Abstract
Background
Peripheral endothelial function is recognized to be impaired in patients with heart failure with reduced ejection fraction (HFrEF), but the peripheral vascular effects of continuous-flow left ventricular assist device (LVAD) implantation, now employed as either a bridge-to-transplantation or as a destination therapy, remain unclear. Using flow-mediated vasodilation (FMD) and reactive hyperemia (RH), this study aimed to provide greater insight into LVAD-induced changes in peripheral vascular function.
Methods and Results
Sixty-eight subjects (13 New York Heart Association (NYHA) Class II HFrEF patients, 19 NYHA Class III/IV HFrEF patients, 20 NYHA Class III/IV HFrEF patients post-LVAD implantation, and 16 healthy age-matched controls) underwent FMD and RH testing in the brachial artery with blood flow velocity, artery diameters, and pulsatility index (PI) assessed by ultrasound Doppler. PI was significantly lower in the LVAD group (2.0 ± 0.4) compared to both the HFrEF II, (8.6 ± 0.8) and HFrEF III/IV (8.1 ± 0.9) patients, who, in turn, were significantly lower than the controls (12.8 ± 0.9). Likewise, LVAD %FMD/shear rate (0.09 ± 0.01 %Δ/s−1) was significantly reduced compared to all other groups (controls, 0.24 ± 0.03; HFrEF II, 0.17 ± 0.02 and HFrEF III/IV, 0.13 ± 0.02 %Δ/s−1) and %FMD/shear rate was significantly correlated with PI (r=0.45). RH was unremarkable across groups.
Conclusions
Although central hemodynamics are improved in patients with HFrEF by a continuous-flow LVAD, peripheral vascular function is further compromised, likely due, at least in part, to the reduction in pulsatility that is a characteristic of such a mechanical assist device.
Keywords: HFrEF, mechanical assist, flow-mediated vasodilation, blood flow, pulsatility
INTRODUCTION
Impaired peripheral vascular function has long been associated with various cardiovascular diseases including hypertension(1), coronary artery disease (2), and is well-known to accompany heart failure (HF) (3–5). Of significance, peripheral vascular dysfunction may also precede the development of these conditions (6–8). Indeed, evidence of peripheral vascular dysfunction is clinically relevant as patients that are at-risk for cardiovascular events who also exhibit a low flow-mediated dilation (FMD) have increased cardiac morbidity and mortality compared to the at-risk patients with normal or mildly abnormal FMD (9). Additionally, an attenuated FMD is also an independent predictor of hospitalization, left ventricular assist device (LVAD) implantation, heart transplantation (HTx), and ultimately death (10–12). Reactive hyperemia (RH), another research tool used to quantify changes in peripheral vascular function (13,14), has also been linked to reduced microvascular function in HF (15,16).
Over the last several decades, advances in mechanical circulatory support devices have drastically improved the clinical management of HF. LVADs are not only a standard bridge-to-transplant therapy, but have also become a destination therapy for individuals that may be ineligible for HTx (17,18). Although the second-generation continuous-flow LVADs have many advantages over the first-generation pulsatile devices, the current LVADs produce continuous blood flow with variable and often diminished pulsatility (19). Given that the nature and magnitude of shear stress is influenced by pulsatility and, generally, plays a positive role in the structure and function of blood vessels over time (20,21), the anticipated reduction in pulsatility in patients receiving a continuous-flow LVAD may negatively impact peripheral vascular function. However, the long-term effects of continuous (i.e. non-pulsatile) blood flow, induced by a LVAD, on peripheral vascular function remain largely unexplored and may be potentially important in terms of end-organ health, and long-term functional capacity of these patients.
Accordingly, using ultrasound Doppler to assess FMD, RH, and pulsatility index (PI), this study sought to determine peripheral vascular function in patients with New York Heart Association (NYHA) Class II and III/IV HFrEF patients, Class III/IV HFrEF patients following continuous-flow LVAD implantation, and healthy age-matched controls. We hypothesized that a) PI would be lowest in the class III/IV HFrEF patients with a LVAD and progressively improve across the HFrEF patients to the healthy controls, b) peripheral vascular function, as assessed by FMD and RH, would be lowest in the class III/IV HFrEF patients with an LVAD and progressively improve across the HFrEF patients to the healthy controls, and therefore c) there would be a significant positive relationship between peripheral vascular function and PI. A better understanding of the peripheral vascular consequences of LVAD implantation and the role of pulsatility, afforded by this study, may help guide the clinical care and rehabilitation of these patients as well as guiding LVAD design.
