Abstract
Commonly used adjuvant systemic therapies harbor high rates of severe short-term and long-term side effects but are often justified to patients because of curative intent in early stage breast cancer. One of the oldest and least toxic adjuvant regimens, oral cyclophosphamide given with intravenous methotrexate and 5-fluourouracil (CMF) has been largely abandoned because of the perception that it underperforms for survival outcomes compared to modern regimens containing anthracycline and/or taxanes. To address this misperception, we performed a review of all consecutive breast cancer patients at the Seattle Cancer Care Alliance over the past decade receiving 6 months of adjuvant CMF as their sole chemotherapy regimen and determined rates for relapse-free survival (RFS), overall survival (OS), and major organ toxicity. From January 2003 to August 2013, 248 patients (median age of 52 years at the start of chemotherapy) met criteria for inclusion in this series and had a median follow-up of 67 months. RFS and OS at 5 years was 94.5% (91.3-97.9%) and 98% (96-100%), respectively. The only major organ toxicity occurring in more than 5% of patients was Grade III neutropenia (18.1%). One patient died during therapy from pneumocystis pneumonia attributed to previously undiagnosed AIDS. In a modern cohort of patients thoroughly characterized for grade and hormone receptor status, CMF was a well-tolerated and effective adjuvant regimen for early stage breast cancer and should be considered for appropriately selected patients.
Keywords: adjuvant chemotherapy, cyclophosphamide, methotrexate, fluorouracil, CMF
Introduction
In 1976, Bonadonna et al. reported that the adjuvant regimen of oral cyclophosphamide plus intravenous (IV) methotrexate and IV 5-fluorouracil (CMF) was efficacious for decreasing the incidence of relapse after radical mastectomy in women with breast cancer [1]. A total of 391 women with involved axillary lymph nodes were randomized after surgery to receive either CMF for 12 months or no further treatment. With a mean follow-up period of about 14 months, only 5.3% of patients receiving CMF had a recurrence whereas 24.0% of those who received no chemotherapy recurred. The most frequent toxicity associated with the regimen was nausea and vomiting because of oral cyclophosphamide. While leukopenia was observed, there were no increased rates of infection in the CMF-treated group, and authors noted that most working women continued to work during the entire period of chemotherapy. Twenty-year follow-up of the same group of patients showed that CMF-treated patients maintained an improved relapse-free survival (hazard ratio = 0.71, p=0.004) and overall survival (hazard ratio = 0.78, p=0.04) [2]. A disease-free survival benefit was seen with 6 months of adjuvant CMF in node-negative women who were considered at high risk of recurrence by virtue of negative estrogen receptor status (ER−) or positive estrogen receptor status (ER+) with primary tumor size greater than 3 cm [3]. Despite these excellent outcomes, the use of CMF has declined in the recent decades with the rise of anthracycline and taxane-based regimens which showed in some studies to have an incremental benefit for decreasing risk of recurrence. At the Seattle Cancer Care Alliance (SCCA; the clinical partnership of the University of Washington and the Fred Hutchinson Cancer Research Center), we continue to use a metronomically-delivered adjuvant CMF regimen for early stage breast cancer based on the original 1976 Bonadonna regimen, and CMFVP as given by the Southwest Oncology Group [4]. We have now systematically reviewed our outcomes with this regimen to report on efficacy and safety.
