Abstract
Objective
Cardiac changes of hypertensive pregnancy include left ventricular hypertrophy (LVH) and diastolic dysfunction. These are thought to regress postpartum. We hypothesized that women with a history of hypertensive pregnancy would have altered left ventricular (LV) geometry and function when compared to women with only normotensive pregnancies.
Methods
In this cohort study, we analyzed echocardiograms of 2637 women who participated in the Family Blood Pressure Program (FBPP). We compared LV mass and function in women with hypertensive pregnancy compared to those with normotensive pregnancies.
Results
Women were evaluated at a mean age of 56 years: 427 (16%) had at least one hypertensive pregnancy; 2210 (84%) had normotensive pregnancies. Compared to women with normotensive pregnancies, women with hypertensive pregnancy had a greater risk of LVH (OR: 1.42, 95% CI 1.01-1.99, p=0.05), after adjusting for age, race, research network of the FBPP, education, parity, BMI, hypertension and diabetes. When duration of hypertension was taken into account, this relationship was no longer significant (OR: 1.19, CI 0.08-1.78 p=0.38). Women with hypertensive pregnancies also had greater left atrial size and lower mitral E/A ratio after adjusting for demographic variables. The prevalence of systolic dysfunction was similar between the groups.
Conclusions
A history of hypertensive pregnancy is associated with LVH after adjusting for risk factors; this might be explained by longer duration of hypertension. This finding supports current guidelines recommending surveillance of women following a hypertensive pregnancy, and sets the stage for longitudinal echocardiographic studies to further elucidate progression of LV geometry and function after pregnancy.
Keywords: Hypertension, Pregnancy, Left ventricular hypertrophy, Women, Diastolic function
Introduction
Left ventricular hypertrophy (LVH), as diagnosed by echocardiography, is associated with an increased risk for cardiovascular mortality [1], independent of other traditional risk factors [2]. LVH is the typical cardiac response to chronic ventricular pressure overload, such as with hypertension, or volume overload. Women are more likely to develop LVH in response to hypertension than men [3]. This, along with their tendency for higher background left ventricular (LV) ejection fraction (EF) [4], may contribute to the increased risk for heart failure with preserved EF and other conditions associated with diastolic dysfunction noted in women [5]. Given these sex differences, predictors of LVH in women are needed.
Pregnancy is considered a ‘stress test’ for cardiovascular disease in women: those who develop a hypertensive pregnancy disorder are at greater risk for chronic hypertension, ischemic heart disease, stroke, and cardiovascular death later in life [6-8]. However, little is known about the impact of a history of hypertensive pregnancy on subsequent LVH and LV function in women. The hypertensive pregnancy disorders include gestational hypertension, preeclampsia-eclampsia, chronic hypertension and chronic hypertension with superimposed preeclampsia. Immediate cardiac effects of a hypertensive pregnancy include eccentric and concentric ventricular remodeling [9], as well as impaired contractility and diastolic dysfunction [10]. Increases in left atrial size are accompanied by increases in atrial natriuretic peptide levels [11]. Recent studies have questioned the traditional wisdom that these changes regress postpartum. One study demonstrated that LV geometric changes and diastolic dysfunction persisted 1 year following a preeclamptic pregnancy [12]. A second demonstrated that women with a history of hypertensive pregnancies are more likely to develop heart failure and arrhythmias approximately 8 years postpartum [13]. These findings suggest that changes in myocardial structure and function persist postpartum.
Our objective was to examine the relationship between a history of hypertensive pregnancy and LV geometry and function, while controlling for traditional risk factors and duration of hypertension. We hypothesized that women with a history of hypertensive pregnancy would be more likely to LVH and diastolic dysfunction decades after pregnancy, compared to those who had normotensive pregnancies.
Methods
Participants
The NHLBI Family Blood Pressure Program (FBPP) consists of four separate multicenter networks: GenNet, GENOA, (Genetic Epidemiology Network of Atherosclerosis), HyperGEN (Hypertension Genetic Epidemiology Network), and SAPPHIRe (Stanford Asian Pacific Program in Hypertension and Insulin Resistance). The FBPP was established in 1995 to investigate the genetic determinants of hypertension [14]. Each network collected data on multiple racial and ethnic groups. While recruitment criteria differed among the networks (Supplemental table 1), all recruited subjects were from families who had multiple members with elevated blood pressure or had a genetic disposition to hypertension. The study design and recruitment have been previously described [14]. The four networks used common definitions and standardized measurement protocols to facilitate pooling of results [15]. This current study included all women from all networks who reported at least one pregnancy lasting more than 6 months, and who had an echocardiogram. Echocardiograms were performed at the field sites in Maywood, IL (GenNet); Jackson, MS and Starr County, TX (GENOA); Forsyth County, NC, Minneapolis, MN, Salt Lake City, UT, and Birmingham, AL (HyperGEN); and Hawaii (SAPPHIRe). Imaging was performed and Doppler evaluation was recorded using standardized protocols and methods as previously described; studies were read centrally at Cornell Medical Center, with >90% of the studies verified or primarily read by a single experienced investigator [16-18]. Principal sonographers received training at the center, and feedback on studies was provided by the Reading Center to monitor compliance with the protocol.
