Abstract
Schizophrenia, a major psychiatric disorder defined by delusions and hallucinations, among other symptoms, often with onset in early adulthood, is potentially associated with molecular and cellular alterations in parvalbumin-expressing fast spiking interneurons and other constituents of the cortical inhibitory GABAergic circuitry. The underlying mechanisms, including the role of disease-associated risk factors operating in adolescence such as drug abuse and social stressors, remain incompletely understood. Here, we summarize emerging findings from animal models, highlighting the ability of parvalbuminergic interneurons (PVI) to induce, during the juvenile period, long-term plastic changes in prefrontal and visual cortex, thereby altering perception, cognition and behavior in the adult. Of note, molecular alterations in PVI from subjects with schizophrenia, including downregulated expression of a subset of GABAergic genes, have also been found in juvenile stress models of the disorder. Some of the transcriptional alterations observed in schizophrenia postmortem brain could be linked to changes in the epigenetic architecture of GABAergic gene promoters, including dysregulated DNA methylation, histone modification patterns and disruption of promoter-enhancer interactions at site of chromosomal loop formations. Therefore, we predict that, in the not-to-distant future, PVI- and other cell-type specific epigenomic mappings in the animal model and human brain will provide novel insights into the pathophysiology of schizophrenia and related psychotic diseases, including the role of cortical GABAergic circuitry in shaping long-term plasticity and cognitive function of the cerebral cortex.
Keywords: Epigenome, Parvalbuminergic Interneuron, Critical Period, Cortical Plasticity, Schizophrenia
1. Introduction
Schizophrenia (SCZ), a mental disorder associated with delusions, hallucinations, disorganized thought, social withdrawal and various other symptoms is not defined by unifying neuropathology (Catts, Fung, Long, Joshi, Vercammen, Allen, Fillman, Rothmond, Sinclair, Tiwari, Tsai, Weickert, and Shannon Weickert, 2013; Dorph-Petersen and Lewis, 2011) or narrowly defined genetic risk architectures (Andreassen, Thompson, and Dale, 2014; Rodriguez-Murillo, Gogos, and Karayiorgou, 2012). However, clinical and preclinical research is beginning to identify major building blocks that contribute to the complex pathophysiology of SCZ. One such building block is the GABAergic circuitry in the cerebral cortex. GABAergic interneurons provide a major and critical source of inhibition to cortical networks, as animal models of disrupted GABAergic signaling show deficits in cortical plasticity (Fagiolini, Fritschy, Low, Mohler, Rudolph, and Hensch, 2004; Hensch, 2005; Hensch, Fagiolini, Mataga, Stryker, Baekkeskov, and Kash, 1998; Iwai, Fagiolini, Obata, and Hensch, 2003; Katagiri, Fagiolini, and Hensch, 2007), synchronous oscillations (Gonzalez-Burgos, Fish, and Lewis, 2011; Lodge, Behrens, and Grace, 2009) and cognition (Gonzalez-Burgos et al., 2011; Gruber, Calhoon, Shusterman, Schoenbaum, Roesch, and O’Donnell, 2010). Importantly, similar phenotypes are encountered in SCZ (Gonzalez-Burgos et al., 2011; Inan, Petros, and Anderson, 2013; Lewis, Hashimoto, and Volk, 2005). GABAergic interneurons, however, are an extremely diverse population that can be molecularly classified into three non-overlapping groups based on the expression of either Parvalbumin (PV), Somatostatin (SST) or serotonin receptor 3a (5-HT3AR) that together encompass nearly 100% of all cortical interneurons (Rudy, Fishell, Lee, and Hjerling-Leffler, 2011). Among these subpopulations, fast-spiking interneurons expressing PV provide inhibition to the cell bodies of pyramidal neurons to control their output. This inhibition influences rhythmic synchrony and facilitates information processing during cognitive tasks. Importantly, molecular alterations in PV interneurons (PVI) have been reported in prefrontal cortex and other cortical areas of SCZ subjects (Fung, Webster, Sivagnanasundaram, Duncan, Elashoff, and Weickert, 2010; Mellios, Huang, Baker, Galdzicka, Ginns, and Akbarian, 2009; Volk and Lewis, 2013), including downregulated expression of GABA synthesis enzyme GAD1/GAD67 (Hashimoto, Volk, Eggan, Mirnics, Pierri, Sun, Sampson, and Lewis, 2003), potassium channel subunits (Georgiev, Arion, Enwright, Kikuchi, Minabe, Corradi, Lewis, and Hashimoto, 2014) and transcription factors (Volk, Matsubara, Li, Sengupta, Georgiev, Minabe, Sampson, Hashimoto, and Lewis, 2012a), among various others (Volk, Chitrapu, Edelson, and Lewis, 2014). In addition to PV, low-threshold spiking SST+ neurons also demonstrate altered gene expression in SCZ cortex and hippocampus (Akbarian and Huang, 2006; Fung, Fillman, Webster, and Shannon Weickert, 2014; Fung et al., 2010; Konradi, Yang, Zimmerman, Lohmann, Gresch, Pantazopoulos, Berretta, and Heckers, 2011; Mellios et al., 2009; Schmidt and Mirnics, 2012). According to some estimates, up to 30–40% of subjects with schizophrenia show robust decreases in expression in a subset of RNAs specifically expressed in GABA neurons (Volk, Matsubara, Li, Sengupta, Georgiev, Minabe, Sampson, Hashimoto, and Lewis, 2012b). The underlying mechanisms of GABAergic deficits, just like SCZ as a disorder, are complex and heterogeneous. However, functional hypoactivity and a decrease in neurotrophin levels and signaling are likely to be important drivers for the observed deficits in GABAergic gene expression (Akbarian and Huang, 2006; Hashimoto, Bergen, Nguyen, Xu, Monteggia, Pierri, Sun, Sampson, and Lewis, 2005; Thompson Ray, Weickert, Wyatt, and Webster, 2011).
