Abstract
Microtubules, one of the major cytoskeletal structures, were previously considered stable and only indirectly involved in synaptic structure and function in mature neurons. However, recent evidence demonstrates that microtubules are dynamic and have an important role in synaptic structure, synaptic plasticity, and memory. In particular, learning induces changes in microtubule turnover and stability, and pharmacological manipulation of microtubule dynamics alters synaptic plasticity and long-term memory. These learning-induced changes in microtubules are controlled by the phosphoprotein stathmin, whose only known cellular activity is to negatively regulate microtubule formation. During the first eight hours following learning, changes in the phosphorylation of stathmin go through two phases causing biphasic shifts in microtubules stability/instability. These shifts, in turn, regulate memory formation by controlling in the second phase synaptic transport of the GluA2 subunit of AMPA receptors. Improper regulation of stathmin and microtubule dynamics has been observed in aged animals and in patients with Alzheimer’s disease and depression. Thus, recent work on stathmin and microtubules has identified new molecular players in the early stages of memory encoding.
Keywords: dynamic, biphasic, stathmin, microtubules, hippocampus, dentate gyrus, memory consolidation, contextual fear conditioning, AMPAR
1. Introduction
An organism’s success and survival are critically dependent on its ability to process and transmit information related to a certain stimulus or threat in the environment. Therefore, it is crucial to unravel activity-dependent intracellular processes, as they provide the means for neural circuits to control adaptive behaviors. Increasing evidence demonstrates that extracellular stimuli initiate a chain of intracellular events with temporal patterns and signaling dynamics, which reflect the identity and intensity of information received from the environment (Behar and Hoffmann, 2010; Purvis and Lahav, 2013). For neurons, propagating intracellular signals with temporal patterns and signaling dynamics is especially challenging. Signals often travel long distances along neuronal projections, which are required for communication between the synapse and neuronal cell body and nucleus. This results in the strengthening of connections between synapses and thus, memory formation (Mayford et al., 2012).
Among many mechanisms mediating synaptic transport (Ch’ng and Martin, 2011), cytoskeletal structures – actin filaments and microtubules – play a major role in motor-driven import and export (Bredt and Nicoll, 2003; Hirokawa et al., 2010; Wenthold et al., 2003). Known to be dynamic and present in dendritic spines, actin filaments are essential in synaptic function and memory formation (Hotulainen and Hoogenraad, 2010; Tada and Sheng, 2006). In contrast, the role of microtubules, and, in particular, microtubule dynamics, in synaptic function and memory is less clear. Microtubule dynamics is a process involving a constant shift between microtubule stability and instability. There is a clearly established role for dynamic microtubules in cell division, axonal pathfinding during development, and axonal regeneration (Conde and Caceres, 2009). In contrast, microtubules in mature neurons are generally viewed as stable cytoskeletal structures present in dendritic shafts, but not in dendritic spines. Recently, work in hippocampal and cortical primary neuronal cell cultures has shown that microtubules, like actin filaments, can also be dynamically regulated during neuronal activity, move from the dendritic shaft to dendritic spines and be directly involved in synaptic structure and function (Gu et al., 2008; Jaworski et al., 2009; Kapitein et al., 2011; Merriam et al., 2011; Mitsuyama et al., 2008; Penzes et al., 2009). Thus, it is likely that microtubule dynamics in the dendritic spines and surrounding areas play important roles in synaptic plasticity and memory formation. Indeed, recent work in animals has begun to show that microtubule dynamics at synaptic sites are controlled by activity and learning, and that these changes are critical for long-term potentiation (LTP) and memory (Fanara et al., 2010; Uchida et al., 2014).
Here, we will summarize recent progress in the studies of the role of microtubules in synaptic structure, synaptic function and memory formation. In particular, we will focus on some recent findings that link activity-dependent changes in microtubule stability/instability and activity of stathmin, a negative regulator of microtubule formation.
2. Post-translational modifications (PTMs) of tubulin
Microtubules are composed of heterodimers of α- and β-tubulins. They are heterogeneous in length and highly dynamic in vitro and in vivo, undergoing cycles of polymerization and rapid depolymerization. Microtubule dynamics and function are highly controlled by the intrinsic GTPase activity of tubulins as well as various PTMs, such as tyrosination, detyrosination, acetylation, Δ2 modification, glutamylation, glycylation, palmitoylation, and phosphorylation (Fukushima et al., 2009; Janke and Bulinski, 2011; Yu et al., 2015). Some of these PTMs occur both on α-tubulin and β-tubulin. Most of tubulin PTMs happen on the C-terminal tails that are critical for interaction with various molecular motors and microtubule-associated proteins (Yu et al., 2015). Modifications on the C-terminal tails include detyrosination/tyrosination of α-tubulin, the removal of the penultimate glutamate of α-tubulin (forming Δ2-tubulin), and glutamylation and glycylation of α- and β-tubulin tails (Yu et al., 2015). Although PTMs of tubulin have been known for more than 40 years, we are only now starting to understand their roles, as they emerge as crucial controllers of microtubule properties, defining microtubule diverse cellular functions in various biological systems.
In this review we will focus mostly on the tyrosination/tyrosination of tubulin because recent work has shown its critical role in memory formation (Uchida et al., 2014). α-tubulin undergoes the tyrosination/detyrosination cycle. It is initiated by the removal of a Tyr functional group (detyrosination). Re-addition of Tyr (tyrosination) then reverses the modification and returns tubulin to its nascent state. All tubulins, except for α4A- and α8-tubulin, possess a Tyr residue at the C-terminus immediately after translation, which is a marker of newly generated unstable microtubules (Khawaja et al., 1988; Paturle-Lafanechere et al., 1994). After assembly, tyrosinated tubulin in microtubules is detyrosinated. Detyrosinated tubulin is present in stable, long-lived microtubules (Khawaja et al., 1988; Paturle-Lafanechere et al., 1994). As discussed below, detyrosination and tyrosination of tubulin are highly regulated by learning (Table 1).
Table 1.
Brief summary of activity-dependent changes in microtubule stability in neuronal cells
| Microtubule dynamics | |||
|---|---|---|---|
| Targets | System | Findings | References |
| Tyrosinated tubulin |
Primary neuron |
Tyrosinated tubulin extended into dendritic protrusions. Potassium chloride treatment increases microtubule invasions of dendritic protrusions. |
(Hu et al., 2008) |
| Tubulin EB3 |
Primary neuron |
Microtubule invasions of dendritic protrusions. BDNF-induced increase of spine number is blocked by microtubule destabilizer (nocodazole) treatment. |
(Gu et al., 2008) |
| EB3 | Primary neuron |
Microtubule invasions of dendritic protrusions. | (Jaworski et al., 2009) |
| Tubulin | Hippoca mpal slices |
Electron microscopic analysis shows that there is redistribution of microtubules in dendritic spines following LTP induction. |
(Mitsuyama et al., 2008) |
| Acetylated tubulin |
Hippoca mpal slices |
Potassium chloride or NMDA treatment enhances the level of acetylated tubulin. |
(Pandey and Sharma, 2011) |
| Tyrosinated tubulin |
Mouse dentate gyrus (contextu al fear condition ing) |
Tyrosinated tubulin increases 0.5-1 h and decreases 8 h following context-shock exposure. Increased tyrosinated tubulin is observed in mice received context only or immediate shock. |
(Uchida et al., 2014) |
| Detyrosinate d tubulin |
Mouse dentate gyrus (contextu al fear condition ing) |
Detyrosinated tubulin decreases 0.5-1 h and increases 8 h following context-shock exposure. Decreased detyrosinated tubulin is observed in mice received context only or immediate shock. |
(Uchida et al., 2014) |
| Microtubule s |
Mouse hippoca mpus (contextu al fear condition ing) |
Microtubule stabilizer (paclitaxel) injection immediately after contextual fear conditioning reduces long-term memory. Paclitaxel and microtubule destabilizer (nocodazole) injection 8 h after contextual fear conditioning enhances or reduces long-term memory, respectively. |
(Uchida et al., 2014) |
| Microtubule s |
Mouse hippoca mpus (contextu al fear condition ing) |
Microtubule turnover is changed following contextual fear conditioning. Nocodazole injection reduces contextual fear memory and spine number. |
(Fanara et al., 2010) |
| Microtubule s |
Mouse (Y maze) |
Microtubule stabilizer (epothilone D) treatment rescues deficits in axonal microtubule density and spatial learning observed in aged PS19 mice. |
(Zhang et al., 2012) |
| Microtubule s |
Mouse (Morris water maze) |
Paclitaxel treatment leads to reduced microtubule dynamics and deficits in spatial learning. |
(Atarod et al., 2015) |
Please note that this is not an exclusive survey and that the findings listed here do not cover all the contents of the reference papers.