Methods
Patient selection
We identified 299 consecutive women with breast cancer (there were no men) who received metronomic CMF between January 2003 and August 2013 at the University of Washington/Seattle Cancer Care Alliance. This regimen consisted of: oral cyclophosphamide (60mg/m2 daily), IV methotrexate (15 mg/m2 weekly), and 5-fluorouracil (300 mg/m2 weekly) for a total 24 weeks. CMF was administered as a standard institutional protocol outside the context of a clinical trial, and dose adjustments were made at the discretion of the treating provider. Clinical chart records and follow-up data for these patients were reviewed and a research medical record abstract was created for each patient. Patients who received CMF as therapy for recurrent disease and de novo metastatic disease were excluded from further analyses as were patients who received CMF in the neoadjuvant setting or in combination with other chemotherapy agents. Patients requiring radiation therapy were allowed a treatment break between weeks 12 and 13 of the regimen to complete radiation. Characteristics including age, pathologic stage, hormone receptor status, HER2 status, grade, Oncotype Dx® score, menopausal status, adjuvant hormonal therapy, grade 3 and 4 toxicities, treatment delays, total weeks of treatment completed, the use of myeloid growth factors, and any hospitalizations related to treatment were abstracted. All clinic notes during all courses of treatment were individually reviewed for each patient to gather toxicity information, and NCI-CTCAE v4.03 was used for grading. The status of patients with information on recurrence and death was obtained from medical records. Our investigation was approved by the Cancer Consortium Institutional Review Board.
Pathology
Tumors were classified as ER+ and PR+ by standard immunohistochemistry in accordance with CAP/ASCO guidelines. ER and PR stains were interpreted using a modified H-score system (Allred) based on the proportion of cells staining and intensity of staining: >1% of cells with weak staining intensity (Allred score 3 of 8) was the clinically validated threshold for a positive result [5]. HER2 status of primary tumors was determined using either IHC method and/or gene amplification method using fluorescent in situ hybridization (FISH) technique. Tumors were classified as HER2+ if the protein was overexpressed by IHC (score ≥ 3+) or amplified by FISH (HER2/CEP17 ratio 2). Oncotype Dx® was ordered at the discretion of the treating physician and performed by Genomic Health, Inc.
Statistical methods
Relapse-free survival (RFS) and overall survival (OS) were estimated using the Kaplan-Meier analysis from the date of first CMF dose to date of relapse (RFS), death from any cause (OS), or last visit (RFS and OS). The association between recurrence and the covariate of interest was assessed using hazard ratios estimated by univariate Cox proportional hazard models, where p<0.05 was considered statistically significant.
Results
A total of 248 patients with localized breast cancer who received metronomic CMF as adjuvant therapy were identified. Of the 51 patients excluded from the analysis, 28 received CMF for metastatic disease, 11 received CMF in addition to other systemic chemotherapy agents as part of planned adjuvant therapy, 10 received CMF in the neoadjuvant setting, 1 received non-standard dosing for morbid obesity, and 1 patient had no traceable medical records documenting receipt of chemotherapy. Patient characteristics including stage and histology are summarized in Table 1. Either or both hormone receptors were positive in 235 patients (94.8%) and HER2 expression was negative in all but 1 patient where HER2 status was not explored. There were 13 patients (5.2%) with triple negative disease. Axillary lymph nodes were positive in 130 patients (52.4%). Of the 118 patients with node-negative disease, 42 patients had OncotypeDx® testing with mean reported score of 28 [90% CI 26-30]. Three patients had low scores (<18), 26 patients had intermediate scores (18-30) and 13 patients had high scores (>30). The median age at start of treatment was 53, and the median duration of follow up was 67 months.
Table 1. Patient and Tumor Characteristics.