METHODS
Subjects
A total of 68 subjects (13 NYHA Class II HFrEF patients, 19 NYHA Class III/IV HFrEF patients, 20 NYHA Class III/IV HFrEF patients post-LVAD implantation, and 16 healthy age-matched controls) were recruited from the HF clinics at the University of Utah and the Salt Lake City VA Medical Center, and the controls by word of mouth. The protocol was approved by the Institutional Review Board of the University of Utah and the Salt Lake City Veterans Affairs Medical Center and written informed consent was obtained from all subjects. The healthy controls were normotensive (<140/90), not taking any prescription medication, and free of overt cardiovascular disease, as determined by health history. Exclusion criteria for the healthy controls included a diagnosis of cardiovascular disease, diabetes mellitus, hypercholesterolemia, and hypertension. Inclusion criteria for the patients with HFrEF included NYHA classification and an ejection fraction of < 35%. All patients were considered to be on optimal medical therapy by their physicians and these medications were not withheld at the time of the study. All studies were performed in a temperature controlled environment (~23C). Subjects reported to the laboratory in a fasted state, and without caffeine, alcohol, or exercise for 12 hours.
Flow-mediated Dilation (FMD) and Reactive Hyperemia (RH) Measurements
Details of the FMD procedure have been described previously (22) and were performed in accordance with current recommendations (23). Briefly, a blood pressure cuff was placed on the right arm distal to both the elbow and the placement of the ultrasound Doppler probe on the brachial artery. The brachial artery was insonated approximately midway between the antecubital and axillary regions, and measurements of brachial artery diameter and blood velocity (Vmean) measurements were obtained continuously at rest and for two minutes after cuff deflation (Logiq 7, GE Medical Systems, Milwaukee, WI).
Analyses
Mean blood velocities (Vmean), maximal blood velocities (Vmax), and minimum blood velocities (Vmin) in the brachial artery were automatically calculated using commercially available software (Logiq 7). End-diastolic electrocardiogram (ECG) R-wave gated images were collected from the video output of the Logiq 7 for offline analysis of brachial artery vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). %FMD was quantified as the maximal percentage change in brachial artery diameter after cuff release. Shear rate was calculated as: Shear rate (s−1) = 8Vmean/arterial diameter. Blood flow was calculated as: Blood flow = Vmeanπ (arterial diameter/2)2 × 60. For both shear rate and blood flow, cumulative area under the curve values were integrated with the trapezoidal rule and calculated as follows: Σ(yi(x(i+1) − xi) + (1/2)(y(i+1) − yi)(x(i+1) − xi)). RH was quantified as cumulative brachial artery blood flow for two minutes (area under the curve) following the cessation of cuff occlusion. Normalized FMD was calculated by dividing %FMD by the cumulative shear rate area under the curve until the time of peak brachial artery vasodilation. Pulsatility index (PI) was calculated as: PI = (Vmax − Vmin)/Vmean.
Statistical Analyses
Statistics were performed using commercially available software (SPSS 17.0, Chicago, IL). A one-way ANOVA 1×4 (α < 0.05) was used to determine differences in vascular function (FMD and RH), PI, and subject characteristics. Tukey’s HSD test was conducted to evaluate pairwise differences among the means. All data are expressed as mean ± standard error (SE).
RESULTS
Subject Characteristics
The three distinct HFrEF patient groups and the healthy controls were well matched for age and most other physical characteristics that were assessed (Table 1). The healthy controls were not currently taking any medications, and the disease-specific characteristics and medications of the patients with HFrEF and LVAD are displayed in Table 2.
Table 1.
Subject Characteristics
| Variable | Controls | HFrEF II | HFrEF III/IV | LVAD |
|---|---|---|---|---|
| N | 16 | 13 | 19 | 20 |
| Male/Female | 13/3 | 12/1 | 18/1 | 17/3 |
| Age (yrs) | 62 ± 3 | 59 ± 2 | 61 ± 2 | 58 ± 3 |
| Weight (kg) | 77 ± 4 | 100 ± 7* | 90 ± 5* | 86 ± 3*† |
| Height (cm) | 176 ± 2 | 177 ± 2 | 176 ± 2 | 175 ± 2 |
| Body mass index (kg/m2) | 25 ± 1 | 32 ± 2* | 29 ± 2* | 28 ± 1*† |
| Systolic blood pressure (mmHg) | 125 ± 4 | 116 ± 3 | 112 ± 3* | - |
| Diastolic blood pressure (mmHg) | 75 ± 3 | 71 ± 2 | 67 ± 2* | - |
| Glucose (mg/dL) | 92 ± 3 | 122 ± 9* | 119 ± 18* | 120 ± 7* |
| Cholesterol (mg/dL) | 185 ± 9 | 159 ± 11 | 129 ± 8* | 134 ± 11* |
| HDL (mg/dL) | 49 ± 3 | 41 ± 2* | 34 ± 2*† | 37 ± 3* |
| LDL (mg/dL) | 118 ± 8 | 98 ± 9 | 74 ± 6*† | 75 ± 8*† |
| Triglycerides (mg/dL) | 129 ± 19 | 130 ± 16 | 142 ± 18 | 134 ± 14 |
| Hemoglobin (g/dL) | 14.9 ± 0.4 | 15.1 ± 0.5 | 13.8 ± 0.5 | 12.5 ± 0.3*† |
| Hematocrit (%) | 44.5 ± 1.0 | 44.7 ± 1.4 | 41.3 ± 1.3 | 38.7 ± 0.8*† |
| RBC (M/uL) | 5.0 ± 0.1 | 4.9 ± 0.2 | 4.6 ± 0.2 | 4.3 ± 0.1*† |
| WBC (K/uL) | 5.2 ± 0.3 | 7.8 ± 0.5* | 6.6 ± 0.4*† | 6.8 ± 0.5* |
Mean ± standard error; N, number of subjects; HDL, high density lipoprotein; LDL, low density lipoprotein; RBC, red blood cells; WBC, white blood cells. Systolic and diastolic pressures were not able to be reliably measured in the LVAD patients.