Acetylation of Lys40 on α-tubulin (L’Hernault and Rosenbaum, 1985), similar to the detyrosination, takes place on the microtubule polymer (Maruta et al., 1986). Acetylated tubulin exists in stable, long-lived microtubules, although acetylation does not seem to cause microtubule stabilization (Palazzo et al., 2003). Although the role of tubulin PTMs in microtubule dynamics is not well understood, it is important to mention that some of the critical microtubule functions are dependent on microtubule dynamics, which in turn are controlled by PTMs of tubulin.
3. Activity-dependent changes in microtubules in dendritic spines
Dendritic spines are the major sites of excitatory synaptic input in the mammalian central nervous system. Their morphological changes are believed to be the basis of learning and memory (Kandel, 2001). Dendritic spines of neurons are enriched with actin filaments, and both spine structure and function are regulated by actin (Hotulainen and Hoogenraad, 2010; Tada and Sheng, 2006). Dynamics changes in actin filaments are important for structural modification in neurons. In contrast to what is known about actin filaments, microtubules were generally thought to be stable in mature neurons and not present in the spines. However, recent studies have shown that microtubules enter the spines under physiological conditions in cultured hippocampal and cortical primary neurons (Gu et al., 2008; Hu et al., 2008; Jaworski et al., 2009) and in the hippocampal slices after tetanic stimulation (Mitsuyama et al., 2008) (Table 1). In addition, tubulin posttranslational modifications such as tyrosination and acetylation are associated with neuronal activity (Table 1). Using time-lapse total internal reflection fluorescence microscopy in cultured hippocampal and cortical neurons, Dent and colleagues have shown that dynamic microtubules invade dendritic spines (Hu et al., 2008). They showed that treatment with BDNF increases tyrosinated-tubulin in spines, whereas paclitaxel, a microtubule-stabilizing drug, abolishes this effect. This result indicates that BDNF increases microtubule invasion of dendritic spines, and that these invasions are dependent on microtubule dynamics. In addition, by imaging microtubule dynamics, they found that 10% of dendritic protrusions are targeted by microtubules in an hour. Interestingly, they found that microtubule invasion occurs in very mature hippocampal neurons (DIV63 or 63 days in vitro), suggesting that microtubule dynamics in spines appears to be an ongoing process throughout life of an organism. Zheng and colleagues also reported that microtubules can be present in spines of cultured hippocampal neurons using immunocytochemistry (Gu et al., 2008). They showed that a knockdown of EB3, a microtubule end-binding protein, leads to a decrease in the number of dendritic spines. Furthermore, they showed that blocking microtubule dynamics with nocodazole inhibits the BDNF-induced increase in the number of spines in neuronal cultures. This was confirmed by another study, which also found microtubule invasions in hippocampal dendritic spines (Jaworski et al., 2009). Importantly, microtubule entry into spines was increased 3-fold after transient stimulation of cultured hippocampal neurons with potassium chloride, and this increase was blocked by treatment with tetrodotoxin, indicating that microtubule dynamics in neurons are changing in an action potential-dependent manner (Hu et al., 2008). Finally, it was shown that the microtubule entry into dendritic spines is synaptic activity-dependent (Mitsuyama et al., 2008). These studies clearly demonstrate invasion of microtubules in dendritic spines in an activity-dependent manner and provide the first important clues regarding the role of microtubule dynamics in post-mitotic neurons.
4. Microtubule-mediated intracellular transport
In neurons, the transport between the cell body and synapses is fundamental for synaptic function and for memory. Microtubules play a critical role in transport of various molecules and organelles, both pre- and post-synaptically. Importantly, PTMs of tubulin are associated with intracellular transport through the regulation of microtubule stability and motor protein activity. This suggests that activity-dependent changes in PTMs of tubulin might control synaptic transport, thereby mediating synaptic plasticity and memory formation. Molecular motors from the kinesin, dynein, and myosin superfamilies are normally involved in transport of various cargoes, and two of those, kinesins and dyneins, are utilized by microtubules (Hirokawa et al., 2010). Microtubule stability has been reported to affect the activity of motor proteins, thereby influencing intracellular transport.
4.1. Role of KIF in dendritic transport
In dendrites, cargo is transported by kinesin superfamily of proteins (KIFs) (Hirokawa et al., 2010). Some of the cargo includes the N-methyl-D-aspartate receptors (NMDARs), which are critical for synaptic plasticity, learning and memory. KIF17 is a motor protein that transports GluN2B, a subunit of NMDAR-containing vesicles (Hirokawa et al., 2010). The disruption of the kif17 gene inhibits GluN2B transport, resulting in a loss of synaptic GluN2B (Yin et al., 2011). Critically, NMDA receptor-mediated synaptic currents, early and late long-term potentiation (LTP), long-term depression (LTD), and CREB responses are attenuated in KIF17 knockout mice, which is consistent with hippocampus-dependent memory impairment (Yin et al., 2011). Another kinesin, KIF5, associates with GRIP, a scaffolding protein that binds to α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) and transports them in dendrites (Setou et al., 2002). In addition, microtubule motor function of KIF5 is required for AMPAR-mediated synaptic transmission (Hoerndli et al., 2013). Moreover, γ-aminobutyric acid receptors (GABARs), a critical component of the inhibitory system in the brain, are transported by KIF5 via huntingtin-associated protein 1 (HAP1) (Twelvetrees et al., 2010). In conditional KIF5A knockout mice, in which KIF5A protein is postnatally deleted in neurons, impaired GABA(A) receptor-mediated synaptic transmission leads to an epileptic phenotype, thus demonstrating that KIF5A may regulate inhibitory transmission (Nakajima et al., 2012).
4.2. Microtubule stability influences motor proteins activity
Regulating the activity of molecular motors via microtubule PTMs provides a mechanism for transporting cargo to the corresponding synaptic sites (Janke and Bulinski, 2011). Tubulin detyrosination has been shown to regulate the binding and motor activity of the KIF5 to microtubules (Dunn et al., 2008; Konishi and Setou, 2009; Liao and Gundersen, 1998). Tubulin detyrosination increases the affinity of KIF5 for microtubules ~2.8-fold (Dunn et al., 2008; Konishi and Setou, 2009; Liao and Gundersen, 1998) affecting long-distance transport processes. Although all PTMs of tubulin are found on neuronal microtubules (Janke and Bulinski, 2011), their role in synaptic plasticity and memory has remained unclear. Knockout and transgenic approaches to study individual microtubule-modifying enzymes will provide direct evidence for their function in vivo.