| Characteristic | # of patients | % |
|---|---|---|
| Age, years | ||
| Median | 53 | |
| Range | 25-82 | |
| Menopausal status | ||
| Premenopausal | 105 | 42.3 |
| Peri/postmenopausal | 140 | 56.5 |
| Unknown | 3 | 1.2 |
| Pathologic stage | ||
| I | 79 | 31.9 |
| IIA | 93 | 37.5 |
| IIB | 50 | 20.2 |
| IIIA | 22 | 8.9 |
| IIIB | 2 | 0.8 |
| IIIC | 2 | 0.8 |
| Histologic grade | ||
| I | 69 | 27.8 |
| II | 119 | 48.0 |
| III | 57 | 23.0 |
| unknown | 3 | 1.2 |
| Hormone receptors | ||
| ERpos PRpos | 200 | 80.1 |
| ERpos PRneg | 34 | 13.7 |
| ERneg PRpos | 1 | 0.4 |
| HER2 status | ||
| Negative | 247 | 99.6 |
| Unknown | 1 | 0.4 |
| Triple-negative disease | 13 | 5.2 |
| Positive Nodes | ||
| 0 | 118 | 47.6 |
| 1-3 | 115 | 46.4 |
| 4-9 | 13 | 5.2 |
| ≥10 | 2 | 0.8 |
Efficacy
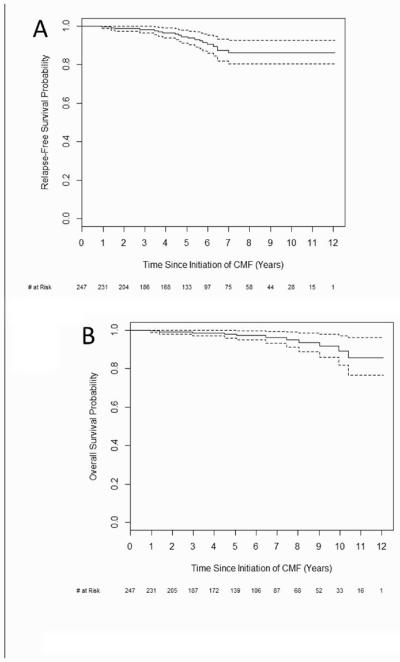
There were a total of 15 (6%) recurrences, 9 local (4%) and 6 distant (2%). There were 11 deaths (4%), 3 of which were unrelated to breast cancer. Kaplan-Meier curves are shown below for RFS and OS are shown in Figure 1. Relapse-free survival (RFS) at 3 years was 98.2% (95% confidence interval 96.4-100%) and at 5 years was 94.5% (91.3-97.9%). Overall survival (OS) at 5 years was 98% (96-100%). Events used to calculate RFS, OS and sites of relapse are listed in Table 2. Kaplan-Meier curve for OS by number of nodes involved is shown in Figure 2. Median duration RFS by stage is shown in Table 3. For patients with relapse, the median time to relapse was 57.7 months. Two of the patients with relapse had TNBC (13%) and the other 13 had hormone-positive disease (87%). Other characteristics of relapsed patients including use of adjuvant hormonal therapy, when applicable, are shown in supplementary Table A.1.
Fig 1.
Kaplan-Meier analyses of A) Relapse-free survival and B) Overall survival. 95% confidence intervals denoted by dotted lines. CMF, cyclophosphamide, methotrexate, fluorouracil.
Table 2. Relapse-Free Survival.
| Event | # of patients | % |
|---|---|---|
| Breast cancer relapse | 15 | 6.0 |
| Locoregional alone | 9 | 3.6 |
| Distant (± locoregional) | 6 | 2.4 |
| Death, any cause | 11 | 4.4 |
| Death on treatment* | 1 | 0.4 |
| Death without relapse | 3 | 1.2 |
One death from pneumocystis pneumonia in a patient with previously undiagnosed AIDS
Fig 2.
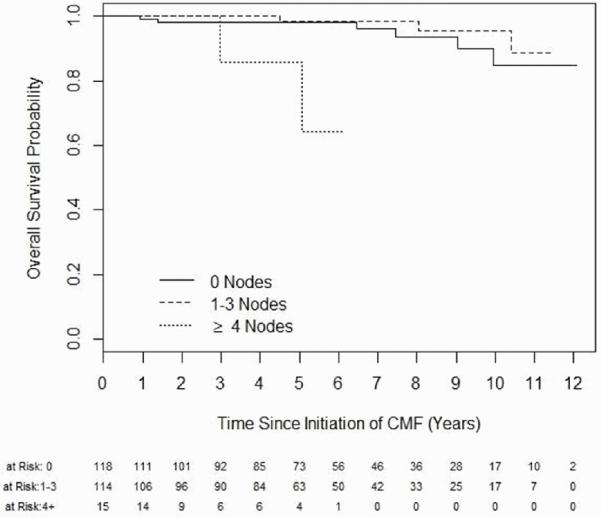
Kaplan-Meier analysis of overall survival stratified by number of lymph nodes involved. CMF, cyclophosphamide, methotrexate, fluorouracil.
