Abstract
The endocytosis and catabolism of large quantities of host cell hemoglobin is a hallmark of the intraerythrocytic asexual stage of the human malaria parasite Plasmodium falciparum. It is known that the parasite’s production of amino acids from hemoglobin far exceeds its metabolic needs. Here, we show that P. falciparum effluxes large quantities of certain non-polar (Ala, Leu, Val, Pro, Phe, Gly) and polar (Ser, Thr, His) amino acids to the external medium. That these amino acids originate from hemoglobin catabolism is indicated by the strong correlation between individual amino acid efflux rates and their abundances in hemoglobin, and the ability of the food vacuole falcipain inhibitor E-64d to greatly suppress efflux rates. We then developed a rapid, sensitive and precise method for quantifying flux through the hemoglobin endocytic-catabolic pathway that is based on leucine efflux. Optimization of the method involved the generation of a novel amino acid-restricted RPMI formulation as well as the validation of D-norvaline as an internal standard. The utility of this method was demonstrated by characterizing the effects of the phosphatidylinositol-3-kinase inhibitors wortmannin and dihydroartemisinin on the kinetics of Leu efflux. Both compounds rapidly inhibited Leu efflux, which is consistent with a role for phosphtidylinositol-3-phosphate production in the delivery of hemoglobin to the food vacuole; however, wortmannin inhibition was transient, which was likely due to the instability of this compound in culture medium. The simplicity, convenience and non-invasive nature of the Leu efflux assay described here makes it ideal for characterizing the in vivo kinetics of hemoglobin endocytosis and catabolism, for inhibitor target validation studies, and for medium-throughput screens to identify novel inhibitors of cytostomal endocytosis.
Keywords: malaria, hemoglobin, amino acid, phosphatidylinositol-3-kinase, wortmannin, dihydroartemisinin
1. INTRODUCTION
Asexual replication of Plasmodium spp. within human erythrocytes gives rise to the clinical symptoms of malaria. During its intraerythrocytic residence, P. falciparum, the most virulent human malaria species, degrades up to three-quarters of the host erythrocyte’s hemoglobin [1, 2]. Hemoglobin endocytosis takes place at the cytostome, a specialized pore-like structure of unknown composition that is associated with invaginating parasitophorous vacuole and parasite plasma membranes [3–6]. Catabolism of hemoglobin occurs in the food vacuole, an acidic organelle that is formed through the fusion of endocytic structures beginning at about 14 hours post-invasion [3]. The mechanistic details of hemoglobin transport from the cytostome to the food vacuole remain obscure. There is some experimental support for a model for hemoglobin transport in which an unconventional double-membrane transport vesicle pinches off from the cytostome and fuses with the food vacuole membrane [3, 5–8]. Potential non- cytostomal phagotropic structures have also been observed [5, 8]; however, the extent of their contribution to hemoglobin endocytosis remains to be established.
The food vacuole contains endo- and exo-peptidases that catalyze the hydrolysis of α- and β-globin to amino acids [9–11]. The quantities of amino acids generated through hemoglobin catabolism are sufficient to satisfy the parasite’s needs for protein synthesis and other metabolic activities requiring amino acids; only isoleucine, which is absent from human hemoglobin, needs to be obtained from an exogenous source [12]. Intriguingly, only about one-fifth of globin-derived amino acids are used by P. falciparum [2]. Studies with intraerythrocytic malaria parasites have revealed that substantial quantities of amino acids diffuse out of the parasitized erythrocyte into the external milieu [13–15]. The relative amounts of amino acids released correlate well with their abundance in hemoglobin [13, 14]. Thus, it appears that many of the amino acids generated during the catabolism of hemoglobin exit the parasite.
We hypothesized that measurement of the rate of release of amino acids from blood-stage P. falciparum could serve as the basis for a non-invasive and sensitive readout of flux through the hemoglobin endocytic-catabolic pathway. Such an assay could bolster efforts to study endocytic process in P. falciparum and to identify and characterize the effects of inhibitors of hemoglobin endocytosis and catabolism in vivo. Existing methods are either insufficiently sensitive to probe the kinetics of hemoglobin endocytosis and/or catabolism over short (30 minutes to 3 hours) timeframes, or are difficult to scale up to medium- to high-throughput capacity. For example, polyacrylamide gel-based approaches to monitor the accumulation of undegraded hemoglobin in P. falciparum have been employed to characterize the in vivo effects of cysteine protease inhibitors, quinoline- and artemisinin-family anti- malarials and actin-perturbing agents on hemoglobin endocytosis and catabolism [16–20]. These methods can deliver quantitative data over short time periods [16]; however they are not readily scalable to medium- or high-throughput capacity. Quantitation of inhibition of endocytosis has typically relied on the uptake from resealed erythrocytes of endocytic tracers such as biotin- or fluorophore-labeled dextran or horse radish peroxidase [3, 17, 18]. While this approach has yielded valuable insights, it suffers from the significant disadvantage that the resealing process necessarily alters the composition of the host erythrocyte cytosol, including the concentration of hemoglobin, which likely affects rates of hemoglobin endocytosis and catabolism.
In this report, we describe a convenient, sensitive and non-invasive method for quantitation of the flux through the hemoglobin endocytic/catabolic pathway at high temporal resolution. We demonstrate the utility of this approach by characterizing the effects of wortmannin and dihydroartemisinin (DHA), two inhibitors of P. falciparum phosphatidylinositol-3-kinase, on the kinetics of hemoglobin endocytosis and catabolism in vivo.
