Abstract
Estrogens, predominantly 17β-estradiol, exert diverse effects throughout the body in both normal and patho-physiology, during development and in reproductive, metabolic, endocrine, cardiovascular, nervous, musculoskeletal and immune systems. Estrogen and its receptors also play important roles in carcinogenesis and therapy, particularly for breast cancer. In addition to the classical nuclear estrogen receptors (ERα and ERβ) that traditionally mediate predominantly genomic signaling, the G protein-coupled estrogen receptor GPER has become recognized as a critical mediator of rapid signaling in response to estrogen. Mouse models, and in particular knockout (KO) mice, represent an important approach to understand the functions of receptors in normal physiology and disease. Whereas ERα KO mice display multiple significant defects in reproduction and mammary gland development, ERβ KO phenotypes are more limited, and GPER KO exhibit no reproductive deficits. However, the study of GPER KO mice over the last six years has revealed that GPER deficiency results in multiple physiological alterations including obesity, cardiovascular dysfunction, insulin resistance and glucose intolerance. In addition, the lack of estrogen-mediated effects in numerous tissues of GPER KO mice, studied in vivo or ex vivo, including those of the cardiovascular, endocrine, nervous and immune systems, reveals GPER as a genuine mediator of estrogen action. Importantly, GPER KO mice have also revealed roles for GPER in breast carcinogenesis and metastasis. In combination with the supporting effects of GPER-selective ligands and GPER knockdown approaches, GPER KO mice demonstrate the therapeutic potential of targeting GPER activity in diseases as diverse as obesity, diabetes, multiple sclerosis, hypertension, atherosclerosis, myocardial infarction, stroke and cancer.
Keywords: Estrogen, Receptors, Cancer, Metabolism, Obesity, Cardiovascular, Immune, Diabetes
Introduction
The physiology of estrogen and its multiple receptors is diverse and highly complex [1–4]. Estrogen is recognized predominantly for its function in the female reproductive system including the development of secondary sex characteristics, with actions predominantly in the uterus and mammary glands [5]. However, estrogen also plays multiple and critical roles in most physiological systems of the body [6], and importantly not only in women but also in men [7, 8]. Functions for estrogen in metabolism [9, 10], endocrine [11], cardiovascular [12], nervous [13], musculoskeletal [14] and the immune [15] system are all well documented. The actions of estrogen were traditionally thought to be mediated by a single estrogen receptor, now named estrogen receptor α (ERα, ESR1), first identified in the 1960’s [16, 17]. Not until 1996 was a second structurally homologous estrogen receptor identified [18], now named estrogen receptor β (ERβ, ESR2). These receptors are both members of the family of nuclear hormone receptors and are generally described to function primarily as ligand-regulated transcription factors [6, 19, 20]. However, as the earliest described cellular signaling activities of estrogen included cAMP production [21] and calcium uptake [22], it is clear that estrogen mediates rapid cellular signaling events as well as acting as a transcriptional regulator [23]. The status quo regarding estrogen receptor biology was upended in the 2000’s with the discovery and characterization of a third estrogen receptor, structurally unrelated to ERα/β [24–26]. This receptor, originally named GPR30, based on its orphan designation, and now named G protein-coupled estrogen receptor (GPER) [3], belongs to the family of 7-transmembrane G protein-coupled receptors (GPCRs), which classically mediates rapid cellular responses involving kinases, ion channels and second messengers [27].
Studies of these three estrogen receptors have enhanced our understanding of the extensive and complex activities of estrogen throughout the body [2, 4, 28, 29]. However, given that all three estrogen receptors mediate both rapid signaling and transcriptional regulation in response to estrogen [3, 30, 31], there remain many questions regarding the functions of each individual receptor, as well as their interactions, in the complex physiology of estrogen. One approach to address the functions of individual receptors has been through the development of receptor-selective pharmacological agents. Many compounds have been identified that exhibit selective activity within the classical estrogen receptors, either with respect to ER function in different tissues or between ERα and ERβ. An example of the former is the selective estrogen receptor modulator (SERM), which includes molecules such as tamoxifen and raloxifene, that act as antagonists in the breast but agonists in bone, based on the fundamental actions of estrogen in each of these tissues [32, 33]. ER antagonists that result in ER degradation in all tissues are referred to as selective estrogen receptor downregulators (SERDs) [32, 33]. Both types of agents have found widespread use in the treatment of breast cancer (SERMs and SERDs) as well as osteoporosis (SERMs), although they exhibit virtually no selectivity between ERα and ERβ. Compounds that do exhibit such selectivity include 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT), which exhibits ~400-fold selectivity for ERβ over ERα [34], and 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN), which exhibits ~70-fold selectivity for ERβ over ERα [35]. Studies of GPER however have revealed that both SERMs [25, 36] and SERDs [24], as well as PPT [36] function as GPER agonists, thus complicating our understanding of estrogen action based on the use of such compounds [3]. In contrast, both a GPER-selective agonist (G-1, [37]) and antagonists (G15 and G36, [38, 39]) have been described that exhibit virtually no activity towards the classical estrogen receptors [3] and over 1000-fold selectivity towards a panel of 25 GPCRs [40], facilitating pharmacological studies of GPER function in cells, tissues and animals [2–4]. Although no “off-target” effects have been reported for G15 or G36, G-1 has been suggested to interact with microtubules at high concentrations [41–43]. It is important to remember however that every small molecule pharmacologic agent will likely exhibit “off-target” effects, particularly at “high” concentrations (≫EC50 of the intended target) [44]. As in the words of Paracelsus: “The dose makes the poison”.
To complement pharmacological methods, a second approach to reveal the functions of individual receptors has been the development of null mice, through targeted gene disruption or deletion. Multiple null or “knockout” (KO) mice have been created targeting ERα [45, 46], ERβ [46–48] and most recently GPER [49–52]. As no overt or extreme phenotypes in GPER KO mice similar to those of ERα KO mice were initially observed, questions arose as to the functions of GPER as an estrogen receptor [53]. However, many recent studies have revealed significant phenotypic alterations in GPER KO mice in both normal and patho-physiology. Importantly, these studies not only reveal the effects of GPER deficiency, they also demonstrate that numerous estrogen-mediated effects are absent in GPER KO mice, underscoring the role of GPER as a mediator of estrogen action. In this review, we will provide an overview of the recent discoveries regarding the roles of GPER in estrogen-related physiology and disease based on the use of GPER KO mice, highlighting the physiological, pathological and therapeutic implications of GPER expression and activity.
Estrogen Receptor Knockout Mice
Several exhaustive reviews have described the phenotypes of mice lacking ERα, ERβ, and ERα/β double knockout mice [54–57]. The phenotypes exhibited in ERα KO mice affect multiple organ systems in adult mice, although embryonic organogenesis is normal [58]. Loss of ERα leads to complete infertility in both males and females, attributed to a combination of altered mating behavior and physiological deficiencies in the gonads of both sexes that prevent ovulation and spermatogenesis [58–62]. The female reproductive tract is characterized by a hypoplastic uterus and a lack of responsiveness (proliferation, imbibition) to estrogen [63]. Similarly, the mammary glands in ERα KO mice develop normally during the fetal period, but no further ductal elongation occurs at puberty [64]. Other phenotypes include impaired long bone growth [65–67], loss of vascular/cardiac protection to injury [68], increase in white adipose tissue (adipocyte hypertrophy and hyperplasia) and associated glucose intolerance and insulin resistance in males and females [69].
ERβ KO mice display few overt phenotypes compared to ERα knockouts, and gene expression analysis demonstrates that each receptor regulates expression of distinct genes [70], suggesting that the two classical estrogen receptors have little redundancy. ERβ KO females are subfertile due to reduced ovulation [47], an ovarian phenotype distinct from that of ERα KO females. Other more subtle phenotypes that do not interfere with normal physiology include impaired terminal differentiation of mammary epithelia during late pregnancy [71] and prostate hyperplasia in older males [72, 73]. Both of these phenotypes are associated with increased Ki-67 expression, suggesting that ERβ functions to restrict proliferation and promote differentiation.
A second set of ERα and ERβ knockout mice were created [46] in light of the fact that a truncated but transcriptionally competent ERα transcript is detectable at low levels in the ERα KO mice [74]. These mice phenocopy the original ERα and ERβ KO mice, as well as the ERα/β double KO mice, in most respects [46]; however, functional redundancy was observed in ERα/β double KO ovaries, along with a transformation of granulosa cells into a cell type resembling Sertoli cells in the testis. Remarkably, alternatively spliced transcripts have been detected in both the original [47] and the second [46] ERβ KO mice. To resolve the function of ERβ, a third KO was created and confirmed to be a true null [48]. Female mice display infertility due to ovulation defects, while males are infertile due to unknown causes [48].