Significantly different from Controls;
Significantly different from HFrEF II.
Table 2.
Characteristics pertinent to the HFrEF patients and LVAD recipients
| Variable | HFrEF II | HFrEF III/IV | LVAD |
|---|---|---|---|
| N | 13 | 19 | 20 |
| Disease Specific Characteristics | |||
| Diagnosis (ischemic cardiomyopathy) | 7/13 | 14/19 | 11/20 |
| Diagnosis (nonischemic cardiomyopathy) | 6/13 | 5/19 | 9/20 |
| Left ventricular ejection fraction, % | 26 ± 3 | 22 ± 1 | - |
| Diabetic | 4/13 | 6/19 | 10/20 |
| Medications, number of all cases | |||
| β-Blocker | 13/13 | 16/19 | 10/20 |
| ACE inhibitor | 10/13 | 8/19 | 3/20 |
| Angiotensin receptor blocker | 2/13 | 6/19 | 0/20 |
| Aldosterone antagonist | 6/13 | 11/19 | 4/20 |
| Statin | 11/13 | 11/19 | 14/20 |
| Diuretic | 8/13 | 18/19 | 13/20 |
| Antiarrhythmic | 0/13 | 6/19 | 1/20 |
| α-Blocker | 1/13 | 0/19 | 0/19 |
| Anticoagulant | 0/13 | 8/19 | 20/20 |
| Type of continuous-flow LVAD | |||
| Heartmate II (Thoratec) | - | - | 17/20 |
| Heartware (Heartware) | - | - | 2/20 |
| Levacor (WorldHeart) | - | - | 1/20 |
| Time post-LVAD implantation, months | - | - | 5 ± 1 |
N, number of subjects; Left ventricular ejection fraction is not presented for the LVAD group as it is post-LVAD implantation.
Resting Blood Flow and Pulsatility Index (PI)
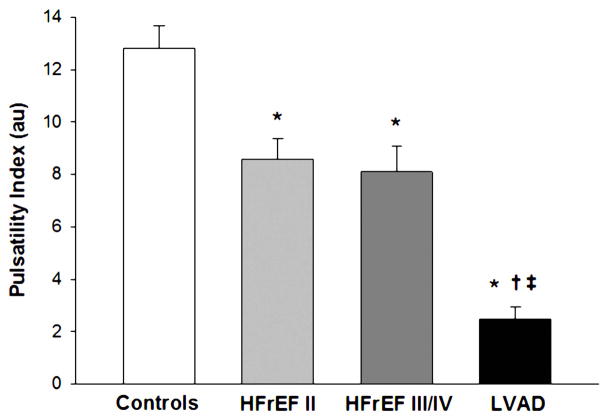
Resting brachial artery blood flow, blood velocity, shear rate, and diameter were not different between groups (Table 3). PI was significantly lower in the LVAD group compared to both the HFrEF II, and HFrEF III/IV patients, who, in turn, were significantly lower than the controls (12.8 ± 0.9) (Table 3, Figure 1).
Table 3.
Brachial artery baseline and post-cuff occlusion hemodynamics in healthy controls, HFrEF patients and LVAD recipients.