5. Microtubules in synaptic plasticity and memory formation
Microtubules are critical for synaptic transport, but how dynamically changing microtubules are involved in learning and memory has remained unclear. As LTP is one of the forms of synaptic plasticity and a leading model of the cellular mechanisms responsible for memory formation (Bliss and Lomo, 1973; Kandel et al., 2014), it is important to note that drugs inhibiting microtubule dynamics interfere with LTP. For example, applying a microtubule-stabilizing drug, paclitaxel, leads to LTP deficits in the cortico-amygdala pathway in mouse brain slices (Shumyatsky et al., 2005). Also, an inhibitor of microtubule dynamics, nocodazole, decreases mossy fiber LTP (Barnes et al., 2010) and Schaffer collateral LTP (Jaworski et al., 2009). Since drug interfering with microtubule dynamics affect LTP, it is likely that microtubules are also involved in memory formation.
Indeed, recent evidence in live animals has revealed a previously unknown feature of microtubules: learning induces biphasic shifts in microtubule stability in the dentate gyrus of hippocampus (Uchida et al., 2014). The level of detyrosinated tubulin (marker of stable microtubules) was changed in a biphasic manner, first decreasing within 30–60 min following contextual fear conditioning and then increasing at the time point of 8 h following conditioning, whereas tyrosinated tubulin (marker of unstable microtubules) levels were changed in the opposite direction (Table 1). This suggests that microtubules become unstable 0.5–1 h and hyperstable 8 h following learning (Figure 1). Exposure to context only or immediate shock led to a reduction of the detyrosinated tubulin and an increase in tyrosinated tubulin 0.5 h, but not 8 h, following the exposure (Uchida et al., 2014). These results suggest that the increase in microtubule stability at 8 h is specific to associative learning.
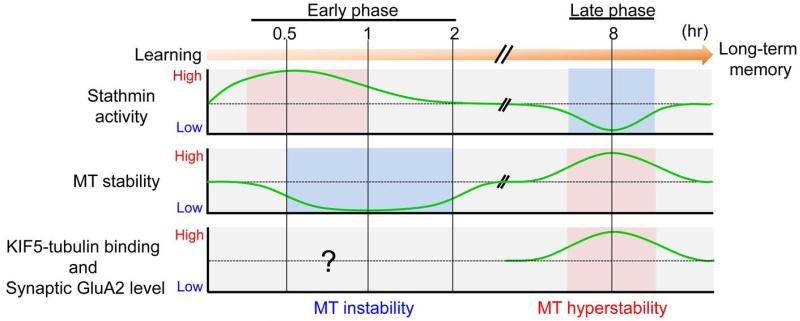
Figure 1. The early and late phases of microtubule stability and stathmin activity following learning.
In the early phase, stathmin is dephosphorylated and binds to tubulin during the first 15-60 min following contextual fear conditioning. This leads to microtubule disassembly by activated stathmin. 0.5-2 h after learning, tyrosinated-tubulin-enriched microtubules (labile, newly generated microtubules) are formed. Although it is suggested that the activity-dependent dynamic microtubules (eg., tyrosinated tubulin) can entry into the spine in primary neuronal cell cultures (see reviews (Dent et al., 2011; Penzes et al., 2009)), it is still unclear whether this also occurs in the mouse brain. In the late phase (8 hours) after the learning, phosphorylation of stathmin is increased and the stathmin is dissociated from tubulin. Subsequently, the amount of detyrosinated-tubulin-enriched microtubules (stable microtubules) is increased. Tubulin detyrosination activates the transport of GluA2 subunit of AMPARs from the soma to synaptic sites by enhancing the binding of KIF5-GluA2 complex to microtubules.
Pharmacological manipulation of hippocampal microtubule activity further supports an important role of biphasic changes in microtubule stability in memory formation. Intra-hippocampal injection of nocodazole 8 h following contextual fear conditioning causes a decrease in long-term memory. By contrast, infusion of paclitaxel, a microtubule stabilizer, increases memory (Uchida et al., 2014). These pharmacological experiments demonstrate that memory can be enhanced or disrupted by changing microtubule hyperstability 8 h following learning. They also suggest that during this time period microtubule-dependent intracellular processes involved in memory consolidation are sensitive to manipulation (see also Section 9).
The learning-induced destabilization of microtubules during the early phase is also important for memory formation. Intra-hippocampal injection of paclitaxel immediately after training disrupts long-term memory in contextual fear conditioning (Uchida et al., 2014). This suggests that the early phase has a significant role in associative memory formation. To examine the role of the early phase of microtubule changes in memory, further work needs to be performed (also see Chapter 8).
A study by Fanara et al. (2010) also showed a role of microtubules in neuronal plasticity and memory. Using stable isotope labeling to measure the turnover of tubulin in defined microtubule populations in the mouse brain, they found increased turnover of hippocampal MAP2-associated and cold-stable microtubules following contextual fear conditioning. Nocodazole infusion into the brain prevented learning-induced increase in MAP2-associated microtubules and reduced contextual fear memory. In addition, nocodazole infusion blocked learning-dependent enhancement of spine density in the hippocampus, demonstrating a link between spine structural plasticity and microtubule dynamics in vivo.
6. Stathmin, a microtubule-destabilizing protein
Although several lines of evidence have shown that microtubule dynamics are important for synaptic plasticity and memory formation, the molecular mechanisms underlying learning-mediated control of microtubule dynamics are largely unknown. Proteins that act as both microtubule stabilizers and destabilizers have been identified in neurons. These include microtubule-interacting proteins, microtubule-associated proteins, microtubule-destabilizing proteins, microtubule-severing proteins, microtubule plus-end tracking proteins (+TIPs), and motor proteins (Akhmanova and Steinmetz, 2008; Conde and Caceres, 2009; Dent and Gertler, 2003; Fukushima et al., 2009; Janke and Bulinski, 2011; Rodriguez et al., 2003). This review will focus on a microtubule destabilizer stathmin/oncoprotein 18 (also known as stathmin 1). Stathmin (stathmin 1) is a member of the stathmin family of phosphoproteins, which also includes superior cervical ganglia neural-specific 10 protein (SCG10; stathmin 2), SCG10-like protein (SCLIP; stathmin 3), and RB3 (stathmin 4). Among them, stathmin is the most abundantly expressed in the nervous system (Chauvin and Sobel, 2015). In addition, stathmin is highly expressed in the rodent brain during development and in adulthood, whereas the expression of SCG10, SCLIP and RB3 gradually decrease during development and stay low or at not detectable level in the adult rodent brain (Boekhoorn et al., 2014). Thus, stathmin (stathmin 1) may be the only member of the stathmin family controlling microtubule dynamics in the adult brain.
Stathmin has four Ser residues (Ser16, Ser25, Ser38 and Ser63), all of which can be phosphorylated (Di Paolo et al., 1997; Larsson et al., 1997). Unphosphorylated or hypophosphorylated stathmin binds tubulin heterodimers, preventing microtubule assembly. After its phosphorylation, stathmin releases tubulin dimers, allowing microtubules to be formed. Although phosphorylation of stathmin at all Ser residues is important for inactivation of its depolymerization activity (Lawler, 1998; Melander Gradin et al., 1997), phosphorylation at Ser16 strongly reduces the binding affinity of stathmin to tubulin heterodimers (Di Paolo et al., 1997; Larsson et al., 1997; Manna et al., 2009). It should be noted that Ser16 can be phosphorylated by cAMP-dependent protein kinase A (PKA) and Ca2+/Calmodulin-dependent kinases II and IV (CaMKII/IV) (Beretta et al., 1993; le Gouvello et al., 1998; Marklund et al., 1994), which are critically involved in brain function and in memory (Abel et al., 1997; Fukushima et al., 2008; Kang et al., 2001; Malleret et al., 2010; Mayford et al., 1996; Silva et al., 1992). Thus, these kinases may regulate memory formation by phosphorylating stathmin at Ser16 and thus controlling microtubule stability. Other three Ser residues are also likely to be involved in microtubule-depolymerizing activity of stathmin following behavior or brain activity.