Despite the widespread phenotypes associated with loss of ERα, a small subset of estrogenic responses have been reported in ERα KO or ERα/β double KO mice, raising the intriguing possibility that another estrogen receptor such as GPER may mediate these activities. Some of these responses can be attributed to the residual truncated or alternative spliced transcripts present in the original ER KO mice [75]; in other instances classical ER involvement has been convincingly ruled out. For example using mRNA differential display, the expression of several genes was increased or decreased by estrogen treatment in ERα KO uteri [76, 77]. The gene products include proteins involved in calcium homeostasis, chaperone proteins, and proteins that regulate the Wnt signaling pathway, although a subsequent microarray analysis of a global deletion of ERα exon 3 revealed minimal residual estrogenic responses in the uterus [78]. In a model of cardioprotection during ischemia/reperfusion, wild type mice or mice lacking ERα or ERβ responded to estrogen treatment with improved recovery and reduced infarct size, but estrogen treatment did not lead to cardioprotection in GPER KO mice [79]. Moreover, estrogen attenuated early-stage atherosclerosis in an ERα-independent manner [80], although ERα is involved in the protective effects of estrogen in late-stage disease [68]. ERβ involvement was not assessed; however, other studies have found no evidence for ERβ function in atherosclerosis protection [81]. Finally, estrogen protects pancreatic β-cells from apoptosis, but in ERα/β double KO mice, estrogen continues to afford partial protection, whereas GPER KO mice demonstrate increased sensitivity to apoptotic stress, with the GPER-selective agonist G-1 affording protection in wild type mice similar to that of estrogen [82]. Collectively, these results reinforce the notion that while estrogen exerts a plethora of physiological effects through the classical estrogen receptors, specific estrogen-dependent activities are independent of ERα and ERβ.
GPER Knockout Mice
The identification of a G protein-coupled estrogen receptor, together with the realization that some estrogen-dependent physiological outcomes occur independently of classical ERs, prompted several groups to create genetically modified models of GPER through targeted gene deletion. A total of four such mice have been reported, each using a slightly different approach. Three of these used a strategy that deleted the entire GPER coding region, which is contained within one exon. All three knockouts are confirmed to be true nulls, while the fourth, which also encodes a lacZ reporter, retains expression of the C-terminal portion of the protein. For simplicity, in this review, we will refer to the GPER knockout mouse models as GPER KO1 [52], GPER KO2 [50], GPER KO3 [49], and GPER-LacZ [51].
GPER KO1 was created by disrupting the GPER gene using traditional positive (Neomycin resistance)/negative (thymidine kinase sensitivity) selection methods [52]. The full GPER gene, including coding and non-coding regions, was excised during targeted deletion, leaving the Neo cassette behind. Correct targeting was confirmed with restriction digest mapping and Southern blotting and sequencing of cloning junctions. GPER KO2 was created using a targeting vector containing the complete GPER open reading frame flanked by loxP sites, a loxP-flanked neo cassette for positive selection, and an upstream diphtheria toxin (PDK-DTA) negative selection cassette [50]. Once correct targeting was confirmed by Southern blotting and PCR, GPER and neo were excised by Cre recombinase. GPER KO3 was created using a strategy similar to that used to create GPER KO2, in that the complete GPER open reading frame (flanked by loxP sites) and neo selection cassette (flanked by frt sites) were completely removed following confirmation of homologous recombination [49]. Another difference between the three complete knockouts is that homologous recombination was performed in embryonic stem (ES) cells derived from the C57Bl/6N strain to create GPER KO3, while ES cells derived from the 129 strain were used for GPER KO1 (129 SvEvTac) and GPER KO2 (129X1/SvJ x 129S1), followed typically but not always by a minimum of 5–6 (or better 10) generations of backcrossing to the C57Bl/6 strain.
The fourth GPER knockout line, GPER-lacZ, was created through homologous recombination that replaced the first 349 bp of the GPER open reading frame (amino acids 20-136) with an insertion cassette that includes the full-length lacZ transcript and the neo resistance cassette [51]. Like GPER KO1 and GPER KO2, homologous recombination was accomplished in 129 ES cells, and once germline transmission was confirmed, mice were backcrossed 6 generations onto the C57Bl/6 strain. In addition to lacZ expression, real-time PCR reveals the presence of a neoR:5′-terminal truncated GPER fusion transcript. Therefore the C-terminal ~65% of the GPER protein, including transmembrane domains 3–7, is expressed in this knockout model. It is not clear if this truncated protein would be non-functional, partially functional, or functionally dominant-negative.
Initial analyses of the four GPER KO mouse models revealed no overt phenotypes in viability or reproductive function. Nevertheless, as will be described in detail herein, more detailed histopathological analysis and response to various physiologic challenges has revealed several phenotypes in metabolism and disease progression. At first glance it may appear that the different KO models display distinct phenotypes; however, in most cases different protocols and analyses have been performed, and in only a few instances have similar or identical studies been conducted on multiple knockouts by different labs.
Reproductive Functions
As estrogen was discovered in studies aimed at identifying sex hormones, it is of no surprise that the role of estrogen in the female reproductive system is one of its best-characterized functions. As described above, ERα is responsible for the majority of estrogen’s effects on the female and male reproductive tracts, including maintenance of the hypothalamic-pituitary-gonadal axis, uterine estrogenicity (epithelial proliferation, water imbibition, and induction of estrogen-responsive genes), ductal elongation in the mammary gland, and spermatogenesis. ERβ mediates some reproductive functions, mainly to orchestrate ovulation and male fertility through an unknown mechanism. In contrast, all of the GPER KO mouse models exhibit normal fertility and reproduction, suggesting that GPER does not contribute to normal reproductive physiology [49–52], although GPER mRNA expression was detected in the uterus, ovary, and mammary gland of wild type mice [49]. More detailed inspection of the GPER KO3 mouse model revealed no differences in uterine or mammary gland histopathology. GPER KO3 uteri responded to ovariectomy and estrogen challenge as well as wild type uteri as measured by water imbibition, epithelial cell height and proliferation, and expression of estrogen-responsive genes that are reported to be ERα-independent [76, 77]. Similarly, GPER KO3 and wild type mammary gland response to hormonal stimulation (estrogen or estrogen + progesterone following ovariectomy) in terms of morphometry, proliferation, and estrogen-dependent gene expression was equivalent [49].
Three studies have examined the role of GPER in uterine function using pharmacologic perturbation with GPER-selective compounds. In one study no difference was seen in proliferation using the GPER-selective agonist G-1 in wild type mice [83]; however, quantitation of the proliferation results was not described. In a second study, estrogen and G-1 significantly increased proliferation 18 hours after administration, although the magnitude of the increase compared to sham-treated mice was distinct for the two agonists (~17-fold for estrogen vs. ~3-fold for G-1). Importantly, the GPER antagonist G15 blocked estrogen-dependent proliferation by about 50% in a dose-dependent manner [39], suggesting that GPER contributes to estrogen-dependent proliferation. G-1 treatment did not increase in water imbibition in either study. The differing observations of the two studies may be due to the length of agonist treatment. It has been reported that peak uterine epithelial proliferation occurs approximately 18 hours following estrogen treatment (Newbold et al, 2001), and therefore this time point was used by Dennis et al. [39], as opposed to 3 treatments, 24 hours apart [49, 83]. Indeed, measured estrogen-induced proliferation reached 60% of total epithelial nuclei 18 hours after a single estrogen injection [39], but only ~16% after 3 estrogen injections over 3 days [49], potentially resulting in an undetectable effect of G-1. Finally, a third study found evidence for GPER-ERα crosstalk in uterine responses. Very high concentrations of G-1 (1,000-fold greater than the concentration used to detect an increase in proliferation) displayed an inhibitory effect on estrogen-induced proliferation and imbibition, in part due to phosphorylation of ERα (serine 118; [84]). Collectively, these studies demonstrate that estrogen-dependent uterine epithelial proliferation is in part GPER-dependent, while many other estrogen-dependent uterine responses attributed to ERα were intact in GPER KO mice.
Immune functions
Estrogens, as well as therapeutic anti-estrogens, regulate the development, maturation and function of the immune system [85–87]. GPER expression has been documented in various immune cells, including B and T cells, monocytes/macrophages and neutrophils, suggesting that certain actions of estrogens in the immune system could be mediated by GPER [40, 52, 88, 89]. The first study to describe the creation of (GPER KO1) mice in 2008 used these mice to determine whether GPER played a role in estrogen-induced thymic atrophy, a condition that can occur during pregnancy or prolonged estrogen exposure and is characterized by reduced thymus weight (atrophy) and cellularity, as well as alterations in CD4 and CD8 T cell populations [90]. Previous studies with ERα KO mice had indicated that ERα only partially mediated the effects of estrogen on the thymus [91], whereas studies with ERβ KO mice revealed little effect of this receptor [92]. Interestingly, although GPER KO1 mice displayed no gross immunological defects, a role for GPER in estrogen-mediated thymic atrophy was identified [52]. In a direct comparison of ERα KO to GPER KO1 mice, estrogen-induced thymic atrophy was attenuated not only in ERα KO mice as previously observed, but also in GPER KO1 mice [52]. More detailed analyses revealed that estrogen-induced thymic atrophy involved both a developmental block as well as apoptosis. In fact, whereas ERα exclusively mediated the early developmental blockage of thymocyte development (specifically CD4/CD8 double-negative thymocytes), GPER was required for apoptosis of double-positive thymocytes. This result was further supported through the use of the GPER-selective agonist G-1, which induced thymic atrophy through thymocyte apoptosis without the developmental blockage ascribed to ERα. Although another report has observed an increased apoptosis rate of naïve T cells in GPER-LacZ reporter mice not challenged with estrogen [51], a lack of global effects of G-1 [83] and GPER expression [93] on the thymus has also been reported. The reasons for these apparently differing results remain unclear.