2. MATERIALS AND METHODS
2.1. P. falciparum culture and formulation of amino acid-restricted media
P. falciparum clone 3D7 was routinely cultured in human O+ erythrocytes (Interstate Blood Bank; 2% hematocrit) in RPMI 1640 medium (Life Technologies) supplemented with 27 mM sodium bicarbonate, 11 mM glucose, 0.37 mM hypoxanthine, 10 μg/mL gentamicin, and 5 g/L Albumax I (Invitrogen). For all experiments, parasite cultures were maintained in a 5% CO2 incubator at 37 °C. We note that a reduced-oxygen gas was not used, as we have found that the 3D7 line replicates with a ~44 hour cycle at ambient oxygen levels in a 5% CO2 environment. Parasites were synchronized by 5% sorbitol treatment [21].
A variation of RPMI containing isoleucine as the only proteinogenic amino acid (“Ile+Nva”) was formulated as previously described ([12]; Table 1). The medium designated “RPMI-VL+Nva” contained all proteinogenic amino acids except Ala, Leu and Val at the concentrations indicated in Table 1. In both media, 100 μM D-norvaline (Nva) was added to serve as an internal standard.
Table 1.
Amino acid compositions of RPMI formulations used in this study.
| Amino Acid1 | Concentration (μM)
|
||
|---|---|---|---|
| RPMI2 | Ile+Nva | RPMI-VL+Nva | |
| Ala | 0 | 0 | 0 |
| Arg | 1148 | 0 | 200 |
| Asn | 379 | 0 | 200 |
| Asp | 150 | 0 | 150 |
| Cystine | 208 | 0 | 200 |
| Glu | 136 | 0 | 136 |
| Gln | 2052 | 0 | 200 |
| Gly | 133 | 0 | 133 |
| His | 97 | 0 | 97 |
| HydroxyPro3 | 153 | 0 | 0 |
| Ile | 381 | 174 | 150 |
| Leu | 381 | 0 | 0 |
| Lys | 219 | 0 | 200 |
| Met | 101 | 0 | 101 |
| Phe | 91 | 0 | 91 |
| Pro | 174 | 0 | 174 |
| Ser | 285 | 0 | 200 |
| Thr | 168 | 0 | 168 |
| Trp | 24 | 0 | 24 |
| Tyr | 111 | 0 | 111 |
| Val | 171 | 0 | 0 |
| D-norvaline | 0 | 100 | 100 |
Enantiomeric amino acids are L-isomers unless indicated.
As formulated in “RPMI 1640 Medium”, Life Technologies, product number 23400–021.
4-hydroxyproline
2.2. Amino acid efflux assays
Synchronized, mid-trophozoite stage parasites (one to two nuclei, ~28 hours post-invasion) were used for all efflux assays. Parasites at 5–10% parasitemia (infected RBC, or iRBC) or uninfected erythrocytes (uRBC) were washed three times with amino-acid restricted medium and aliquotted into 96-well plates (200 μL per well, 2% hematocrit). At the prescribed times, aliquots were removed to a separate plate and centrifuged at 860 x g for 3 minutes. The conditioned medium was removed and stored at 4 °C until the end of the experiment, at which time all media samples were transferred to an Ultracel-10 MultiScreen filter plate (10 kDa cutoff, Millipore) and centrifuged at 1940 x g for 30 minutes. The flow-through, depleted of proteins, was progressed to amino acid analysis or frozen at −20 °C.
Quantitation of amino acids was carried out on a Waters Ultra-High Pressure Liquid Chromatography (UPLC) system equipped with an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm particle size). Mobile phases were AccQ-Tag Ultra Eluent A and B (Waters) and the column temperature was 55 °C. Programs for the separation of all amino acids in Ile+Nva medium or for the rapid quantitation of Leu and D-norvaline in RPMI-VL+Nva are provided in Tables S1 and S2, respectively. Pre-column derivatization was conducted by mixing 5 μL of filtered conditioned medium with 15 μL of 500 mM sodium borate pH 9.5 and 5 μL of AccQ-Tag reagent (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, [22]; Waters). Control experiments indicated that the quantity of amino acids in RPMI-VL+Nva fell within the linear range of the assay. After one minute, samples were heated to 55 °C for 10 minutes to convert a labile di-derivative of Tyr to the mono-derivative [22]. Injection volume was 1 μL and derivatized amino acids were detected at 260 nm. Peak areas were determined by manual integration using Empower2 software (Waters). Concentrations of amino acids in media were calculated by reference to the peak area of the 100 μM D-norvaline internal standard.
To determine amino acid efflux rates, aliquots of medium were collected after 2, 4, 6 and 8 hours incubation. Rates were determined by linear regression fit to a concentration vs. time plot (e.g., Fig. 1B). The first two hours were considered to be an equilibration period and were not included in the fit. To normalize amino acid efflux rates to parasite numbers, aliquots of P. falciparum culture and uninfected erythrocyte controls were fixed by mixing with an equal volume of 0.2% glutaraldehyde in phosphate buffered saline. After permeabilization with 0.25% Triton X-100, samples were incubated with 400 nM YOYO-1 (Life Technologies) in phosphate buffered saline. Parasite density per unit volume was measured using an Accuri C6 flow cytometer (BD Biosciences) with manual gating to define the population of infected erythrocytes. Background was determined from samples of uninfected erythrocytes.
