, Adamson JD, Prosnitz LR, Horton JK. Preoperative single fraction partial breast radiotherapy for early-stage breast cancer. International journal of radiation oncology, biology, physics. 2012;82(1):37–42. doi: 10.1016/j.ijrobp.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Iaccarino G, Pinnaro P, Landoni V, et al. Single fraction partial breast irradiation in prone position. Journal of experimental & clinical cancer research : CR. 2007;26(4):543–552. [PubMed] [Google Scholar]
- 15.Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who's at risk? International journal of radiation oncology, biology, physics. 2008;72(5):1283–1286. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Leonard KL, Hepel JT, Hiatt JR, Dipetrillo TA, Price LL, Wazer DE. The effect of dose-volume parameters and interfraction interval on cosmetic outcome and toxicity after 3-dimensional conformal accelerated partial breast irradiation. International journal of radiation oncology, biology, physics. 2013;85(3):623–629. doi: 10.1016/j.ijrobp.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 17.(P089) A Phase III Randomized Trial of MRI-Mapped Dose-Escalated Salvage Radiotherapy Postprostatectomy: The Maps Trial-An Initial Dosimetric Assessment. Oncology (Williston Park) 2014;28(Suppl 1) [Google Scholar]
- 18.Miwa K, Matsuo M, Ogawa S, et al. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. doi: 10.1186/1748-717X-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regini F, Gourtsoyianni S, Cardoso De Melo R, et al. Rectal tumour volume (GTV) delineation using T2-weighted and diffusion-weighted MRI: Implications for radiotherapy planning. European journal of radiology. 2014;83(5):768–772. doi: 10.1016/j.ejrad.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 20.MacFadden D, Zhang B, Brock KK, et al. Clinical evaluation of stereotactic target localization using 3-Tesla MRI for radiosurgery planning. International journal of radiation oncology, biology, physics. 2010;76(5):1472–1479. doi: 10.1016/j.ijrobp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Nichols EM, Mohiuddin M, Flannery T, Dhople AA, Yu C, Regine WF. Comparative analysis of the post-lumpectomy target volume versus the pre-lumpectomy tumor volume for early stage breast cancer: implications for the future. San Antonio Breast Cancer Symposium:; 2008. 2008 doi: 10.1016/j.ijrobp.2009.04.063. http://www.abstracts2view.com/sabcs/sessionindex.php; [DOI] [PubMed]
- 22.Grimsby GM, Gray R, Dueck A, et al. Is there concordance of invasive breast cancer pathologic tumor size with magnetic resonance imaging? American journal of surgery. 2009;198(4):500–504. doi: 10.1016/j.amjsurg.2009.07.012. [DOI] [PubMed] [Google Scholar]