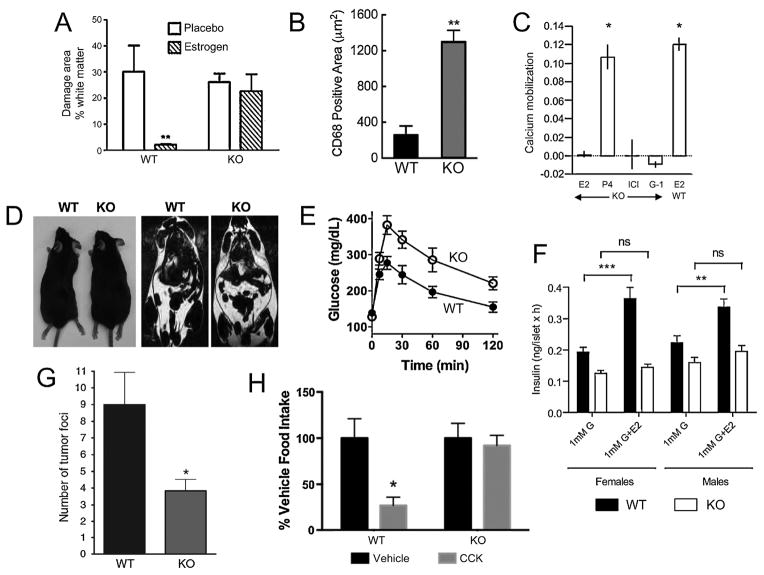
Estrogens are also receiving increased attention as anti-inflammatory therapeutic agents, particularly in multiple sclerosis, where disease symptoms are attenuated during pregnancy, suggesting a protective effect of estrogen(s) [94]. Consequently, a clinical trial of estriol for multiple sclerosis therapy is ongoing (ClinicalTrials.gov, [95]). Employing the murine experimental autoimmune encephalomyelitis (EAE) model, in which estrogens exhibit both protective (i.e. preventive) [96] and therapeutic [97] effects, estrogen-mediated protection, as assessed by clinical score, was substantially decreased in GPER KO1 mice, with a complete absence of estrogen-mediated protection from white matter damage in GPER KO1 mice (Fig. 1A) [98]. A role for GPER was further supported employing G-1 treatment with the EAE model, which resulted in protection against both the clinical and histological manifestations of EAE, similar to the effects observed with estrogen [40, 98]. Although the effects of G-1 were completely absent in GPER KO1 mice, demonstrating a requirement for GPER and the selectivity of G-1 for GPER, the protective effects of estrogen itself were only partially absent, suggesting that estrogen acts through mechanisms involving both ERα and GPER. Importantly however, activation of GPER alone (with G-1) in wild type mice completely reproduced the effects of estrogen, suggesting that ERα activation is not essential when stimulating only GPER [98]. The effects of G-1 have not been examined in ERα KO mice, which would reveal whether G-1-mediated protection requires ERα expression and perhaps basal activity. Multiple mechanisms of GPER-mediated protection have been observed including enhanced suppressive activity of regulatory T cells through upregulation of programmed death 1 [98] as well as inhibition of macrophage inflammatory cytokine production [40]. In addition to the protective effects of estrogen itself, the therapeutic effect of ethinyl estradiol in established EAE was absent in GPER KO1 mice but remained in ERα KO mice, and was associated with increased IL-10 production [99]. Finally, the estrogen-dependent protective effects of vitamin D3 in EAE, were also absent in GPER KO1 mice and correlated with decreased anti-inflammatory cytokines, again suggesting GPER mediates the estrogen-dependent effects in this model [100]. Consistent with the anti-inflammatory role of GPER activation in EAE, aged GPER KO1 mice exhibit a pro-inflammatory state [101], possibly secondary to other metabolic dysfunctions (see section below on metabolic functions). Furthermore, G-1 stimulates de novo IL-10 production in pro-inflammatory Th17-polarized cells in vitro as well as following G-1 administration in vivo [102], and elicits Foxp3 expression (a marker of regulatory T cells) under Th17-polarizing conditions [103], all of which support an anti-inflammatory role of GPER activation.
Figure 1.
GPER deficiency leads to multiple phenotypes and loss of estrogen responsiveness of multiple tissues. A. The estrogen-mediated decrease in white matter damage, a result of immune infiltration and demyelination, in a murine model of multiple sclerosis (experimental autoimmune encephalomyelitis, EAE) is absent in GPER KO1 mice [98]. B. GPER KO1 mice fed an atherogenic diet show increased vascular infiltration of macrophages (CD68-positive cells) associated increased atherosclerosis [124]. C. Renal intercalated cells from GPER KO2 mice are deficient in estrogen (E2)-, ICI182,780 (ICI)- and G-1-mediated calcium mobilization. Calcium mobilization to progesterone (P4) was unaltered in GPER KO2 mice, similar in magnitude to estrogen-mediated calcium mobilization in WT cells [132]. D. GPER KO1 mice exhibit increased body size/obesity (left) and adiposity (right, as measured by MRI) [101]. E. Upon aging, male GPER KO1 become glucose intolerant [101]. F. Estrogen (E2)-mediated insulin secretion from islets is absent in both male and female GPER KO2 mice [50]. G, glucose. G. GPER KO1 mice exhibit reduced metastasis in a PyMT-MMTV model of mammary tumorigenesis [160]. H. Female GPER KO1 mice display a lack of cholecystokinin (CCK)-mediated satiety/appetite suppression (an estrogen-sensitized effect) as measured by food intake [139]. Reproduced (in some cases with minor modifications for clarity) with permission from the indicated sources.
Cardiovascular and Renal Functions
Estrogens play important roles in cardiovascular and renal function in both health and disease. The lack of estrogen resulting from menopause or oophorectomy is associated with an increased incidence of hypertension and cardiovascular disease compared to premenopausal women [104–106]. Premenopausal women also exhibit increased cardiovascular health compared to age-matched men, further indicating a protective role for estrogen. However, although the beneficial effects (cardiovascular, in particular) of estrogen came under question as a result of the Women’s Health Initiative (WHI) Trial of hormone replacement using conjugated equine estrogens (combined with medroxyprogesterone acetate, a synthetic progestin analog) [107, 108], more recent analyses, particularly for recently menopausal women, largely confirm the safety and efficacy of estrogen replacement therapy [109, 110].
The first reports to describe a cardiovascular role for GPER employing GPER KO mice were published in 2009 by Barton and colleagues [111] and Leeb-Lundberg and colleagues [50]. In the former study, the vasodilatory effect of the selective GPER agonist G-1, which was similar to that of estrogen, was absent in carotid arteries of GPER KO1 mice. Furthermore, the inhibitory effects of G-1 on serotonin-mediated contractions were absent in GPER KO1 mice. Consistent with the observed vasodilatory effects on isolated rings, acute intravenous injection of G-1 into normotensive Sprague-Dawley rats induced rapid reductions in mean arterial pressure (MAP). In the latter study, GPER KO2 mice displayed increased MAP at 9 but not 6 months of age, due in large part to a decrease in the WT mice at 9 vs. 6 months of age. Interestingly, acetylcholine relaxed K+-preconstricted aortic rings similarly in WT and GPER KO2 mice, unlike later results employing physiologically preconstricted rings (see below in this section). Differences in MAP were ascribed to a lack of increase in lumen circumference in mesenteric resistance vessels of older GPER KO2 mice, suggesting a deficit in vessel diameter growth in GPER KO2 mice with age. Reports have also described that male, but not female, GPR30 KO3 or GPER-LacZ mice exhibit either impaired left ventricular cardiac function with enlarged left ventricles displaying both impaired contractility and relaxation [112] or decreased ejection fraction and fractional shortening, specifically in aging (male) mice [113].