Figure 1. Characterization of amino acid efflux by P. falciparum trophozoites.
(A) UPLC chromatograms of amino acids in Ile+Nva medium after an eight hour incubation with iRBC (black) or uRBC (red). C–C, cystine; Cit, citrulline; oxMet; Met sulfoxide; O, ornithine; oxGlut, oxidized glutathione; Nva, D-norvaline. The asterisk indicates a peak originating from the derivatization reagent. Peak assignments were inferred from amino acid standards. (B) Rates of amino acid efflux to the culture medium are highly linear over eight hours. Lines indicate linear regression fits to the data points. (C) Comparison of mean efflux rates for 13 proteinogenic amino acids and ornithine (O), which is likely not effluxed but rather derived from extracellular Arg. Error bars are standard deviations from three independent biological replicates. (D) Relationship between amino acid abundance and efflux rates of selected amino acids for human α- and β-globin (top panel), human myoglobin (middle panel) and the UniProt database (lower panel). Lines indicate linear regression fits. (E) Inhibition of amino acid efflux upon treatment with 10 μM E-64d for four hours. Inhibition is reported as percentage of a 0.1% DMSO control. Error bars are standard deviations from three independent biological replicates.
The effect of E64-d on amino acid efflux was determined in a four-hour endpoint assay. Immediately after washing into amino acid-restricted medium, 10 μM E64-d or 0.1% DMSO was added to the cultures. Four hours later, medium was collected and prepared for analysis of amino acid concentrations as described above.
2.3. Parasite growth rate assays
Asynchronous parasites were washed into the following media: regular RPMI (section 2.1); RPMI-VL+Nva (Table 1); RPMI-VL (identical to RPMI-VL+Nva except D-norvaline was absent); and RPMI-VL supplemented with 200 μM Leu and 171 μM Val, designated RPMI-VL+VL. Every two days for 8 days, an aliquot was taken for determination of parasitemia by flow cytometry as described in section 2.2 and a separate aliquot was subcultured. The natural log of the product of parasitemia and cumulative dilution factor was plotted against time in hours and fit by linear regression to the equation , where p = parasitemia x cumulative dilution factor, a = starting parasitemia and τ = doubling time. For each medium, mean doubling times and standard deviations were calculated from three separate growth rate determinations in the same experiment.
2.4. Validation of D-norvaline as an internal standard
iRBC (16% parasitemia, trophozoite stage; 2% hematocrit) and uRBC (2% hematocrit) cultures were washed into RPMI-VL+Nva and divided into twelve 200 μL aliquots in a 96-well culture plate. In addition, twelve 200 μL aliquots of medium alone were added to the plate. Samples were incubated for four hours, at which time media were collected, filtered and derivatized as described in section 2.2. Amino acids were resolved using the UPLC program in Table S2 and areas of the Ile, Leu and Nva peaks were determined by manual integration. For each analyte, mean peak area and standard deviation were calculated from the values obtained for the twelve samples. Statistical analysis was conducted using a two-tailed Student’s t-test.
2.5. Wortmannin and dihydroartemisinin inhibition of Leu efflux
iRBC cultures were washed into RPMI-VL+Nva, supplemented with 10 μM wortmannin (EMD Millipore), 50 or 500 nM DHA, or 0.1% DMSO, and aliquotted into 200 μL volumes in a 96 well culture plate. At regular intervals, aliquots were removed and Leu concentrations were determined as described in section 2.2.
3. RESULTS AND DISCUSSION
3.1. Chromatographic separation of amino acids
Methods to resolve and quantify pre-column derivatized amino acids by high-pressure liquid chromatography have been available for many years. A factor limiting their application has been the long run times that were required to resolve all of the proteinogenic amino acids. The recent development of ultra-high pressure liquid chromatography (UPLC) and sub-2 μm particle columns has brought the time of amino acid analysis down to less than ten minutes while improving sensitivity and peak resolution [23]. We investigated the potential of this technology to rapidly quantify amino acid levels in conditioned medium from P. falciparum cultures. Using standard solutions of amino acids, we found that of the twenty proteinogenic amino acids (including both reduced and oxidized cysteine), all were resolved except Arg and Gln (Table 2). We also determined the retention times of ornithine and citrulline, two metabolites of Arg that have been shown to accumulate in culture medium [24], of 4-hydroxyproline, and of methionine sulfoxide and methionine sulfone, two oxidation products of Met (Table 2). Ornithine, 4-hydroxyproline and Met sulfone were resolved from the proteinogenic amino acids; however, citrulline co-eluted with Glu. Attempts to resolve these two amino acids by modifying the chromatography conditions were not successful. In addition, Met sulfoxide co-eluted with Asp. In summary, under the UPLC conditions used here, we are able to quantify the levels of all proteinogenic amino acids except Arg, Gln, Asp and Glu.
Table 2.
