Abstract
It is unknown whether or why genetic test reporting confers benefits in the understanding and management of cancer risk beyond what patients learn from counseling based on family history. A prospective nonexperimental control group study compared participants from melanoma-prone families who underwent CDKN2A/p16 (p16) genetic testing (27 carriers, 38 noncarriers) to participants from equivalently melanoma-prone families known not to carry a deleterious p16 mutation (31 no-test controls). All participants received equivalent counseling concerning elevated lifetime melanoma risk and corresponding recommendations for prevention and screening. Both immediately and one month after counseling, participants receiving a genetic test result reported greater understanding of their risk, decreased derogation of the risk information, and greater personal applicability of prevention recommendations than no-test controls. Decreased derogation of risk information after test reporting predicted further increases in understanding of melanoma risk and applicability of prevention recommendations one month later. Results suggest unique benefits of genetic test reporting in promoting understanding and acceptance of information about hereditary cancer risk and its management.
Keywords: genetic testing, melanoma, illness coherence, defensive processing, understanding of risk, CDKN2A/p16
Introduction
Interventions providing personalized risk information are designed to motivate improvements in medical adherence prior to disease development. The provision of genetic information, because it is highly personalized, objective, and exact, holds particular promise for accomplishing these goals. However, research has yet to determine whether any beneficial outcomes are due specifically to the provision of a genetic test result, rather than the counseling that typically accompanies test results. Such counseling includes detailed education about family history, elevated disease risk, and corresponding management recommendations (Aspinwall, Taber, Kohlmann, et al., 2013). As we will review, research suggests that receiving an explanation for one’s risk increases the acceptance of health-risk information. Because a genetic test result provides an explanation that is highly personalized and objective, we predicted that genetic test reporting would have advantages over counseling based on family history alone in promoting increased understanding and acceptance of information about highly elevated risk and its management among members of cancer-prone families.
Previous research on the outcomes of genetic test reporting has focused primarily on adherence to screening recommendations and psychological distress (see Aspinwall, Taber, Kohlmann, et al., 2013, for review). As such, relatively little is known about other potentially important outcomes of testing, including reactions to or evaluations of the information presented about cancer risk and behaviors recommended to manage that risk. In the present study, we focused on the impact of genetic testing on participant-reported understanding of personal risk, beliefs about the accuracy of the risk information, and perceived personal applicability of the accompanying recommendations for prevention and screening. These constructs were selected as key health cognitions that may be related to subsequent adherence.
One potential benefit of receiving a genetic test result is that its seemingly incontrovertible nature might reduce tendencies to respond defensively to health-risk information, especially when the stated risk is quite high and therefore likely to be threatening. Defensive responses, defined as a strategy to control fear that arises from threatening health information (McQueen et al., 2013; van’t Riet & Ruiter, 2013; Wiebe & Korbel, 2003; Witte, 1994), are a major barrier to the effective provision of risk information to people at elevated risk (Croyle et al., 2006; Jemmott et al., 1986; Liberman & Chaiken, 1992; Reed & Aspinwall, 1998). These defensive responses may take multiple forms, including derogating the accuracy of risk information or believing that management recommendations are exaggerated or not personally applicable (de Hoog et al., 2007; Good & Abraham, 2007; McQueen et al., 2013; van’t Riet & Ruiter, 2013, Witte, 1994).
To date, little research has examined whether the provision of genetic test results conferring highly elevated risk leads to defensive responding. In studies comparing participants’ risk estimates to those provided during counseling, unaffected mutation carriers have been found to underestimate their risk (Aspinwall, Taber, Kohlmann et al., 2014; Claes et al., 2005; Lipkus et al., 2004), but it is unknown whether individuals who receive information based on family history alone underestimate their risk similarly or to an even greater degree. Personalized cancer risk estimates (not based on genetic testing) may be perceived as inaccurate, particularly by individuals at higher risk (Scherer et al., 2013). Further research is necessary to test whether risk estimates conferred by genetic test results are more or less likely to invoke defensive responses than other kinds of risk estimates, such as those based on family history.