Detailed studies of vascular function and reactivity in GPER KO1 mice were first described by Meyer et al. in 2012, comparing the effects of chronic global GPER deficiency to acute vascular effects with the GPER antagonist G15, with respect to both endothelium-dependent and -independent reactivity [114, 115]. In the aorta, GPER deficiency was associated with increased endothelial prostanoid-medicated vasoconstriction with no effect on endothelial nitric oxide (NO) activity. Direct thromboxane prostanoid (TP) receptor-mediated contraction was also increased in GPER KO1 mice. Acute GPER inhibition with G15 enhanced endothelium-dependent contractions and reduced NO activity (both basal and acetylcholine-induced) but had no effect on direct TP receptor activation [114]. In pressurized carotid arteries, GPER KO1 show enhanced constriction to endothelin, despite a decrease in vascular smooth muscle cell calcium mobilization, suggesting that GPER activation mediates a reduced myofilament force sensitivity to calcium [115]. In another study, the vasoconstrictor activity of peri-vascular adipose, a cyclooxygenase-derived thromboxane, was first identified in obese GPER KO mice [116]. In addition to studies of GPER KO mice, many additional studies have employed the selective GPER agonist G-1 to underscore the vasodilatory actions of GPER [117–121]. Finally, supporting a role for GPER in the regulation of vascular tone, a common (22%) hypofunctional genetic variant of GPER has been associated with increased blood pressure in women [122].
In epidemiological studies and animal models of cardiovascular diseases such as atherosclerosis and ischemia/reperfusion injury resulting from myocardial infarction and stroke, estrogen plays a highly protective role [123]. Interestingly, the anti-atherogenic effects of estrogen, particularly in the early stages of atherosclerosis, are independent of ERα [80]. Recent results demonstrate that GPER KO1 mice exhibit increased early atherosclerosis progression when fed a high-fat atherogenic diet, under both ovary-intact and ovariectomized conditions [124]. This corresponded with increased vascular macrophage (Fig. 1B) and T cell infiltration and decreased basal NO activity. Importantly, treatment with G-1 reduced plaque formation and macrophage accumulation, consistent with a protective role for GPER. The cardioprotective effects of estrogen following ischemia/reperfusion have also been shown to be dependent on GPER expression, with GPER KO2 mice, but not ERα KO or ERβ KO mice, lacking estrogen-mediated protection [79, 125]. Similarly, G-1 mediates cardioprotective effects through mechanisms involving ERK-mediated inhibition of mitochondrial transition pore opening [126], Akt activation [127] and reductions in inflammatory cytokines [128]. Activation of GPER also improves diastolic function and left ventricular remodeling in hypertensive rats [129] and ameliorates cardiac function in isoproterenol-induced heart failure [130].
GPER activity is also involved in the renoprotective effects of estrogen. In the kidney, GPER is expressed predominantly in distal convoluted tubules and the Loop of Henle, with lower expression in proximal convoluted tubules and little expression in collecting ducts, with varying expression levels and patterns throughout the estrus cycle [131]. Estrogen and G-1 in addition to the SERD ICI182,780 (Fulvestrant), an ER antagonist, stimulated rapid calcium signaling and H+-ATPase activity in renal tubules and isolated intercalating cells, with the effects of all three ligands absent in tubules and cells isolated from GPER KO2 mice (Fig. 1C) [132]. In a model of salt-induced hypertension with commensurate renal damage, G-1 reduced renal hypertrophy, improved creatinine clearance and reduced proteinuria, all in the absence of blood pressure effects, presumably due to the presence of endogenous estrogen [133, 134]. G-1 reduced tubular oxidative stress and induced megalin expression leading to improved protein reabsorption [134]. The acute renoprotective effect of estrogen following cardiac arrest and cardiopulmonary resuscitation, which involves neither ERα no ERβ [135], was recently shown also not to involve GPER, based on the use of GPER KO1 mice and G-1 [136], suggesting that the renoprotective functions of GPER may be context-specific.
Metabolic Functions: Obesity and Diabetes
Among the first phenotypes reported for GPER KO mice was that they were obese (Fig. 1D) [111], glucose intolerant (Fig. 1E) [50] and exhibited increased sensitivity to diabetes-inducing treatments [82]. Estrogen is an important mitigant of these and other metabolic disorders in both humans and animal models [137] and thus it is not unexpected that GPER plays a role in multiple aspects of metabolic regulation.
In 2009, Barton and colleagues reported the observation that, in addition to the cardiovascular effects described above, both male and female GPER KO1 mice were obese, exhibiting excess abdominal adipose [111]. Interestingly, at about the same time, Leeb-Lundberg and colleagues reported that female (but not male) GPER KO2 mice exhibited a ~10% decrease in body weight at 19 weeks of age, with a similar decrease in overall body size [50], whereas Otto and colleagues observed no differences in body weight at 5 months of age in GPER KO3 mice [49]. Finally, Noppinger and colleagues reported that the overall body weight as well as lean and fat mass was unaltered in GPER-LacZ fusion mice [51], although female GPER-LacZ mice fed a high-fat diet exhibited lower HDL levels and excess fat accumulation in the liver [113]. Some of these apparent discrepancies may be due to the age of the mice studied, targeting strategy, genetic background, diet or possibly environmental factors. For example, a more in depth study by Sharma et al. of aging male GPER KO1 mice revealed that whereas the body weight difference was only about 7% in 6 month-old mice, this increased to almost 20% in 12 month-old mice, with unaltered body size (tibia length) [101]. A similar but slightly reduced weight difference was maintained through 24 months of age. In these mice both visceral and subcutaneous adipose increased by ~30–40% [101], with a three-fold increase in the peri-aortic adipose [116]. Dyslipidemia in male GPER KO1 mice, as evidenced by increased serum levels of total cholesterol, LDL cholesterol and triglycerides, occurred as the mice aged (24 vs. 12 months) [101]; whereas in young female GPER KO1 mice, an atherogenic diet resulted in increased total and LDL cholesterol levels [124]. Consistent with these observations in mice, epidemiological evidence for the regulation of LDL cholesterol through GPER activity in humans derives from carriers of a hypofunctional GPER polymorphism that correlates with higher circulating levels of plasma LDL cholesterol, potentially through the regulation of LDL receptor expression in the liver [138].
Similar levels of obesity in GPER-deficient mice have also been observed by Clegg and colleagues [139]. Importantly, the estrogen-mediated reductions in body weight and adipocyte size (which increase following ovariectomy) were greatly attenuated in GPER KO1 mice, consistent with a role for GPER in mediating certain metabolic actions of estrogen [139]. Decreased energy expenditure (measured as oxygen consumption) was also reported for GPER KO1 mice, along with decreased UCP1 and β3-adrenergic receptor expression in brown adipose [139], although no difference in food intake or locomotion was observed [101, 139], suggesting specific roles in peripheral energy metabolism. Nevertheless, central effects of GPER in the hypothalamus may also contribute to effects on overall obesity [139, 140] (see below). Additionally, the GPER-selective agonist G-1 inhibits adipognesis [141] and decreases fatty acid synthesis and triglyceride accumulation in human and rodent islets and β-cells, suggesting roles in the development of adipose tissue and lipid metabolism in the pancreas [142].
In addition to lipid metabolism and homeostasis, extensive data indicate that GPER also plays important roles in estrogen-mediated glucose homeostasis [11], particularly with respect to pancreas/islet/β-cell function and survival. Estrogen exhibits numerous important effects in the pancreas, including stimulating insulin synthesis [143, 144] and secretion [145, 146], enhancing β-cell survival [11, 147] and islet survival following transplantation [148], and reducing lipotoxicity [142, 149]. In 2009, Mauvais-Jarvis and colleagues employed a streptozotocin-induced model of type 1 diabetes with ERα, ERβ and GPER KO mice to demonstrate that both ERα and GPER mediated pancreatic islet survival [82]. Specifically, female GPER KO1 mice exhibited a loss in protection from streptozotocin-induced diabetes by endogenous estrogens and the GPER-selective agonist G-1 protected from ROS-induced apoptosis as effectively as estrogen in both murine and human islets, with the protective effect of G-1 absent in islets isolated from GPER KO1 mice [82]. Furthermore, G-1, as well as ERα- and ERβ-selective estrogenic ligands, increase survival of human islets following xenotransplantation into mice, but only the ER-selective ligands improved islet revascularization [148].