| Variable | Controls | HFrEF II | HFrEF III/IV | LVAD |
|---|---|---|---|---|
| Baseline | ||||
| BA diameter, mm | 4.8±0.2 | 5.1±0.3 | 4.7±0.2 | 5.1±0.2 |
| BA blood velocity, cm/s | 8.7±1.3 | 9.1±1.0 | 9.4±0.8 | 9.1±1.0 |
| BA blood flow, mL/min | 89±14 | 124±22 | 95±9 | 106±10 |
| BA shear rate, s−1 | 140±21 | 142±18 | 165±18 | 150±19 |
| BA Pulsatility Index (PI), au | 12.8±0.9 | 8.6±0.8* | 8.1±0.9* | 2.0±0.4*†‡ |
| Post-Cuff Occlusion | ||||
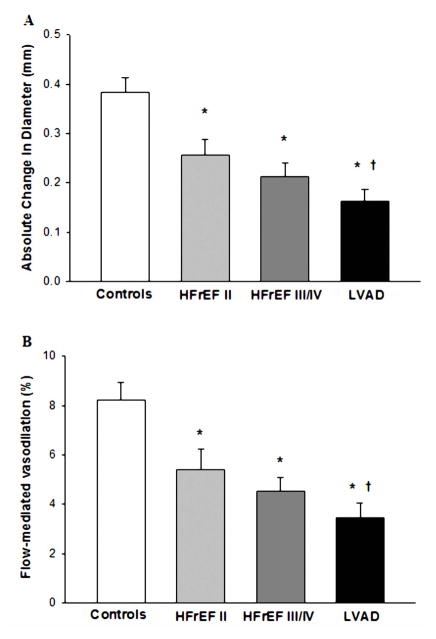
| Change in BA diameter, mm | 0.38±0.02 | 0.27±0.03* | 0.21±0.03* | 0.16±0.02*† |
| Change in BA diameter, % | 8.2±0.7 | 5.4±0.8* | 4.5±0.5* | 3.4±0.6*† |
| Time to peak BA vasodilation, s | 71±6 | 61±7 | 64±5 | 72±6 |
| BA Blood flow AUC, mL | 562±70 | 608±76 | 509±42 | 599±31 |
| BA Peak shear rate, s−1 | 962±83 | 880±78 | 878±46 | 1032±84 |
| BA shear rate AUC at peak vasodilation | 37535±3994 | 35593±5350 | 39767±4393 | 39128±4050 |
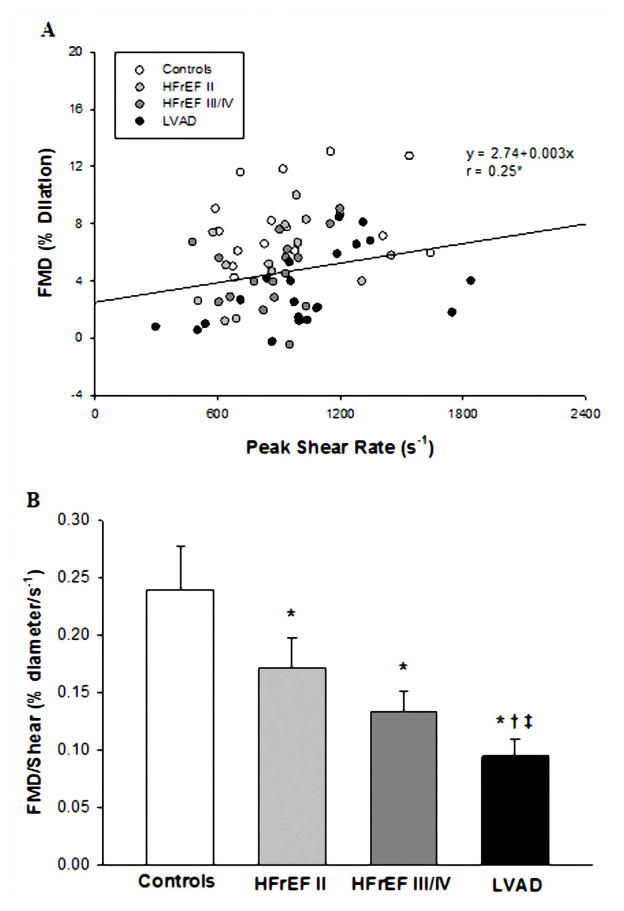
| BA FMD/shear, %/s−1 | 0.23±0.04 | 0.17±0.03* | 0.13±0.02* | 0.09±0.02*†‡ |
Mean ± standard error; BA, brachial artery; AUC, area under the curve.
Significantly different from Controls;
Significantly different from HFrEF II.
Significantly different from HFrEF III/IV.
Figure 1.
Brachial artery PI in NYHA Class II HFrEF patients, NYHA Class III/IV HFrEF patients, NYHA Class III/IV HFrEF patients post-LVAD implantation, and healthy controls. (*) Significantly different from Controls; (†) Significantly different from HFrEF II. (‡) Significantly different from HFrEF III/IV. Values are mean ± SE.
Flow-mediated Dilation (FMD), and Reactive Hyperemia (RH)
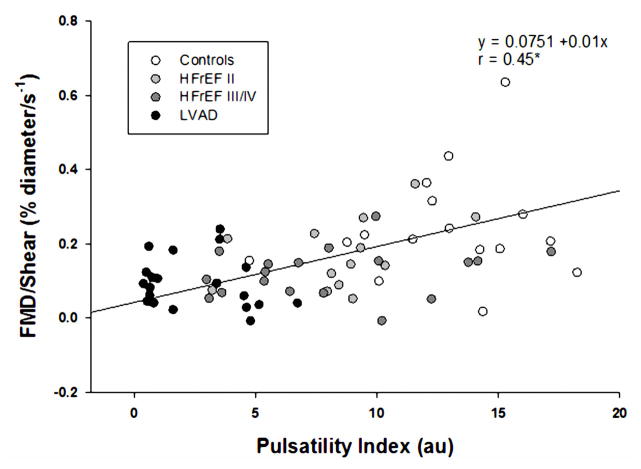
Peripheral vascular function, as measured by both absolute change in brachial artery diameter and %FMD, was significantly lower in all patient groups compared to the controls. The absolute change in brachial artery diameter and %FMD for the patients with the LVAD were also significantly lower than the HFrEF II group, but were not lower than the HFrEF III/IV group (Table 3, Figure 2A and B). However, there was a significant positive relationship between %FMD and peak shear rate (Figure 3A) and when %FMD was normalized for shear rate, it was evident that %FMD/shear in the LVAD group was significantly attenuated compared to all other groups (Table 3, Figure 3B). There was also a significant relationship between PI and both %FMD and %FMD/shear (Figure 4). Within the LVAD group there was no relationship between time post-implantation, absolute change in brachial artery diameter, %FMD or %FMD/shear. Although not a main focus of the current study, there were no significant differences in %FMD or %FMD/shear when comparing patients with an ischemic versus non-ischemic disease etiology in any of the patient groups. RH, measured as blood flow area under the curve, for two minutes following cuff release, was not different between groups (Table 3).