All subjects provided written informed consent prior to participating. The protocol was approved by each site’s institutional review board.
Questionnaires
Questionnaires were administered by a trained interviewer. The Phase 1 (1996-2000) questionnaire examined personal and family medical histories. An additional standardized, previously validated questionnaire concerning hypertension occurring during pregnancy [19] was added in Phase 2 (2000-2004); therefore, only Phase 2 data were included in the analysis. In the pregnancy questionnaire, women were asked, ‘Have you had at least one pregnancy lasting more than 6 months?’ Women who responded ‘yes’ were asked to enumerate their pregnancies, and to state whether they developed hypertension in any pregnancy that lasted more than 6 months (thus categorizing them into the hypertensive pregnancy group).
Clinical and laboratory variables
Hypertension was diagnosed if the participant reported using a prescription anti-hypertensive medication, or if average blood pressures were ≥140 mm Hg systolic and/or ≥90 mm Hg diastolic at the study examination. Blood pressure was measured by trained technicians using an automated oscillometric device after 5 minutes of rest. Values were calculated as the average of the 2nd and 3rd of three readings, taken at least 2 minutes apart. Duration of hypertension was calculated as the time between the self-reported onset of hypertension and the study visit. For those with an unknown duration of hypertension, we imputed duration as the mean duration (among those for whom the duration of hypertension was known).
Body mass index (BMI) was defined as the weight divided by height (kg/m2). Weight was obtained in the fasting state. Height was measured with the subject standing with heels together, without shoes, against a vertically mounted ruler. ‘Ever’ smoking was defined as a lifetime history of having smoked at least 100 cigarettes. Diabetes was defined by self-report of a physician diagnosis.
Echocardiographic variables
All echocardiograms were interpreted in a blinded manner. LV dimensions were measured at end-diastole and end-systole in accordance with American Society of Echocardiography (ASE) recommendations on up to 3 cycles [20]. When optimal orientation of the LV could not be obtained for M-mode echocardiography, 2-dimensional linear dimensions were obtained by the leading-edge ASE convention at the time [21]. Mitral inflow pulsed wave Doppler recordings were traced along the black-white interface to measure peak E-wave and A-wave velocities and deceleration time of early inflow. The isovolumic relaxation time (IVRT) was measured from the closure spike of the aortic valve to the onset of mitral inflow. LV outflow tract (LVOT) pulsed wave Doppler recording was traced along the black-white interface to measure the time-velocity integral (TVI).
End diastolic LV dimensions were used to calculate LV mass by a necropsy-validated method.[22] LV mass was then indexed to body surface area (LV mass index, LVMI). The definition of LVH was a LVMI >95 g/m2. Relative wall thickness (RWT) was calculated as [end-diastolic ventricular septal thickness (IVSd) + end-diastolic posterior wall thickness (PWTd)] ÷ [end-diastolic LV internal dimension (LVIDd)]. An increased RWT (> 0.42) was used to classify LV geometric patterns. Normal geometry was present when both LVMI and RWT were normal; concentric remodeling referred to the presence of a normal LVMI with an increased RWT; eccentric hypertrophy was defined as a normal RWT plus an increased LVMI, and concentric hypertrophy was defined as an increased RWT plus an increased LVMI.
The EF was calculated from linear LV dimensions using the Teichholz [23] method. Systolic dysfunction was defined as an EF < 50%. The cardiac index was calculated as the product of the time-velocity integral of the transaortic flow at the aortic annular level, annular cross sectional area and heart rate, indexed to body surface area.
Diastolic parameters reported were mitral inflow E/A ratio, IVRT and deceleration time, and left atrial dimensions. We present these as continuous variables, with lower E/A ratio, longer IVRT and longer deceleration times suggesting slower relaxation times, and larger atrial dimensions suggesting a higher prevalence of diastolic dysfunction. Echocardiographic data in this study were obtained before the wide availability of tissue Doppler analysis. Given our current understanding of diastolic function grading, in the absence of tissue Doppler measurements, more precise characterization of diastolic function (normal vs. mild to severe diastolic dysfunction, or grades 1-4) was not attempted.