α-Amino acid retention times and efflux rates from P. falciparum-infected erythrocytes.
| Amino acid1 | Representative retention time2 (min) | Co-elution3 | Efflux rate (nmol//h/107 parasites)4 |
|---|---|---|---|
| Ala | 5.1 | 7.5 ± 0.4 | |
| Arg | 3.5 | Gln | NR |
| Asn | 2.7 | 0.5 ± 0.4 | |
| Asp | 3.9 | Met sulfoxide | NR |
| Citrulline | 4.5 | Glu | NR |
| Cys | 5.6 | ND | |
| Cystine | 6.6 | trace | |
| Glu | 4.4 | Citrulline | NR |
| Gln | 3.5 | Arg | NR |
| Gly | 3.6 | 2.9 ± 0.2 | |
| His | 2.5 | 3.6 ± 0.2 | |
| 4-hydroxyproline | 2.3 | ND | |
| Ile | 7.7 | NA | |
| Leu | 7.8 | 6.3 ± 0.3 | |
| Lys | 6.6 | 0.2 ± 0.1 | |
| Met | 6.9 | trace | |
| Methionine sulfoxide | 4.0 | Asp | NR |
| Methionine sulfone | 4.0 | ND | |
| Norvaline | 7.1 | NA | |
| Ornithine | 6.3 | 0.3 ± 0.1 | |
| Phe | 7.9 | 2.6 ± 0.1 | |
| Pro | 5.7 | 2.9 ± 0.2 | |
| Ser | 3.3 | 1.4 ± 0.1 | |
| Thr | 4.8 | 2.3 ± 0.1 | |
| Trp | 8.0 | 0.3 ± 0.1 | |
| Tyr | 6.8 | 0.6 ± 0.1 | |
| Val | 7.0 | 5.6 ± 0.3 |
Enantiomeric amino acids are L-isomers except for norvaline (D-isomer).
We have seen retention times vary up to ~ ±0.2 minutes from these values.
Identifies any amino acids that are not baseline-resolved. If blank, no co-eluting species were identified.
Mean ± standard deviation from three independent analyses. ND, not detected; trace: efflux rate < 0.2 nmol/107 parasites/h; NR, not resolved, therefore could not determine rate; NA, not applicable, amino acid was present in the medium.
3.2 Characterization of amino acid efflux by cultured P. falciparum trophozoites
Our studies of amino acid efflux focused on the trophozoite stage of the asexual cycle, as this is when the rate of hemoglobin uptake and catabolism is at its peak [1]. To quantify efflux rates, synchronized P. falciparum 3D7 trophozoites and uninfected erythrocyte controls were washed into an amino acid-restricted, RPMI-based medium that contained L-Ile as the only proteinogenic amino acid [12]; 100 μM D-norvaline was included in the medium as an internal standard to permit accurate quantitation of effluxed amino acids (I+Nva medium, Table 1; see section 3.3 for validation of D-norvaline as an internal standard). Chromatograms of amino acids released after 8 hours by iRBC and uRBC are shown in Fig. 1A. Both uRBC and iRBC released amino acids into the medium; however, for most of these, the amounts of amino acids effluxed from iRBC greatly exceeded those of uRBC. In the analyses that follow, iRBC efflux rates are corrected for the contribution of uRBC to the observed concentration of amino acid in the medium.
The efflux of amino acids from synchronized trophozoite cultures was highly linear over eight hours, which permitted the determination of efflux rates by linear regression (Fig. 1B). To permit comparisons between experiments, efflux rates were normalized to parasite numbers (determined by flow cytometry) and were reported as nmol/h/107 parasites (Table 2). This approach afforded highly reproducible efflux rates, as indicated by the relatively small standard deviations from three independent biological replicates (Table 2). A comparison of efflux rates for all amino acids exhibiting rates over 0.2 nmol/h/107 parasites (the estimated lower limit for reliable quantitation) is shown in Fig. 1C. The non-polar amino acids Ala, Val and Leu exhibited the highest efflux rates. Substantial rates of efflux were also observed for Gly, Phe, Pro, Ser, Thr and His. Efflux rates were low but measurable for Tyr, Trp, Asn, Lys and ornithine (the latter was presumably derived from effluxed Arg [24]). Negligible/no efflux was detected for the sulfur-containing amino acids Cys, cystine, and Met. Efflux rates were not determined for Asp, Glu, Arg and Gln due to incomplete resolution from other species (Table 2); however, inspection of chromatograms suggests that parasites efflux very little of these amino acids (Fig. 1A). The efflux rate for Ile could not be determined because it is a component of the culture medium.
Because hemoglobin catabolism has been reported to make a major contribution to amino acid efflux [13, 14], we asked whether the rates of the more highly effluxed amino acids (9 amino acids with efflux rates of ≥1 nmol/h/107 parasites) correlate with their abundance in human α- and β-globin (Fig. 1D). The coefficient of determination (R2) value for a linear regression fit was used as a measure of the correlation between the two variables. There was a strong correlation between efflux rates and amino acid abundance in α- and β-globin, with an R2 value of 0.90. To determine whether this correlation simply reflects general trends in the use of amino acids in proteins, we examined the correlation of efflux rates with amino acid abundance in human myoglobin. Myoglobin is distantly related to hemoglobin and has a similar oxygen-binding function. The correlation was much weaker with myoglobin, with an R2 value of 0.29. We also examined the correlation with amino acid abundance in the UniProt database release 2014_5, which is derived from 228,536 protein sequences. Here too, the correlation between efflux rates and abundance was poor (Fig. 1D). These results are consistent with erythrocyte hemoglobin serving as a dominant source of the effluxed amino acids included in the analysis. We note, however, that the correlation breaks down for amino acids such as Asp and Lys; these residues are effluxed at much lower rates (if at all) than would be predicted by their abundance in human hemoglobin. Thus, there appears to be an element of selectivity in the efflux of amino acids from the parasitized erythrocyte.