Figure 2.
Brachial artery FMD expressed as an absolute change in diameter (A) and percentage change from pre-cuff baseline (B) in NYHA Class II HFrEF patients, NYHA Class III/IV HFrEF patients, NYHA Class III/IV HFrEF patients post-LVAD implantation, and healthy controls. (*) Significantly different from Controls; (†) Significantly different from HFrEF II. (‡) Significantly different from HFrEF III/IV. Values are mean ± SE.
Figure 3.
Relationship between brachial artery FMD and brachial artery peak shear rate in all subjects (A) and brachial artery FMD expressed as a percentage change from precuff baseline after normalizing for shear rate (B) in NYHA Class II HFrEF patients, NYHA Class III/IV HFrEF patients, NYHA Class III/IV HFrEF patients post-LVAD implantation, and healthy controls. (*) Significantly different from Controls; (†) Significantly different from HFrEF II. (‡) Significantly different from HFrEF III/IV.
Figure 4.
Relationship between brachial artery PI and brachial artery %FMD/shear.
DISCUSSION
This study sought to determine peripheral vascular function in NYHA Class III/IV HFrEF patients following continuous-flow LVAD implantation in comparison with NYHA Class II and III/IV HFrEF patients and healthy age-matched controls. Using FMD to assess vascular function, we documented attenuated vasodilatory capacity in the LVAD patients compared to both the HFrEF II patients and the healthy controls in terms of absolute change in brachial artery diameter and %FMD. However, when %FMD was normalized for shear rate, the LVAD patients exhibited attenuated vascular function compared to both the HFrEF II and HFrEF III/IV patients and controls. Of note, more than half of the HFrEF III/IV patients were scheduled to receive a LVAD within a few days. Thus, although cross-sectional in design, the findings of this study suggest that, despite the central hemodynamic improvements afforded by a continuous-flow LVAD, peripheral vascular function in these patients becomes further compromised following LVAD implantation. Further, evaluation of pulsatility by PI revealed a significant attenuation in all HFrEF groups, but, of importance, PI was also significantly lower in the LVAD patients compared to both HFrEF groups and there was a significant positive relationship between PI and %FMD/shear across all subjects. This decline in vascular function suggests a negative physiologic response to diminished vascular pulsatility and has significant implications for the clinical care and rehabilitation of these patients.
FMD in HFrEF and Following LVAD Implantation
Peripheral vascular function is known to be reduced in HFrEF patients (3–5) and the results of the present study further substantiate this dogma. Due to the increasing incidence of HF and a lack of suitable donor organs for HTx, LVADs have become a standard bridge-to-transplant therapy. Recently the FDA also approved LVADs as a destination therapy for individuals that may be ineligible for HTx (17,18). However, somewhat surprisingly, considering the predictive value of assessing vascular function in patients (9–12), to date, there is only one study that has utilized FMD to assess the consequences of LVAD implantation on peripheral vascular health (24). This study by Amir et al. (24) revealed that approximately 4 months after surgery, individuals with a first generation pulsatile LVAD exhibited what they considered “normal” vascular function, as measured by FMD. In contrast, those individuals who received a second generation continuous flow LVAD had a significantly lower FMD. Unfortunately, this study did not include healthy controls or other HFrEF groups and the “normal” values used for comparison were simply extracted from previous FMD literature (25,26), which is fraught with high variability and differences in technique. Additionally, although the pulsatile LVADs assessed in the study by Amir et al. (24) appeared to support and possibly even enhance peripheral vascular function, unfortunately, these pulsatile-flow devices are no longer available due to their large size and poor durability, which was largely a result of the many moving parts required to generate the pulsatile blood flow (18).
To our knowledge, this is the first comprehensive peripheral vascular function study to include healthy age-matched controls, two groups of HFrEF patients with differing severity, and a group of continuous-flow LVAD recipients. With this approach, using brachial artery FMD, we have clearly documented attenuated peripheral vascular function in the LVAD recipients compared to the controls and the HFrEF II patients (Figure 2). As shear stress is an important mechanical force that stimulates the release of vasoactive substances such as nitric oxide (20,21) and because there was a significant relationship between %FMD and peak shear rate (Figure 3) in the current study; %FMD was normalized for shear rate. This correction revealed that the LVAD recipients, that were approximately 5 months post-implantation, a time point similar to the Amir et al. study (24), peripheral vascular function, as assessed by %FMD/shear, was attenuated compared to the healthy controls and, more importantly, both of the HFrEF patient groups. Although, cross sectional in design, recognition that more than half of the patients in the HFrEF III/IV group were scheduled to receive a LVAD suggests that this study has considerable clinical relevance. Specifically, these findings suggest that the already compromised vascular function present in the NYHA Class III/IV HFrEF patients is actually exaggerated following LVAD implantation. This is in stark contrast to previous work by our group (5) and others (27) that documents an improvement in peripheral vascular function within the first few months and years following HTx.