In 2009, Leeb-Lundberg and colleagues reported that whereas ovary-intact female GPER KO2 mice were glucose intolerant at 6 months of age, male mice at the same age were unaffected [50]. However, Sharma et al. subsequently revealed that male GPER KO1 mice are insulin resistant at 6 months of age and only become glucose intolerant as they age [101], possibly explaining the lack of observed glucose tolerance phenotypes in other studies of GPER KO mice [51, 82]. In female GPER KO2 mice, impaired glucose tolerance was associated with decreased insulin expression and glucose-stimulated secretion in pancreatic islets [50]. Furthermore, following ovariectomy, estrogen replacement failed to preserve serum insulin levels [50] in GPER KO 2 mice and glucose tolerance [139] in GPER KO1 mice as in wild type mice. In order to determine the effects of GPER specifically on pancreatic insulin release, both isolated islets [50, 150] and β-cells [150] have been examined. Whereas estrogen stimulated insulin and inhibited glucagon secretion in islets isolated from wild type male and female mice, these effects were absent in islets from GPER KO2 mice (Fig. 1F) [50, 150]. Furthermore, the GPER-selective agonist G-1, similar to estrogen, stimulated insulin secretion from islets isolated from wild type but not GPER KO1 mice; in addition, the GPER-selective antagonist G15 inhibited insulin secretion by both estrogen and G-1 [150]. Taken together, these results demonstrate extensive roles for GPER in metabolic regulation, and specifically in many of the effects mediated acutely or chronically by estrogen.
Cancer
Estrogen promotes the proliferation of breast epithelial cells at puberty, leading to epithelial ductal outgrowth, as well as tumor cell proliferation in breast cancer. Extensive evidence supports a role for ERα in these activities [151, 152], and indeed standard medical practice includes determination of ERα expression following a breast cancer diagnosis, in order to evaluate suitability of anti-estrogen therapy (e.g., SERMs) [153]. However, the greater benefits observed from aromatase inhibitor (AI) therapy [154], which prevents estrogen production, compared to tamoxifen therapy, which inhibits estrogen activating classical ERs, suggest the possibility that estrogen promotes breast cancer progression through additional mechanisms.
The question of whether GPER might contribute to breast cancer progression was explored in several ways. In vitro studies have demonstrated that GPER activation stimulates MAPK, PI3K/Akt and related survival/proliferation signaling pathways, concomitantly inducing cell growth, in multiple GPER-expressing breast cancer cell lines [24, 25, 155–157]. Examination of GPER expression in human breast tumors has revealed a correlation between GPER expression level with both tumor size and metastasis [158]. Furthermore, an inverse correlation was observed between GPER expression and disease-free survival in patients initially presenting with GPER-positive tumors, and treated only with tamoxifen [159]. Collectively these results support the notion that GPER may contribute to breast cancer progression, and importantly given that tamoxifen is a GPER agonist, may contribute to some forms of tamoxifen resistance.
To date, only one study has investigated GPER function in breast cancer progression in a physiologically intact model: GPER KO1 mice (backcrossed onto the FVB strain for 10 generations) interbred with mice transgenic for the polyoma middle T antigen (PyMT), expressed under the control of the mouse mammary tumor virus (MMTV) long terminal repeat [160]. MMTV-PyMT transgenic (Tg) mice express the PyMT oncoprotein in the mammary gland, and develop mammary tumors with high fidelity [161]. The MMTV-PyMT Tg model of breast cancer progression mimics human breast cancer in terms of histopathology, temporal gene expression changes during tumor progression, loss of/decrease in ERα expression [162, 163] and estrogen-responsiveness [163, 164], multistep tumor progression, metastasis to the lung, and gene expression patterns [165, 166]. GPER deficiency in this model did not affect tumor latency or hyperplasia in early stages of tumor onset [160]. However, GPER KO1 had smaller tumors at later stages of tumorigenesis, with reduced proliferation and histologic features of less aggressive tumors. Importantly, GPER KO1 mice displayed decreased metastasis to the lung (Fig. 1G) [160]. This represents the first study to provide direct evidence for a functional role of GPER in breast cancer progression. Collectively these results, combined with in vitro experiments and retrospective human breast cancer analysis, suggest that GPER expression positively influences breast tumor progression in certain settings, and also suggest that GPER should be investigated as a therapeutic target.
GPER has also been linked to the progression of other cancers, including endometrial and ovarian cancer, where estrogen exposure increases risk [167, 168]. GPER expression strongly correlated with poor prognosis in endometrial and ovarian cancer in women [169, 170]. The Hec50 cell line expresses GPER but not ERα or ERβ, and in a xenograft model of endometrial tumorigenesis, Hec50 tumor growth increased with G-1 and estrogen treatment, while estrogen-mediated growth was inhibited by the GPER antagonist G36 [36]. Studies of endometrial or ovarian cancer utilizing GPER KO models have not been reported; however; they may one day illuminate the role of GPER in these hormone-responsive cancers as with the breast cancer model.
Neurological Functions
Estrogens, both systemic and synthesized locally in nervous tissue [171], mediate effects throughout the central and peripheral nervous system. Functions include regulation of the hypothalamus-pituitary-gonadal axis, sexual/reproductive behavior, synaptic plasticity, mood, memory, cognition, pain sensation, energy metabolism, temperature regulation and neuroprotection from injury [172]. GPER expression has been documented in multiple regions of the central and peripheral nervous system of both female and male rodents, including the cortex, hippocampus, hypothalamus, nuclei of the midbrain, the trigeminal nuclei and cerebellum Purkinje layer of the hindbrain, the anterior, intermediate and neural lobes of the pituitary, as well as the spinal cord and dorsal root ganglia [173–175]. Kelly and colleagues have described a putative membrane-associated estrogen receptor that is coupled to desensitization of GABAB receptors in hypothalmic proopiomelanocortin neurons. Having previously demonstrated the effect was maintained in ERα KO, ERβ KO and ER double KO mice [176], in 2008, they also demonstrated that GPER KO1 were not defective in this estrogen-mediated effect, suggesting the involvement of another as-of-yet unidentified estrogen receptor [177].
Clegg and colleagues, in examining the metabolic effects of GPER deficiency, observed that the weight gain observed in female GPER KO1 mice (discussed above) was associated with a decreased anorectic response to leptin [139]. Importantly, plasma insulin and leptin levels, as well as estrogen and testosterone levels, and hypothalmic leptin receptor levels were unaltered although there was a ~25% decrease in hypothalmic ERα mRNA expression. Furthermore, the prandial effect of CCK, which is sexually dimorphic and greater in females, and results in anorexia, was reduced in female but not male GPER KO1 mice (Fig. 1H). In addition, whereas food intake varies across the female estrus cycle, GPER KO1 mice displayed no such variations, suggesting GPER plays a role in rapid estrogenic enhancement of satiety. A potential mechanism for this resulted from the demonstration that the estrogen-induced phosphorylation of ERK1/2 in the basal medial hypothalamus is absent in ovariectomized female GPER KO1 mice [139].
Roles for GPER in anxiety and stress responses have also been demonstrated employing GPER KO mice. Kastenberger and Schwarzer have recently described highly sexually dimorphic effects of GPER deficiency [178]. In particular, male GPER KO3 mice displayed increased exploratory drive in multiple tests, whereas female GPER KO3 mice displayed estrous-stage dependent anxiolytic effects in the open field test with a shift in the cycle-dependent fluctuations of basal cortisone levels and stress coping responses. Thus, the effects of GPER deficiency on behavior are clearly complex and require further investigation.
In support of studies with GPER KO mice, a plethora of studies have investigated the behavioral functions of GPER using GPER-selective agonists and antagonists. For example, Kwon et al. recently demonstrated that estrogen stimulated STAT3 phosphorylation in GPER-expressing, but not in ERα- or ERβ-expressing cells, and that estrogen- (and G-1)-stimulated phosphorylation in hypothalmic neurons was blocked by the GPER antagonist G15 [140]. Furthermore, cerebroventricular injection of G-1 increased STAT3 phosphorylation in the arcuate nucleus, and was associated with a decrease in food intake and body weight gain [140]. G-1 also induces depolarization of spinal cord neurons [174], stimulates mechanical hyperalgesia, [179] and mediates visceral hypersensitivity [180]. In vivo studies further demonstrated that G-1 replicates the effects of estrogen in enhancing neuronal survival following global or local ischemia in the brain [181, 182], improves cerebral microvascular function following hypoxia and reperfusion [183] and improves immunosuppression following stroke [184]. G-1 also attenuates serotonin receptor signaling in the paraventricular nucleus of the hypothalamus and reduces responses to oxytocin and adrenocorticotropic hormone, consistent with a role for GPER in mood disorders [185]. In addition, G-1, like estrogen, exhibited antidepressant properties in a mouse model of depression, with effects by both ligands inhibited by the GPER-selective antagonist G15 [39]. Although G-1 did not alter the estrogen-mediated inhibition of negative feedback by luteinizing hormone, and furthermore knockdown of GPER in the mediobasal hypothalamus did not alter lordosis behavior in rats [186], a recent report did in fact demonstrate that G-1 promotes lordosis in mice [187]. Together, these results suggest that GPER plays extensive roles in neurological function and that GPER agonists could represent a new therapeutic approach for stroke, behavioral, as well as chronic neurodegenerative diseases [188].
