Abstract
Few studies have evaluated the role of antibacterial prophylaxis during neutropenia in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation (HSCT). At our center, levofloxacin prophylaxis was initiated in June 2006 in patients with myeloma who were undergoing autologous HSCT. We compared the incidence of bloodstream infection (BSI) and fever and neutropenia (FN) within 30 days of transplantation before (January 2003 - May 2006) and after (June 2006 - April 2010) the initiation of levofloxacin prophylaxis in patients undergoing autologous HSCT for myeloma. We also compared rates of BSI and FN during the same time periods in autologous HSCT recipients with lymphoma who did not receive antibacterial prophylaxis during either time period. After the initiation of levofloxacin prophylaxis, the BSI rate decreased from 41.2% (49/119) to 14.7% (23/156) and the rate of FN decreased from 91.6% to 60.9% in patients with myeloma (P < 0.001, for each). In contrast, rates of BSI (43.1% vs. 47.3%; P = 0.50) and FN (98.8% vs. 97.1%; P = 0.63) did not change in patients with lymphoma. Levofloxacin prophylaxis was independently associated with decreased odds of BSI (odds ratio [OR] 0.27; 95% confidence interval [CI] 0.14–0.51; P < 0.001) and FN (OR 0.18, 95% CI 0.09–0.36; P < 0.001) in multivariate analysis. Patients with myeloma had a non-significant increase in the risk of BSI due to levofloxacin-resistant Enterobacteriaceae (5% vs. 1%, P = 0.08) and Clostridium difficile infection (7% vs. 3%, P = 0.12) after the initiation of levofloxacin prophylaxis, but did not have higher rates of BSI due to other resistant bacteria. Levofloxacin prophylaxis is associated with decreased risk of BSI and FN in patients with myeloma undergoing autologous HSCT.
INTRODUCTION
High-dose chemotherapy combined with autologous hematopoietic stem cell transplantation (HSCT) is an important component of the treatment of multiple myeloma [1]. Neutropenia is a universal complication of autologous HSCT and combines with chemotherapy-induced mucositis to establish a high-risk setting for bacteremia [2, 3]. In order to decrease the risk of bacterial infection, Guidelines of the Infectious Diseases Society of America and American Society for Blood and Marrow Transplantation recommend considering the administration of antibacterial prophylaxis during chemotherapy-induced neutropenia in patients with anticipated neutropenic periods of at least 7 days [4, 5].
These recommendations are largely based on two randomized, placebo-controlled trials of levofloxacin in patients with cancer and neutropenia that were conducted from 1999–2003 and demonstrated lower rates of fever and neutropenia (FN) and bacterial infections, but not decreased mortality, in patients receiving levofloxacin prophylaxis [6, 7]. However, during the decade since these trials were conducted, fluoroquinolone resistance has become increasingly common [8, 9] and a new virulent strain of fluoroquinolone-resistant Clostridium difficile has emerged [10]. These developments merit a reassessment of the role of levofloxacin prophylaxis in patients with neutropenia. Furthermore, the applicability of results from these landmark trials to patients with multiple myeloma undergoing autologous HSCT is unclear, as very few of these patients were evaluated in these trials. Two subsequent single-center, randomized trials have been conducted to assess the role of fluoroquinolone prophylaxis in patients receiving autologous HSCT, but these studies are limited by small sample sizes and/or the use of multiple prophylactic antimicrobial agents [11, 12].
Prior to June 2006, antibacterial prophylaxis was not administered to patients undergoing autologous HSCT at our center. In response to two deaths in patients with multiple myeloma from septic shock due to fluoroquinolone-susceptible Gram-negative bacteria, levofloxacin prophylaxis was initiated in June 2006 in patients with myeloma undergoing autologous HSCT, but not in patients receiving autologous HSCT for other indications, such as lymphoma. This selective intervention established a unique setting to evaluate the efficacy and adverse effects related to the use of levofloxacin prophylaxis in patients undergoing autologous HSCT.
MATERIALS AND METHODS
Study design
This is a single-center, retrospective cohort study at New York-Presbyterian Hospital/Weill Cornell Medical Center that consists of two study periods. In Period 1 (January 2003 - May 2006), neither patients with multiple myeloma nor patients with lymphoma received antibacterial prophylaxis during their autologous transplant admission. In Period 2 (June 2006 - April 2010), patients with myeloma who underwent autologous HSCT received 500 mg of oral levofloxacin daily from one day prior to their stem cell infusion until recovery from neutropenia. Patients with lymphoma who underwent autologous HSCT continued not to receive antibacterial prophylaxis during Period 2. Multiple transplants involving individual patients were eligible for analysis, provided that the patient did not have a prior transplant within the previous 90 days.
The primary objective of the study was to compare the incidence of 1) bloodstream infection (BSI) and 2) FN within 30 days of transplantation in Period 1 versus Period 2 in patients with myeloma and in patients with lymphoma. We also assessed the incidence of BSI and FN during each year of the study in both patient populations. Secondary objectives of the study were to compare rates of BSIs due to specific bacteria, including multidrug-resistant (MDR) pathogens, and rates of Clostridium difficile infection within 90 days of transplantation between time periods in both patient groups.