Table 3. Median recurrence-free survival by stage.
| Stage | Median time (months) | No. patients | No. patients without relapse (%) |
|---|---|---|---|
| I | 72.2 | 79 | 71 (90) |
| IIA | 67.8 | 93 | 89 (96) |
| IIB | 60.4 | 50 | 45 (90) |
| III | 37.7 | 26 | 23 (88) |
Effect of hormone receptor status, tumor grade, and age on RFS
The association of RFS and hormone receptor status was calculated by Cox proportional hazard ratios. ER+PR+ was the reference group. ER+PR− patients had a significantly increased hazard ratio for relapse at 3.93 [95% CI 1.45-10.63, p=0.0071], and ER−PR− patients (i.e., triple negative patients) had a trend toward increased hazard ratio at 2.81 [95% CI 0.62-12.66, p=0.18]. There was only one patient with ER−PR+ disease. Each point increase in Nottingham grade was associated with an increased risk of relapse (HR=1.79, p=0.053). Age did not have a significant association with relapse.
Treatment Compliance, Density and Intensity
The median number of weeks of CMF given was 24 (range: 1-26). 160 patients (64.5%) completed 24 or more weeks of therapy. Reasons for early discontinuation included toxicities and patient choice. 133 patients (53.6%) required at least one dose hold or dose modification for toxicities. 100 women (40.3%) took a scheduled break after the first 12 weeks of CMF for radiation therapy (intended ‘sandwich’ approach). 80 patients (32.3%) completed at least 24 weeks of therapy without any dose modifications. Recurrence rate in those patients who discontinued treatment early was 3.4% (3/88) and 5% (4/80) in those who completed a course of full therapy at intended dose.
Chemotherapy-induced menopause
Prior to initiation of therapy, there were 105 women who were pre-menopausal, 140 women who were post-menopausal, and 3 women for whom menopausal status could not be determined from the records. Of the 105 women who were pre-menopausal, 29 (28%) had resumption of menses following chemotherapy with a median age of 39. Seventy women (67%) had chemotherapy-induced amenorrhea with a median age of 47, and menopausal status as a result of CMF exposure could not be determined in 18 women (17%) either due to limited follow-up, oophorectomy, or prolonged medical ovarian suppression as part of adjuvant hormonal therapy.
Adverse Events
All Grade 3 and 4 adverse events are shown in Table 4. The most frequent grade 3 to 4 hematologic adverse event, neutropenia was observed in 51 patients (20.6%). No patient received prophylactic myeloid growth factor but 38 patients (15.3%) were initiated on myeloid growth factor during their treatment course at the discretion of their treating oncologist. Grade 3 non-hematologic toxicities were rare, and there were no grade 4 non-hematologic toxicities. Ten women had hospitalizations during treatment (7.7%) and there was one death during therapy (0.4%) from pneumocystis pneumonia from previously undiagnosed AIDS.
Table 4. Grade 3 and 4 Toxicity.