2. Role of PVIs in the postnatal maturation of cortical circuits
Cortical PVIs show a protracted developmental trajectory across adolescence (Hoftman and Lewis, 2011; O’Donnell, 2011). In prefrontal cortex, a brain region frequently affected by dysfunction and hypoactivity in subjects with SCZ, preclinical work strongly points to a period of heightened sensitivity of PVI during postnatal development (including childhood and juvenile stages). Disruption during this period results in subsequent deviation from the normal course of development into maladaptive trajectories ultimately resulting in long-lasting functional alterations (Powell, Sejnowski, and Behrens, 2012; Steullet, Cabungcal, Monin, Dwir, O’Donnell, Cuenod, and Do, 2014). These central features of PVI during juvenile age are not limited to the prefrontal cortex. Role of PVI on developmental critical period for experience-dependent cortical plasticity has been most extensively studied in visual cortex (Hensch, 2005; Takesian and Hensch, 2013). In the following, we review the recent findings in both prefrontal and visual cortex highlighting the key roles of PVIs during postnatal development in health and disease.
2.1. PVI-mediated juvenile plasticity in prefrontal cortex and lasting alterations relevant to SCZ
Maturation of PVIs in prefrontal cortex extends beyond the second decade of life and such protracted developmental trajectory may play a key role in the pathophysiology of many psychiatric disorders including SCZ with a typical onset around adolescence (Hoftman and Lewis, 2011; O’Donnell, 2011). Accumulating preclinical works strongly points to a period of heightened vulnerability of PVIs during postnatal development (including childhood and juvenile stages), which when perturbed, results in lasting deficits in the expression of neuropsychiatric risk genes, including some of the genes with a key role in ordinary inhibitory networks (Bharadwaj, Jiang, Mao, Jakovcevski, Dincer, Krueger, Garbett, Whittle, Tushir, Liu, Sequeira, Vawter, Gardner, Casaccia, Rasmussen, Bunney, Mirnics, Futai, and Akbarian, 2013; Chao, Chen, Samaco, Xue, Chahrour, Yoo, Neul, Gong, Lu, Heintz, Ekker, Rubenstein, Noebels, Rosenmund, and Zoghbi, 2010; Curley, Eggan, Lazarus, Huang, Volk, and Lewis, 2013; Guidotti, Dong, Tueting, and Grayson, 2014; Hashimoto et al., 2003; Huang, Matevossian, Whittle, Kim, Schumacher, Baker, and Akbarian, 2007; Hyde, Lipska, Ali, Mathew, Law, Metitiri, Straub, Ye, Colantuoni, Herman, Bigelow, Weinberger, and Kleinman, 2011; Jaaro-Peled, Hayashi-Takagi, Seshadri, Kamiya, Brandon, and Sawa, 2009; Jeevakumar, Driskill, Paine, Sobhanian, Vakil, Morris, Ramos, and Kroener, 2015; Karam, Ballon, Bivens, Freyberg, Girgis, Lizardi-Ortiz, Markx, Lieberman, and Javitch, 2010; Rico and Marin, 2011; Volk, Edelson, and Lewis, 2014) (Figure 1A). Some of the genes highly expressed in PVIs, such as GAD1, PV, and ERBB4 (Del Pino, Garcia-Frigola, Dehorter, Brotons-Mas, Alvarez-Salvado, Martinez de Lagran, Ciceri, Gabaldon, Moratal, Dierssen, Canals, Marin, and Rico, 2013; Mitchell, Janssen, Karavanova, Vullhorst, Furth, Makusky, Markey, and Buonanno, 2013; Neddens, Fish, Tricoire, Vullhorst, Shamir, Chung, Lewis, McBain, and Buonanno, 2011; Yang, Zhang, Chen, Geng, Ye, Spitzer, Luo, Duan, and Li, 2013), have been implicated in dysfunction of cortical networks and psychosis-related behavior in the adult animal (Behan, Hryniewiecka, O’Tuathaigh, Kinsella, Cannon, Karayiorgou, Gogos, Waddington, and Cotter, 2012; Behrens, Ali, and Dugan, 2008; Belforte, Zsiros, Sklar, Jiang, Yu, Li, Quinlan, and Nakazawa, 2010; Cabungcal, Counotte, Lewis, Tejeda, Piantadosi, Pollock, Calhoon, Sullivan, Presgraves, Kil, Hong, Cuenod, Do, and O’Donnell, 2014; Cabungcal, Steullet, Kraftsik, Cuenod, and Do, 2013a; Jeevakumar and Kroener, 2014; Thomases, Cass, and Tseng, 2013), long after the period of risk exposure has ceased. Examples of risk exposures transiently impacting adolescent PVI that subsequently lead to long-lasting functional alterations include drugs-of-abuse such as cannabis (Behan et al., 2012), and ketamine (Behrens et al., 2008; Jeevakumar and Kroener, 2014) and other NMDA antagonists (Thomases et al., 2013), as well as social isolation in conjunction with NMDA hypofunction (Belforte et al., 2010). This type of pathophysiology, starting with early life transient exposure to risk factor(s), with subsequent deviation from the normal course of development into maladaptive trajectories ultimately resulting in psychosis and depression in adult life, is a key concept in psychiatry.