Furthermore, for autologous HSCT recipients with multiple myeloma, we reviewed medical records to compare the following variables between patients who received and did not receive levofloxacin prophylaxis: demographics, myeloma characteristics, comorbidities [13], baseline serum albumin and creatinine levels, recent C. difficile infection, conditioning regimen, central venous catheter type, number of CD34 cells infused, and duration of neutropenia. We then conducted multivariate analyses to determine whether levofloxacin prophylaxis was independently associated with the risk of BSI or FN in patients with myeloma. Finally, we compared the following additional outcomes for myeloma patients who received and did not receive levofloxacin prophylaxis: developing a BSI that was associated with severe sepsis or an intensive care unit (ICU) admission, a microbiologically documented infection other than bacteremia or an invasive fungal infection, duration of hospitalization, readmission within 90 days of the transplant, mortality within 30 and 90 days of the transplant, and mortality related to sepsis.
Definitions and study procedures
Fever was defined as a temperature ≥ 38.0°C and neutropenia was defined as an absolute neutrophil count ≤ 500 cells/µL. Common skin commensals (coagulase-negative staphylococci, Bacillus and Corynebacterium spp. other than C. jeikeium) were only considered causes of BSI if isolated from at least two sets of blood cultures collected on the same day or on consecutive days.
During both study periods, HSCT recipients with myeloma and lymphoma were placed in private rooms on the same inpatient transplant unit until neutrophil engraftment. They were cared for by the same medical staff and received the same supportive care practices (other than antibacterial prophylaxis), including infection control practices recommended by the Centers for Disease Control and Prevention [14]. Intravenous melphalan was administered as a conditioning regimen at a dose of 200 mg/m2, divided into 2 doses on days −2 and days −1. The melphalan dose was reduced to 140 mg/m2 in frail elderly patients and in patients with a creatinine clearance < 60 mL/min.
For initial work-up of FN, blood cultures (one aerobic and one anaerobic bottle per set) were obtained from peripheral blood and each central venous catheter lumen. Subsequent blood cultures were drawn daily for persistent fever. Piperacillin-tazobactam was the primary agent used for FN during both time periods and broad-spectrum β-lactam therapy was typically continued until resolution of fever and neutropenia. All patients received prophylactic fluconazole and valacyclovir and daily filgastrim injections until resolution of neutropenia. All patients had central venous catheters that were typically placed on the day of admission for transplant and removed upon discharge from the transplant admission, unless intravenous medications were required after discharge.
BacT/ALERT® 3D (BioMérieux Inc., Durham, NC) was the automated blood culture system used during both study periods. Species identification and antimicrobial susceptibility testing of bloodstream isolates were primarily performed by Vitek II (BioMérieux Inc.), according to Clinical and Laboratory Standards Institute recommendations [15]. From 2003–2009, the Wampole™ Clostridium difficile Tox A/B Microplate Assay (ELISA) was used to detect C. difficile toxin from stool. In 2010, more sensitive assays were employed to detect C. difficile toxin, and thus patients who received a transplant in 2010 were excluded from the analysis of C. difficile infection rates.
Statistical analysis