Relationship between Pulsatility and Vascular Function
Under normal conditions, in most areas of the vasculature, the blood vessels and endothelial cells experience pulsatile shear stress that corresponds to the cardiac cycle. Although the current LVADs leave patients with some degree of pulsatility, the pulse pressure is still typically quite minimal and does not actually approach the “normal” native level of pulsatility induced by a healthy heart. This study clearly revealed that although brachial artery pulsatility, measured by PI, is attenuated with HFrEF, the pulsatility in the LVAD patients is several magnitudes less (Figure 1). Therefore, with the implantation of a LVAD, the shear stress experienced by the endothelial cells is very different which likely results in a fall in endothelial nitric oxide synthase (eNOS) expression and subsequently nitric oxide bioavailability. Given that shear stress plays an integral role in the structure and function of blood vessels over time it seems likely that peripheral vascular function will change in patients following implantation of a continuous-flow LVAD. In fact, in this study, we document a significant positive relationship between PI and peripheral vascular function across all subjects (Figure 4). This supports previous findings that pulsatile flow produces greater wall shear stresses and superior vascular responsiveness (28,29) and, therefore, may be more effective in restoring peripheral hemodynamics (30) than continuous flow. Overall, our findings are supportive of the concept that peripheral vascular function is dependent upon pulsatility, and the associated shear stresses, and when these are disrupted, such as with a continuous-flow LVAD, there are negative vascular consequences.
Additionally, although previous work has reported that the pulsatile LVADs can improve sympathetic innervation (31), a recent study reported that patients with second generation continuous-flow LVADs have higher muscle sympathetic nerve activity than patients with pulsatile LVADs (32). This is likely due, at least in part, to baroreceptor unloading with reduced pulsatility. Another recent study reported that plasma renin activity and aldosterone were both significantly elevated in patients with continuous compared to pulsatile devices (33). Therefore, it is possible that both baroreceptor unloading and an augmented renin-angiotensin-aldosterone system, via increased sympathetic nerve activation, may have contributed to the current finding of diminished vascular function in continuous-flow LVAD recipients, however this needs to be verified.
RH in HFrEF and Following LVAD Implantation
RH, which is thought to reflect a conglomerate of both endothelial-dependent and –independent vasodilation of the microvasculature (34,35), is often reported to be attenuated in animal models of HF (15,36), but is not as clear in human HF patients (5,16,37). We hypothesized that RH would be reduced in all the patient groups, but particularly the LVAD recipients. Somewhat surprisingly, resting blood flow in the brachial artery and the RH response, both in terms of peak and area under the curve, were not different in any of the current subject groups (Table 3). Therefore, in comparison to the healthy controls, there was no evidence of microvascular dysfunction in either of the HFrEF patients or the LVAD recipients. Again, there appears to only be one other study, to date, that has evaluated the effects of continuous-flow LVAD support on microvascular function. In that study, Lou et al. (38) reported that 1–4 months of LVAD support did not negatively affect microvascular function, as measured by the reactive hyperemia index, compared to a group of HFrEF patients that were awaiting LVAD implantation. These findings support the results of the current study, however, given the increased incidence of LVAD implantation as a destination therapy and the potential for vascular stiffening to occur with long-term ventricular support (39), further studies are necessary to better examine blood flow distribution and the consequences in the microcirculation with LVAD use.
Clinical Implications for Patients with LVAD Support
Over the past decade, survival following LVAD-implantation has greatly improved, which is partially due to more experienced LVAD management teams and the introduction of the smaller, more reliable continuous-flow pumps. However, mortality and especially morbidity are still considerable following LVAD-implantation. A recent prospective study by Jorde et al. (40) revealed that in the first 247 patients that received a Heartmate II continuous-flow LVAD for destination therapy, the Kaplan-Meier survival was only 74 ± 3% and 61 ± 3% at 12 and 24 months, respectively. Even more importantly, only 43% of the patients were alive and free of a serious adverse event two years following LVAD implantation. The assessment of peripheral vascular function is highly clinically relevant as cardiovascular patients with a low FMD have increased cardiac morbidity and mortality compared to patients with a normal or mildly abnormal FMD (9). However, up until this point, the effects of continuous, non-pulsatile blood flow on peripheral vascular reactivity have remained largely unexplored. Given that the continuous-flow LVAD may have even more deleterious effects on the already compromised peripheral vasculature, it is important to understand the true impact and mechanisms responsible, particularly in the patients receiving an LVAD as a destination therapy.