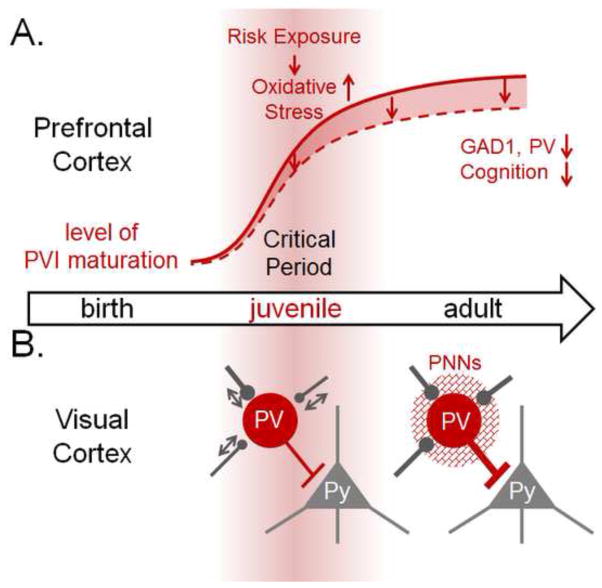
Figure 1. Central role of PVIs in developmental critical period for cortical plasticity.
(A) Protracted maturation of PVIs in prefrontal cortex plays a key role in the pathophysiology of SCZ. A heightened vulnerability of PVIs during juvenile development to exposure to risk factors, which collectively cause oxidative stress, results in subsequent deviation from the normal course of development (red line) into maladaptive trajectories (dotted line). This leads to lasting deficits in the expression of neuropsychiatric risk genes (e.g. GAD1, PV), and schizophrenia-related cognitive behaviors in the adult. Red background represents the extent of plasticity. (B) Protracted postnatal maturation of perisomatic innervation of PVIs triggers the onset of experience-dependent plasticity in visual cortex. During critical period, PVIs and their inputs are plastic (arrows). Visual deprivation rapidly leads to the reduction of excitatory inputs onto PVIs and PVI activity within one day leading to the disinhibition of pyramidal (Py) neurons. This in turn triggers the expression of global ocular dominance plasticity after a few days in visual cortex. After the critical period, expression of molecular brakes including perineuronal nets (PNNs: mesh around PVIs) limits rapid PVI plasticity as well as subsequent global plasticity. These mechanisms of plasticity associated with PVIs will provide a novel framework for the future investigation of PVI-dependent regulation of prefrontal cortex maturation in health and disease.
Interestingly, these different developmental insults collectively cause oxidative stress, which itself leads to disruption in prefrontal PVIs (Cabungcal et al., 2013a; Powell et al., 2012; Steullet et al., 2014), with juvenile anti-oxidant treatment preventing future PVI defects and psychosis (Cabungcal et al., 2014). Importantly, prefrontal PVIs are more vulnerable to oxidative stress during adolescence than later in life, and oxidative stress during this period is linked to long-lasting deficits in social behavior and other cognition. High metabolic demands render fast-spiking PVIs particularly vulnerable to oxidative stress (Behrens, Ali, Dao, Lucero, Shekhtman, Quick, and Dugan, 2007). A recent study demonstrated that one of the mechanisms that makes immature prefrontal PVIs particularly vulnerable to oxidative stress is the absence of fully mature perineuronal nets (PNNs) enwrapping PVIs, which protect PVIs from oxidative stress (Cabungcal et al., 2013a; Cabungcal, Steullet, Morishita, Kraftsik, Cuenod, Hensch, and Do, 2013b). An excess oxidative stress also leads to the breakdown of PNNs themselves (Cabungcal et al., 2013b; Morishita, Cabungcal, Chen, Do, and Hensch, In press). Of note, reduced PNNs have been found in the human post mortem amygdala, entorhinal and prefrontal cortices of Schizophrenia patients (Berretta, 2012; Mauney, Athanas, Pantazopoulos, Shaskan, Passeri, Berretta, and Woo, 2013; Pantazopoulos, Woo, Lim, Lange, and Berretta, 2010). As further discussed below, future studies are essential to further understand the molecular and epigenetic mechanism underlying the enduring effect of transient adolescent insult of PVIs on adult cognitive behaviors.