Statistical analyses
Differences in age, race, network, and education between the hypertensive pregnancy and normotensive pregnancy groups were assessed using chi-square tests for categorical and two-sample t-tests for continuous variables. Because of the differences in recruitment methods across networks, linear and logistic regression models adjusting for age, race, network, and education were used to assess between-group differences in participant characteristics and echocardiography variables. In order to account for family relationships among the women, all models were fit with generalized estimating equations. Data are presented as adjusted mean (SD) or percentage within group. Additionally, we used logistic regression to assess associations between a history of hypertensive pregnancy and LVH, after adjusting for age, race, network, education, parity (categorized as 1, 2, or 3 or more pregnancies) BMI, diabetes, and hypertension and hypertension duration. For the 8% of hypertensive subjects with an unknown duration, the duration of hypertension was imputed as the mean duration of hypertension among subjects with known duration. However, we also included an “unknown duration” indicator variable in the final model. We also performed analyses replacing current hypertension with systolic blood pressure in view of the strong association of current systolic BP with LV mass [24]. Analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was defined as p< 0.05.
Results
Participant characteristics
There were 2637 women from 1449 families who participated in Phase 2, reported at least one pregnancy lasting more than 6 months, and had an echocardiogram. Of these, 427 (16%) reported having had at least one hypertensive pregnancy. The mean age of the entire group was 56 ± 13 years at the time of echocardiography. The mean age at the first hypertensive pregnancy among women who reported a hypertensive pregnancy was 27 ± 7 years, which was 26 ± 14 years prior to the echocardiography.
There were differences in age, race, and education by network due to recruitment criteria and network location (p<0.001 for all). As the proportion of subjects from each network differed between the pregnancy groups, age and race also differed among the pregnancy groups (Table 1). Women who had a hypertensive pregnancy were younger than women who had normotensive pregnancies. These age differences remained significant after adjusting for network. Women who had a history of a hypertensive pregnancy were more likely to be non-Hispanic Black and less likely to be Japanese compared to women who had normotensive pregnancies. All further variables are presented adjusted for age, race, network, and education level.
Table 1.
Demographic Characteristics
| Variable | Normotensive Pregnancy (n=2210) | Hypertensive Pregnancy (n=427) | P |
|---|---|---|---|
| Age | 56.0 ±13.0 | 53.5 ±13.1 | <0.001 |
| Race | <0.001 | ||
| Non-Hispanic White | 145 (7%) | 29 (7%) | |
| Hispanic | 681 (31%) | 135 (32%) | |
| Non-Hispanic Black | 983 (44%) | 226 (53%) | |
| Japanese | 401 (18%) | 37 (9%) | |
| Network | <0.001 | ||
| GENOA | 1346 (61%) | 294 (69%) | |
| GenNet | 210 (10%) | 43 (10%) | |
| HyperGEN | 253 (11%) | 53 (12%) | |
| SAPPHIRe | 401 (18%) | 37 (9%) | |
| Education | 0.13 | ||
| Less than High School (≤ 8 years) | 518 (23%) | 91 (21%) | |
| Partial High School (9-11 years) | 261 (12%) | 65 (15%) | |
| High School Graduate or GED (12 years) | 505 (23%) | 106 (25%) | |
| Post High School (> 12 years) | 926 (42%) | 165 (39%) |
Values shown are mean ± standard deviation or count (percentage)
GENOA: Genetic Epidemiology Network of Atherosclerosis; HyperGEN: Hypertension Genetic Epidemiology Network; SAPPHIRe: Stanford Asian Pacific Program in hypertension and Insulin Resistance; GED: General educational development
Women with at least one hypertensive pregnancy had a higher mean BMI and were more likely to have diabetes, hypertension and a family history of hypertension than women with normotensive pregnancy (Table 2). Smoking status and renal function did not significantly differ between the groups.
Table 2.