Skeletal Functions
Estrogen plays multiple roles in the growth and metabolism of bone [189]. This is exemplified by the loss of bone mass and density (osteoporosis) in women following menopause, and the effectiveness of hormone replacement therapies, as well as SERMs, to prevent or limit osteoporosis [190]. In men as well as mouse models, deficiency in ERα or aromatase, which converts testosterone into estrogen, is associated with low bone density and delayed growth plate closure [191]. GPER expression has been demonstrated in growth plate cartilage, declining as puberty progresses [192], and in osteoblasts, osteocytes and osteoblasts [193]. In preosteoblasts, GPER expression was upregulated by Runx2, a regulator of osteoblast differentiation, and GPER knockdown inhibited osteoblast proliferation [194]. Together, these results suggested a role for GPER in bone metabolism.
Surprisingly, few studies have examined the role(s) of GPER in skeletal metabolism and physiology. In 2009, Leeb-Lundberg and colleagues, in their original characterization of GPER KO2 mice, reported a small age-dependent decrease in femur length and overall size (crown-rump) in female but not male GPER KO2 mice [50]. Subsequently, the same group demonstrated that in ovariectomized female GPER KO2 mice, estrogen treatment failed to reduce longitudinal skeletal growth or decrease growth plate height as it did in wild type mice, although other parameters (bone mineral density and cortical bone thickness were unaffected in the GPER KO2 mice [93]. In 2011, Oz and colleagues reported that male GPER KO1 mice not only exhibited increased lean body mass and body fat, but greater femur length, bone mineral density, trabecular bone volume, cortical thickness and surface mineralization [195]. In addition, the growth plate of male GPER KO1 mice showed greater proliferation and under osteogenic conditions, bone marrow cells produced fewer alkaline phosphatase-positive colonies in early differentiating cultures, but increased mineralized nodule deposition in mature osteoblast cultures. No effects on osteoclast function were observed. Importantly, levels of IGF-1 in the serum were unaltered, suggesting that in male mice, GPER regulates multiple aspects of bone mass, size and microarchitecture [195]. Taken together, these results demonstrate sexually dimorphic effects of GPER in bone that require further study.
Conclusions
Our understanding of the roles of GPER in physiology and disease has expanded greatly in the last decade, and even more so in the last five years. In part, this has been a result of the development and more widespread use of GPER KO mice. Importantly, numerous functions of estrogen, though clearly not all, have been shown to be absent in GPER KO mice, in support of its designation as a physiologically relevant estrogen receptor (Table I). Furthermore, the effects of G-1 in animal models are absent in GPER KO mice, revealing the specificity of G-1 for GPER in vivo. Nevertheless, studies using knockout mice have inherent limitations. For example, effects in global KO mice are prone to complex interactions when a target is expressed in multiple interacting tissues. Compensatory changes during development may also obscure certain aspects of a protein’s inherent function(s). Genetic differences in strain background can impact phenotypes [196]. Furthermore, multiple approaches to the deletion of the Gper gene as well as environmental factors (chow, phytoestrogens, climate control, altitude, etc.) could result in mice with somewhat varying phenotypes in different labs. The development of inducible tissue-specific conditional KO mice will be an important addition to the arsenal of tools available to study GPER function, allowing tissue-specific deletion of GPER in adult mice, reducing compensatory developmental effects and the complexity of multiple interacting systems. The creation of double (ERα/GPER) or even triple (ERα/ERβ/GPER) KO mice, though challenging, could provide additional information regarding the possible overlapping functions of these receptor systems. To date, the use of pharmacological agents has provided an important complimentary approach to the study of GPER KO mice. In many cases, GPER-selective antagonists recapitulate the effects observed in GPER KO mice, whereas GPER-selective agonists demonstrate the opposite effect, often mimicking the effects of estrogen. Importantly, pharmacological agents can be used with greater ease in vivo, and in species not amenable to genetic manipulation, though with their own caveats (stability, half-life, metabolism, selectivity, etc.). Nevertheless, when used in combination, these multiple approaches are slowly but surely unmasking the physiological and potential therapeutic roles of GPER.
Table I.
Loss of Estrogen-mediated Effects in GPER KO mice
| System | Measurement | Observation in GPER KO* | GPER KO model and Reference(s) |
|---|---|---|---|
| Nervous | Prevention in EAE# | E2
 , G-1 (−) , G-1 (−) |
KO1 [98] |
| Therapy in EAE | Ethinyl estradiol (−) | KO1 [99] | |
| pERK activation in basal medial hypothalamus (ovx#) | E2 (−) | KO1 [139] | |
| Cholecystokinin- and leptin-mediated satiety | (−) (females) | KO1 [139] | |
| Cardiovascular | Vascular dilation | E2
 , G-1 (−) , G-1 (−) |
KO1 [111, 197] |
| Reduction in myocardial infarct size | E2 (−) | KO2 [79] | |
| atherosclerosis |
 (females) (females) |
KO1 [124] | |
| Kidney | Calcium flux in renal intercalated cells | E2 (−), G-1 (−), ICI182,780 (−) | KO2 [132] |
| Pancreas | STZ-induced diabetes# |
 (females) (females) |
KO1 [82] |
| Insulin secretion by islets and β-cells | E2 (−), G-1 (−) | KO1 & KO2 [50, 150] | |
| Decreased glucagon secretion | E2 (−) | KO2 [50] | |
| Islet cell survival | G-1 (−), E2 effect compensated by ERα | KO1 [82] | |
| Metabolism | Glucose tolerance (ovx) | E2 (−) | KO1 [139] |
| Serum insulin (ovx) | E2 (−) | KO2 [50] | |
| Body weight (ovx) | E2

|
KO1 [139] | |
| Adipocyte size (ovx) | E2

|
KO1 [139] | |
| Immune | Thymocyte apoptosis | E2 (−) | KO1 [52] |
| Skeletal | Longitudinal bone growth (ovx) | E2 (−) | KO2 [93] |
| Decreased growth plate height (ovx) | E2 (−) | KO2 [93] | |
| Bone mineral density, trabecular bone volume |
 (males) (males) |
KO1 [195] | |
| Cancer | Mammary tumor growth and metastasis |

|
KO1 [160] |
E2/G-1 (−),
 : effect of estrogen (E2) or G-1 is lost (−) or diminished
: effect of estrogen (E2) or G-1 is lost (−) or diminished
 in GPER KO mice;
in GPER KO mice;
 ,
,
 , (−) (females/males): the indicated estrogen-responsive disease state or physiological effect is increased
, (−) (females/males): the indicated estrogen-responsive disease state or physiological effect is increased
 , decreased
, decreased
 , or absent (−) in female/male GPER KO mice.
, or absent (−) in female/male GPER KO mice.
ovx, ovariectomized. EAE, experimental autoimmune encephalomyelitis; STZ, streptozotocin.
Highlights.
GPER KO mice reveal multiple roles for GPER in estrogen physiology.
Pharmacological manipulation of GPER supports many of these functions.
GPER-selective agonists and antagonists represent novel therapeutic agents.
GPER targets include obesity, diabetes, cardiovascular diseases, stroke and cancer.
Acknowledgments
The authors have been supported by NIH grants CA116662, CA118743 CA127731 and CA163890 (ERP) and the UNM Cancer Center (CA118100).