Receiving a genetic test result compared to counseling alone is also likely to influence subjective understanding of one’s disease risk and its implications (Read et al., 2005). For members of cancer-prone families in which a genetic cause for disease has been identified, a genetic test result not only confirms to what degree a known familial risk applies to them and to their biological children but also provides an explanation of why a particular individual is at increased risk. This information about a disease’s causes may enhance comprehension of risk information (Rothman & Kiviniemi, 1999). In cancer-prone families in which a genetic cause has not yet been identified, disease mechanisms may be less likely to be made clear to counselees who are given risk estimates based on family history alone. In such cases, information about the magnitude of risk may be presented, but information about why one’s family members are at such elevated risk or whether oneself in particular is at especially high risk remains unspecified. Counseling about disease risk may also improve understanding of the illness itself, known as illness coherence (Leventhal et al., 2003). Illness coherence is important as it predicts other beneficial consequences of test reporting and counseling, such as greater perceived risk, greater perceived control, less passive coping, lower cancer worry, and less distress (Gould et al., 2010; Kaptein et al., 2007; Van Oostrom et al., 2007).
Genetic counseling for hereditary cancer frequently confers lifelong risk and corresponding needs for continued education and sustained prevention and screening behaviors, and it is thus important to understand whether beliefs about the accuracy of risk information and the applicability of management recommendations are sustained over time. Most studies of defensive processing of health-risk information are experimental laboratory studies in which level of risk can be experimentally manipulated in order to study the effects of risk status on the processing of risk information. Participants are typically debriefed at the end of the experimental session. As a result, there is relatively little research examining defensive responses over time or in field settings (van’t Riet & Ruiter, 2013). The prospective relation of defensive processing to other beliefs about risk and its management is similarly understudied. For example, defensive processing following exposure to risk information may influence downstream cognitions, such as understanding of risk. There is some evidence that defensive responding may increase over time among participants given high risk estimates, as shown by increasingly biased recall for specific medical test results (Croyle et al., 2006) or recall for risk-inconsistent information (Reed & Aspinwall, 1998). The provision of particular explanations for risk may offset these tendencies, as people who learned how their risk estimate was obtained reported enhanced perceptions of its personalization and applicability to the self (Scherer et al., 2013). In this case, increased understanding of risk following the presentation of genetic test results could promote subsequent acceptance of risk information and management recommendations.
The Present Study: Overview and Hypotheses
Members of melanoma-prone families face highly elevated lifetime risk (i.e., 35–70x population risk, up to 76% lifetime risk) and are recommended to engage in daily ultraviolet radiation (UVR) avoidance and monthly skin self-examinations. Because management recommendations involve prevention and screening behaviors that can be personally undertaken, genetic testing for familial melanoma provides a unique model for studying the impact of providing genetic test results on how members of cancer-prone families process both risk information and corresponding management recommendations. In the present study, a novel nonexperimental control group design was used to compare the effects of genetic counseling and test reporting to the effects of equivalent counseling based on family history alone.
All participants were recruited from families with high melanoma rates, defined as three or more melanoma cases. Participants were recruited from either (a) families with a known mutation in the CDKN2A/p16 (or simply, p16) tumor suppressor that confers 28–76% lifetime risk to US residents (Begg et al., 2005; Bishop et al., 2002) or (b) other melanoma-prone families in which magnitude of risk is similar (35–70x approximate relative risk; Dutton-Regester & Hayward, 2012; Kefford et al., 1999), but for whom the melanoma risk cannot be attributed to currently known melanoma predisposition genes. Importantly, based on prior genetic testing of family members, these control families were known not to carry a p16 mutation, and thus new participants recruited from these families served as “no-test controls.”
Participants who tested positive for a p16 mutation conferring elevated melanoma risk (carriers) and no-test controls were given comparable estimates of up to 70 times population risk for melanoma (70 in 100 for carriers and 30–70 in 100 for no-test controls). Participants from p16 families who tested negative for a p16 mutation (noncarriers) were given estimates of up to two times population risk. With the exception of additional information about pancreatic cancer risk associated with a p16 mutation, only the provision of a genetic test result and the more specific point versus range estimate made possible by the genetic test result differed between p16 carriers and no-test controls (see Methods). All participants received identical recommendations for sun-protection and screening (see Methods).