2.2. PVI-mediated control of developmental critical period for experience-dependent plasticity in visual cortex
One of the key roles of PVIs during postnatal maturation is regulation of the critical period of experience-dependent cortical plasticity (Hensch, 2005; Takesian and Hensch, 2013). Critical period of cortical plasticity is also thought to be important for the etiology of cognitive developmental disorders such as autism (Gogolla, Leblanc, Quast, Sudhof, Fagiolini, and Hensch, 2009; LeBlanc and Fagiolini, 2011) and schizophrenia (Morishita et al., In press; Morishita and Hensch, 2008). Neuronal circuits are refined by extraordinary levels of plasticity during critical periods in early development, while subsequently plasticity becomes diminished in adulthood (Hensch, 2004; Knudsen, 2004). It is during these “critical periods” that neurons acquire multiple functional properties in the context of experience-dependent maturation. To this end, ocular dominance plasticity, defined as the change in responsiveness to eye-specific (left versus right) input of neurons in primary visual cortex following monocular deprivation is one of the most characterized models of critical period (Hensch, 2004). Visual deprivation during adolescence critical period can eventually lead to an enduring loss of responsiveness to an eye deprived of vision, a condition called amblyopia, or persistent reduced visual acuity, affecting about 1–5% of the human population (Morishita and Hensch, 2008).
Over the last 15 years, rodents have emerged as a valuable model system for genetic manipulation to dissect the mechanisms of critical period for ocular dominance plasticity in primary visual cortex (Espinosa and Stryker, 2012; Levelt and Hubener, 2012; Morishita and Hensch, 2008; Nabel and Morishita, 2013; Takesian and Hensch, 2013). Several of these studies identified PVI as a key regulator of critical period timing (Hensch, 2005) (Figure 1B). Specifically, critical period could be precociously initiated through GABAA receptor α1 subunit, which is mainly located at the soma-proximal dendrite region of pyramidal cells, where PV-basket cells preferentially target (Fagiolini et al., 2004). Consistently, a non-cell autonomous homeoprotein Otx2, crucial for the maturation of PV-cells, is required for critical period initiation (Sugiyama, Di Nardo, Aizawa, Matsuo, Volovitch, Prochiantz, and Hensch, 2008). With age, PV-cells become preferentially enwrapped in chondroitin sulphate proteoglycans that form PNNs. Interestingly, removal of these nets can reactivate critical period plasticity in adult animals (Pizzorusso, Medini, Berardi, Chierzi, Fawcett, and Maffei, 2002) (Figure 1B). In addition to PNNs, several molecular brakes of relevance to schizophrenia have been identified to close the window of the critical period, including myelin signaling through a NgR-PirB complex (Insel, 2010; McGee, Yang, Fischer, Daw, and Strittmatter, 2005; Stephany, Chan, Parivash, Dorton, Piechowicz, Qiu, and McGee, 2014; Syken, Grandpre, Kanold, and Shatz, 2006) or dampened nicotinic receptor signaling by Lynx1 (Morishita, Miwa, Heintz, and Hensch, 2010; Stark, Xu, Bagchi, Lai, Liu, Hsu, Wan, Pavlidis, Mills, Karayiorgou, and Gogos, 2008), which all converge on PVI function as a hub of vulnerability.