Although continuous-flow LVAD support has been documented to significantly improve functional capacity and quality of life compared to pre-implantation values (41), physical activity and overall energy expenditure (42), and peak VO2 (43,44) are all attenuated when compared to patients that received a HTx, considered to be the gold standard treatment for end-stage HF. Exercise intolerance in LVAD patients likely involves many factors that include device type, inability to increase cardiac output during exercise, right ventricular dysfunction, chronotropic incompetence, impaired pulmonary function, skeletal myopathy, and endothelial dysfunction (45). As several of these factors are currently very difficult to control, the peripheral vasculature, extremely important in oxygen transport to skeletal muscle, represents a target area where reversal of disease-related symptoms can occur, improving overall physical activity and exercise tolerance.
Experimental Considerations
We acknowledge several experimental considerations that may be perceived as limitations to this study. First, the study was cross-sectional in experimental design which raises the possibility that other factors, such as pre-LVAD vascular function and other co-morbidities such as history of hyperglycemia, hyperlipidemia, and hypertension influenced the results. Although this study did not include a longitudinal pre to post LVAD assessment, more than 50% of the patients in the HFrEF III/IV group were imminently awaiting LVAD implantation, suggesting that this group and the LVAD recipients were inherently very similar. Additionally, as we chose not to remove these HFrEF patients from their current medications, we cannot rule out the possibility of a pharmacological effect on the hemodynamic responses assessed in the current study. Specifically, only a small number of LVAD patients were receiving either an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) compared to the HFrEF groups. However, it should be noted that within each of our patient groups there was no significant difference in any of the main outcome variables between those on an ACE/ARB therapy and those that were not. Also, due to a need to maintain patient safety, the investigators were not blinded to the health status of the subjects (Controls vs. HFrEF vs. LVAD), although the analyses were blinded. Finally, our post-LVAD measurements were made on average 5 months following implantation and although this is relatively soon following surgery our group (5) and others (27) have reported improvement in peripheral vascular function within the first few months and years following HTx.
Conclusions
LVAD implantation is an accepted therapy in patients with advanced HF as either a bridge-to-transplantation or as a destination therapy. This study has, for the first time, revealed that peripheral vascular function in patients with a continuous-flow LVAD, as assessed by brachial artery %FMD/shear rate, was significantly reduced compared to other patients with HFrEF and healthy controls. As brachial artery PI was significantly attenuated in the LVAD patients and PI was positively correlated with %FMD/shear across all subjects, it seems likely that peripheral vascular dysfunction in the LVAD patients is a consequence of the lower pulsatility, and associated changes in shear stress, due to the continuous-flow mechanical support. This is of clinical significance and has implications for LVAD design requirements. Specifically, although the continuous-flow LVAD clearly helps to support the failing heart, the periphery likely represents the area where reversal of other disease-related symptoms, such as improved tissue perfusion, can occur, that will improve exercise tolerance and subsequently quality of life in these patients.
Perspectives.
Competency in Medical Knowledge
Peripheral vascular function in patients with a continuous-flow LVAD is attenuated compared to other patients with HFrEF and this is partially related to the diminished pulsatility experienced by these patients.
Translational Outlook
Future research should explore the timing and magnitude of the changes in peripheral vascular function following LVAD implantation.
Acknowledgments
Sources of Funding: This study was supported in part by grants from the NIH (RSR: P01 HL-091830; CHS: 4R01 HL089592), and the VA (RSR: RR&D Merit Grant E6910R and SPiRe Grant E1433-P). Advanced Fellowships in Geriatrics from the Department of Veterans Affairs supported MAW and RSG.