| Adverse Event | Grade 3 # of patients (%) | Grade 4 # of patients (%) |
|---|---|---|
| Hematologic | ||
| Anemia | 0 | 0 |
| Febrile Neutropenia | 1 (0.4) | 0 |
| Neutropenia | 45 (18.1) | 6 (2.4) |
| Thrombocytopenia | 1 (0.4) | 0 |
| Nonhematologic | ||
| Bronchial infection | 1 (0.4) | 0 |
| Diarrhea | 1 (0.4) | 0 |
| Mucositis | 1 (0.4) | 0 |
| Nausea | 1 (0.4) | 0 |
| Pneumonitis | 1 (0.4) | 0 |
| Thrombosis/embolism | 1 (0.4) | 0 |
Discussion
For some women with localized breast cancer, adjuvant chemotherapy can target undetected micrometastatic disease to reduce risk of recurrence. The first adjuvant therapy showing a dramatic benefit in disease free survival and OS in node-positive and node-negative breast cancer was CMF with oral cyclophosphamide given for one year [1, 6]. In subsequent years, a number of trials were conducted evaluating anthracycline-containing regimens to improve on the results of CMF, and the results were inconsistent with some studies showing improvement in outcomes with the addition of anthracyclines and others showing no difference [7-11]. The Early Breast Cancer Trialists’ Collaborative Group overview in 1998 reviewed 11 studies of anthracycline-based regimens compared with CMF. The meta-analysis showed that compared with CMF (any schedule), anthracycline-based regimens produced a 12% proportional reduction in recurrence (p=0.006) and 11% proportional reduction in mortality (p=0.002) [12]. However, the patient population in this meta-analysis included both pre- and postmenopausal women, women with both hormone receptor-positive and hormone receptor-negative disease, and several variations of CMF administration including both oral and parenteral cyclophosphamide. Of note, an all-parenteral CMF has been shown to be inferior to CMF with oral cyclophosphamide in the metastatic setting although never directly compared in the adjuvant setting [13]. Also, subsequent analyses have shown that the incremental benefit of adding anthracyclines to adjuvant chemotherapy may have been limited to patients with HER2+ breast cancers [14-16]. A meta-analysis evaluating the addition of taxanes to anthracycline-based adjuvant regimens found an additional benefit in recurrence-free survival, breast cancer mortality, and overall survival [17]. Taxanes are also used routinely without anthracyclines in the adjuvant setting [18]. There have been no direct comparisons between taxane-based regimens and CMF. However, the use of CMF has diminished in the past few decades in favor of anthracycline- and/or taxane-based regimens.
Review of our institutional experience shows that RFS and OS are excellent among our patients treated with adjuvant CMF delivered in a metronomic schedule. We can indirectly compare outcomes of our high-risk node-negative patients (as defined by 1998 St. Gallen criteria of age <35, tumor >2 cm, negative hormone receptor, histologic grade 2 or 3) to those in the GEICAM/2003-02 study. Our retrospective experience herein included 97 patients who fit this criteria with a median age of 52 and median follow-up of 66 months. There have been 7 recurrences (7.2%) which compares favorably to both the fluorouracil, doxorubicin, and cyclophosphamide (FAC) arm and FAC plus weekly paclitaxel (wP) arm, which had recurrence rates of 9.3% and 7%, respectively [19]. Our incidence of grade 3 and 4 neutropenia in this group was 18.5%, which is lower than the 21.8% reported for FAC and 25.4% reported for FAC-wP. Our incidence of febrile neutropenia was 1%, which is lower than 2.7% and 3.6% for FAC and FAC-wP, respectively. Results on outcomes and toxicities from other landmark adjuvant trials are compared to results from our patients in Table 5.
Table 5. Selected Landmark Adjuvant Trials in Breast Cancer.