7. Role of stathmin in synaptic plasticity and behavior
The first evidence that members of the stathmin family are involved in activity-dependent processes in the adult brain came with the demonstration of RB3 protein being induced in the dentate gyrus granule layer of the hippocampus by electrically induced seizure activity or by stimuli that lead to LTP (Beilharz et al., 1998). While both basic synaptic transmission and NMDAR function are normal, LTP is deficient in the cortico-amygdala pathway in stathmin knockout mice (Shumyatsky et al., 2005). In addition, mice overexpressing the unphosphorylatable Stathmin4A mutant have deficiency in perforant path-dentate gyrus LTP (Uchida et al., 2014).
Other studies also have shown that stathmin may play a role in neuronal plasticity (Table 2). Stimulation of cultured primary neurons with BDNF increases phosphorylation of stathmin (Cardinaux et al., 1997). In addition, phosphorylation of stathmin is induced by treatment with dopamine or forskolin (Cardinaux et al., 1997). Given that BDNF and forskolin are widely known to influence neuronal plasticity and memory, phosphorylation of stathmin might be an underlying mechanism of synaptic plasticity and memory (see Sections 8 and 9).
Table 2.
Brief summary of activity-dependent changes in stathmin phosphorylation in neuronal cells
| Stathmin phosphorylation | |||
|---|---|---|---|
| Targets | System | Findings | References |
| Stathmin | Primary neuron | Phosphorylation of stathmin in response to BDNF is inhibited by the specific inhibitor of the MAP kinase cascade. |
(Cardinaux et al., 1997) |
| Stathmin | Primary neuron | Phosphorylation of stathmin is induced by the treatment with dopamine or forskolin. |
(Chneiweiss et al., 1992) |
| Stathmin 2 (SCG10) | Primary neuron | Phosphorylation of SCG10 in response to kainic acid is inhibited by the NMDA receptor antagonist. |
(Morii et al., 2006) |
| Stathmin | Mouse dentate gyrus (contextual fear conditioning) |
Phosphorylation of stathmin at Ser16 increases 2-8 h following context-shock exposure. Phosphorylation of stathmin at Ser25 decreases 0.5-1 h following context-shock exposure. Phosphorylation of stathmin at Ser38 decreases 0.5-1 h and increases 8 h following context-shock exposure. Exposure of either context only or immediate shock reduces stathmin phosphorylation at Ser 25 and Ser38. |
(Uchida et al., 2014) |
Accumulated evidence has indicated which brain areas and behaviors are dependent on stathmin function. Stathmin was first found to be functionally important for cortico-amygdala LTP and behaviors dependent on the basolateral amygdala-associated neural circuitry, including the anatomical pathways relaying both conditioned and unconditioned stimuli information to the basolateral amygdala (Shumyatsky et al., 2005). This expression pattern allows stathmin to control innate and learned fear. In addition, stathmin knockout mice display enhanced extinction of cued fear conditioning, which was accompanied by an increase in neural activity in the prefrontal cortex and dentate gyrus with a simultaneous decrease in amygdala activity (Martel et al., 2012). Stathmin knockout mice also show an increase in social interactions but a decrease in social recognition memory and affiliative maternal care, which were associated with stathmin expression in the basolateral amygdala (Martel et al., 2008). Stathmin transgenic mice expressing Stat4A, an unphosphorylatable stathmin mutant, show reduced contextual fear memory (Uchida et al., 2014), suggesting that stathmin phosphorylation plays an important role in brain function.
8. Biphasic changes in stathmin activity following learning
Stathmin function is regulated by various stimuli in cultured neurons, but what about its role in vivo following learning? A recent study has found that learning biphasically regulates stathmin phosphorylation and tubulin-binding activity at synaptic sites (Table 2) (Uchida et al., 2014). During the first phase, phosphorylation of synaptosomal stathmin at the Ser16, Ser25 and Ser38 sites was rapidly decreased 15–60 min following training in fear conditioning. During the second phase – 8 h following fear conditioning – phosphorylation of synaptosomal stathmin at Ser16 and Ser38 sites was increased. There was no effect of either the context only or the immediate shock on stathmin phosphorylation at Ser16, suggesting a specific role of Ser16 phosphorylation in memory formation. Importantly, stathmin-tubulin binding was increased 30 min following 8 h after training and it was decreased 8 h following training. In mice exposed to context only, stathmin binding to α-tubulin was increased 30 min following exposure, but not after 8 h,. These data suggest that during associative learning, stathmin may regulate microtubule stability by changing its affinity to α-tubulin.
It should be noted that during both the early and late phases, shifts in learning-dependent microtubule stability are accompanied by parallel changes in stathmin phosphorylation and in the microtubule-destabilizing activity of stathmin (Figure 1), suggesting that stathmin can directly control the shifts in microtubule stability during memory encoding and can be critically involved in memory formation. Supporting this idea, both gain- and loss-of-function stathmin mutations disrupt learning-dependent microtubule dynamics and memory in contextual fear conditioning (Uchida et al., 2014). Changes in stathmin activity and microtubule stability are seen in synaptosomal fractions, but not in whole-cell extracts. Because these changes accompany deficits in synaptic plasticity and contextual fear memory, the synaptosomal localization of the changes suggests the importance of stathmin-microtubule interactions at the synaptic sites for memory processing.
Microtubule-depolymerizing activity of stathmin (measured by its phosphorylation status) is induced 15 min after learning and then followed by an increase in microtubule instability 15 min later (30 min after learning; Figure 1). Initial microtubule disassembly caused by a decrease in stathmin phosphorylation, and thus an increase in stathmin activity, is likely to be followed by a subsequent, new microtubule assembly, since the level of tyrosinated tubulin, a marker of labile newly generated microtubules, is increased. Exposure to only context or shock dephosphorylates stathmin to the extent that is not as pronounced as that after context-US exposure, which represents associative memory. However, the changes during the early phase are seen both after context-US association and after exposure to context or immediate shock. Thus, they might reflect molecular events related to attention or arousal, which is somewhat similar to induction of expression observed in immediate-early genes (Kubik et al., 2007). It is plausible that the early phase is related to an arousal/attention or memory trace, which can be transferred to a long-lasting form of memory if it becomes biologically relevant (for example after being associated with a shock).
Learning-induced changes in stathmin phosphorylation seem to directly regulate its microtubule-destabilizing activity and thus microtubule stability. This is illustrated by the dysregulation of changes in microtubule stability in Stat4A transgenic mice, overexpressing the unphosphorylatable Stathmin4A mutant. While in wild-type mice, an increase in detyrosinated tubulin normally occurs 8 h following contextual fear conditioning, in Stat4A mice, it was disrupted, suggesting improper regulation of microtubule stability (Uchida et al., 2014). This result indicates that phosphorylation of stathmin is essential for microtubule hyperstability following learning. In addition, stathmin knockout mice showed suppression of changes in microtubule stability during the early and late phase and were deficient in contextual fear memory as tested 24 h after training (Uchida et al., 2014). These data indicate that in the absence of stathmin, both early instability and late hyperstability in microtubules are deficient following learning, leading to deficits in memory formation.
The changes in stathmin phosphorylation within the first eight hours following learning described above do not involve changes in total amount of stathmin protein. However, it appears that the total levels of stathmin mRNA and protein in the dentate gyrus of the rat hippocampus are increased 48 h following contextual fear conditioning (Federighi et al., 2013). This finding suggests that following learning stathmin may be involved in other intracellular processes in addition to the synapse-specific events (Uchida et al., 2014).