To further examine whether hemoglobin is the source of effluxed amino acids, the effect of 10 μM E-64d on amino acid concentrations in the medium was assessed. E-64d is a cell-permeant cysteine protease inhibitor that blocks the proteolytic activity of food vacuole falcipains and causes the accumulation of undegraded hemoglobin in the vacuole [25]. In a four-hour endpoint assay, E-64d reduced the concentrations of amino acids in the medium to about 20% of control values (Fig. 1E), which is consistent with hemoglobin catabolism serving as the dominant source of effluxed amino acids. The residual level of amino acids observed after four hours could accrue from incomplete falcipain inhibition, from hemoglobin catabolism by other vacuolar endopeptidases such as the plasmepsins, or from contributions by other proteolytic pathways, such as the proteasome.
Finally, we asked whether parasites efflux a substantial fraction of the amino acids generated from hemoglobin catabolism. Val and Leu were used for this analysis because they are effluxed at high rates and the parasite lacks biosynthetic pathways for these amino acids. Using an adult human mean corpuscular hemoglobin value of 30 pg/cell [26] and the efflux rates for Val and Leu in Table 2, and assuming that these rates are sustained for a third of the 44 hour asexual cycle during which 70% of erythrocyte hemoglobin is degraded [1], we calculate that 41% of Val and 40% of Leu generated from hemoglobin catabolism is effluxed. This exercise suggests that a large fraction of the amino acids generated through hemoglobin catabolism is lost from the parasite and is consistent with a previous report that asexual P. falciparum uses only one-fifth of the amino acids generated from hemoglobin catabolism for protein synthesis [2].
3.3 Reformulation of the amino acid composition of RPMI medium for sensitive and accurate quantitation of Leu and Val efflux
Taken together, our studies indicate that amino acids that are both highly abundant in hemoglobin and that are effluxed from parasitized erythrocytes at high rates (e.g., Ala, Leu, Val) can be useful probes of in vivo flux through the hemoglobin endocytic-catabolic pathway. Because Ala does not elute near the D-norvaline internal standard (Fig. 1A), we focused on Leu and Val as the analytes of choice. The use of two analytes rather than one provides redundancy, which could be advantageous when conducting inhibitor screens; should a screening compound interfere with one of the analytes, the other will be available for quantitation. In this section, we describe the development of a robust and rapid assay for quantifying Leu and Val efflux.
While Ile+Nva medium was useful for profiling global amino acid efflux in the above experiments, we have observed that many laboratory P. falciparum lines grow more slowly in this medium, and is was reported that some lines such as HB3 fail to grow at all [12]. To develop an amino acid efflux assay that can be used with the widest range of parasite lines, we reformulated the standard RPMI recipe such that it contained all proteinogenic amino acids except Ala, Val and Leu (regular RPMI lacks Ala but contains Val and Leu; Table 1). In addition to removing these amino acids, the concentrations of many other amino acids were reduced such that the highest concentration of any amino acid was 200 μM (Table 1). The rationale for these changes was to reduce the total amine content of the medium to avoid exceeding the linear range of the assay. The concentration of Ile was reduced from 381 μM to 100–150 μM to enhance baseline separation between Ile and the neighboring Leu peak. Finally, the non-proteinogenic amino acid 4-hydroxyproline, a component of regular RPMI, was omitted. The reformulated medium was named “RPMI-VL” to reflect the removal of Val and Leu.
An internal standard was considered to be necessary to account for evaporation of cultures during incubation and for variability introduced during pipetting and sample injection steps. D-norvaline appeared to be a promising candidate based on its structural similarity to the analytes, its resolution from all proteinogenic amino acids (Table 2) and its proximity in the chromatogram to the analytes Val and Leu (Fig. 1A). The D-isomer was chosen to avoid any potential toxic effects arising from misincorporation into proteins [27]. 100 μM D-norvaline was added to RPMI-VL; this medium was termed “RPMI-VL+Nva”.
To determine the whether these changes to the standard RPMI formula had any effect on parasite viability or cell cycle duration, growth rate assays were carried out with P. falciparum clone 3D7. Doubling times in the following media were determined: i) standard RPMI; ii) RPMI-VL; iii) RPMI-VL supplemented with 200 μM Leu and 171 μM Val (designated RPMI-VL+VL); and iv) RPMI-VL+Nva. Growth in the reformulated media was as robust as it was in standard RPMI (Fig. 2A). Thus, there appeared to be no deleterious effect on proliferation rate arising from the removal of Leu and Val or the addition of D-norvaline.
Figure 2. Validation of reformulated RPMI and the use of D-norvaline as an internal standard.
(A) Doubling times of 3D7 parasites in regular RPMI and in RPMI-VL, RPMI-VL supplemented with Val and Leu (RPMI-VL+VL), and RPMI-VL with D-norvaline (RPMI-VL+NVA) as assessed in a 6-day growth experiment. Error bars are the standard deviation for three separate doubling time determinations in one experiment. (B) Comparison of peak areas for Ile, Leu and D-norvaline in the absence of added cells (“medium”) or in the presence of uRBC (2% hematocrit) or iRBC (2% hematocrit, 16% parasitemia) after 4 hours. Bars are the mean values obtained from analysis of twelve samples (see section 2.4). Error bars indicate the standard deviation.