| Trial & Year published |
N | Follow -up (years) |
Setting | Regimen (IV or oral cyclophosphamide) |
Outcome | Toxicity | Ref |
|---|---|---|---|---|---|---|---|
| Our CMF experience |
248 | 5.6 | pre& post-menopausal T1-4 LN pos in 52% HR pos in 95% |
CMF (oral C) |
5-year RFS of 94.5%, 5- year OS of 98% |
Febrile neutropenia in <1%, Gr 4 neutropenia in 2.4% |
|
| ICCG 1996 |
759 | 4.5 | premenopausal LN pos in all HR status unknown |
CMF vs FEC (oral and IV C) |
FEC better than CMF with IV C for OS (87% vs 74%) but not different from CMF with oral C |
More nausea/vomiting and alopecia in FEC |
[26] |
| MA.5 1998 |
710 | 5 | pre- and peri-menopausal T1-3 LN pos in all HR status unknown |
CMF vs FEC (oral C) |
FEC better than CMF for DFS (HR = 0.77) |
Febrile neutropenia in 1.1% for CMF, 8.5% for FEC |
[11] |
| INT-0102 2005 |
2690 | 10 | pre & post-menopausal T1-T3a LN neg in all HR pos in 46% |
CMF vs CAF (+/− Tam) (oral C) |
No difference in DFS, slightly improved OS with CAF (85% vs 82%) |
Gr 4 in CAF = 59-62%, CMF = 39% |
[9] |
| BIG 02-98 2008 |
2887 | 5.2 | pre & post-menopausal T1-T3 LN pos in all HR pos in 76% |
A→CMF, AC→CMF, A→T→CMF, AT→CMF (oral C) |
T improved DFS (HR=0.86) |
Gr 3 or 4 in 32% with A→T→CMF vs 20% with A→CMF |
[27] |
| USOR 9735 2006 |
1016 | 5.5 | pre & post-menopausal T1-T3 LN pos in 53% HR pos in 72% |
AC vs TC (IV C) |
TC with improved DFS (86% vs 80%) |
Gr 4 neutropenia in 51% TC, 43% AC |
[18] |
| GEICAM/ 2003-02 2013 |
1917 | 5.2 | pre & post-menopausal T1-3 LN neg in all HR pos in 65% |
FAC vs FAC→P (IV C) |
FAC→P with improved DFS (93% vs 90.3%) |
Febrile neutropenia in 2.7% with P, 3.6% without P, Gr 3 or 4 neutropenia in 22% with P, 25% without P |
[19] |
| SWOG- 8814 2009 |
1477 | 10 | Post-menopausal T1-3 LN pos in all HR pos in all |
CAF+Tam vs Tam (oral C) |
Improved 10-year DFS with CAF (57% vs 48%) |
Gr 4 neutropenia with CAF of 44%. |
[21] |
| BCIRG 001 2005 |
1491 | 4.6 | pre & post-menopausal T1-3 LN pos in all HR pos in 76% |
TAC vs FAC (IV C) |
Improved 5-yr DFS in TAC vs FAC (75% vs 68%) |
Febrile neutropenia in 24.7% of TAC and 2.3% of FAC. Gr 3 or 4 non- heme tox 36.3% vs 26.6%. |
[20] |
C, cyclophosphamide; M, methotrexate; F, fluorouracil; LN, lymph node; HR, hormone receptor; RFS, relapse free survival; OS, overall survival; Gr, grade; E, epirubicin; IV, intravenous; A, Adriamycin; DFS, disease free survival; Tam, tamoxifen; T, docetaxel; P, paclitaxel
Recurrence rate among node-positive patients was also low at 6% (8/130) at a median follow-up of 60 months. All but 4 of the node positive patients had hormone receptor positive disease (97%). The node positive patients in our cohort did not have recurrence score testing but they were recommended to receive adjuvant chemotherapy based on their pathologic lymph node involvement. We had a higher percentage of hormone-receptor positive patients in our series compared to the BCIRG study and therefore it is difficult to compare outcomes but our RFS rates are very low, 6% versus the 19% for FAC and 26% for TAC seen in the BCIRG study [20]. Comparison of our results to those seen in SWOG-8814 using the anthracycline-based regimen of CAF and tamoxifen is also quite favorable [21]. In that study, all patients had hormone-positive, node-positive disease and at 10 years, DFS was 57% for the group receiving CAF plus tamoxifen, and DFS at 5 years was approximately 75% by extrapolation from the Kaplan-Meier curve.