Proportions were compared using two-tailed χ2 or Fisher’s exact tests and p ≤ 0.05 was considered statistically significant. Continuous variables were expressed as median values with interquartile ranges and compared by the Wilcoxon rank-sum test. Factors associated with developing a BSI or FN in patients with multiple myeloma were evaluated in univariate and multivariate logistic regression models. All variables with a p-value ≤ 0.1 in the univariate model, as well as age, years since myeloma diagnosis, prior HSCT, and duration of neutropenia were included in the multivariate model. STATA, version 12.0 (StataCorp, College Station, TX) was used for statistical analysis.
RESULTS
Patients and rates of BSI and FN in Period 1 and Period 2
There were 475 autologous HSCTs performed during the study period. Of these, 275 transplants for multiple myeloma and 190 transplants for lymphoma were eligible for analysis. None of the 119 patients with myeloma and none of the 88 patients with lymphoma in Period 1 received antibacterial prophylaxis. In Period 2, 148 of the 156 myeloma patients received levofloxacin prophylaxis (eight patients had a levofloxacin allergy or prior intolerance) and none of the 102 lymphoma patients received antibacterial prophylaxis.
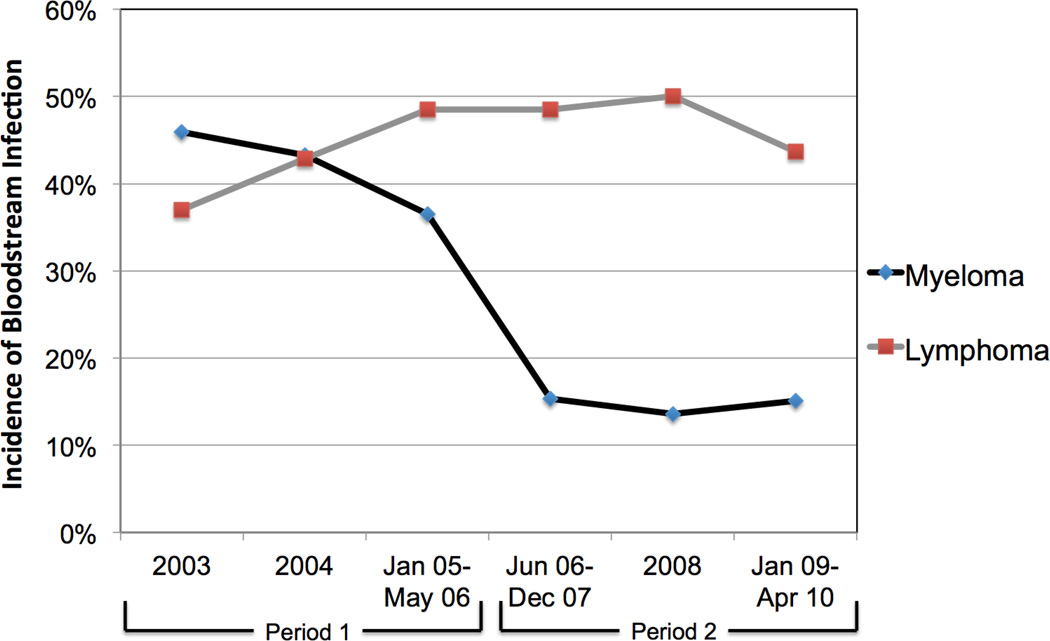
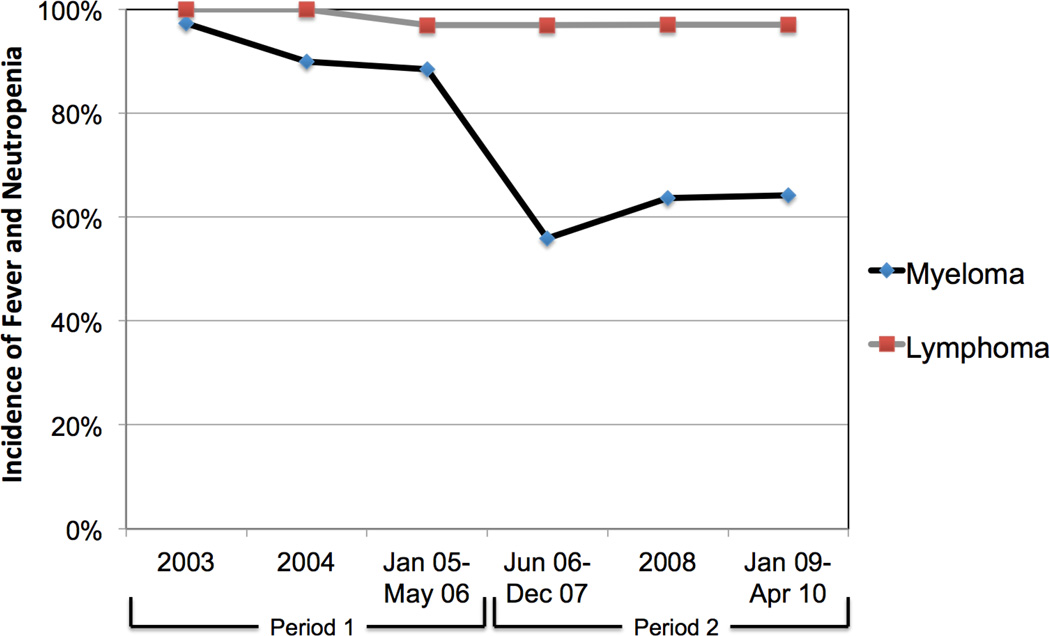
The incidence of BSI within 30 days of transplantation in patients with myeloma decreased from 41.2% in Period 1 (before levofloxacin prophylaxis) to 14.7% in Period 2 (after levofloxacin prophylaxis; P < 0.001; Table 1). Patients with lymphoma who did not receive antibacterial prophylaxis during either time period had no significant change in BSI incidence (43.1% in Period 1 vs. 47.3% in Period 2; P = 0.50). Similarly, the incidence of FN in patients with myeloma decreased from 91.6% in Period 1 to 60.9% in Period 2 (P < 0.001). The incidence of FN did not change in patients with lymphoma (98.8% vs. 97.1%; P = 0.63). The decreases in rates of BSI and FN in patients with myeloma occurred immediately after the intervention of fluoroquinolone prophylaxis and were sustained during each subsequent study year (Figures 1a and 1b).
Table 1.
Comparisons of the microbiology of bloodstream infections and Clostridium difficile infection rates between Period 1 (January 2003 – May 2006) and Period 2 (June 2006 – April 2010) in patients with multiple myeloma and lymphoma who underwent autologous stem cell transplantation.
| Infection types | Myeloma Period 1 (n = 119) |
Myeloma Period 21 (n = 156) |
P2 | Lymphoma Period 1 (n = 88) |
Lymphoma Period 2 (n = 102) |
P2 |
|---|---|---|---|---|---|---|
| Any bloodstream infection | 49 (41) | 23 (15) | <0.001 | 38 (43) | 49 (47) | 0.50 |
| Gram-positive bacteremia | 37 (31) | 15 (10) | <0.001 | 24 (27) | 26 (26) | 0.78 |
| Staphylococcus aureus | 6 (5) | 4 (3) | 1 (1) | 5 (5) | ||
| Methicillin-susceptible | 2 (2) | 0 | 1 (1) | 4 (4) | ||
| Methicillin-resistant (MRSA) | 4 (3) | 4 (3) | 0 | 1 (1) | ||
| Coagulase-negative staphylococci | 11 (9) | 0 | <0.001 | 7 (8) | 4 (4) | |
| Viridans group streptococci (VGS) | 12 (10) | 4 (3) | 0.008 | 5 (6) | 10 (10) | |
| Penicillin-non-susceptible VGS | 3 (3) | 3 (2) | 3 (3) | 2 (2) | ||
| Enterococci | 6 (5) | 6 (5) | 10 (11) | 9 (9) | ||
| Vancomycin-susceptible | 2 (2) | 1 (1) | 3 (3) | 2 (2) | ||
| Vancomycin-resistant (VRE) | 4 (3) | 5 (3) | 7 (8) | 7 (7) | ||
| Gram-negative bacteremia | 13 (11) | 11 (7) | 0.26 | 28 (32) | 34 (33) | 0.82 |
| Enterobacteriaceae | 11 (9) | 11 (7) | 26 (30) | 30 (29) | ||
| Escherichia coli | 7 (6) | 8 (5) | 9 (10) | 14 (14) | ||
| Enterobacter cloacae | 2 (2) | 2 (1) | 2 (2) | 1 (1) | ||
| Klebsiella pneumoniae | 2 (2) | 0 | 13 (15) | 16 (16) | ||
| Levofloxacin-resistant | 1 (1) | 8 (5) | 0.08 | 7 (8) | 2 (2) | 0.08 |
| Ceftriaxone-resistant | 0 | 1 (1) | 5 (6) | 1 (1) | 0.10 | |
| Carbapenem-resistant | 0 | 1 (1) | 0 | 0 | ||
| Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | ||
| Candidemia | 2 (2) | 0 | 0 | 1 (1) | ||
| Clostridium difficile infection | 3 (3) | 9 (7)3 | 0.12 | 4 (5) | 6 (6)3 | 0.75 |
Variables are presented as No. (%) of total.