Participant Characteristics
| Variable | N | Unadjusted
|
Adjusted for age, race, education, and network
|
|||
|---|---|---|---|---|---|---|
| Normotensive Pregnancy (n=2210) | Hypertensive Pregnancy (n=427) | Normotensive Pregnancy (n=2210) | Hypertensive Pregnancy (n=427) | P | ||
| Parity | 2636 | <0.001 | ||||
| 1 pregnancy | 289 (13%) | 41 (10%) | 13% | 9% | ||
| 2 pregnancies | 562 (25%) | 87 (20%) | 25% | 21% | ||
| 3+ pregnancies | 1358 (61%) | 299 (70%) | 62% | 70% | ||
| BMI (kg/m2) | 2629 | 30.7 ± 6.9 | 33.9 ± 7.7 | 30.8 ± 6.4 | 33.2 ± 7.3 | <0.001 |
| BMI ≥ 30 (kg/m2) | 2629 | 1076 (49%) | 284 (67%) | 50% | 62% | <0.001 |
| Ever smoked | 2637 | 631 (29%) | 111 (26%) | 28% | 26% | 0.38 |
| Diabetes | 2635 | 614 (28%) | 151 (35%) | 28% | 35% | <0.001 |
| Hypertension | 2634 | 1164 (53%) | 294 (69%) | 53% | 71% | <0.001 |
| Duration of hypertension, years* | 1458 | 11.6 ± 11.0 | 18.0 ± 11.8 | 11.3 ± 9.9 | 18.9 ± 10.4 | <0.001 |
| SBP (mmHg) | 2555 | 129.4 ± 23.8 | 136.5 ± 25.0 | 129.6 ± 19.5 | 135.7 ± 22.1 | <0.001 |
| DBP (mmHg) | 2555 | 71.6 ± 11.6 | 74.5 ± 12.5 | 71.8 ± 10.4 | 73.4 ± 11.7 | 0.006 |
| Antihypertensive medications | 2633 | 960 (43%) | 261 (62%) | 44% | 63% | <0.001 |
| Family history of hypertension | 2637 | 1555 (70%) | 348 (81%) | 71% | 78% | <0.001 |
| Heart rate (bpm) | 2636 | 67.5 ± 10.4 | 68.6 ± 11.1 | 67.6 ± 10.3 | 68.3 ± 11.1 | 0.24 |
| Log(creatinine + 1) | 2359 | 0.57 ± 0.15 | 0.57 ± 0.19 | 0.57 ± 0.14 | 0.58 ± 0.18 | 0.12 |
| Creatinine (mg/dL)** | 2359 | 0.79 ± 0.38 | 0.80 ± 0.45 | 0.77 | 0.79 | |
Values shown are mean ± standard deviation or count (percentage)
BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; bpm: beats per minute.
Duration of hypertension is shown among women with current hypertension
Adjusted models were fit with log-transformed values and the adjusted means have been back-transformed to raw units for interpretability
The age of onset of hypertension was 40.9 ±11.2 years in women with hypertensive pregnancy compared to 50.6 ± 11.9 years in women with normotensive pregnancies (p<0.001). Consequently, the duration of current hypertension was longer in women with hypertensive pregnancy compared to women with normotensive pregnancies (18.0 ± 11.8 vs 11.6 ± 11.0, p<0.001). Among women with hypertensive pregnancy, the age of onset of hypertension was 13.2 ± 12.2 years after the age at which the first hypertensive pregnancy occurred.
Left ventricular geometry
Women who had a hypertensive pregnancy had greater ventricular size, LV wall thickness, and LV mass than women who had a normotensive pregnancy (Table 3). The frequency of LVH was significantly higher in the hypertensive pregnancy group compared to the normotensive pregnancy group (15.8% vs. 10.3%). Concentric geometry (RWT > 0.42) was rare, occurring in only 67 women in the entire cohort (2.7%). This included 19 (0.8%) women with concentric hypertrophy, of whom 3 reported a history of hypertensive pregnancies.
Table 3.
LV Geometric Findings
| Variable | N | Normotensive Pregnancy (n=2210) | Hypertensive Pregnancy (n=427) | P |
|---|---|---|---|---|
| PWTd (cm) | 2552 | 0.75 ± 0.10 | 0.77 ± 0.10 | <0.001 |
| IVSd (cm) | 2552 | 0.82 ± 0.11 | 0.85 ± 0.11 | <0.001 |
| RWT | 2551 | 0.316 ± 0.043 | 0.323 ± 0.044 | 0.002 |
| Log(LVIDd) | 2552 | 1.60 ± 0.08 | 1.61 ± 0.09 | 0.027 |
| LVIDd (cm)* | 4.97 | 5.02 | ||
| Log(LVMI) | 2500 | 4.28 ± 0.21 | 4.31 ± 0.23 | 0.005 |
| LVMI (g/m2)* | 71.9 | 74.5 | ||
| LVMI > 95 g/m2 | 2500 | 10.3% | 15.8% | 0.002 |
| LV geometry** | 2500 | |||
| Normal geometry | 87.9% | 82.3% | ||
| Concentric remodeling | 1.9% | 2.3% | ||
| Concentric hypertrophy | 0.8% | 0.8% | ||
| Eccentric hypertrophy | 9.5% | 14.8% |
Values shown are means ± standard deviation or percentages adjusted for age, race, education, and network.
LV: Left ventricular; PWTd: diastolic posterior wall thickness; IVSd: diastolic intraventricular septal thickness; LVIDd: End-diastolic LV internal dimension; LVMI: LV mass index (indexed to body surface area); RWT: Relative wall thickness (PWTd+IVSd)/LVIDd
Models were fit with log-transformed values. The means have been back-transformed to raw units for interpretability.
Unadjusted percentages are shown. Analyses were not done to compare LV geometry across the groups adjusting for covariates due to the limited number of subjects with concentric geometry types.