Grade 3 neutropenia was present in 24 patients (18%) and grade 4 neutropenia was present in 4 patients (3%). There was one case of febrile neutropenia. Our rates of significant toxicities such as febrile neutropenia, hospitalization and death are much lower than reported in the Breast Cancer International Research Group (BCIRG) trial comparing FAC to docetaxel plus doxorubicin plus cyclophosphamide (TAC) [20]. Rates of serious (grade 3 or 4) hematologic and nonhematologic toxicities were low in our experience. Although the treating physicians routinely checked blood work during the course of therapy including complete blood count with differential, electrolyte panel, and liver function tests, there were no cases of grade 3 or 4 elevated transaminases. Though no formal financial assessments were made in our review, financial toxicities of treatment are presumed to be lower than other regimens. Many patients are also able to keep working throughout their chemotherapy course. Routine advanced diagnostics as echocardiograms to monitor cardiac function were not necessary for CMF as it is not associated with risk of cardiomyopathy. Newer generation, high-cost, antiemetics such as fosaprepitant were also not needed to control chemotherapy-induced nausea. Indeed most patients were not offered first-cycle prophylactic parenteral or oral anti-emetics. Prophylactic use of myeloid growth factors, common with or even required for some anthracycline and taxane-based regimens, was also not necessary. Other known advantages include no alopecia and a higher rate of permanent amenorrhea as a result of therapy (in premenopausal) patients given metronomic CMF compared to anthracycline and/or taxane containing regimens. Reported rates of chemotherapy-induced amenorrhea for CMF are 61% in women under age 40 and 95% in women over age 40 [22], whereas reported rate for doxorubicin/cyclophosphamide is 34% [23]. Younger women with hormone-positive disease who experience chemotherapy-induced amenorrhea have improved survival compared to those who retain ovarian function [24, 25]. For premenopausal women offered CMF, counseling regarding ovarian function is required in women who desire to become pregnant after therapy.
Conclusions
Based on our institutional experience, adjuvant metronomic CMF is a well-tolerated regimen with excellent survival outcomes in hormone receptor positive early-stage breast cancer with high-risk features such as node positivity, pre-menopausal status, and elevated Oncotype Dx® scores. It should remain a relevant alternative to anthracycline and/or taxane-containing regimens for similar patients to those we treated in our series.
Supplementary Material
Clinical Practice Points.
The combination of oral cyclophosphamide, intravenous methotrexate and intravenous fluorouracil (CMF) has been established since the 1970’s as an efficacious adjuvant regimen for breast cancer with low toxicities. However, in recent years, the use of CMF has been largely abandoned because of the perception that it underperforms for survival outcomes compared to modern regimens containing anthracycline and/or taxanes.
The objective of this single-institution retrospective review was to address this misconception by determining rates for relapse-free survival (RFS), overall survival (OS), and major organ toxicity for patients treated with CMF as their sole adjuvant chemotherapy regimen. Hormone receptors were positive in 235 patients (94.8%) and HER2 expression was negative in all but 1 patient where HER2 status was not explored. Axillary lymph nodes were positive in 130 patients (52.4%).
The results for 248 consecutive patients treated with CMF indicated a 5-year RFS of 94.5% and OS of 98%. The most frequent grade 3 to 4 hematologic adverse event, neutropenia was observed in 51 patients (20.6%). Grade 3 non-hematologic toxicities were rare, and there were no grade 4 non-hematologic toxicities.
The results presented here suggest that adjuvant metronomic CMF is a well-tolerated regimen with excellent survival outcomes in hormone receptor positive early-stage breast cancer.
Acknowledgements
We would like to recognize the many donors to the VK Gadi Research Fund for their generous contributions, which have made this manuscript possible. Eunpi Cho is the recipient of a National Cancer Institute funded postdoctoral fellowship (T32 CA009515). The work was also supported through NCRR/NIH UL1TR000423.
Footnotes
Disclosure: None.