ABBREVIATIONS LIST
- FMD
flow-mediated vasodilation
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HTx
heart transplantation
- LVAD
left ventricular assist device
- NYHA
New York heart association
- PI
pulsatility index
- RH
reactive hyperemia
- Vmax
maximal blood velocity
- Vmean
mean blood velocity
- Vmin
minimum blood velocity
Footnotes
Disclosures: There are no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 2.Monnink SH, van Haelst PL, van Boven AJ, et al. Endothelial dysfunction in patients with coronary artery disease: a comparison of three frequently reported tests. J Investig Med. 2002;50:19–24. doi: 10.2310/6650.2002.33513. [DOI] [PubMed] [Google Scholar]
- 3.Katz SD, Biasucci L, Sabba C, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–25. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 4.Drexler H, Hayoz D, Munzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 5.Witman MA, Fjeldstad AS, McDaniel J, et al. Vascular Function and the Role of Oxidative Stress in Heart Failure, Heart Transplant, and Beyond. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer D, Sorensen K, Spiegelhalter D, Georgakopoulos D, Robinson J, Deanfield J. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 9.Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–23. doi: 10.1016/j.jacc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–9. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 11.Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail. 2009;11:588–93. doi: 10.1093/eurjhf/hfp053. [DOI] [PubMed] [Google Scholar]
- 12.Tarro Genta F, Eleuteri E, Temporelli PL, et al. Flow-mediated dilation normalization predicts outcome in chronic heart failure patients. J Card Fail. 2013;19:260–7. doi: 10.1016/j.cardfail.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vac Biol. 2007;27:2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–9. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 15.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. The American journal of physiology. 1992;262:H411–9. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 16.Morgan DR, Dixon LJ, Hanratty CG, et al. Impaired endothelium-dependent and -independent vasodilation in elderly patients with chronic heart failure. Eur J Heart Fail. 2004;6:901–8. doi: 10.1016/j.ejheart.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Badiwala MV, Rao V. Left ventricular device as destination therapy: are we there yet? Curr Opin Cardiol. 2009;24:184–9. doi: 10.1097/HCO.0b013e328323f58f. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 19.Wever-Pinzon O, Selzman CH, Drakos SG, et al. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device HeartMate II. Circ Heart Fail. 2013;6:517–26. doi: 10.1161/CIRCHEARTFAILURE.112.000206. [DOI] [PubMed] [Google Scholar]
- 20.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vac Biol. 1998;18:677–85. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 22.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol. 2009;107:445–53. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 23.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amir O, Radovancevic B, Delgado RM, 3rd, et al. Peripheral vascular reactivity in patients with pulsatile vs axial flow left ventricular assist device support. J Heart Lung Transplant. 2006;25:391–4. doi: 10.1016/j.healun.2005.11.439. [DOI] [PubMed] [Google Scholar]
- 25.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 26.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. The American journal of cardiology. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 27.Roig E, Cuppoletti A, Masotti M, et al. Assessment of peripheral endothelial-dependent vasodilatation within the first year after heart transplantation. J Heart Lung Transplant. 2009;28:299–304. doi: 10.1016/j.healun.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. The American journal of physiology. 1991;261:H257–62. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- 29.Nakata M, Tatsumi E, Tsukiya T, et al. Augmentative effect of pulsatility on the wall shear stress in tube flow. Artif Organs. 1999;23:727–31. doi: 10.1046/j.1525-1594.1999.06411.x. [DOI] [PubMed] [Google Scholar]
- 30.Drakos SG, Charitos CE, Ntalianis A, et al. Comparison of pulsatile with nonpulsatile mechanical support in a porcine model of profound cardiogenic shock. ASAIO J. 2005;51:26–9. doi: 10.1097/01.mat.0000150323.62708.35. [DOI] [PubMed] [Google Scholar]
- 31.Drakos SG, Athanasoulis T, Malliaras KG, et al. Myocardial sympathetic innervation and long-term left ventricular mechanical unloading. JACC Cardiovasc Imaging. 2010;3:64–70. doi: 10.1016/j.jcmg.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Markham DW, Fu Q, Palmer MD, et al. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Heart Fail. 2013;6:293–9. doi: 10.1161/CIRCHEARTFAILURE.112.969873. [DOI] [PubMed] [Google Scholar]
- 33.Welp H, Rukosujew A, Tjan TD, et al. Effect of pulsatile and non-pulsatile left ventricular assist devices on the renin-angiotensin system in patients with end-stage heart failure. The Thoracic and cardiovascular surgeon. 2010;58 (Suppl 2):S185–8. doi: 10.1055/s-0029-1240709. [DOI] [PubMed] [Google Scholar]
- 34.Loscalzo J, Vita JA. Ischemia, hyperemia, exercise, and nitric oxide. Complex physiology and complex molecular adaptations. Circulation. 1994;90:2556–9. doi: 10.1161/01.cir.90.5.2556. [DOI] [PubMed] [Google Scholar]
- 35.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. The American journal of physiology. 1996;270:H1435–40. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 36.McAllister RM, Laughlin MH, Musch TI. Effects of chronic heart failure on skeletal muscle vascular transport capacity of rats. The American journal of physiology. 1993;264:H689–91. doi: 10.1152/ajpheart.1993.264.3.H686. [DOI] [PubMed] [Google Scholar]
- 37.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 38.Lou X, Templeton DL, John R, Dengel DR. Effects of continuous flow left ventricular assist device support on microvascular endothelial function. J Cardiovasc Transl Res. 2012;5:345–50. doi: 10.1007/s12265-011-9321-z. [DOI] [PubMed] [Google Scholar]
- 39.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010;25:490–4. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 40.Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the Destination Therapy Post-Food and Drug Administration Approval Study With a Continuous Flow Left Ventricular Assist Device: A Prospective Study Using the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2014;63:1751–7. doi: 10.1016/j.jacc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 41.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Jakovljevic DG, McDiarmid A, Hallsworth K, et al. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. The American journal of cardiology. 2014;114:88–93. doi: 10.1016/j.amjcard.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kugler C, Malehsa D, Tegtbur U, et al. Health-related quality of life and exercise tolerance in recipients of heart transplants and left ventricular assist devices: a prospective, comparative study. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:204–10. doi: 10.1016/j.healun.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail. 2014;20:548–54. doi: 10.1016/j.cardfail.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loyaga-Rendon RY, Plaisance EP, Arena R, Shah K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014 doi: 10.1016/j.healun.2014.12.006. [DOI] [PubMed] [Google Scholar]