9. How do stathmin-mediated microtubule dynamics affect memory process?
9.1. Stathmin-mediated control of microtubule stability is associated with intracellular transport
As mentioned earlier, increased stability of microtubules and hyper-phosphorylation of stathmin during the late phase following learning are specifically observed in the memory process. This fact raises the possibility that increased detyrosination of tubulin during the late phase may influence motor protein function, which in turn affect memory formation (Figure 2). Indeed, in hippocampal primary cell cultures, tubulin detyrosination enhances the motor activity of KIF5 and its binding to microtubules (Konishi and Setou, 2009). In agreement with this, there is an increase in the interaction between KIF5 and α-tubulin 8 h following learning (Uchida et al., 2014). In addition, learning induces KIF5-GluA2 interactions and increased GluA2 levels in the synaptosomal and microtubule fractions during the late phase (Uchida et al., 2014), which is consistent with the report that the dendritic GluA2 is transported by KIF5 (Setou et al., 2002). Confirming these interactions, Stat4A transgenic mice expressing the constitutively active Stathmin4A mutant, show a lack of dendritic transport of GluA2 with KIF5 following learning (Uchida et al., 2014). The finding that KIF5-GluA2 binding is regulated by stathmin-microtubule interactions is in agreement with work showing that the gain-of-function stathmin mutation leads to a decrease in KIF5 levels in the cerebellar dendrites (Ohkawa et al., 2007), further supporting the notion that stathmin-mediated microtubule dynamics influence KIF5-driven intracellular transport. The functional importance of the change in microtubule-mediated GluA2 synaptic transport is confirmed by the ability of the TAT-GluA23Y peptide, a blocker of GluA2 endocytosis, to rescue contextual fear memory when injected into the dentate gyrus area of Stat4A mice and is in agreement with the role of AMPAR transport in synaptic plasticity (Correia et al., 2008) and contextual memory (Matsuo et al., 2008; Mitsushima et al., 2011; Rao-Ruiz et al., 2011). This evidence suggests an important role for stathmin-mediated dendritic transport of the GluA2 along microtubules in synaptic plasticity and memory formation.
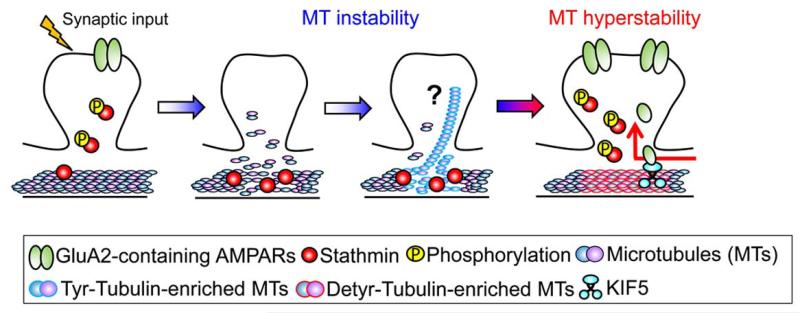
Figure 2. Proposed Model for the Role of Dynamic Microtubules in Memory Formation.
During the early phase (see Figure 2), microtubules become unstable due to the increased binding of stathmin to the tubulin caused by the hypophosphorylation of stathmin. Microtubule instability during the early phase is important for memory formation because treatment with microtubule stabilizer drug paclitaxel disrupts long-term memory formation (see text for details). During the late phase, phosphorylation of stathmin is increased and tubulin is dissociated from stathmin. Subsequently, the amount of detyrosinated-tubulin-enriched microtubules (stable microtubules) is increased. Tubulin detyrosination activates the transport of the GluA2 subunit from the soma to synaptic sites by enhancing the binding of the KIF5-GluA2 complex to microtubules. The microtubule hyperstability during the late phase is also important for memory, since treatment with microtubule stabilizer drug paclitaxel or microtubule destabilizer drug nocodazole 8 h following learning enhances or reduces long-term memory, respectively.
9.2. Possible role of stathmin and microtubules in tagging of synapses
Accumulated evidence suggests that individual synapses are “tagged” by previous synaptic input, leading them to be selectively recognized as targets for recruitment of new synaptic molecules (reviewed in Martin and Kosik, 2002). Kinesin-mediated transport of proteins, mRNA, or organelles that are involved in synaptic development and plasticity seem to be associated with synaptic tagging (Dent et al., 2011). Thus, it is plausible that learning-associated changes in microtubule dynamics at synaptic sites are involved in the “tagging” of the synapses for further modifications by GluA2. It would be interesting, in the future, to investigate whether the same synaptic sites undergo changes in microtubule stability during the early and late phases following learning. Further studies are required to understand the role of microtubule dynamics in synaptic homeostasis in the dentate gyrus, as well as other brain regions.
Learning-dependent regulation of dynamic microtubules by stathmin at synaptic sites in the adult mammalian brain plays an important role in memory formation (Figure 2). Learning induces changes in phosphorylation of stathmin and its ability to bind tubulin, which in turn leads to shifts in microtubule dynamics and regulates microtubule-mediated intracellular localization of the GluA2 subunit of AMPARs. These intercellular changes control synaptic plasticity and memory formation. Thus, learning-dependent control of microtubule dynamics by stathmin represents a new signaling pathway crucially involved in memory formation.
10. The biphasic shifts in stathmin and microtubules confirm the hypothesis of multiple waves of neuronal plasticity during memory consolidation
It is interesting to note that several other cellular processes show biphasic changes following learning. For example, training in the water maze leads to biphasic changes in phosphorylation of cAMP-response element-binding protein (CREB) in the CA1 area of the hippocampus, with two different peaks occurring 15 min and 8 h post-acquisition (Porte et al., 2011). Also, in the CA1, training in tone-shock fear conditioning leads to a monophasic increase in phosphorylation (15 min following training) of extracellular signal-regulated kinase 1/2 (ERK) and CREB, while context-shock conditioning leads to a biphasic increase in ERK/CREB phosphorylation (Trifilieff et al., 2006). The monophasic change in ERK/CREB phosphorylation in CA1 following tone-shock is somewhat reminiscent to the early phase of the changes in stathmin and microtubules in the dentate gyrus following exposure to tone or shock only. The immediate-early gene coding for activity-regulated cytoskeleton-associated protein (Arc), which regulates AMPAR trafficking, shows a biphasic increase in protein expression (0.5-1 and 8 h) in the hippocampus following spatial exploration of a novel environment (Ramirez-Amaya et al., 2005). In another study, hippocampal Arc levels after contextual fear conditioning increase in a biphasic manner immediately and 12 h after conditioning, and selectively blocking late Arc expression interferes with persistence, but not formation, of contextual fear memory (Nakayama et al., 2015). Similar biphasic changes in gene transcription- or protein translation-related processes have been observed in other studies (Bourtchouladze et al., 1998; Izquierdo et al., 2002; Martel et al., 2007; Stanciu et al., 2001; Swank and Sweatt, 2001; Trifilieff et al., 2007). Thus, the biphasic shift in stathmin activity and microtubule stability resembles previous observations of biphasic changes following exploration or learning, which led to the suggestion that multiple waves of neuronal plasticity are involved in memory consolidation (Rose, 2000). Therefore, there is now evidence for multiple time windows during memory formation. These waves and their timing seem to depend on the exact task used, the brain region studied and the molecular process being examined. Another general concern with time windows is the potential for circadian effects. For example, large time-of-day effects on gene expression in the hippocampus were found, suggesting that some of the reported changes may be circadian regulated (Peixoto et al., 2015). So, one issue is doing appropriate time of day controls. This might also relate to the role of sleep in memory.
Given all these concerns, biphasic changes in stathmin and microtubules are unique in a sense that the first phase leads to instability and the second phase leads to hyperstability. Most of the other biphasic transcription/translation processes show an increase or decrease in both phases.