REFERENCES
- 1.Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, De Lena M, Tancini G, Bajetta E, Musumeci R, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. The New England journal of medicine. 1976;294(8):405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. The New England journal of medicine. 1995;332(14):901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 3.Mansour EG, Gray R, Shatila AH, Osborne CK, Tormey DC, Gilchrist KW, Cooper MR, Falkson G. Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer. An intergroup study. The New England journal of medicine. 1989;320(8):485–490. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- 4.Rivkin SE, Green S, Metch B, Glucksberg H, Gad-el-Mawla N, Constanzi JJ, Hoogstraten B, Athens J, Maloney T, Osborne CK, et al. Adjuvant CMFVP versus melphalan for operable breast cancer with positive axillary nodes: 10-year results of a Southwest Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1989;7(9):1229–1238. doi: 10.1200/JCO.1989.7.9.1229. [DOI] [PubMed] [Google Scholar]
- 5.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 6.Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni L, Valagussa P. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. Bmj. 2005;330(7485):217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, Bowman D, Wolmark N, Wickerham DL, Kardinal CG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1990;8(9):1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 8.Moliterni A, Bonadonna G, Valagussa P, Ferrari L, Zambetti M. Cyclophosphamide, methotrexate, and fluorouracil with and without doxorubicin in the adjuvant treatment of resectable breast cancer with one to three positive axillary nodes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1991;9(7):1124–1130. doi: 10.1200/JCO.1991.9.7.1124. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins LF, Green SJ, Ravdin PM, Lew D, Martino S, Abeloff M, Lyss AP, Allred C, Rivkin SE, Osborne CK. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(33):8313–8321. doi: 10.1200/JCO.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 10.Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N, National Cancer Institute of Canada Clinical Trials G Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(22):5166–5170. doi: 10.1200/JCO.2005.09.423. [DOI] [PubMed] [Google Scholar]
- 11.Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, Findlay B, Warr D, Bowman D, Myles J, et al. National Cancer Institute of Canada Clinical Trials Group Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(8):2651–2658. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352(9132):930–942. [PubMed] [Google Scholar]
- 13.Engelsman E, Klijn JC, Rubens RD, Wildiers J, Beex LV, Nooij MA, Rotmensz N, Sylvester R, An EORTC Breast Cancer Co-operative Group Phase III Trial (10808) “Classical” CMF versus a 3-weekly intravenous CMF schedule in postmenopausal patients with advanced breast cancer. European journal of cancer. 1991;27(8):966–970. doi: 10.1016/0277-5379(91)90259-g. [DOI] [PubMed] [Google Scholar]
- 14.Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. Journal of the National Cancer Institute. 2008;100(1):14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 15.Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, Chia SK, Shepherd LE, Levine MN, Pritchard KI, Davies S, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(8):2402–2412. doi: 10.1158/1078-0432.CCR-11-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard KI, Shepherd LE, O’Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN, National Cancer Institute of Canada Clinical Trials G HER2 and responsiveness of breast cancer to adjuvant chemotherapy. The New England journal of medicine. 2006;354(20):2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative G. Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(34):5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 19.Martin M, Ruiz A, Ruiz Borrego M, Barnadas A, Gonzalez S, Calvo L, Margeli Vila M, Anton A, Rodriguez-Lescure A, Segui-Palmer MA, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(20):2593–2599. doi: 10.1200/JCO.2012.46.9841. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, et al. Adjuvant docetaxel for node-positive breast cancer. The New England journal of medicine. 2005;352(22):2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 21.Albain KS, Barlow WE, Ravdin PM, Farrar WB, Burton GV, Ketchel SJ, Cobau CD, Levine EG, Ingle JN, Pritchard KI, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldhirsch A, Gelber RD, Castiglione M, The International Breast Cancer Study Group The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 1990;1(3):183–188. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 23.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1996;14(5):1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 24.Swain SM, Jeong JH, Geyer CE, Jr., Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. The New England journal of medicine. 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(36):5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 26.Coombes RC, Bliss JM, Wils J, Morvan F, Espie M, Amadori D, Gambrosier P, Richards M, Aapro M, Villar-Grimalt A, et al. The International Collaborative Cancer Group Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1996;14(1):35–45. doi: 10.1200/JCO.1996.14.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Francis P, Crown J, Di Leo A, Buyse M, Balil A, Andersson M, Nordenskjold B, Lang I, Jakesz R, Vorobiof D, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. Journal of the National Cancer Institute. 2008;100(2):121–133. doi: 10.1093/jnci/djm287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.