To be an effective internal standard, D-norvaline levels should not change appreciably in the presence of a high parasite load. To test whether this was the case, peak areas for D-norvaline, Leu and Ile in RPMI-VL+Nva were determined after four hours incubation of: i) medium alone; ii) medium containing P. falciparum-infected erythrocytes at 16% parasitemia, 2% hematocrit; and iii) medium containing uninfected erythrocytes at 2% hematocrit (Fig. 2B). As expected, high levels of Leu were only observed in the iRBC sample. Consumption of Ile, which is not present in human hemoglobin, was also observed only for the iRBC sample and was consistent with the use of this amino acid for parasite protein synthesis. Levels of D-norvaline were very similar in the three samples, but did appear to be elevated by about 5% in the iRBC samples. Assuming a normal distribution and equal variance, this difference was statistically significant, with a p-value <0.001 for the difference in the means of the iRBC and media alone samples. The reason for this slight elevation is not known; it is possible that a small amount of an unknown parasite metabolite co-elutes with D-norvaline. In most cases, this small deviation will be inconsequential. We examined the possibility of using α-aminoadipic acid as an alternate internal standard; however, this additive had a slight suppressive effect on parasite growth (data not shown) and was not further pursued.
3.4. Effect of wortmannin and dihydroartemisinin on Leu efflux
To examine the utility of the amino acid efflux assay in evaluating the kinetics of in vivo inhibition of hemoglobin endocytosis and catabolism, we selected wortmannin as a challenging test case. Wortmannin is a potent and selective inhibitor of phosphatidylinositol-3-kinases (PI3Ks), enzymes that catalyze a phosphotransfer reaction from ATP to phosphatidylinositol to generate phosphatidylinositol-3-phosphate (PI3P) [28]. Wortmannin has been shown to inhibit P. falciparum PI3K in vitro and to substantially reduce parasite synthesis of PI3P [29, 30]. However, wortmannin is highly unstable in RPMI medium, forming adducts with a number of amino acids that have reduced inhibitory activity [31]. Perhaps because of this lability, we have found that wortmannin at 10 μM concentration does not impair parasite proliferation, despite genetic evidence that PI3K is an essential enzyme [29]. The inability of wortmannin to kill parasites at single doses as high as 25 μM has been previously reported [29]; in contrast, wortmannin was found to “stall” parasite growth over six days of treatment [30].
In mammalian and yeast cells, PI3P is predominantly located on early endosomes and plays critical roles in endocytosis, endosome fusion and motility, and protein traffic to the vacuole or lysosome [32]. In P. falciparum, PI3P has been localized to the membranes of the food vacuole, the apicoplast and the lumen of the endoplasmic reticulum [29, 33]. The presence of PI3P on the food vacuole membrane implies a role in the transport of endocytosed hemoglobin. Consistent with this idea, wortmannin has been shown to impair hemoglobin delivery to the vacuole. Vaid et al have reported that a five-hour incubation with wortmannin caused an accumulation of undegraded hemoglobin in the parasite, which appeared to be contained in vesicular structures [30]. In a separate study, wortmannin promoted food vacuole fragmentation and disruption of the vacuolar membrane [34]. On the basis of these findings, a reduction in the rate of hemoglobin catabolism was expected upon wortmannin treatment.
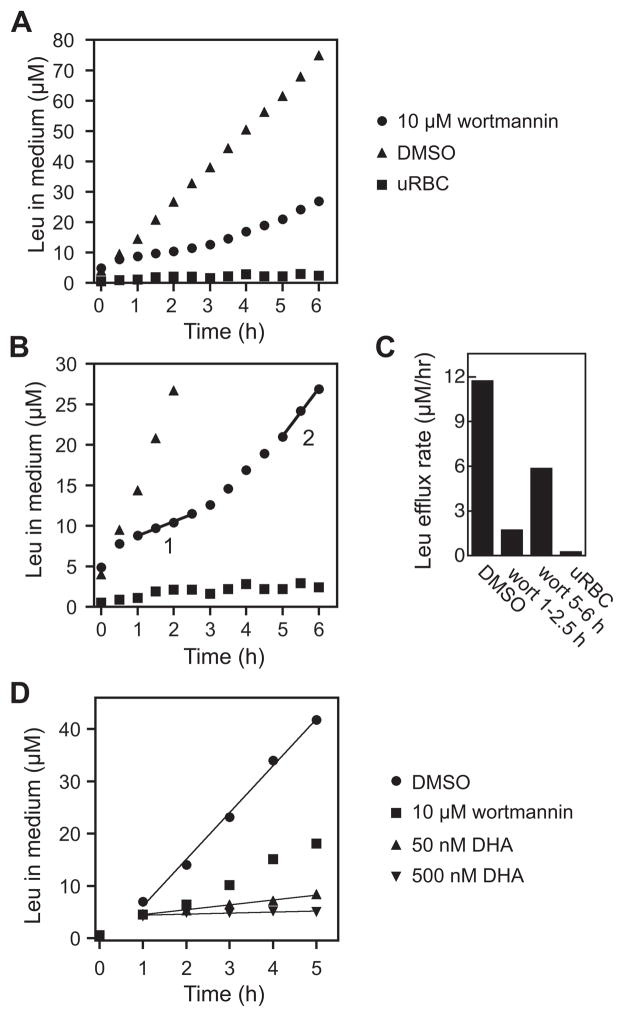
To determine whether Leu efflux could serve as an indicator of wortmannin-induced alterations in rates of hemoglobin uptake and catabolism in vivo, iRBC were washed into RPMI-VL+Nva and supplemented with either 0.1% DMSO (control) or 10 μM wortmannin. Culture media were sampled every 30 minutes for six hours and concentrations of Leu were determined. Suppression of Leu efflux by wortmannin was evident after 60 minutes and continued for the duration of the experiment (Fig. 3A). Interestingly, there appeared to be two phases to the kinetics of wortmannin inhibition of Leu efflux (Fig. 3B). In the first phase (“1” in Fig. 3B), lasting from 1 to 2.5 hours, the rate of Leu efflux in the presence of wortmannin was reduced to a steady-state value that was 15% of that in the control (Fig. 3C). Over time, however, the rate of Leu efflux in the presence of wortmannin increased, reaching a value corresponding to half that of the control in the time period of 5 – 6 hours (Fig. 3C). These data support a role for PI3K-catalyzed de novo PI3P synthesis in hemoglobin endocytosis and delivery to the food vacuole; however, they also suggest that wortmannin inhibition of PI3K is transient, likely due to the instability of wortmannin in culture medium [31]. The “recovery” Leu efflux kinetics that we observe may be due to the biosynthesis of PI3K once wortmannin is exhausted from the medium, and they provide an explanation for the lack of wortmannin lethality when administered in single doses [29].