11. Implication of stathmin and microtubule in neuropsychiatric disorders
All previous sections in this review discussed the role of stathmin and microtubules in regulating neuronal function in the healthy brain. But is there any evidence implicating stathmin and microtubules in brain dysfunction? We will review several examples of these molecules being involved in mental states and discuss how stathmin, by controlling microtubule dynamics, may be responsible for the pathophysiology of cognitive dysfunction in the adult brain, as well as in age-related disorders.
11.1. Age-related memory loss and Alzheimer’s disease
Stathmin-microtubule interactions seem to be one of possible mechanisms underlying age-dependent memory loss. Interestingly, stathmin expression in the brain is decreased with aging (Saetre et al., 2011) and this decrease is accelerated in Alzheimer’s disease (Hayashi et al., 2006; Jin et al., 1996; Saetre et al., 2011), linking stathmin to aging processes.
The dentate gyrus has been implicated in normal aging in humans and primates (Burke and Barnes, 2006; Small et al., 2004). Also, reduced levels of the synaptic GluA2 in the dentate gyrus were associated with memory loss in aged monkeys (Hara et al., 2012). In support of the hypothesis that stathmin and microtubules are involved in aged-dependent memory loss, aged wild-type mice show reduced stathmin levels as well as reduced levels of GluA2 at the synaptic sites and in microtubule fractions of the dentate gyrus (Uchida et al., 2014). Furthermore, blocking the GluA2 endocytosis reverses the deficits in contextual fear memory in aged mice, suggesting that the deficit in the GluA2 transport has a crucial role in age-dependent memory loss. This implicates stathmin and microtubules involvement in the age-related changes in GluA2 transport (Uchida et al., 2014). Since changes in stathmin-mediated microtubule dynamics influence GluA2 intracellular transport from the cell body to synaptic sites (Uchida et al., 2014), the stathmin-microtubule-GluA2 signaling pathway might be associated with age-dependent memory loss and might use a similar mechanism as that in young adult mice.
Other work also suggests a role for microtubules in memory and cognition. Paclitaxel-like drug Epothilone D (which crosses the blood-brain barrier) improves memory in an Alzheimer’s disease mouse model when used in a very low dose. Phase I clinical trials with low doses of Epothilone D in Alzheimer’s patients were initiated (Barten et al., 2012).
11.2 The role of stathmin and microtubules in cognitive impairments
Many signaling pathways involved in memory and cognition are also found to be disrupted in various mental disorders (Ebert and Greenberg, 2013; Pittenger and Duman, 2008). Thus, it is not surprising that growing evidence links dysfunction in the adult brain to stathmin and microtubules. Stathmin has been implicated in abnormal states of fear, anxiety, cognition, social behavior, and brain trauma in rodents and humans (Brocke et al., 2010; Ehlis et al., 2011; Elder et al., 2012; Martel et al., 2012; Martel et al., 2008; Shumyatsky et al., 2005), suggesting microtubule involvement in these processes. Common single nucleotide polymorphisms (SNPs) in the STMN1 gene significantly impacted fear and anxiety responses in humans, measured with the startle and cortisol stress response (Brocke et al., 2010). In another human study, carriers of the SNP rs182455 of STMN1 showed altered cognitive-affective processing with the effects more pronounced in females (Ehlis et al., 2011). In a rat model of mild traumatic brain injury (mTBI) that leads to post-traumatic stress disorder (PTSD), animals exposed to repetitive blast injury displayed elevated levels of stathmin in the amygdala (Elder et al., 2012). Finally, stathmin knockout mice show increased extinction of cued fear conditioning, which affects neuronal activity as measured with c-fos in the dentate gyrus, amygdala and prefrontal cortex (Martel et al., 2012).
Recent evidence also links depressive-like behaviors to cytoskeleton-related changes, both in actin filaments and microtubules (Wong et al., 2013). Chronic unpredictable stress, which is a laboratory model for studying depression, leads to changes in the ratio of tyrosinated tubulin and acetylated tubulin in rat hippocampus, suggesting changes in microtubule stability (Yang et al., 2009). Also, in a proteomics study, changes in tubulin have been reported in an animal model of depression (Piubelli et al., 2011). An increase in microtubule acetylation in the hippocampus is associated with decreased neuronal plasticity and dendritic retraction by chronic stress (Bianchi et al., 2003; Yang et al., 2009). Tubulin becomes less tyrosinated in a depression rat model (Bianchi et al., 2003), and there is an increase in tyrosinated tubulin in sleep-deprived rats, indicating microtubule instability (Basheer et al., 2005).
In post-mortem hippocampus of depressed subjects there are changes in microtubule-associated proteins, MAP1A, MAP1B and MAP2, as well as in genes, which protein transport is dependent on microtubules, such as GluA1 and GluA3 subunits of AMPARs (Duric et al., 2013). As the mechanisms responsible for learning and depression overlap, it is intriguing that we reported learning-dependent synaptic accumulation of the GluA2 subunit and its motor protein, KIF5, to be deficient in transgenic mice expressing a constitutively active Stathmin4A mutant, which leads to deficits in microtubule dynamics (Uchida et al., 2014). Also, MAP2, which modulates microtubule dynamics, is proposed to be a target of drugs that stimulate tubulin assembly and act as antidepressants having advantages over selective serotonin re-uptake inhibitor fluoxetine (Bianchi and Baulieu, 2012).
In addition, recent evidence has suggested that deficits in stathmin and microtubules might be involved in schizophrenia and Huntington disease (Cao et al., 2013; Colin et al., 2008; Dompierre et al., 2007; Hayashi et al., 2006). Thus, stathmin and dynamic microtubules may represent a convergent pathway for different biological processes in health and disease of the nervous system. As a result of these similarities, changes in microtubule stability and/or improving microtubule dynamics may be a beneficial approach to treat memory deficits in aging, Alzheimer’s disease, as well as establish axonal and synaptic connections in spinal cord injury and central nervous system lesions (Barten et al., 2012; Hellal et al., 2011; Witte et al., 2008). Thus, elucidating the molecular mechanisms of stathmin-microtubule interactions or downstream/upstream signaling of microtubule dynamics may help better understand the pathophysiology of neuropsychiatric disorders and may lead to better design of novel approaches for their treatment.
12. Conclusion
A recent finding demonstrates that learning-induced changes in stathmin-dependent dynamic microtubules regulate GluA2 synaptic intracellular transport and this process is crucial for memory formation (Uchida et al., 2014). Taken together, the above studies indicate that there are emerging roles for stathmin in microtubule stability, protein transport, synaptic plasticity and memory formation. Throughout this review we have focused on the stathmin-mediated regulation of microtubule dynamics in memory formation. However, it is still unclear how stathmin activity is controlled in response to synaptic input. Also, stathmin-interacting proteins, which regulate stathmin microtubule-destabilizing activity, are still not well-characterized. Finally, control of localization of the GluA2 subunit of AMPARs is likely not the only process controlled by stathmin-microtubule interactions following learning. Thus, it will be instructive to dissect further both upstream and downstream signaling in the stathmin-microtubules interactions to fully understand the role of this pathway in brain function.
Highlights.