After adjusting for age, race, network, education, parity, BMI, diabetes and current hypertension, women who had a hypertensive pregnancy still had greater odds of LVH than women who had normotensive pregnancies (OR 1.42, 95% CI 1.01-1.99, p=0.046) (Table 4). There was no significant interaction between current hypertension and hypertensive pregnancy on LVH in the final model. Replacing current hypertension with systolic blood pressure showed similar results (OR 1.42, CI 1.00-2.01, p=0.051), again with no significant interaction. Inclusion of duration of hypertension in the model was associated with a marked attenuation of the effect of pregnancy hypertension, (OR 1.19, CI 0.80-1.78, p=0.38). When subjects with unknown duration of hypertension were excluded, the results were similar: (OR 1.23, CI 0.81-1.85, p=0.33).
Table 4.
Logistic Regression Models for Left Ventricular Hypertrophy, including duration of hypertension
| Full multivariable model without duration of hypertension | Full multivariable model with duration of hypertension | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Hypertensive Pregnancy (vs. Normotensive) | 1.42 (1.01, 1.99) | 0.046 | 1.19 (0.80, 1.78) | 0.38 |
| Network | 0.07 | 0.06 | ||
| GENOA | ref. | ref. | ||
| GenNet | 1.50 (0.70, 3.21) | 0.30 | 1.47 (0.67, 3.26) | 0.34 |
| HyperGEN | 3.16 (1.32, 7.58) | 0.010 | 3.31 (1.37, 7.99) | 0.008 |
| SAPPHIRe | 1.93 (0.36, 10.48) | 0.45 | 1.96 (0.36, 10.54) | 0.43 |
| Race | 0.12 | 0.12 | ||
| Non-Hispanic White | ref. | ref. | ||
| Hispanic | 4.77 (0.93, 24.33) | 0.06 | 5.16 (1.02, 26.27) | 0.048 |
| Non-Hispanic Black | 5.13 (1.06, 24.90) | 0.042 | 5.17 (1.07, 24.86) | 0.041 |
| Age, per 10 years | 1.50 (1.27, 1.78) | <0.001 | 1.45 (1.23, 1.72) | <0.001 |
| Education Level | 0.27 | 0.28 | ||
| High School Graduate or GED (12 years) | ref. | ref. | ||
| Less than High School Education (≤ 8 years) | 0.96 (0.65, 1.41) | 0.82 | 0.95 (0.65, 1.39) | 0.79 |
| Partial High School Education (9-11 years) | 0.84 (0.55, 1.27) | 0.41 | 0.84 (0.56, 1.28) | 0.42 |
| Post High School Education (> 12 years) | 0.71 (0.50, 1.02) | 0.06 | 0.71 (0.50, 1.02) | 0.06 |
| Parity | 0.26 | 0.22 | ||
| 1 | ref. | ref. | ||
| 2 | 1.35 (0.75, 2.45) | 0.32 | 1.32 (0.73, 2.39) | 0.36 |
| 3 or more | 1.55 (0.90, 2.64) | 0.11 | 1.57 (0.92, 2.68) | 0.10 |
| BMI, per 5 kg/m2 | 0.98 (0.88, 1.09) | 0.67 | 0.96 (0.87, 1.07) | 0.47 |
| Diabetes | 1.56 (1.14, 2.13) | 0.005 | 1.51 (1.11, 2.07) | 0.009 |
| Current Hypertension | 3.79 (2.43, 5.91) | <0.001 | 2.87 (1.64, 5.02) | <0.001 |
| Log(Duration of hypertension) | 1.16 (1.00, 1.35) | 0.05 | ||
| Unknown duration of hypertension | 1.26 (0.72, 2.22) | 0.42 | ||
GENOA: Genetic Epidemiology Network of Atherosclerosis; HyperGEN: Hypertension Genetic Epidemiology Network; SAPPHIRe: Stanford Asian Pacific Program in hypertension and Insulin Resistance; GED: General educational development; BMI: Body mass index.
Left ventricular systolic and diastolic function
EF and cardiac output did not differ between the two groups (Table 5). Women who had a hypertensive pregnancy had lower E/A ratios (1.07 vs. 1.10) and longer deceleration times when compared to the normotensive pregnancy group (207 ms vs. 202 ms). Left atrial systolic dimensions were also greater in the hypertensive pregnancy group.
Table 5.