Levofloxacin prophylaxis was administered to these patients from one day before stem cell infusion until neutrophil engraftment.
Blank values indicate a P value of > 0.1.
In Period 2, the incidence of C. difficile infection was only evaluated in patients who underwent transplantation from June 2006 – Dec 2009 because testing for C. difficile changed in 2010 from an ELISA-based to a PCR-based method.
Figure 1.
Incidence of a) bloodstream infection and b) fever and neutropenia within 30 days of autologous stem cell transplantation in patients with multiple myeloma and patients with lymphoma, by year of transplant.
Etiologies of BSI and rates of C. difficile infection in Period 1 vs. Period 2
The incidence of Gram-positive bacteremia in patients with myeloma decreased from 31% in Period 1 to 10% in Period 2 (P < 0.001; Table 1). This decrease was largely due to decreases in rates of bacteremia caused by coagulase-negative staphylococci (9% vs. 0%; P < 0.001) and viridans group streptococci (VGS; 10% vs. 3%; P = 0.008). There were no changes in rates of bacteremia caused by methicillin-resistant Staphylococcus aureus (MRSA), penicillin-non-susceptible VGS, or vancomycin-resistant enterococci (VRE). There was no significant change in the incidence of Gram-negative bacteremia in myeloma patients between Period 1 and Period 2 (11% vs. 7%; P = 0.26). There was a trend towards an increase in the rate of BSI due to levofloxacin-resistant Enterobacteriaceae (1% vs. 5%; P = 0.08), but no trends towards increased rates of bacteremia caused by other MDR Gram-negative bacteria. In patients with lymphoma, there were no significant changes in the incidence of bacteremia due to any specific bacterial pathogen between Period 1 and Period 2.
The incidence of Clostridium difficile infection within 90 days of transplantation in patients with myeloma increased from 3% in Period 1 to 7% in Period 2 (P = 0.12; Table 1). This incidence did not change during these two periods in patients with lymphoma.
Characteristics and outcomes of patients with multiple myeloma
Patients with myeloma who received levofloxacin prophylaxis (n = 148) received a median of 9 days of levofloxacin. These patients were older (median age: 59 years vs. 56 years; P = 0.009; Table 2) and had lower baseline serum albumin levels (3.2 vs. 3.6 mg/dL; P < 0.001) than those who did not receive levofloxacin prophylaxis (n = 127). There were no statistically significant differences between groups in myeloma characteristics, rates of prior HSCT or C. difficile infection, Charlson comorbidity index score, and baseline serum creatinine. Melphalan was used as the sole conditioning agent in > 90% of transplants in both groups, although patients who received levofloxacin prophylaxis were less likely to receive a 200 mg/m2 dose of melphalan (88% vs. 76%; P = 0.02). Patients who received levofloxacin prophylaxis had a lower median number of CD34 cells infused (5.1×106/kg vs. 6.4×106/kg, P < 0.001). Despite receiving fewer CD34 cells, they had a marginally decreased duration of neutropenia, although the median duration was 7 days in both groups.
Table 2.
Characteristics of patients with multiple myeloma who received and did not receive prophylactic levofloxacin during neutropenia after autologous hematopoietic stem cell transplantation (HSCT).1,2
| Characteristics | No levofloxacin prophylaxis (n = 127) |
Levofloxacin prophylaxis (n = 148) |
P |
|---|---|---|---|
| Demographics | |||
| Age (years) | 56 (49–61) | 59 (53–64) | 0.009 |
| Female sex | 56 (44) | 74 (50) | 0.33 |
| Myeloma characteristics | |||
| Immunoglobulin type | 0.12 | ||
| IgG | 66 (52) | 93 (63) | |
| IgA | 27 (21) | 22 (15) | |
| None (light chain) | 34 (27) | 31 (21) | |
| Other | 0 (0) | 2 (1) | |
| Light chain type | |||
| Kappa | 80 (63) | 85 (57) | 0.35 |
| Lambda | 47 (37) | 63 (43) | |
| Years since diagnosis | 1 (1–3) | 1 (1–2) | 0.72 |
| Previous HSCT | 23 (18) | 16 (11) | 0.08 |
| Comorbidities | |||
| Charlson comorbidity index score | 2 (2–3) | 2 (2–3) | 0.37 |
| Myocardial infarction | 13 (10) | 5 (3) | 0.02 |
| Congestive heart failure | 4 (3) | 1 (1) | 0.19 |
| Cerebrovascular disease | 3 (2) | 3 (2) | 1.00 |
| COPD | 1 (1) | 2 (1) | 1.00 |
| Connective tissue disease | 1 (1) | 5 (3) | 0.22 |
| Liver disease | 1 (1) | 5 (3) | 0.22 |
| Diabetes | 16 (13) | 13 (9) | 0.31 |
| Kidney disease | 13 (10) | 8 (5) | 0.13 |
| Solid tumor | 6 (5) | 10 (7) | 0.47 |
| Baseline serum creatinine (mg/dL) | 0.9 (0.7–1.3) | 0.8 (0.7–1.1) | 0.19 |
| Baseline serum albumin (mg/dL) | 3.6 (3.3–4.0) | 3.2 (2.9–3.5) | <0.001 |
| Clostridium difficile infection within 90 days prior to HSCT |
1 (1) | 1 (1) | 1.00 |
| Transplant characteristics | |||
| Conditioning regimen | 0.02 | ||
| Melphalan (200 mg/m2) | 112 (88) | 112 (76) | |
| Melphalan (dose < 200 mg/m2) | 8 (6) | 24 (16) | |
| Melphalan + (bendamustine or carmustine) |
7 (6) | 12 (8) | |
| Central venous catheter (CVC) type | 0.30 | ||
| PICC line | 29 (23) | 42 (28) | |
| Tunneled CVC | 98 (77) | 106 (72) | |
| # of CD34 cells infused (x106/kg) | 6.5 (4.7–9.2) | 5.1 (4.1–7.2) | <0.001 |
| Duration of neutropenia, days | 7 (6–9) | 7 (6–8) | 0.03 |
All categorical variables are expressed as No. (%) of total. All continuous variables are expressed as median (interquartile range). COPD, chronic obstructive pulmonary disease; PICC, peripherally inserted central catheter.