In addition to the role of PVIs in controlling the timecourse of critical period, most recent studies revealed that the experience-dependent changes within PVIs and connected circuits themselves trigger the progress of ocular dominance plasticity during the critical period (Aton, Broussard, Dumoulin, Seibt, Watson, Coleman, and Frank, 2013; Gandhi, Yanagawa, and Stryker, 2008; Hengen, Lambo, Van Hooser, Katz, and Turrigiano, 2013; Kameyama, Sohya, Ebina, Fukuda, Yanagawa, and Tsumoto, 2010; Kuhlman, Olivas, Tring, Ikrar, Xu, and Trachtenberg, 2013; Yazaki-Sugiyama, Kang, Cateau, Fukai, and Hensch, 2009). Particularly, a recent intriguing study identified that just one day of visual deprivation during the critical period results in a rapid reduced activity of PVIs (Aton et al., 2013; Hengen et al., 2013; Kuhlman et al., 2013) at least in part due to the weakening and reduction of excitatory synaptic inputs onto PVIs (Kuhlman et al., 2013). This PV cell-mediated disinhibition subsequently permits competitive ocular dominance plasticity in visual cortex (which takes 3–4 days), suggesting a major role for PVI in gating plasticity (Kuhlman et al., 2013). Importantly, this rapid plasticity of PVIs is limited to the critical period but not observed in the adult (Kuhlman et al., 2013). The PVI-triggered juvenile plasticity can have a long lasting impact in the adult brain as a transient shift in ocular dominance, induced by monocular deprivation during critical period, is known to render the adult visual cortex highly susceptible to visual deprivation (Hofer, Mrsic-Flogel, Bonhoeffer, and Hubener, 2006). Identification of underlying molecular and epigenetic mechanisms that trigger and confine this rapid plasticity of PV-circuits in critical period is an important area of future investigation. Mechanisms of PVI-dependent critical period plasticity in the visual cortex may also provide a new framework for the future exploration of critical period mechanisms in prefrontal circuitry and behaviors.
3. Epigenetic regulation in cortical interneurons
The regulatory networks governing the molecular architectures of cortical inhibitory circuitry are exceedingly complex and include a diverse array of transcriptional and post-transcriptional mechanisms. To mention just one recent example from the SCZ literature, prefrontal deficits in the expression of a subset of GABA neuron-specific mRNAs were found to be dependent on the regional supply of Brain-derived Neurotrophic Factor (BDNF), which in turn was subject to post-transcriptional control by a microRNA-dependent mechanism (Mellios et al., 2009). Chromatin-associated mechanisms, regulating genome organization and function including gene expression, are broadly relevant for all phases of brain development (Bagot, Labonte, Pena, and Nestler, 2014; Vogel-Ciernia and Wood, 2014) and for proper learning and plasticity (Fischer, Sananbenesi, Mungenast, and Tsai, 2010; Lopez-Atalaya and Barco, 2014; Peixoto and Abel, 2013). Epigenetic regulators, including DNA methylation, hydroxymethylation and various other derivatives, and an estimated 100 post-translational histone modifications, histone variants and non-random spatial organization of chromosomal fibers (chromosomal loopings) are likely to be of critical importance for the function of PVI during and beyond the critical period of cortical plasticity. Importantly, however, next to nothing is known about the epigenome of PVI. This is because most epigenetic assays do not have single cell resolution, and given the enormous cellular heterogeneity of the cerebral cortex, PVI-specific chromatin studies would require cell-type specific extraction and enrichment procedures. While these are not insurmountable challenges, they would require significant effort such as generation of transgenic mice expressed GFP-tagged histones under the control of PVI-specific promoters, or application of reliable antibodies to immunotag PVI nuclei for subsequent fluorescence-activated sorting (Jiang, Matevossian, Huang, Straubhaar, and Akbarian, 2008). We predict that there will be PVI-specific chromatin studies in the not-to-distant future, with the critical period of cortical plasticity as a major focus. Indeed, there is already early evidence that epigenetic mechanisms regulate critical period plasticity in the visual system. Histone acetylation, an epigenetic modification that is experience dependent and typically enriched at open chromatin sites of active gene expression, becomes down-regulated after critical period plasticity has subsided (Putignano, Lonetti, Cancedda, Ratto, Costa, Maffei, and Pizzorusso, 2007). Histone acetylation is regulated by balanced activity of histone acetyl-transferases (HAT) and deacetylases (HDAC), and administration of HDAC inhibitors in adult mice reactivates visual cortex plasticity (Putignano et al., 2007) and induces recovery from amblyopia in rodent model (Silingardi, Scali, Belluomini, and Pizzorusso, 2010). Although the effect of HDAC inhibitors is intriguing, the changes in genetic expression profiles that HDAC inhibitors produce especially in PVIs, and subsequent downstream effects of visual cortical plasticity, are still unknown and should be a focus for future exploration. Similarly, while alterations in levels and activity of neuronal HDACs in prefrontal cortex are highly relevant for the neurobiology and treatment of SCZ (Jakovcevski, Bharadwaj, Straubhaar, Gao, Gavin, Jakovcevski, Mitchell, and Akbarian, 2013; Kurita, Holloway, Garcia-Bea, Kozlenkov, Friedman, Moreno, Heshmati, Golden, Kennedy, Takahashi, Dietz, Mocci, Gabilondo, Hanks, Umali, Callado, Gallitano, Neve, Shen, Buxbaum, Han, Nestler, Meana, Russo, and Gonzalez-Maeso, 2012), to date there is little knowledge on PVI-specific regulation of histone acetylation in that brain region.