We evaluated the impact of the provision of genetic test results versus family history-based counseling alone on several key health cognitions: understanding of melanoma and one’s risk for it (general melanoma coherence, understanding of risk) and defensive versus accepting responses to the specific risk information and management recommendations provided (derogation of risk information, perceived personal applicability and exaggeration of management recommendations). We expected similar gains in general understanding of melanoma (melanoma coherence) among all participants following the counseling session, given the education about melanoma etiology and risk factors that all participants received, but we predicted that the provision of a genetic test result (whether positive or negative) would specifically increase perceived understanding of one’s melanoma risk compared to counseling based on family history alone. Because a genetic test result is objective and irrefutable and/or because a genetic test result provides a concrete, personalized explanation for one’s elevated risk, we predicted that those who received a genetic test result (whether a positive result conferring high risk or a negative result conferring relatively low risk) would respond less defensively to information about disease risk and its management than those receiving high risk estimates based on family history alone. Importantly, this prediction differs from what one might predict based on the literature on defensive responding in other contexts. A typical finding is that participants informed they are at high risk for an illness are more likely than those at lower stated risk to engage in efforts to minimize the risk and the relevance of the recommended behavior changes. Based on this prior work, the strongest evidence of high risk (i.e., a definitive genetic test result) would be expected to promote the greatest defensive processing. Thus, both groups of participants receiving highly elevated melanoma risk estimates (carriers and no-test controls) would be predicted to show greater defensive processing than noncarrier participants receiving lower risk estimates, and participants receiving positive test results would be expected to engage in the most defensive processing.
Finally, we examined reciprocal relations among these constructs immediately and one month after counseling. As reviewed earlier, little is known about defensive responding to either genetic test results or counseling based on family history over time. A cross-lagged panel model was used to examine whether the increased understanding of melanoma risk immediately following counseling predicted to occur among participants receiving a positive genetic test result was related to subsequent increases in acceptance of risk information and management recommendations, as well as whether the predicted decrease in defensive responses to risk information and management recommendations among participants receiving a positive genetic test result predicted greater subsequent understanding of disease risk.
Methods