Learning induces early and late phases in stathmin activity and microtubule stability
Contextual fear conditioning is controlled by dentate gyrus stathmin
In the late phase stathmin and microtubules control synaptic localization of AMPARs
Aging and mental disorders may involve stathmin and microtubule changes
Acknowledgments
We thank Ted Abel and members of the Shumyatsky lab for comments on the manuscript. Work described here was supported by Whitehall Foundation, March of Dimes, and the New Jersey Commission on Brain Injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Atarod D, Eskandari-Sedighi G, Pazhoohi F, Karimian SM, Khajeloo M, Riazi GH. Microtubule Dynamicity Is More Important than Stability in Memory Formation: an In Vivo Study. J Mol Neurosci. 2015 doi: 10.1007/s12031-015-0535-4. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Opitz T, Merkens M, Kelly T, von der Brelie C, Krueppel R, Beck H. Stable mossy fiber long-term potentiation requires calcium influx at the granule cell soma, protein synthesis, and microtubule-dependent axonal transport. J Neurosci. 2010;30:12996–13004. doi: 10.1523/JNEUROSCI.1847-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PY, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Brown R, Ramesh V, Begum S, McCarley RW. Sleep deprivation-induced protein changes in basal forebrain: implications for synaptic plasticity. J Neurosci Res. 2005;82:650–658. doi: 10.1002/jnr.20675. [DOI] [PubMed] [Google Scholar]
- Behar M, Hoffmann A. Understanding the temporal codes of intra-cellular signals. Current opinion in genetics & development. 2010;20:684–693. doi: 10.1016/j.gde.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz EJ, Zhukovsky E, Lanahan AA, Worley PF, Nikolich K, Goodman LJ. Neuronal activity induction of the stathmin-like gene RB3 in the rat hippocampus: possible role in neuronal plasticity. J Neurosci. 1998;18:9780–9789. doi: 10.1523/JNEUROSCI.18-23-09780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta L, Dobransky T, Sobel A. Multiple phosphorylation of stathmin. Identification of four sites phosphorylated in intact cells and in vitro by cyclic AMP-dependent protein kinase and p34cdc2. J Biol Chem. 1993;268:20076–20084. [PubMed] [Google Scholar]
- Bianchi M, Baulieu EE. 3beta-Methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders. Proc Natl Acad Sci U S A. 2012;109:1713–1718. doi: 10.1073/pnas.1121485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Heidbreder C, Crespi F. Cytoskeletal changes in the hippocampus following restraint stress: role of serotonin and microtubules. Synapse. 2003;49:188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoorn K, van Dis V, Goedknegt E, Sobel A, Lucassen PJ, Hoogenraad CC. The microtubule destabilizing protein stathmin controls the transition from dividing neuronal precursors to postmitotic neurons during adult hippocampal neurogenesis. Dev Neurobiol. 2014;74:1226–1242. doi: 10.1002/dneu.22200. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brocke B, Lesch KP, Armbruster D, Moser DA, Muller A, Strobel A, Kirschbaum C. Stathmin, a gene regulating neural plasticity, affects fear and anxiety processing in humans. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:243–251. doi: 10.1002/ajmg.b.30989. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cao C, Wang L, Wang R, Dong C, Qing Y, Zhang X, Zhang J. Stathmin genotype is associated with reexperiencing symptoms of posttraumatic stress disorder in Chinese earthquake survivors. Progress in neuro-psychopharmacology & biological psychiatry. 2013;44:296–300. doi: 10.1016/j.pnpbp.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Magistretti PJ, Martin JL. Brain-derived neurotrophic factor stimulates phosphorylation of stathmin in cortical neurons. Brain research Molecular brain research. 1997;51:220–228. doi: 10.1016/s0169-328x(97)00241-6. [DOI] [PubMed] [Google Scholar]
- Ch’ng TH, Martin KC. Synapse-to-nucleus signaling. Curr Opin Neurobiol. 2011;21:345–352. doi: 10.1016/j.conb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin S, Sobel A. Neuronal stathmins: A family of phosphoproteins cooperating for neuronal development, plasticity and regeneration. Prog Neurobiol. 2015;126:1–18. doi: 10.1016/j.pneurobio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H, Cordier J, Sobel A. Stathmin phosphorylation is regulated in striatal neurons by vasoactive intestinal peptide and monoamines via multiple intracellular pathways. J Neurochem. 1992;58:282–289. doi: 10.1111/j.1471-4159.1992.tb09308.x. [DOI] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Curr Opin Neurobiol. 2011;21:175–181. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Antonsson B, Kassel D, Riederer BM, Grenningloh G. Phosphorylation regulates the microtubule-destabilizing activity of stathmin and its interaction with tubulin. FEBS Lett. 1997;416:149–152. doi: 10.1016/s0014-5793(97)01188-5. [DOI] [PubMed] [Google Scholar]
- Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Morrison EE, Liverpool TB, Molina-Paris C, Cross RA, Alonso MC, Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci. 2008;121:1085–1095. doi: 10.1242/jcs.026492. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlis AC, Bauernschmitt K, Dresler T, Hahn T, Herrmann MJ, Roser C, Romanos M, Warnke A, Gerlach M, Lesch KP, et al. Influence of a genetic variant of the neuronal growth associated protein Stathmin 1 on cognitive and affective control processes: An event-related potential study. Am J Med Genet B Neuropsychiatr Genet. 2011 doi: 10.1002/ajmg.b.31161. [DOI] [PubMed] [Google Scholar]
- Elder GA, Dorr NP, De Gasperi R, Gama Sosa MA, Shaughness MC, Maudlin-Jeronimo E, Hall AA, McCarron RM, Ahlers ST. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma. 2012;29:2564–2575. doi: 10.1089/neu.2012.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanara P, Husted KH, Selle K, Wong PY, Banerjee J, Brandt R, Hellerstein MK. Changes in microtubule turnover accompany synaptic plasticity and memory formation in response to contextual fear conditioning in mice. Neuroscience. 2010;168:167–178. doi: 10.1016/j.neuroscience.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Federighi G, Traina G, Macchi M, Ciampini C, Bernardi R, Baldi E, Bucherelli C, Brunelli M, Scuri R. Modulation of gene expression in contextual fear conditioning in the rat. PLoS One. 2013;8:e80037. doi: 10.1371/journal.pone.0080037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, Wu LJ, Xu H, Zhao MG, Ueda K, et al. Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 2008;28:9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima N, Furuta D, Hidaka Y, Moriyama R, Tsujiuchi T. Post-translational modifications of tubulin in the nervous system. J Neurochem. 2009;109:683–693. doi: 10.1111/j.1471-4159.2009.06013.x. [DOI] [PubMed] [Google Scholar]
- Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Punsoni M, Yuk F, Park CS, Janssen WG, Rapp PR, Morrison JH. Synaptic distributions of GluA2 and PKMzeta in the monkey dentate gyrus and their relationships with aging and memory. J Neurosci. 2012;32:7336–7344. doi: 10.1523/JNEUROSCI.0605-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Pan Y, Shu H, Ohshima T, Kansy JW, White CL, 3rd, Tamminga CA, Sobel A, Curmi PA, Mikoshiba K, et al. Phosphorylation of the tubulin-binding protein, stathmin, by Cdk5 and MAP kinases in the brain. J Neurochem. 2006;99:237–250. doi: 10.1111/j.1471-4159.2006.04113.x. [DOI] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Maxfield DA, Brockie PJ, Mellem JE, Jensen E, Wang R, Madsen DM, Maricq AV. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron. 2013;80:1421–1437. doi: 10.1016/j.neuron.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid e Silva T, Choi H, Moletta B, Medina JH, Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cellular and molecular neurobiology. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Jin LW, Masliah E, Iimoto D, Deteresa R, Mallory M, Sundsmo M, Mori N, Sobel A, Saitoh T. Neurofibrillary tangle-associated alteration of stathmin in Alzheimer’s disease. Neurobiology of aging. 1996;17:331–341. doi: 10.1016/0197-4580(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci. 2011;31:8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- L’Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- Larsson N, Marklund U, Gradin HM, Brattsand G, Gullberg M. Control of microtubule dynamics by oncoprotein 18: dissection of the regulatory role of multisite phosphorylation during mitosis. Molecular and cellular biology. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S. Microtubule dynamics: if you need a shrink try stathmin/Op18. Curr Biol. 1998;8:R212–214. doi: 10.1016/s0960-9822(98)70128-9. [DOI] [PubMed] [Google Scholar]