Footnotes
Disclosures
E.R.P. holds a US patent on GPER-selective ligands and imaging agents.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58(4):773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 2.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology: G protein-coupled estrogen receptor (GPER) and its pharmacologic modulators. Pharm Rev. 2015 doi: 10.1124/pr.114.009712. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prossnitz ER, Barton M. Estrogen biology: New Insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389(1–2):71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi G, Zarrilli S, Colao A, Paesano L, Di Somma C, Rossi F, De Rosa M. Estrogens and health in males. Mol Cell Endocrinol. 2001;178(1–2):51–55. doi: 10.1016/s0303-7207(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rettberg JR, Yao J, Brinton RD. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2013;35(1):8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Cho HT, Kim YJ. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014 doi: 10.1507/endocrj.ej14-0262. [DOI] [PubMed] [Google Scholar]
- 11.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135(1):54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai Y, Youn MY, Inoue K, Takada I, Kouzmenko A, Kato S. Nuclear receptors in bone physiology and diseases. Physiol Rev. 2013;93(2):481–523. doi: 10.1152/physrev.00008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 16.Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182(108):126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- 17.Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414. [Google Scholar]
- 18.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20(8):1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 20.Schultz-Norton JR, Ziegler YS, Nardulli AM. ERalpha-associated protein networks. Trends Endocrinol Metab. 2011;22(4):124–129. doi: 10.1016/j.tem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253(5490):357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- 23.Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev. 2013;65(2):710–778. doi: 10.1124/pr.112.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 25.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153(7):2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 30.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308(1–2):32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando L, Schiavone P, Fedele P, Calvani N, Nacci A, Rizzo P, Marino A, D’Amico M, Sponziello F, Mazzoni E, Cinefra M, Fazio N, Maiello E, Silvestris N, Colucci G, Cinieri S. Molecularly targeted endocrine therapies for breast cancer. Cancer Treat Rev. 2011;36(Suppl 3):S67–71. doi: 10.1016/S0305-7372(10)70023-2. [DOI] [PubMed] [Google Scholar]
- 33.McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10(6):620–628. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 35.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 36.Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 38.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127(3–5):358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5(6):421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214(1–2):67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gui Y, Shi Z, Wang Z, Li JJ, Xu C, Tian R, Song X, Walsh MP, Li D, Gao J, Zheng XL. The GPER Agonist G-1 Induces Mitotic Arrest and Apoptosis in Human Vascular Smooth Muscle Cells Independent of GPER. J Cell Physiol. 2015;230(4):885–895. doi: 10.1002/jcp.24817. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Lv X, He C, Hua G, Tsai MY, Davis JS. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013;4:e869. doi: 10.1038/cddis.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holm A, Grande PO, Luduena RF, Olde B, Prasad V, Leeb-Lundberg LM, Nilsson BO. The G protein-coupled oestrogen receptor 1 agonist G-1 disrupts endothelial cell microtubule structure in a receptor-independent manner. Mol Cell Biochem. 2012;366(1–2):239–249. doi: 10.1007/s11010-012-1301-3. [DOI] [PubMed] [Google Scholar]
- 44.Lounkine E, Keiser MJ, Whitebread S, Mikhailov D, Hamon J, Jenkins JL, Lavan P, Weber E, Doak AK, Cote S, Shoichet BK, Urban L. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486(7403):361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266(5190):1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 46.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 47.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105(7):2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80(1):34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 50.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 51.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22(3):636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology. 2009;150(4):1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couse JF, Korach KS. Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Ann N Y Acad Sci. 2001;948:1–8. doi: 10.1111/j.1749-6632.2001.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14(1):3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 57.Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 2004;45(4):455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 58.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 60.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138(1):507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 61.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17(6):1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 62.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 63.Couse JF, Curtis SW, Washburn TF, Eddy EM, Schomberg DW, Korach KS. Disruption of the mouse oestrogen receptor gene: resulting phenotypes and experimental findings. Biochem Soc Trans. 1995;23(4):929–935. doi: 10.1042/bst0230929. [DOI] [PubMed] [Google Scholar]
- 64.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mamm Gland Biol Neopl. 1997;2(4):323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 65.Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord. 2002;3(3):193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- 66.Vidal O, Lindberg M, Savendahl L, Lubahn DB, Ritzen EM, Gustafsson JA, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun. 1999;265(2):569–571. doi: 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 67.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci U S A. 2000;97(10):5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107(3):333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21(6):1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 71.Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA. 2002;99(24):15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson JA. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci U S A. 2004;101(25):9375–9380. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98(11):6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 75.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143(5):1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 76.Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. Estrogen targets genes involved in protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-alpha and -beta. J Biol Chem. 2000;275(37):28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- 77.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18(12):3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J. 2010;24(12):4660–4667. doi: 10.1096/fj.10-163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bopassa JC, Lu R, Singh H, Olde B, Krust A, Leeb-Lundberg LF, Toro L, Stefani E. G protein-coupled Estrogen Receptor 1, but not Estrogen Receptors alpha and beta, mediates rapid estrogen-induced cardioprotection during ischemia/reperfusion Iinjury in male mice. Biophys J. 2012;102(3 Suppl 1):141a. [Google Scholar]
- 80.Villablanca AC, Tenwolde A, Lee M, Huck M, Mumenthaler S, Rutledge JC. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res. 2009;2(3):289–299. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143(12):4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- 82.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58(10):2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 84.Gao F, Ma X, Ostmann AB, Das SK. GPR30 Activation Opposes Estrogen-Dependent Uterine Growth via Inhibition of Stromal ERK1/2 and Estrogen Receptor Alpha (ER{alpha}) Phosphorylation Signals. Endocrinology. 2011;152(4):1434–1447. doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 86.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13(1):92–99. doi: 10.1097/ACI.0b013e32835a6dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ray A, Ficek M. Immunomodulatory effects of anti-estrogenic drugs. Acta Pharm. 2012;62(2):141–155. doi: 10.2478/v10007-012-0012-3. [DOI] [PubMed] [Google Scholar]
- 88.Rettew JA, McCall SHt, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328(1–2):87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 89.Cabas I, Rodenas MC, Abellan E, Meseguer J, Mulero V, Garcia-Ayala A. Estrogen signaling through the G protein-coupled estrogen receptor regulates granulocyte activation in fish. J Immunol. 2013;191(9):4628–4639. doi: 10.4049/jimmunol.1301613. [DOI] [PubMed] [Google Scholar]
- 90.Pernis AB. Estrogen and CD4+ T cells. Curr Opin Rheumatol. 2007;19(5):414–420. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 91.Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163(8):4168–4174. [PubMed] [Google Scholar]
- 92.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103(1):17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296(3):E490–496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 94.Niino M, Hirotani M, Fukazawa T, Kikuchi S, Sasaki H. Estrogens as potential therapeutic agents in multiple sclerosis. Cent Nerv Syst Agents Med Chem. 2009;9(2):87–94. doi: 10.2174/187152409788452054. [DOI] [PubMed] [Google Scholar]
- 95.Derwenskus J, Lublin FD. Future treatment approaches to multiple sclerosis. Handb Clin Neurol. 2014;122:563–577. doi: 10.1016/B978-0-444-52001-2.00024-8. [DOI] [PubMed] [Google Scholar]
- 96.Matejuk A, Bakke AC, Hopke C, Dwyer J, Vandenbark AA, Offner H. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004;77(1):119–126. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- 97.Offner H. Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. J Neurosci Res. 2004;78(5):603–624. doi: 10.1002/jnr.20330. [DOI] [PubMed] [Google Scholar]
- 98.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182(5):3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Subramanian S, Miller LM, Grafe MR, Vandenbark AA, Offner H. Contribution of GPR30 for 1,25 dihydroxyvitamin D(3) protection in EAE. Metab Brain Dis. 2012;27(1):29–35. doi: 10.1007/s11011-011-9266-6. [DOI] [PubMed] [Google Scholar]
- 101.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brunsing RL, Prossnitz ER. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology. 2011;134(1):93–106. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brunsing RL, Owens KS, Prossnitz ER. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J Immunother. 2013;36(3):190–196. doi: 10.1097/CJI.0b013e31828d8e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54(1):11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 105.Chakrabarti S, Morton JS, Davidge ST. Mechanisms of Estrogen Effects on the Endothelium: An Overview. Can J Cardiol. 2013;30(7):705–712. doi: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Maric-Bilkan C, Gilbert EL, Ryan MJ. Impact of ovarian function on cardiovascular health in women: focus on hypertension. Int J Womens Health. 2014;6:131–139. doi: 10.2147/IJWH.S38084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S C. Women’s Health Initiative Steering. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 108.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J I. Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 109.Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. The Women’s Health Initiative trial and related studies: 10 years later: a clinician’s view. J Steroid Biochem Mol Biol. 2014;142:4–11. doi: 10.1016/j.jsbmb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 110.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20(2):133–138. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104(3):288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Delbeck M, Golz S, Vonk R, Janssen W, Hucho T, Isensee J, Schafer S, Otto C. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Rep. 2011;4(1):37–40. doi: 10.3892/mmr.2010.402. [DOI] [PubMed] [Google Scholar]