Unaffected members of two kinds of melanoma-prone families (those known to carry a p16 mutation and those known not to carry a p16 mutation, but who have a significant family history of melanoma) were recruited to BRIGHT (Behavior, Risk Information, Genealogy, and Health Trial). BRIGHT was designed to provide a comprehensive assessment of the impact of melanoma genetic test reporting and counseling on health cognitions and prevention behaviors among unaffected members of melanoma-prone families, compared to equivalent counseling about risk and its management based on family history alone. BRIGHT was approved by the University of Utah’s IRB for recruitment of individuals aged 16 to 70. All participants provided written informed consent before beginning the study. Data reported here are part of an ongoing study consisting of four visits over a 13-month period. Participants included in the present analyses completed their first three study visits from March to mid-October of 2012 and 2013.
Recruitment, Eligibility Criteria, and Retention
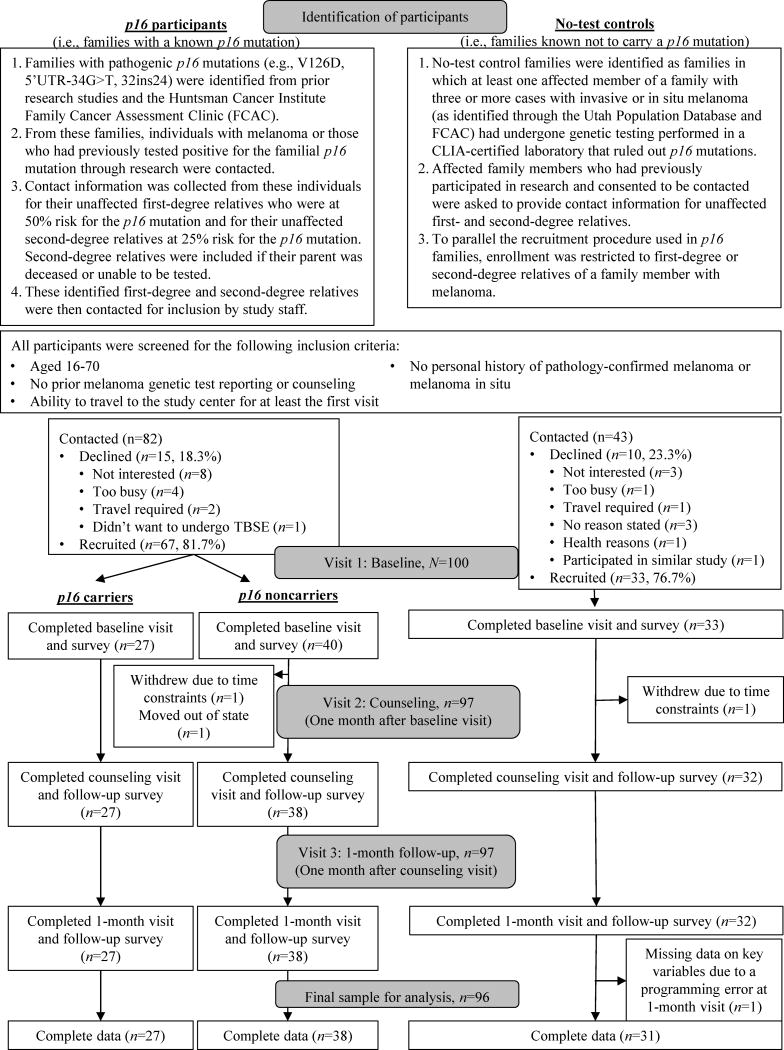
Figure 1 presents the participant identification procedures and recruitment and retention into the BRIGHT study. In brief, eligible participants were identified as 1) unaffected members of families either known to have a p16 mutation (p16 participants) or 2) members of families with three or more cases of invasive or in situ melanoma in which prior genetic testing had ruled out p16 mutations or other high-penetrance causes as the cause of the melanoma (“no-test controls”).
Figure 1.
Participant identification, recruitment and retention for the first three study visits that were completed by participants from March to mid-October of 2012 and 2013.
Additional inclusion criteria for all participants included ages 16–70, no prior melanoma genetic test reporting or counseling, and no personal history of pathology-confirmed melanoma or melanoma in situ, although participants could have had non-melanoma skin cancers or dysplastic nevi. These details were confirmed during a screening telephone call designed to recruit participants. An upper age limit of 70 was used because individuals reaching this age without a melanoma history are at a low likelihood of being p16 mutation carriers, and a lower age limit of 16 was used due to strong interest from these families in having their older adolescent children tested and because they were expected to be developmentally similar to 18-year-olds. Only unaffected individuals were included, as affected members of high-risk families tend to engage in greater prevention behaviors (Aspinwall, Taber, Leaf, et al., 2013, 2014; Geller et al., 2003; Manne et al., 2004). Participants were included regardless of their initial knowledge of melanoma cases in their families.
As shown in Figure 1,125 individuals were contacted. Of these, 100 were recruited (recruitment rate=80%) either from families with a known p16 mutation (n=67, recruitment rate=81.7%) or from families known not to carry a p16 mutation (n=33, recruitment rate=76.7%). Of note, all participants from p16 families elected to receive their genetic test results. The final sample for analysis included 96 respondents (27 carriers, 38 noncarriers, 31 no-test controls), as three withdrew and one participant’s data were lost due to a programming error.