Eight patients in Period 2 did not receive levofloxacin prophylaxis because of allergy or intolerance.
This table analyzes 275 transplants performed among 263 unique patients.
Myeloma patients who received levofloxacin prophylaxis were less likely to develop FN (61% vs. 90%; P < 0.001; Table 3), any BSI within 30 days of the transplant (14% vs. 41%; P < 0.001), and Gram-positive bacteremia (9% vs. 30%; P < 0.001) than those who did not receive levofloxacin prophylaxis. Although there was no difference in rates of Gram-negative bacteremia between Period 1 and Period 2, not all myeloma patients received levofloxacin prophylaxis in Period 2. When myeloma patients who received levofloxacin prophylaxis were compared to those who did not receive antibacterial prophylaxis, the rate of Gram-negative bacteremia was lower in patients who received prophylaxis (5% vs. 13%; P = 0.04). There were no significant differences in rates of BSI associated with severe sepsis [16] or ICU admission, invasive fungal infection, or microbiologically documented infection other than bacteremia within 30 days of the transplant. Seven percent of patients who received levofloxacin prophylaxis developed Clostridium difficile infection within 90 days of the transplant, compared to 3% in patients who did not receive prophylaxis (P = 0.17). There was a shorter median duration of hospitalization in patients who received levofloxacin prophylaxis (18 days vs. 20 days, P = 0.001) and a trend towards a lower readmission rate (16% vs. 25%, P = 0.07), but no differences in 30-day, 90-day, or sepsis-related mortality.
Table 3.
Outcomes of patients with multiple myeloma who received and did not receive prophylactic levofloxacin during neutropenia after autologous hematopoietic stem cell transplantation.
| Outcomes | No levofloxacin prophylaxis (n = 127) |
Levofloxacin prophylaxis (n = 148) |
P |
|---|---|---|---|
| Fever and neutropenia | 114 (90) | 90 (61) | <0.001 |
| Bloodstream infections (BSI) | |||
| BSI before neutrophil engraftment | 48 (38) | 19 (13) | <0.001 |
| BSI within 30 days of transplant | 52 (41) | 20 (14) | <0.001 |
| Gram-positive bacteremia | 38 (30) | 14 (9) | <0.001 |
| Gram-negative bacteremia | 16 (13) | 8 (5) | 0.04 |
| Fungemia | 2 (2) | 0 | 0.21 |
| BSI associated with severe sepsis [16] |
9 (7)1 | 8 (5)2 | 0.56 |
| BSI associated with ICU admission |
8 (6) | 5 (3) | 0.27 |
| Microbiologically documented infection other than bacteremia within 30 days of transplant |
11 (9) | 16 (11) | 0.55 |
| Invasive fungal infection within 30 days | 2 (2) | 2 (1) | 1.00 |
|
Clostridium difficile infection within 90 days of transplant3 |
4 (3)4 | 9 (7)5 | 0.17 |
| Duration of hospitalization, days | 20 (18–27) | 18 (17–21) | 0.001 |
| Readmission within 90 days of transplant |
32 (25) | 24 (16) | 0.07 |
| Mortality | |||
| Within 30 days of transplant | 4 (3) | 4 (3) | 1.00 |
| Within 90 days of transplant | 8 (7) | 4 (3) | 0.13 |
| Sepsis-related mortality6 | 5 (4) | 3 (2) | 0.48 |
All categorical variables are expressed as No. (%) of total. All continuous variables are expressed as median (interquartile range). ICU, intensive care unit.
BSI etiologies associated with severe sepsis in patients who did not receive levofloxacin prophylaxis: polymicrobial (n = 4), Streptococcus mitis (n = 2), vancomycin-resistant Enterococcus faecium (n = 1), Escherichia coli (n = 1), and Klebsiella pneumoniae (n = 1).
BSI etiologies associated with severe sepsis in patients who received levofloxacin prophylaxis: Escherichia coli (n = 3), methicillin-resistant Staphylococcus aureus (n = 2), polymicrobial (n = 2), and Klebsiella pneumoniae (n = 1).