Measures of Systolic and Diastolic Function
| Variable | N | Normotensive Pregnancy (n=2210) | Hypertensive Pregnancy (n=427) | P |
|---|---|---|---|---|
| Systolic function | ||||
| EF | 2549 | 62 ± 7 | 61 ± 7 | 0.42 |
| EF < 50% | 2549 | 4.3% | 5.6% | 0.22 |
| Log(Cardiac index) | 2404 | 7.87 ± 0.19 | 7.88 ± 0.21 | 0.34 |
| Cardiac index (L/min/m2) * | 2.62 | 2.65 | ||
|
| ||||
| Diastolic function | ||||
| LA systolic dimension (cm) | 2629 | 3.4 ± 0.5 | 3.5 ± 0.5 | <0.001 |
| LA/Height | 2621 | 2.14 ± 0.30 | 2.21 ± 0.29 | <0.001 |
| E velocity (cm/s) | 2608 | 78 ± 17 | 81 ± 19 | 0.003 |
| A velocity (cm/s) | 2601 | 71 ± 16 | 75 ± 18 | <0.001 |
| Log(E/A) | 2601 | 0.10 ± 0.25 | 0.07 ± 0.27 | 0.05 |
| E/A* | 1.10 | 1.07 | ||
| E/A ≤ 0.75 | 2601 | 10.2% | 11.9% | 0.34 |
| IVRT (ms) | 2611 | 85 ± 13 | 85 ± 14 | 0.44 |
| Log(Deceleration time) | 2607 | 5.31 ± 0.19 | 5.34 ± 0.21 | 0.019 |
| Deceleration time (ms)* | 202 | 207 | ||
Values shown are means ± standard deviation or percentages, adjusted for age, race, education, and network. EF: ejection fraction; LA: left atrial; LA/Height: Left atrial systolic dimension indexed to height; E: Mitral valve early diastolic filling velocity A: Mitral valve late diastolic filling velocity; IVRT: Isovolumic relaxation time; BSA: Body surface area
Models were fit with log-transformed values. The means have been back-transformed to raw units for interpretability.
Discussion
In this large multi-center echocardiographic study of patients with hypertension or a predisposition to hypertension, a remote history of hypertensive pregnancy was associated with a higher LV mass index and a higher prevalence of LVH compared to women with a history of normotensive pregnancies. The greater left atrial size and the LV filling patterns observed also suggest a higher prevalence of impaired diastolic function after hypertensive pregnancy compared to normotensive pregnancy. Systolic function did not differ between the two groups.
This study suggests that hypertension in pregnancy may be a risk factor for LVH decades after a hypertensive pregnancy. Echocardiographic studies conducted within one year of a preeclamptic pregnancy have reported similar results. Two separate longitudinal studies of patients who had echocardiograms during a preeclamptic pregnancy showed persistent LV geometric changes (concentric remodeling, eccentric and concentric hypertrophy) [12] and diastolic dysfunction at one year postpartum [12 25]. This persistence of LV geometric changes seems to herald the development of hypertension. Ghossein-Doha et al. demonstrated that increased LV mass and left atrial dimension at 10 months postpartum were predictors of the development of hypertension in women whose blood pressure had normalized after a preeclamptic pregnancy [26]. This finding is paralleled by other observations of the predictive value of LV mass in initially normotensive adults [27].
To our knowledge, this is the first study to clearly demonstrate the association of hypertensive pregnancy with subsequent LVH during long-term follow-up. Other long-term studies have yielded conflicting results. A small longitudinal study evaluated cardiac geometry and function 1 year and 14 years following preeclamptic (n=20) or normotensive (n=8) pregnancy, respectively. At 14 years postpartum, 13% of controls and 35% of women who had preeclampsia were hypertensive [28]. There were no significant differences in LVMI or E/A ratio at either time point. Strobl et al. showed no difference in E/A ratio between normotensive women with a history of preeclampsia (n = 17) or HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome (n = 14), compared to controls with normotensive pregnancies (n = 17). Cases and controls were matched for mean blood pressure and BMI, 15 years following the index pregnancy [29]. However, the authors did observe a more abnormal myocardial performance index in the preeclampsia and HELLP groups compared to controls. The myocardial performance index incorporates features of both systolic and diastolic function, indicating that women who had preeclampsia had impaired cardiovascular function compared to women who had normotensive pregnancies.
Several factors could contribute to the stronger effects observed in the present study, compared to previous studies. These include the significantly older age of the participants, greater prevalence of hypertension, and longer exposure to hypertension, high prevalence of diabetes mellitus and obesity and the larger sample size in our study. The multicenter, multiethnic sample of our study is an important strength, as it improves the generalizability of the study.
Taken altogether, these studies suggest that there are several potential pathways to development of LVH and subsequent diastolic dysfunction after a hypertensive pregnancy (Figure 1). In the first pathway, blood pressure and myocardial function normalize after pregnancy, as in gestational hypertension or preeclampsia. However, women with a history of hypertensive pregnancy may develop hypertension years earlier than women with normotensive pregnancies, as was suggested by the earlier age of onset in our study. Alternatively, hypertension may persist following pregnancy, as in women who have chronic hypertension or chronic hypertension with superimposed preeclampsia. Both sets of scenarios expose women with hypertensive pregnancy to many more years of hypertension, increasing their risk of LVH compared to women with normotensive pregnancies.
Figure 1.