Figure 3. Inhibition of Leu efflux by PI3K inhibitors.
(A) Time course of Leu efflux from iRBC treated with 10 μM wortmannin (circles) or 0.1% DMSO (triangles) and from uRBC (squares). Data points are the average of triplicate analyses (iRBC) or represent single analyses (uRBC). Similar results were obtained in two other independent experiments (not shown). (B) Wortmannin inhibition of Leu efflux is transient. The same data as in (A) are shown but with an expanded ordinate. Two kinetic phases are shown: an early inhibition phase (“1”; 1 to 2.5 h) and a later recovery phase (“2”; 5 to 6 h). Lines were generated by linear regression fits. (C) Rates of Leu efflux calculated from the data in panels A and B. iRBC/DMSO and uRBC rates were determined by linear regression fit of data from 0 – 6 h. iRBC/wortmannin rates were determined as shown in panel B. (D) Comparison of effects of wortmannin and dihydroartemisinin (DHA) on Leu efflux. Leu effluxed by uRBC has been subtracted from data points. Lines were generated by linear regression fits of data from 1–5 h.
A recent study has revealed that P. falciparum PI3K is potently inhibited by the artemisinin class of anti-malarials [35]. To further define the consequences of PI3K inhibition on flux through the hemoglobin endocytic/catabolic pathway, we compared the effects of wortmannin and dihydroartemisinin (DHA) on Leu efflux (Fig. 3D). DHA was selected for this analysis because its effects on both parasite PI3K activity (complete inhibition at 4 nM) and 3D7 parasite replication (EC50 value of 5 nM) have been characterized [35, 36]. Leu efflux profiles for parasites incubated with wortmannin (10 μM) and DHA (50 and 500 nM; 10-fold and 100-fold above the EC50 value, respectively) both reveal strong suppression of Leu efflux between one and two hours. However, after two hours, the rate of Leu efflux in the presence or wortmannin begins to increase, while in the presence of DHA it maintains a steady-state value that is well described by a linear fit of the data from 1–5 hours. Comparison to the DMSO control indicates that the steady state rates of Leu efflux are reduced to 10% and 2% of control upon treatment with 50 nM and 500 nM DHA, respectively. These results are consistent with a common mode of action (i.e., inhibition of PI3K) by wortmannin and DHA, with the sustained effect of the latter likely arising from its greater stability.
Together, these experiments provide important “proof-of-principle” evidence that Leu efflux can provide a detailed kinetic picture of the in vivo perturbation of hemoglobin endocytosis and/or catabolism by small molecules. This assay should prove valuable in the characterization of inhibitors that are expected to target these processes.
4. CONCLUSIONS
A sensitive and precise method with high temporal resolution has been developed to interrogate the kinetics of amino acid efflux from cultured P. falciparum. Use of amino acid-restricted culture media, an internal standard, and ultra-high pressure liquid chromatography were key factors in enabling rapid and accurate quantitation of effluxed amino acids. The utility of this method for characterizing the in vivo inhibition of hemoglobin uptake (wortmannin, DHA) and catabolism (E-64d) was demonstrated. We anticipate that this approach will provide valuable insights into the mechanism of action of compounds that are predicted to perturb hemoglobin endocytosis or catabolism. It should also find application in screens for novel inhibitors of these processes.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI077638), the Bill & Melinda Gates Foundation (OPP1067465) and USDA National Institute of Food and Agriculture, project VA-139761. The sponsors had no role in study design, collection and interpretation of data, writing of the manuscript or decision to publish. We are grateful to Waters Corp. for excellent technical support.