GAD1/GAD67, encoding a key enzyme for GABA synthesis, is one of the genes with deficits in expression specifically in PVI of subjects diagnosed with SCZ (Hashimoto et al., 2003; Volk et al., 2012a). To this end, it is important to note that there is evidence for broad epigenetic dysregulation of GAD1/GAD67 in SCZ cerebral cortex, affecting DNA methylation patterns at promoters and proximal introns (Grayson and Guidotti, 2013; Huang and Akbarian, 2007), multiple types of histone modifications (Huang and Akbarian, 2007; Tang, Dean, and Thomas, 2011), and chromosomal loopings that provide structural backbone for GAD1 promoter interactions with cis-regulatory elements positioned 50kb further upstream (Bharadwaj et al., 2013) (Figure 2). Thus, it would be important to explore whether the epigenomic architecture of the GAD1 gene locus on chromosome 2q31, and other genomic sites, becomes dysregulated in PVI during development. Expanding the epigenomic study of GABAergic gene promotors beyond GAD1 is essential to dissect the exceedingly complex interactions of transcriptional and post-transcriptional mechanisms. Some of the transcriptional alterations observed in SZ postmortem brain (i.e., GAD1) could be directly linked to changes in the epigenetic architecture of GABAergic gene promoters, but some of the transcriptional alterations such PV could be in part the consequence of GAD1 epigenetic dysregulation. While developmental regulation has been confirmed for bulk prefrontal cortex tissue, and for neuronal nuclei sorted with a pan-neuronal marker, from the prenatal period to early adulthood and even beyond, it remains to be clarified whether PVI interneurons show dynamic regulation of DNA cytosine and histone lysine methylation at GABAergic gene promoters during critical periods in development (Cheung, Shulha, Jiang, Matevossian, Wang, Weng, and Akbarian, 2010; Huang et al., 2007; Siegmund, Connor, Campan, Long, Weisenberger, Biniszkiewicz, Jaenisch, Laird, and Akbarian, 2007). There is little doubt that epigenetic and other factors resulting in dysregulated expression of GAD1/GAD67 as a key enzyme for GABA synthesis could be highly detrimental to normal PVI function in diseased cortex (Uchida, Furukawa, Iwata, Yanagawa, and Fukuda, 2014). Importantly, conditional PVI-specific Gad1 ablation in juvenile mouse cortex negatively affects dendrite morphology and connectivity of these cells, thereby causing synaptic deficits and pyramidal cell hyperexcitability (Chattopadhyaya, Di Cristo, Wu, Knott, Kuhlman, Fu, Palmiter, and Huang, 2007; Lazarus, Krishnan, and Huang, 2013). Therefore, there can be little doubt that a new generation of epigenetic studies, exploring PVI and other cell-type specific epigenomes in diseased human brain and in preclinical model systems, is likely to provide novel insights into the neurobiology of SCZ and related disease. Furthermore, DNA methylation and histone modification changes at some of the promoters regulating PVI circuitry including REELIN, GAD1 (encoding GAD67 GABA synthesis enzyme) and BDNF (Brain-derived Neurotrophic Factor), not only show epigenetic status in SCZ postmortem brain, were also found in lymphocyte extracts from patients (Aberg, McClay, Nerella, Clark, Kumar, Chen, Khachane, Xie, Hudson, Gao, Harada, Hultman, Sullivan, Magnusson, and van den Oord, 2014; Auta, Smith, Dong, Tueting, Sershen, Boules, Lajtha, Davis, and Guidotti, 2013; Gavin, Kartan, Chase, Jayaraman, and Sharma, 2009; Ikegame, Bundo, Murata, Kasai, Kato, and Iwamoto, 2013). This is interesting because it may provide an opportunity to explore epigenetic alterations in the context of ‘biomarkers’ (defined here as molecular or functional marker for a disease process – SCZ), including coordinated neuronal network synchronizations such as the 40–100 Hz ‘gamma’ oscillations and their cognitive and neuroimaging correlates amenable to exploration in SCZ patients and healthy subjects (Cho, Konecky, and Carter, 2006; Hirano, Oribe, Kanba, Onitsuka, Nestor, and Spencer, 2015; Yoon, Maddock, Rokem, Silver, Minzenberg, Ragland, and Carter, 2010). Given that PVI play a key role in modulating this type of localized synchronized activity in the cerebral cortex (Lewis, 2014; Uhlhaas and Singer, 2014; Volman, Behrens, and Sejnowski, 2011), the study of epigenetic regulators of PVI function and, more broadly, of cortical GABAergic circuitry, could soon move center stage, with promising avenues for postmortem brain and animal research, and even for clinical settings.