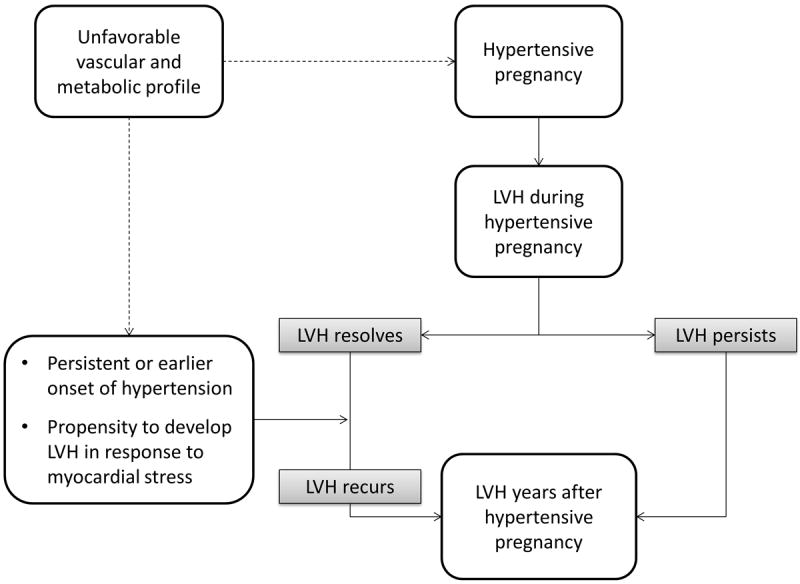
Potential pathways to the development of left ventricular hypertrophy following hypertensive pregnancy.
LVH: Left ventricular hypertrophy
In the second potential pathway, suggested by short-term echocardiographic studies,[12 26] cardiac changes persist following hypertensive pregnancy, despite normalization of blood pressure. This suggests that hypertensive pregnancy itself might be an independent risk factor for LVH and cardiac dysfunction. A longitudinal study specifically designed to follow changes in LV mass would be needed to confirm or disprove this second hypothesis.
The difference in the prevalence of LVH between the hypertensive and normotensive pregnancy groups (16% vs.10%) has significant clinical implications. LVH is an independent prognostic factor for cardiovascular mortality, and our findings emphasize the importance of hypertensive pregnancy as a cardiovascular risk factor and the need for postpartum interventions to reduce cardiovascular risk.
In our analysis, we have opted not to present data for the 13% of women in the cohort who were nulliparous. Our study assesses the response of blood pressure to the stress of pregnancy (a normotensive vs. hypertensive response) and its impact on long-term cardiovascular health. Responses to the stress test of pregnancy are unknown in nulliparous women; hence, this group may be an admixture of the other two groups. The choice for nulliparity may be both a sociological as well as a physiological phenomenon, and without knowledge of the specific reasons for nulliparity, it may be difficult to draw further conclusions about nulliparous women.
Limitations
This study has some limitations. Fifty-five percent of the participants had hypertension, as the FBPP targeted families with strong histories of hypertension. This study may not be generalizable to populations with lower hypertension rates. However, reports from the National Health and Nutrition Examination Survey estimate that the prevalence of hypertension in the United States is 34% among woman ages 45-54, and 52% among women ages 55-64 [30]. This suggests that the prevalence of hypertension in our sample is not that much higher than that of the US population. The inclusion of fewer hypertensive subjects may have resulted in a stronger relationship between altered LV geometry and function and hypertensive pregnancy, as a history of hypertension is a risk factor for both hypertension in pregnancy and LVH. The prevalence of obesity and diabetes mellitus are also significantly higher than the normal population, likely representing a clustering of other metabolic syndrome components in women with hypertension.
Exact characterization of diastolic function was not possible, as data were collected before widespread use of tissue Doppler measurements. Pregnancy history was determined by questionnaire, which introduces the potential for recall bias. However, the questionnaire had 80% sensitivity and 96% specificity for a history of preeclampsia among women who had preeclampsia at least 20 years ago [19]. Trained interviewers were used to limit interviewer bias. We also cannot account for survival bias. Our study does not differentiate between the subsets of hypertensive pregnancy disorders. However, recent evidence suggests that all of the hypertensive pregnancy disorders are associated with increased risk of subsequent cardiovascular morbidity [6], and it is therefore important to study their long-term impact. Finally, this study sampled subjects at a single time point, many years after pregnancy. We therefore have no intermediate data on blood pressure levels, but our questionnaire did capture duration of clinically diagnosed hypertension.