In Period 2, the incidence of C. difficile infection was only evaluated in patients who underwent transplantation from June 2006 – Dec 2009 because testing for C. difficile changed in 2010 from an ELISA-based to a PCR-based method.
Three patients were treated with 10–14 days of oral metronidazole and one was treated with 21 days of oral vancomycin. Three of these patients had C. difficile recurrence after treatment.
Six patients were treated with 10–14 days of oral metronidazole and three were treated with 10–21 days of oral vancomycin. One of these patients had C. difficile recurrence after treatment.
Causes of death unrelated to sepsis were respiratory failure of unknown etiology, acute respiratory distress syndrome after influenza B infection, intraabdominal hemorrhage, and intracerebral hemorrhage.
Factors associated with BSI and FN in patients with multiple myeloma (Table 4)
Table 4.
Factors associated with developing a bloodstream infection (BSI) and fever and neutropenia (FN) within 30 days after autologous hematopoietic stem cell transplantation (HSCT) in patients with multiple myeloma.
| Variables | BSI: Univariate analysis OR (95% Cl) |
P | BSI: Multivariate analysis1 OR (95% CI) |
P | FN: Univariate analysis OR (95% Cl) |
P | BSI: Multivariate analysis1 OR (95% CI) |
P |
|---|---|---|---|---|---|---|---|---|
| Levofloxacin prophylaxis | 0.23 (0.13–0.41) | <0.001 | 0.27 (0.14–0.51) | <0.001 | 0.18 (0.09–0.34) | <0.001 | 0.18 (0.09–0.36) | <0.001 |
| Age, per year increase | 0.98 (0.95–1.01) | 0.17 | 0.98 (0.95–1.02) | 0.29 | 1.00 (0.97–1.03) | 0.83 | 1.02 (0.98–1.05) | 0.36 |
| Female sex | 1.45 (0.85–2.50) | 0.17 | 0.71 (0.41–1.23) | 0.22 | ||||
| Immune-globulin type | ||||||||
| IgG | Reference | Reference | Reference | |||||
| IgA | 1.33 (0.63–2.78) | 0.45 | 1.13 (0.50–2.54) | 0.77 | 1.06 (0.51–2.18) | 0.88 | ||
| Light chain only | 2.30 (1.23–4.30) | 0.009 | 2.09 (1.03–4.21) | 0.04 | 1.39 (0.70–2.77) | 0.34 | ||
| Lambda light chain (vs kappa) | 0.68 (0.39–1.20) | 0.18 | 0.95 (0.55–1.65) | 0.87 | ||||
| Years since diagnosis, per year increase |
1.09 (1.00–1.19) | 0.047 | 0.99 (0.87–1.13) | 0.89 | 0.99 (0.90–1.08) | 0.76 | 0.85 (0.73–0.99) | 0.03 |
| Previous HSCT | 3.28 (1.63–6.59) | 0.001 | 2.11 (0.76–5.87) | 0.15 | 1.41 (0.62–3.23) | 0.42 | 1.15 (0.33–3.96) | 0.82 |
| Charlson comorbidity index score, per unit increase |
0.98 (0.73–1.31) | 0.88 | 1.62 (1.11–2.35) | 0.01 | 1.31 (0.88–1.97) | 0.19 | ||
| Myocardial infarction | 1.88 (0.70–5.05) | 0.21 | 2.94 (0.66–13.1) | 0.16 | ||||
| Congestive heart failure | 0.70 (0.08–6.37) | 0.75 | 1.40 (0.15–12.7) | 0.77 | ||||
| Liver disease | 0.56 (0.06–4.86) | 0.60 | 1.76 (0.20–15.3) | 0.61 | ||||
| Diabetes | 0.89 (0.36–2.17) | 0.79 | 1.11 (0.45–2.71) | 0.83 | ||||
| Kidney disease | 1.83 (0.72–4.61) | 0.20 | N/A2 | |||||
| Solid tumor | 0.64 (0.18–2.30) | 0.49 | 1.54 (0.43–5.58) | 0.51 | ||||
| Baseline serum creatinine, per mg/dL increase |
1.30 (1.05–1.60) | 0.015 | 1.18 (0.94–1.49) | 0.16 | 3.52 (1.56–7.93) | 0.002 | 4.31 (1.56–11.9) | 0.005 |
| Baseline serum albumin, per mg/dL increase |
1.45 (0.87–2.42) | 0.16 | 1.47 (0.89–2.44) | 0.13 | ||||
| Conditioning regimen | ||||||||
| Melphalan 200 mg/m2 | Reference | Reference | ||||||
| Lower dose melphalan | 1.12 (0.49–2.56) | 0.79 | 0.75 (0.34–1.68) | 0.48 | ||||
| Melphalan + other agent | 1.02 (0.35–2.96) | 0.97 | 1.28 (0.41–4.01) | 0.67 | ||||
| PICC line (vs. tunneled CVC) | 0.85 (0.46–1.60) | 0.62 | 0.70 (0.39–1.28) | 0.25 | ||||
| CD34 cells infused, per 106/kg increase |
1.02 (0.98–1.07) | 0.37 | 1.02 (0.97–1.08) | 0.38 | ||||
| Duration of neutropenia, per day increase |
1.16 (1.06–1.27) | 0.001 | 1.13 (1.03–1.25) | 0.012 | 1.15 (1.02–1.31) | 0.02 | 1.14 (1.01–1.29) | 0.04 |
OR, odds ratio; CI, confidence interval; N/A, not applicable; PICC, peripherally inserted central catheter; CVC, central venous catheter.
Variables included in the multivariate model: age, years since diagnosis, previous HSCT, duration of neutropenia, and all variables with P ≤ 0.1 in univariate analysis.