- 113.Meoli L, Isensee J, Zazzu V, Nabzdyk CS, Soewarto D, Witt H, Foryst-Ludwig A, Kintscher U, Noppinger PR. Sex- and age-dependent effects of GPR30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene. 2014;540(2):210–216. doi: 10.1016/j.gene.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 114.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meyer MR, Field AS, Kanagy NL, Barton M, Prossnitz ER. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci. 2012;91(13–14):623–627. doi: 10.1016/j.lfs.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013;8(11):e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2013;81:99–102. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am J Physiol Endocrinol Metab. 2013;305(1):E113–118. doi: 10.1152/ajpendo.00649.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011;57(5):598–603. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2011;5(11):e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150(8):3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feldman RD, Gros R, Ding Q, Hussain Y, Ban MR, McIntyre AD, Hegele RA. A common hypofunctional genetic variant of GPER is associated with increased blood pressure in women. Br J Clin Pharmacol. 2014;78(6):1441–1452. doi: 10.1111/bcp.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24(3):214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 124.Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, Prossnitz ER. G Protein-coupled Estrogen Receptor protects from atherosclerosis. Scientific reports. 2014;4:7564. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bopassa JC, Leeb-Lundberg LF, Olde B, Toro L, Stefani E. Loss of rapid estrogen-Induced cardioprotection in GPER−/− mice after ischemia and reperfusion. Biophys J. 2011;100(3 Suppl 1):294a–295a. [Google Scholar]
- 126.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298(1):H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297(5):H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 2010;148(2):436–443. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res. 2012;94(1):96–104. doi: 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One. 2012;7(10):e48185. doi: 10.1371/journal.pone.0048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, Filardo EJ. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol. 2014;382(2):950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Hofmeister MV, Damkier HH, Christensen BM, Olde B, Fredrik Leeb-Lundberg LM, Fenton RA, Praetorius HA, Praetorius J. 17beta-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am J Physiol Renal Physiol. 2012;302(3):F358–368. doi: 10.1152/ajprenal.00343.2011. [DOI] [PubMed] [Google Scholar]
- 133.Lindsey SH, Chappell MC. GPR30 activation in salt-sensitive mRen2.Lewis females induces beneficial effects independent of alterations in blood pressure. FASEB J. 2009:1017–1018. [Google Scholar]
- 134.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension. 2011;58(4):665–671. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, Murphy SJ, Anderson S, Hurn PD. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology. 2010;112(2):395–405. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hutchens MP, Kosaka Y, Zhang W, Fujiyoshi T, Murphy S, Alkayed N, Anderson S. Estrogen-mediated renoprotection following cardiac arrest and cardiopulmonary resuscitation is robust to GPR30 gene deletion. PLoS One. 2014;9(6):e99910. doi: 10.1371/journal.pone.0099910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203(1):259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hussain Y, Ding Q, Connelly PW, Brunt JH, Ban MR, McIntyre AD, Huff MW, Gros R, Hegele RA, Feldman RD. G-protein estrogen receptor as a regulator of low-density lipoprotein cholesterol metabolism: cellular and population genetic studies. Arterioscler Thromb Vasc Biol. 2015;35(1):213–221. doi: 10.1161/ATVBAHA.114.304326. [DOI] [PubMed] [Google Scholar]
- 139.Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, Clegg DJ. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav. 2014;66(1):196–207. doi: 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kwon O, Kang ES, Kim I, Shin S, Kim M, Kwon S, Oh SR, Ahn YS, Kim CH. GPR30 mediates anorectic estrogen-induced STAT3 signaling in the hypothalamus. Metabolism. 2014;63(11):1455–1461. doi: 10.1016/j.metabol.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 141.Zhu P, Yuen JM, Sham KW, Cheng CH. GPER mediates the inhibitory actions of estrogen on adipogenesis in 3T3-L1 cells through perturbation of mitotic clonal expansion. Gen Comp Endocrinol. 2013;193:19–26. doi: 10.1016/j.ygcen.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 142.Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121(8):3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010;107(29):13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587(Pt 21):5031–5037. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1–2):63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 146.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48(11):2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 147.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 148.Liu S, Kilic G, Meyers MS, Navarro G, Wang Y, Oberholzer J, Mauvais-Jarvis F. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia. 2013;56(2):370–381. doi: 10.1007/s00125-012-2764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tiano JP, Mauvais-Jarvis F. Molecular mechanisms of estrogen receptors’ suppression of lipogenesis in pancreatic beta-cells. Endocrinology. 2012;153(7):2997–3005. doi: 10.1210/en.2011-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152(8):3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47(22):6052–6057. [PubMed] [Google Scholar]
- 152.Higa GM, Fell RG. Sex hormone receptor repertoire in breast cancer. Int J Breast Cancer. 2013;2013:284036. doi: 10.1155/2013/284036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol. 2006;33(6):631–641. doi: 10.1053/j.seminoncol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 154.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 155.Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009;28(5):523–532. doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Girgert R, Emons G, Grundker C. Inactivation of GPR30 reduces growth of triple-negative breast cancer cells: possible application in targeted therapy. Breast Cancer Res Treat. 2012;134(1):199–205. doi: 10.1007/s10549-012-1968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res Treat. 2010;123(1):87–96. doi: 10.1007/s10549-009-0624-6. [DOI] [PubMed] [Google Scholar]
- 158.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12(21):6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 159.Ignatov A, Ignatov T, Weissenborn C, Eggemann H, Bischoff J, Semczuk A, Roessner A, Costa SD, Kalinski T. G-protein-coupled estrogen receptor GPR30 and tamoxifen resistance in breast cancer. Breast Cancer Res Treat. 2011;128(2):457–466. doi: 10.1007/s10549-011-1584-1. [DOI] [PubMed] [Google Scholar]
- 160.Marjon NA, Hu C, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor (GPER) regulates mammary tumorigenesis and metastasis. Mol Cancer Res. 2014;12(11):1644–1654. doi: 10.1158/1541-7786.MCR-14-0128-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163(5):2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Namba R, Young LJ, Maglione JE, McGoldrick ET, Liu S, Wurz GT, DeGregorio MW, Borowsky AD, MacLeod CL, Cardiff RD, Gregg JP. Selective estrogen receptor modulators inhibit growth and progression of premalignant lesions in a mouse model of ductal carcinoma in situ. Breast Cancer Res. 2005;7(6):R881–889. doi: 10.1186/bcr1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dabrosin C, Palmer K, Muller WJ, Gauldie J. Estradiol promotes growth and angiogenesis in polyoma middle T transgenic mouse mammary tumor explants. Breast Cancer Res Treat. 2003;78:1–6. doi: 10.1023/a:1022133219353. [DOI] [PubMed] [Google Scholar]
- 165.Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, Nicholson B, Cardiff RD, MacLeod CL. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61(22):8298–8305. [PubMed] [Google Scholar]
- 166.Fluck MM, Schaffhausen BS. Lessons in signaling and tumorigenesis from polyomavirus middle T antigen. Microbiol Mol Biol Rev. 2009;73(3):542–563. doi: 10.1128/MMBR.00009-09. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Creasman WT, Soper JT, McCarty KS, Jr, McCarty KS, Sr, Hinshaw W, Clarke-Pearson DL. Influence of cytoplasmic steroid receptor content on prognosis of early stage endometrial carcinoma. Am J Obstet Gynecol. 1985;151(7):922–932. doi: 10.1016/0002-9378(85)90671-4. [DOI] [PubMed] [Google Scholar]
- 168.Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114(3):465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196(4):386.e381–311. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 171.Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, Vadlamudi RK, Brann DW. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol Cell Endocrinol. 2014;389(1–2):84–91. doi: 10.1016/j.mce.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 174.Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res. 2009;87:1610–1619. doi: 10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202(2):223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73(9–10):985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kastenberger I, Schwarzer C. GPER1 (GPR30) knockout mice display reduced anxiety and altered stress response in a sex and paradigm dependent manner. Horm Behav. 2014;66(4):628–636. doi: 10.1016/j.yhbeh.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27(7):1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 180.Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009;137(3):1040–1050. doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 181.Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5(1):e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74(7):555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Murata T, Dietrich HH, Xiang C, Dacey RG., Jr G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke. 2013;44(3):779–785. doi: 10.1161/STROKEAHA.112.678177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184(8):4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158(4):1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lebesgue D, Reyna-Neyra A, Huang X, Etgen AM. GPR30 differentially regulates short latency responses of luteinising hormone and prolactin secretion to oestradiol. J Neuroendocrinol. 2009;21(9):743–752. doi: 10.1111/j.1365-2826.2009.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Anchan D, Gafur A, Sano K, Ogawa S, Vasudevan N. Activation of the GPR30 Receptor Promotes Lordosis in Female Mice. Neuroendocrinology. 2014 doi: 10.1159/000365574. [DOI] [PubMed] [Google Scholar]
- 188.Etgen AM, Jover-Mengual T, Suzanne Zukin R. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Front Neuroendocrinol. 2011;32(3):336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Imai Y, Kondoh S, Kouzmenko A, Kato S. Regulation of bone metabolism by nuclear receptors. Mol Cell Endocrinol. 2009;310(1–2):3–10. doi: 10.1016/j.mce.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 190.Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95(8):3569–3577. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ohlsson C, Vandenput L. The role of estrogens for male bone health. Eur J Endocrinol. 2009;160(6):883–889. doi: 10.1530/EJE-09-0118. [DOI] [PubMed] [Google Scholar]
- 192.Chagin AS, Savendahl L. GPR30 Estrogen Receptor Expression in the Growth Plate Declines as Puberty Progresses. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2007-0814. [DOI] [PubMed] [Google Scholar]
- 193.Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197(2):R1–6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 194.Teplyuk NM, Galindo M, Teplyuk VI, Pratap J, Young DW, Lapointe D, Javed A, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Runx2 regulates G protein-coupled signaling pathways to control growth of osteoblast progenitors. J Biol Chem. 2008;283(41):27585–27597. doi: 10.1074/jbc.M802453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. Journal of Bone and Mineral Research. 2011;26(2):298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain Behav Immun. 2009;23(3):318–324. doi: 10.1016/j.bbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fredette NC, Meyer MR, Prossnitz ER. EB14. San Diego, CA: 2014. The G protein-coupled receptor (GPER/GPR30) activates endothelial nitric oxide synthase 1075.5. [Google Scholar]