Despite these limitations, our data clearly indicate that a history of hypertensive pregnancy is associated with LVH even after adjusting for classical risk factors. In addition, the association seems to be mediated through the duration of hypertension following hypertensive pregnancies. Commonly, young women after hypertensive pregnancy are lost to follow up after their hypertension resolves. The identification of hypertension in pregnancy as a female specific risk factor for adverse cardiac remodeling adds to the growing body of evidence linking hypertensive pregnancy and future cardiovascular disease. Our data support the American Heart Association recommendation that women with a history of hypertensive pregnancy undergo heightened surveillance [31] and more aggressive therapy, which may decrease the burden of cardiovascular disease in women later in life.
Supplementary Material
Key questions.
What is already known about this subject?
Small echocardiographic studies show that women with hypertensive pregnancy exhibit concentric and eccentric remodeling, impairment of contractility and diastolic dysfunction. Short-term follow-up studies vary on whether these changes persist or regress postpartum.
What does this study add?
In this large, multicenter, multi-ethnic study, we have demonstrated that a history of hypertensive pregnancy is a risk factor for LVH and alterations in diastolic function later in life.
How might this impact on clinical practice?
This study adds insight into possible mechanisms underlying the increased long-term morbidity associated with a hypertensive pregnancy disorder diagnosis. It underscores the need for vigilance in monitoring of women with a history of hypertensive pregnancy.
Acknowledgments
None
Sources of funding
We would like to note the following funding sources: Award number P50 AG 44170 from the National Institute on Aging, (VDG); The Office of Women’s Health Research: Building Interdisciplinary Careers in Women’s Health award K12HD065987 (TLW); and Grants from the National Heart, Lung, and Blood Institute, National Institutes of Health: U01HL054481, U01HL054471, U01HL054512, and U01HL054498.
Footnotes
Conflicts of Interest/ Disclosures
None
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–9. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72(3):310–3. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 4.Bella JN, Palmieri V, Roman MJ, et al. Gender differences in left ventricular systolic function in American Indians (from the Strong Heart Study) Am J Cardiol. 2006;98(6):834–7. doi: 10.1016/j.amjcard.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 5.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26(6):562–8. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 6.Mannisto T, Mendola P, Vaarasmaki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Therapeutic advances in cardiovascular disease. 2008;2(4):249–59. doi: 10.1177/1753944708094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57(1):85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 10.Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(5):682–6. doi: 10.1002/uog.5311. [DOI] [PubMed] [Google Scholar]
- 11.Tihtonen KM, Koobi T, Vuolteenaho O, Huhtala HS, Uotila JT. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol. 2007;196(4):328 e1–7. doi: 10.1016/j.ajog.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98(15):1136–41. doi: 10.1136/heartjnl-2011-301548. [DOI] [PubMed] [Google Scholar]
- 14.Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39(1):3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 15.Province MA, Boerwinkle E, Chakravarti A, et al. Lack of association of the angiotensinogen-6 polymorphism with blood pressure levels in the comprehensive NHLBI Family Blood Pressure Program. National Heart, Lung and Blood Institute. Journal of hypertension. 2000;18(7):867–76. doi: 10.1097/00004872-200018070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Bella JN, Palmieri V, Kitzman DW, et al. Gender difference in diastolic function in hypertension (the HyperGEN study) Am J Cardiol. 2002;89(9):1052–6. doi: 10.1016/s0002-9149(02)02274-9. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri V, Bella JN, Arnett DK, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103(1):102–7. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Bella JN, Palmieri V, Liu JE, et al. Relationship between left ventricular diastolic relaxation and systolic function in hypertension: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38(3):424–8. doi: 10.1161/01.hyp.38.3.424. [DOI] [PubMed] [Google Scholar]
- 19.Diehl CL, Brost BC, Hogan MC, et al. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008;198(5):e11–3. doi: 10.1016/j.ajog.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37(1):7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 24.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 25.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52(5):873–80. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 26.Ghossein-Doha C, Peeters L, van Heijster S, et al. Hypertension after preeclampsia is preceded by changes in cardiac structure and function. Hypertension. 2013;62(2):382–90. doi: 10.1161/HYPERTENSIONAHA.113.01319. [DOI] [PubMed] [Google Scholar]
- 27.de Simone G, Devereux RB, Chinali M, et al. Left ventricular mass and incident hypertension in individuals with initial optimal blood pressure: the Strong Heart Study. Journal of hypertension. 2008;26(9):1868–74. doi: 10.1097/HJH.0b013e3283050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghossein-Doha C, van Kuijk SM, Spaanderman ME, Delhaas T, Peeters LL. Age-related alterations in cardiac geometry in formerly preeclamptic women and healthy parous controls: an explorative study. Reprod Sci. 2013;20(1):39–44. doi: 10.1177/1933719112459230. [DOI] [PubMed] [Google Scholar]
- 29.Strobl I, Windbichler G, Strasak A, et al. Left ventricular function many years after recovery from pre-eclampsia. Bjog. 2011;118(1):76–83. doi: 10.1111/j.1471-0528.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