- le Gouvello S, Manceau V, Sobel A. Serine 16 of stathmin as a cytosolic target for Ca2+/calmodulin-dependent kinase II after CD2 triggering of human T lymphocytes. Journal of immunology. 1998;161:1113–1122. [PubMed] [Google Scholar]
- Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, Chen IZ, Kandel ER, Shumyatsky GP. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci. 2010;30:3813–3825. doi: 10.1523/JNEUROSCI.1330-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna T, Thrower DA, Honnappa S, Steinmetz MO, Wilson L. Regulation of microtubule dynamic instability in vitro by differentially phosphorylated stathmin. J Biol Chem. 2009;284:15640–15649. doi: 10.1074/jbc.M900343200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund U, Larsson N, Brattsand G, Osterman O, Chatila TA, Gullberg M. Serine 16 of oncoprotein 18 is a major cytosolic target for the Ca2+/calmodulin-dependent kinase-Gr. Eur J Biochem. 1994;225:53–60. doi: 10.1111/j.1432-1033.1994.00053.x. [DOI] [PubMed] [Google Scholar]
- Martel G, Blanchard J, Mons N, Gastambide F, Micheau J, Guillou JL. Dynamic interplays between memory systems depend on practice: the hippocampus is not always the first to provide solution. Neuroscience. 2007;150:743–753. doi: 10.1016/j.neuroscience.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Martel G, Hevi C, Wong A, Zushida K, Uchida S, Shumyatsky GP. Murine GRPR and stathmin control in opposite directions both cued fear extinction and neural activities of the amygdala and prefrontal cortex. PLoS One. 2012;7:e30942. doi: 10.1371/journal.pone.0030942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel G, Nishi A, Shumyatsky GP. Stathmin reveals dissociable roles of the basolateral amygdala in parental and social behaviors. Proc Natl Acad Sci U S A. 2008;105:14620–14625. doi: 10.1073/pnas.0807507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Kosik KS. Synaptic tagging -- who’s it? Nat Rev Neurosci. 2002;3:813–820. doi: 10.1038/nrn942. [DOI] [PubMed] [Google Scholar]
- Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103:571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander Gradin H, Marklund U, Larsson N, Chatila TA, Gullberg M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol. 1997;17:3459–3467. doi: 10.1128/mcb.17.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Lumbard DC, Viesselmann C, Ballweg J, Stevenson M, Pietila L, Hu X, Dent EW. Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS One. 2011;6:e27688. doi: 10.1371/journal.pone.0027688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:12503–12508. doi: 10.1073/pnas.1104558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama F, Niimi G, Kato K, Hirosawa K, Mikoshiba K, Okuya M, Karagiozov K, Kato Y, Kanno T, Sanoe H, et al. Redistribution of microtubules in dendrites of hippocampal CA1 neurons after tetanic stimulation during long-term potentiation. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia. 2008;113:17–27. [PubMed] [Google Scholar]
- Morii H, Yamada T, Nakano I, Coulson JM, Mori N. Site-specific phosphorylation of SCG10 in neuronal plasticity: role of Ser73 phosphorylation by N-methyl D-aspartic acid receptor activation in rat hippocampus. Neurosci Lett. 2006;396:241–246. doi: 10.1016/j.neulet.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yin X, Takei Y, Seog DH, Homma N, Hirokawa N. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron. 2012;76:945–961. doi: 10.1016/j.neuron.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Nakayama D, Iwata H, Teshirogi C, Ikegaya Y, Matsuki N, Nomura H. Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. J Neurosci. 2015;35:819–830. doi: 10.1523/JNEUROSCI.2525-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa N, Hashimoto K, Hino T, Migishima R, Yokoyama M, Kano M, Inokuchi K. Motor discoordination of transgenic mice overexpressing a microtubule destabilizer, stathmin, specifically in Purkinje cells. Neurosci Res. 2007;59:93–100. doi: 10.1016/j.neures.2007.06.1464. [DOI] [PubMed] [Google Scholar]
- Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003;421:230. doi: 10.1038/421230a. [DOI] [PubMed] [Google Scholar]
- Pandey K, Sharma SK. Activity-dependent acetylation of alpha tubulin in the hippocampus. J Mol Neurosci. 2011;45:1–4. doi: 10.1007/s12031-011-9491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturle-Lafanechere L, Manier M, Trigault N, Pirollet F, Mazarguil H, Job D. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107(Pt 6):1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- Peixoto LL, Wimmer ME, Poplawski SG, Tudor JC, Kenworthy CA, Liu S, Mizuno K, Garcia BA, Zhang NR, Giese K, et al. Memory acquisition and retrieval impact different epigenetic processes that regulate gene expression. BMC genomics. 2015;16(Suppl 5):S5. doi: 10.1186/1471-2164-16-S5-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Srivastava DP, Woolfrey KM. Not just actin? A role for dynamic microtubules in dendritic spines. Neuron. 2009;61:3–5. doi: 10.1016/j.neuron.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Piubelli C, Carboni L, Becchi S, Mathe AA, Domenici E. Regulation of cytoskeleton machinery, neurogenesis and energy metabolism pathways in a rat gene-environment model of depression revealed by proteomic analysis. Neuroscience. 2011;176:349–380. doi: 10.1016/j.neuroscience.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Porte Y, Trifilieff P, Wolff M, Micheau J, Buhot MC, Mons N. Extinction of spatial memory alters CREB phosphorylation in hippocampal CA1. Hippocampus. 2011;21:1169–1179. doi: 10.1002/hipo.20844. [DOI] [PubMed] [Google Scholar]
- Purvis JE, Lahav G. Encoding and Decoding Cellular Information through Signaling Dynamics. Cell. 2013;152:945–956. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Rose SP. God’s organism? The chick as a model system for memory studies. Learn Mem. 2000;7:1–17. doi: 10.1101/lm.7.1.1. [DOI] [PubMed] [Google Scholar]
- Saetre P, Jazin E, Emilsson L. Age-related changes in gene expression are accelerated in Alzheimer’s disease. Synapse. 2011;65:971–974. doi: 10.1002/syn.20933. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, et al. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain research Molecular brain research. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci. 2001;21:3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Calandreau L, Herry C, Mons N, Micheau J. Biphasic ERK1/2 activation in both the hippocampus and amygdala may reveal a system consolidation of contextual fear memory. Neurobiol Learn Mem. 2007;88:424–434. doi: 10.1016/j.nlm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Martel G, Pavlowsky A, Takizawa S, Hevi C, Watanabe Y, Kandel ER, Alarcon JM, Shumyatsky GP. Learning-induced and stathmin-dependent changes in microtubule stability are critical for memory and disrupted in ageing. Nature communications. 2014;5:4389. doi: 10.1038/ncomms5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Chang RC, Law AC. A breach in the scaffold: the possible role of cytoskeleton dysfunction in the pathogenesis of major depression. Ageing research reviews. 2013;12:67–75. doi: 10.1016/j.arr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang G, Wang H, Liu Z, Wang X. Cytoskeletal alterations in rat hippocampus following chronic unpredictable mild stress and re-exposure to acute and chronic unpredictable mild stress. Behav Brain Res. 2009;205:518–524. doi: 10.1016/j.bbr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Yin X, Takei Y, Kido MA, Hirokawa N. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 2011;70:310–325. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]
- Yu I, Garnham CP, Roll-Mecak A. Writing and Reading the Tubulin Code. J Biol Chem. 2015;290:17163–17172. doi: 10.1074/jbc.R115.637447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, 3rd, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]