Abbreviations
- DHA
dihydroartemisinin
- iRBC
infected red blood cell
- Nva
D-norvaline
- PI3P
phosphatidylinositol-3-phosphate
- PI3K
phosphatidylinositol-3-kinase
- UPLC
ultra-high pressure liquid chromatography
- uRBC
uninfected red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanssen E, Knoechel C, Dearnley M, Dixon MW, Le Gros M, Larabell C, et al. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J Struct Biol. 2012;177:224–32. doi: 10.1016/j.jsb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krugliak M, Zhang J, Ginsburg H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol Biochem Parasitol. 2002;119:249–56. doi: 10.1016/s0166-6851(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 3.Abu Bakar N, Klonis N, Hanssen E, Chan C, Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–50. doi: 10.1242/jcs.061499. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa M, Hepler PK, Huff CG, Sprinz H. The feeding mechanism of avian malarial parasites. J Cell Biol. 1966;28:355–73. doi: 10.1083/jcb.28.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott DA, McIntosh MT, Hosgood HD, Chen S, Zhang G, Baevova P, et al. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 2008;105:2463–8. doi: 10.1073/pnas.0711067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus MD, Schneider TG, Taraschi TF. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J Cell Sci. 2008;121:1937–49. doi: 10.1242/jcs.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis SE, Sullivan DJ, Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Milani KJ, Schneider TG, Taraschi TF. Defining the morphology and mechanism of the hemoglobin transport pathway in Plasmodium falciparum-infected erythrocytes. Eukaryot Cell. 2015;14:415–26. doi: 10.1128/EC.00267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal S, Klemba M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J Biol Chem. 2007;282:35978–87. doi: 10.1074/jbc.M703643200. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal PJ. Hydrolysis of erythrocyte proteins by proteases of malaria parasites. Curr Opin Hematol. 2002;9:140–5. doi: 10.1097/00062752-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–91. doi: 10.1007/3-540-29088-5_11. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci USA. 2006;103:8840–5. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenedella RJ, Rosen H, Angel CR, Saxe LH. Free amino-acid production in vitro by Plasmodium berghei. Am J Trop Med Hyg. 1968;17:800–3. doi: 10.4269/ajtmh.1968.17.800. [DOI] [PubMed] [Google Scholar]
- 14.Zarchin S, Krugliak M, Ginsburg H. Digestion of the host erythrocyte by malaria parasites is the primary target for quinoline-containing antimalarials. Biochem Pharmacol. 1986;35:2435–42. doi: 10.1016/0006-2952(86)90473-9. [DOI] [PubMed] [Google Scholar]
- 15.Groman NB. Dynamic aspects of the nitrogen metabolism of Plasmodium gallinaceum in vivo and in vitro. J Infect Dis. 1951;88:126–50. doi: 10.1093/infdis/88.2.126. [DOI] [PubMed] [Google Scholar]
- 16.Famin O, Ginsburg H. Differential effects of 4-aminoquinoline-containing antimalarial drugs on hemoglobin digestion in Plasmodium falciparum-infected erythrocytes. Biochem Pharmacol. 2002;63:393–8. doi: 10.1016/s0006-2952(01)00878-4. [DOI] [PubMed] [Google Scholar]
- 17.Hoppe HC, van Schalkwyk DA, Wiehart UI, Meredith SA, Egan J, Weber BW. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:2370–8. doi: 10.1128/AAC.48.7.2370-2378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts L, Egan TJ, Joiner KA, Hoppe HC. Differential effects of quinoline antimalarials on endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:1840–2. doi: 10.1128/AAC.01478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal PJ. Plasmodium falciparum: effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp Parasitol. 1995;80:272–81. doi: 10.1006/expr.1995.1033. [DOI] [PubMed] [Google Scholar]
- 20.Smythe WA, Joiner KA, Hoppe HC. Actin is required for endocytic trafficking in the malaria parasite Plasmodium falciparum. Cell Microbiol. 2008;10:452–64. doi: 10.1111/j.1462-5822.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 22.Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–87. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- 23.Salazar C, Armenta JM, Cortes DF, Shulaev V. Combination of an AccQ·Tag-Ultra Performance Liquid Chromatographic Method with Tandem Mass Spectrometry for the Analysis of Amino Acids. In: Alterman MA, Hunziker P, editors. Amino Acid Analysis: Methods and Protocols. Springer Science; 2012. pp. 13–28. [DOI] [PubMed] [Google Scholar]
- 24.Olszewski KL, Morrisey JM, Wilinski D, Burns JM, Vaidya AB, Rabinowitz JD, et al. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe. 2009;5:191–9. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal PJ. Cysteine proteases of malaria parasites. Int J Parasitol. 2004;34:1489–99. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Vajpayee N, Graham SS, Bem S. Basic examination of blood and bone marrow. In: McPherson RA, Pincus MR, editors. Henry’s clinical diagnosis and management by laboratory methods. 22. Saunders; 2011. pp. 509–35. [Google Scholar]
- 27.Apostol I, Levine J, Lippincott J, Leach J, Hess E, Glascock CB, et al. Incorporation of norvaline at leucine positions in recombinant human hemoglobin expressed in Escherichia coli. J Biol Chem. 1997;272:28980–8. doi: 10.1074/jbc.272.46.28980. [DOI] [PubMed] [Google Scholar]
- 28.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–7. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 29.Tawk L, Chicanne G, Dubremetz JF, Richard V, Payrastre B, Vial HJ, et al. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot Cell. 2010;9:1519–30. doi: 10.1128/EC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood. 2010;115:2500–7. doi: 10.1182/blood-2009-08-238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H, Barnes KR, Weissleder R, Cantley L, Josephson L. Covalent reactions of wortmannin under physiological conditions. Chemistry & biology. 2007;14:321–8. doi: 10.1016/j.chembiol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Raiborg C, Schink KO, Stenmark H. Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic. Febs J. 2013;280:2730–42. doi: 10.1111/febs.12116. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell. 2012;148:201–12. doi: 10.1016/j.cell.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howe R, Kelly M, Jimah J, Hodge D, Odom AR. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot Cell. 2013;12:215–23. doi: 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–7. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duraisingh MT, Roper C, Walliker D, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol. 2000;36:955–61. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.