Unable to calculate odds ratio or P value because all patients with kidney disease per Charlson comorbidity index criteria developed FN.
In univariate analysis, factors associated with developing a BSI within 30 days of the transplant included having light chain only disease, increased number of years since myeloma diagnosis and increased baseline serum creatinine and duration of neutropenia; whereas, levofloxacin prophylaxis was associated with decreased odds of BSI. In a multivariate model, levofloxacin prophylaxis was independently associated with a 73% decrease in odds of developing a BSI (odds ratio [OR] 0.27; 95% confidence interval [CI] 0.14–0.51; P < 0.001).
Factors associated with developing FN within 30 days of the transplant in univariate analysis included having a higher Charlson comorbidity index score and having an increased baseline serum creatinine and duration of neutropenia; whereas levofloxacin prophylaxis was associated with decreased odds of FN. In a multivariate model, levofloxacin prophylaxis was independently associated with an 82% decrease in odds of developing FN (OR 0.18; 95% CI 0.09–0.36; P < 0.001).
DISCUSSION
The selective intervention of initiating levofloxacin prophylaxis in patients with multiple myeloma, but not in patients with lymphoma, created a unique setting to examine the impact of levofloxacin prophylaxis on infectious complications after autologous HSCT. This study design allowed us to not only examine the rates of infectious complications before and after the initiation of levofloxacin prophylaxis in patients with myeloma, but also to make these assessments in a comparator population of patients with lymphoma, who did not receive the intervention during either time period. We found that the introduction of levofloxacin prophylaxis led to a 27% absolute decrease in risk of BSI and a 31% absolute decrease in the risk of FN within 30 days after transplantation. There were no changes in these rates in the comparator population of patients with lymphoma. Levofloxacin prophylaxis was not associated with increased rates of bacteremia due to MDR bacterial pathogens, but was associated with a non-significant increase in the rates of infections due to Clostridium difficile and fluoroquinolone-resistant Enterobacteriaceae.
All non-randomized studies are subject to the potential for confounding bias. In this study, we believe that the finding that rates of BSI and FN were unchanged in a population of lymphoma patients who did not receive the intervention of antibacterial prophylaxis, but who otherwise received the same practices in supportive care and were managed by the same staff as the myeloma patients, strengthens the validity of our findings. Another finding that supports a causal relationship between levofloxacin prophylaxis and decreased rates of BSI and FN is that these decreased rates occurred immediately after the intervention was implemented. If one were to hypothesize that other factors were responsible for the decline in BSI and FN rates, that were specific to patients with myeloma, then it would be expected that these rates would have declined gradually, and would not have immediately decreased after the initiation of levofloxacin prophylaxis. Finally, we conducted multivariate models that adjusted for potential confounding variables, such as age, comorbid illnesses and duration of neutropenia, and found that levofloxacin prophylaxis remained independently associated with substantially lower odds of BSI and FN in these analyses.
Although levofloxacin prophylaxis was strongly associated with lower rates of infectious complications, several caveats warrant discussion. Despite the fact that levofloxacin prophylaxis was associated with decreased BSI rates overall, it was not associated with decreased rates of BSIs complicated by severe sepsis or ICU admission. Perhaps this is related to our observation that levofloxacin prophylaxis had a greater impact on rates of BSI due to coagulase-negative staphylococci (CNS) and viridans group streptococci (VGS) than BSI due to Gram-negative organisms. Bacteremias due to CNS and VGS in HSCT recipients are associated with lower rates of severe sepsis than that which is seen in Gram-negative bacteremias [17]. Consistent with previous studies, we also did not identify a mortality benefit associated with the introduction of levofloxacin prophylaxis. Given the low short-term mortality rates after autologous HSCT [18], even if there was a small incremental mortality benefit to levofloxacin prophylaxis, a larger study would be needed to detect this effect.
Levofloxacin prophylaxis was associated with a non-significant increased risk of developing bacteremia due to levofloxacin-resistant Enterobacteriaceae. However, anti-pseudomonal β-lactam agents, not levofloxacin, are recommended for the treatment of fever in neutropenic patients. Fluoroquinolone use has previously been identified as a risk factor for infections caused by pathogens that are resistant to these β-lactam agents, such extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacteriaceae [19, 20]. However, we did not find an association between levofloxacin prophylaxis and increased rates of bacteremia due to either these MDR Gram-negative pathogens or MDR Gram-positive pathogens, such as MRSA, penicillin-resistant VGS, and VRE. A potential explanation for these findings is that levofloxacin prophylaxis was associated with a lower incidence of FN and BSI, and thus less use of broad-spectrum β-lactam agents. The absence of an increase in bacteremias due to these MDR pathogens is reassuring, but given the ever-changing epidemiology of resistant organisms, centers that administer levofloxacin prophylaxis in the myeloma population should continue to monitor for increasing rates of antibacterial resistance.
Our analysis was inconclusive as to the effect of levofloxacin prophylaxis on the risk of C. difficile infection. There was a non-significant increase from 3% to 7% in the incidence of C. difficile infection rates after initiating levofloxacin prophylaxis in patients with myeloma that was not seen in patients with lymphoma. Although fluoroquinolones are risk factors for C. difficile infection [21, 22], it is possible that lower rates of bacteremia and FN led to less exposure to other antimicrobial agents, which in turn mitigated the effect of fluoroquinolone prophylaxis on rates of C. difficile infection. As with antimicrobial resistance, monitoring for increased rates of C. difficile infection is warranted with the use of levofloxacin prophylaxis, particularly given the emergence of virulent NAP1 strains that are fluoroquinolone-resistant [10].