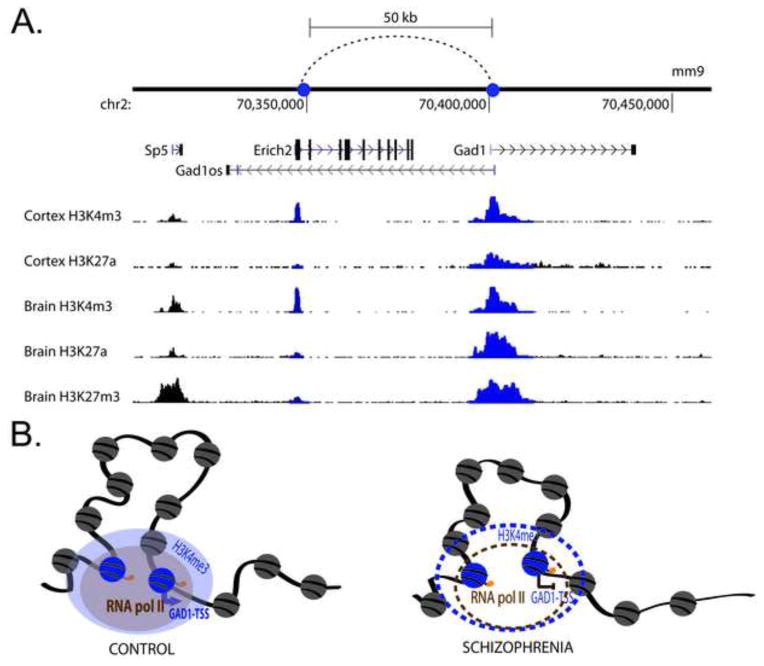
Figure 2. Epigenetic regulation at the GAD1/GAD67 gene locus.
(A) Chromosomal loopings between 5′ end of Gad1-TSS and regulatory sequences 50kb upstream marked by a H3K4me3 peak and additional histone modifications in human and mouse cortex. It is selectively labeled with H3K4me3 sites in cortex (Bernstein, Birney, Dunham, Green, Gunter, and Snyder, 2012). (B) This 50-kb loop also exists in humans and is characterized by RNA polymerase II binding and H3K4me3 sites. The loop is weakened in the prefrontal cortex of subjects with schizophrenia along with decreased GAD1 gene expression, loss of the H3K4me3 mark, altered levels of CpG island, and probably also RNA polymerase II binding.
Highlights.
GABAergic and Parvalbuminergic interneurons (PVIs) are disrupted in Schizophrenia.
PVIs mediate juvenile plasticity and provide long-lasting impact on adult behaviors.
Epigenetic alterations in GABAergic genes are found in Schizophrenia brains.
Epigenomic mapping of PVI is expected to provide novel insights into pathophysiology.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health and the Brain Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg KA, McClay JL, Nerella S, Clark S, Kumar G, Chen W, Khachane AN, Xie L, Hudson A, Gao G, Harada A, Hultman CM, Sullivan PF, Magnusson PK, van den Oord EJ. Methylome-Wide Association Study of Schizophrenia: Identifying Blood Biomarker Signatures of Environmental Insults. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2013.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr Bull. 2014;40:13–17. doi: 10.1093/schbul/sbt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci U S A. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta J, Smith RC, Dong E, Tueting P, Sershen H, Boules S, Lajtha A, Davis J, Guidotti A. DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients. Schizophr Res. 2013;150:312–318. doi: 10.1016/j.schres.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Labonte B, Pena CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16:281–295. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan AT, Hryniewiecka M, O’Tuathaigh CM, Kinsella A, Cannon M, Karayiorgou M, Gogos JA, Waddington JL, Cotter DR. Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on indices of dopaminergic, endocannabinoid and GABAergic pathways. Neuropsychopharmacology. 2012;37:1773–1783. doi: 10.1038/npp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62:1584–1597. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, Garbett K, Whittle C, Tushir JS, Liu J, Sequeira A, Vawter MP, Gardner PD, Casaccia P, Rasmussen T, Bunney WE, Jr, Mirnics K, Futai K, Akbarian S. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. J Neurosci. 2013;33:11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, Calhoon GG, Sullivan EM, Presgraves E, Kil J, Hong LE, Cuenod M, Do KQ, O’Donnell P. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013a;73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013b;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, Tsai SY, Weickert TW, Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, Akbarian S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc Natl Acad Sci U S A. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, Ciceri G, Gabaldon MV, Moratal D, Dierssen M, Canals S, Marin O, Rico B. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Lewis DA. Stereological approaches to identifying neuropathology in psychosis. Biol Psychiatry. 2011;69:113–126. doi: 10.1016/j.biopsych.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Fillman SG, Webster MJ, Shannon Weickert C. Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res. 2014;155:26–30. doi: 10.1016/j.schres.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci U S A. 2008;105:16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin DP, Kartan S, Chase K, Jayaraman S, Sharma RP. Histone deacetylase inhibitors and candidate gene expression: An in vivo and in vitro approach to studying chromatin remodeling in a clinical population. J Psychiatr Res. 2009;43:870–876. doi: 10.1016/j.jpsychires.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Georgiev D, Arion D, Enwright JF, Kikuchi M, Minabe Y, Corradi JP, Lewis DA, Hashimoto T. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2014;171:62–71. doi: 10.1176/appi.ajp.2013.13040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:138–166. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Tueting P, Grayson DR. Modeling the molecular epigenetic profile of psychosis in prenatally stressed mice. Prog Mol Biol Transl Sci. 2014;128:89–101. doi: 10.1016/B978-0-12-800977-2.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame T, Bundo M, Murata Y, Kasai K, Kato T, Iwamoto K. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. J Hum Genet. 2013;58:434–438. doi: 10.1038/jhg.2013.65. [DOI] [PubMed] [Google Scholar]
- Inan M, Petros TJ, Anderson SA. Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis. 2013;53:36–48. doi: 10.1016/j.nbd.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski M, Bharadwaj R, Straubhaar J, Gao G, Gavin DP, Jakovcevski I, Mitchell AC, Akbarian S. Prefrontal cortical dysfunction after overexpression of histone deacetylase 1. Biol Psychiatry. 2013;74:696–705. doi: 10.1016/j.biopsych.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevakumar V, Driskill C, Paine A, Sobhanian M, Vakil H, Morris B, Ramos J, Kroener S. Ketamine administration during the second postnatal week induces enduring schizophrenia-like behavioral symptoms and reduces parvalbumin expression in the medial prefrontal cortex of adult mice. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Jeevakumar V, Kroener S. Ketamine Administration During the Second Postnatal Week Alters Synaptic Properties of Fast-Spiking Interneurons in the Medial Prefrontal Cortex of Adult Mice. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu293. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Matevossian A, Huang HS, Straubhaar J, Akbarian S. Isolation of neuronal chromatin from brain tissue. BMC Neurosci. 2008;9:42. doi: 10.1186/1471-2202-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T. Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. J Neurosci. 2010;30:1551–1559. doi: 10.1523/JNEUROSCI.5025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–390. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han MH, Nestler EJ, Meana JJ, Russo SJ, Gonzalez-Maeso J. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245–1254. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Krishnan K, Huang ZJ. GAD67 Deficiency in Parvalbumin Interneurons Produces Deficits in Inhibitory Transmission and Network Disinhibition in Mouse Prefrontal Cortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JJ, Fagiolini M. Autism: a “critical period” disorder? Neural Plast. 2011;2011:921680. doi: 10.1155/2011/921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Barco A. Can changes in histone acetylation contribute to memory formation? Trends Genet. 2014;30:529–539. doi: 10.1016/j.tig.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo TU. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makusky A, Markey SP, Buonanno A. ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity. Proc Natl Acad Sci U S A. 2013;110:19603–19608. doi: 10.1073/pnas.1312791110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Cabungcal J-H, Chen Y, Do KQ, Hensch TK. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biological Psychiatry. doi: 10.1016/j.biopsych.2014.12.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EM, Morishita H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front Psychiatry. 2013;4:146. doi: 10.3389/fpsyt.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry. 2011;70:636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Rico B, Marin O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21:262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Murillo L, Gogos JA, Karayiorgou M. The genetic architecture of schizophrenia: new mutations and emerging paradigms. Annu Rev Med. 2012;63:63–80. doi: 10.1146/annurev-med-072010-091100. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K. Modeling interneuron dysfunction in schizophrenia. Dev Neurosci. 2012;34:152–158. doi: 10.1159/000336731. [DOI] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31:2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stephany CE, Chan LL, Parivash SN, Dorton HM, Piechowicz M, Qiu S, McGee AW. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J Neurosci. 2014;34:11631–11640. doi: 10.1523/JNEUROSCI.0545-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Monin A, Dwir D, O’Donnell P, Cuenod M, Do KQ. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res. 2014 doi: 10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl Psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 2013;33:26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A. Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry. 2014;4:e371. doi: 10.1038/tp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Oscillations and Neuronal Dynamics in Schizophrenia: The Search for Basic Symptoms and Translational Opportunities. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Wood MA. Neuron-specific chromatin remodeling: a missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology. 2014;80:18–27. doi: 10.1016/j.neuropharm.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Chitrapu A, Edelson JR, Lewis DA. Chemokine receptors and cortical interneuron dysfunction in schizophrenia. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Edelson JR, Lewis DA. Cortical inhibitory neuron disturbances in schizophrenia: role of the ontogenetic transcription factor Lhx6. Schizophr Bull. 2014;40:1053–1061. doi: 10.1093/schbul/sbu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012a;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in Transcriptional Regulators of Cortical Parvalbumin Neurons in Schizophrenia. Am J Psychiatry. 2012b doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Zhang J, Chen XJ, Geng HY, Ye M, Spitzer NC, Luo JH, Duan SM, Li XM. Development of GABA circuitry of fast-spiking basket interneurons in the medial prefrontal cortex of erbb4-mutant mice. J Neurosci. 2013;33:19724–19733. doi: 10.1523/JNEUROSCI.1584-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Cateau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]