In addition to the observational nature of the study, other limitations merit mention. This study was conducted at a single center where approximately 50% of staphylococci, 75% of Klebsiella pneumoniae, and 70% of Escherichia coli were susceptible to levofloxacin during the time of the study. The effectiveness of levofloxacin prophylaxis may be diminished at centers with lower rates of levofloxacin susceptibility among these prominent pathogens and individual centers should monitor for increases in levofloxacin resistance. It is possible that the duration of follow-up after the initiation of fluoroquinolone prophylaxis in this study (3.5 years) was not sufficient to identify a statistically significant increase in antimicrobial resistance. Although we assessed rates of bacteremia due to MDR pathogens and C. difficile infection, we did not assess other potential adverse effects from levofloxacin prophylaxis, such as nausea, diarrhea unrelated to C. difficile, rash, QT prolongation, tendonitis, and central nervous system toxicity. A prospective investigation would be required to properly assess for these potential adverse effects. Additionally, the ELISA-based assay used to detect C. difficile infection in the study had lower sensitivity than current PCR-based tests [23]. It is possible that the lower sensitivity of this test limited our ability to detect a difference in rates of C. difficile infection after levofloxacin prophylaxis was initiated. Finally, we were unable to assess the effectiveness of levofloxacin prophylaxis in patients with lymphoma undergoing autologous HSCT, because these patients did not receive levofloxacin prophylaxis during either time period of the study. We recently started to administer levofloxacin prophylaxis to this population and plan on evaluating the impact of this change in practice in the future.
In summary, we found that the initiation of levofloxacin prophylaxis was independently associated with lower rates of BSI and FN in patients with multiple myeloma who underwent autologous HSCT. Patients with lymphoma who underwent autologous HSCT during the same time period, but did not receive levofloxacin prophylaxis had no changes in rates of BSI and FN. Furthermore, in patients with myeloma, levofloxacin prophylaxis was not associated with increased rates of BSIs due to MDR bacteria, but was associated with a non-significant increase in the rates of infections due to Clostridium difficile and fluoroquinolone-resistant Enterobacteriaceae. Based on these findings, we believe that levofloxacin prophylaxis should be considered in neutropenic patients with myeloma undergoing autologous HSCT, with close monitoring for increases in rates of C. difficile infection and antibacterial resistance.
HIGHLIGHTS.
We evaluated the impact of levofloxacin prophylaxis in SCT recipients with myeloma
Levofloxacin prophylaxis led to less bacteremia and fever and neutropenia
Rates of bacteremia due to multidrug-resistant organisms did not increase
Non-significant increases in C. difficile and levofloxacin resistance were seen
Lymphoma patients who did not receive prophylaxis had no change in infection rates
ACKNOWLEDGMENTS
This work was supported in part by an award (KL2TR000458) to M.J.S. from the National Center for Advancing Translational Sciences/National Institutes of Health, through the Weill Cornell Clinical and Translational Science Center. T.J.W. was supported in part as a Scholar of the Sharp Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: All authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 2.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Bloodstream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49:1211–1225. doi: 10.1086/605664. [DOI] [PubMed] [Google Scholar]
- 4.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guidelines for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 5.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–987. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 7.Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353:988–998. doi: 10.1056/NEJMoa050078. [DOI] [PubMed] [Google Scholar]
- 8.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 9.Macesic N, Morrissey CO, Cheng AC, Spencer A, Peleg AY. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transpl Infect Dis. 2014;16:887–896. doi: 10.1111/tid.12298. [DOI] [PubMed] [Google Scholar]
- 10.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 11.Eleutherakis-Papaiakovou E, Kostis E, Migkou M, et al. Prophylactic antibiotics for the prevention of neutropenic fever in patients undergoing autologous stem-cell transplantation: results of a single institution, randomized phase 2 trial. Am J Hematol. 2010;85:863–867. doi: 10.1002/ajh.21855. [DOI] [PubMed] [Google Scholar]
- 12.Vehreschild JJ, Moritz G, Vehreschild MJ, et al. Efficacy and safety of moxifloxacin as antibacterial prophylaxis for patients receiving autologous hematopoietic stem cell transplantation: a randomized trial. Int J Antimicrob Agents. 2012;39:130–134. doi: 10.1016/j.ijantimicag.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention: Healthcare Infection Control Practices Advisory Committee. [Accessed 03 March 2015];Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006. Available from: www.cdc.gov/hicpac/mdro/mdro_0.html/.
- 15.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: M100-S19. Wayne, PA: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 17.Satlin MJ, Soave R, Racanelli AC, et al. The emergence of vancomycin-resistant enterococcal bacteremia in hematopoietic stem cell transplant recipients. Leuk Lymphoma. 2014;55:2858–2865. doi: 10.3109/10428194.2014.896007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JA, Qazilbash MH, Shih YC, Cantor SB, Cooksley CD, Elting LS. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112:1096–1105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 19.Ha YE, Kang Cl, Cha MK, et al. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42:403–409. doi: 10.1016/j.ijantimicag.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Rafailidis PI, Koftehdis D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J Antimicrob Chemother. 2007;60:1124–1130. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 21.Alonso CD, Dufresne SF, Hanna DB, et al. Clostridium difficile infection after adult autologous stem cell transplantation: a multicenter study of epidemiology and risk factors. Biol Blood Marrow Transplant. 2013;19:1502–1508. doi: 10.1016/j.bbmt.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 23.Peterson LR, Manson RU, Paule SM, et al. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis. 2007;45:1152–1160. doi: 10.1086/522185. [DOI] [PubMed] [Google Scholar]