Procedure and Counseling Protocol
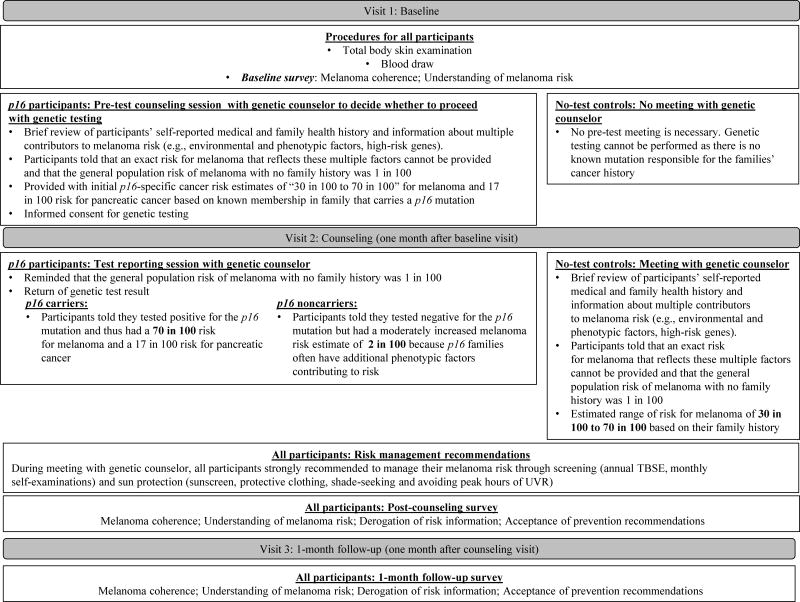
We report survey data from the first three visits over a 2-month period: 1) baseline, 2) counseling, and 3) 1-month follow-up (see Figure 2). The post-counseling survey was completed immediately following the genetic test reporting and/or counseling sessions at the second visit. The 1-month follow-up occurred one month later. All participants were compensated with a $50 gift card upon completing each visit, additional travel compensation dependent on distance traveled, a total body skin examination at baseline, vouchers for two free subsequent annual total body skin examinations, and free genetic counseling. Participants from families with a known p16 mutation also received free genetic testing for the familial mutation. Site-specific p16 genetic testing was performed in a CLIA-certified laboratory.
Figure 2.
Outline of study procedure through three visits in the first year of participation, including key points of information from the genetic counseling protocol and selected information about the content of surveys at each assessment.
Figure 2 provides details about the procedure of each visit, the counseling protocol, and measures administered to all participants regardless of family membership, as well as procedures specific to each kind of family (families with known p16 mutation; no-test control families; see also Supplemental Materials for complete counseling protocol including melanoma genetics education, risk information provision, and detailed management recommendations). Of note, members of p16 families met with one of two certified genetic counselors (CGCs) twice (during the baseline and counseling visits), whereas no-test controls only met with a CGC once (during the counseling visit). As noted in Figure 2, this procedural difference was necessary because members of p16 families needed to participate in a pre-testing information session in order to decide whether to obtain genetic test results and to provide informed consent for genetic testing. For p16 participants only, the second session included return of their genetic test result. Carriers were counseled that they tested positive for a p16 mutation and thus had a 70 in 100 risk for melanoma and a 17 in 100 risk for pancreatic cancer. Noncarriers from p16 families were counseled that they tested negative for the p16 mutation but still had a moderately increased melanoma risk estimate of 2 in 100. No-test controls were provided with an estimated range of risk for melanoma of “30 in 100 to 70 in 100” based on their family history. All participants were given identical recommendations for sun-protection and screening (see Figure 2 and Supplemental Materials).
Measures
Surveys were completed by all participants at baseline, following their genetic counseling session, and one month later. All survey items were completed on scales from 1 (Strongly disagree) to 5 (Strongly agree). Standard demographic factors, namely date of birth (used to calculate age at baseline), education (coded as number of years), household income, sex, and race, were self-reported. Participants reported their number of living and deceased first-degree relatives and second-degree relatives with melanoma both during the telephone screening and as part of their family history review with the CGC at the beginning of their counseling session.
Melanoma coherence was assessed by five items concerning participants’ understanding of melanoma adapted from the Illness Perceptions Questionnaire-Revised (Moss-Morris et al., 2002; e.g., “I have a clear picture or understanding of melanoma,” “I don’t understand melanoma”). Higher scores indicate greater perceived coherence (αs=0.86–0.88).
Understanding of melanoma risk was assessed by the 6-item certainty subscale (αbaseline= .74, αpost=.86, α1month=.70) of the Psychological Adaptation to Genetic Information Scale (PAGIS; Read et al., 2005). The original PAGIS items assessed understanding of carriers’ genetic test results (e.g., “I feel certain that I understand the meaning of having this gene,” “I understand the chances I have of passing this gene along to my children”). To apply to p16 and no-test control families, the underlined portion of each item presented to all participants was modified to “this melanoma risk.” Other items were, “I understand how I came to have this melanoma risk,” and “I feel that I can explain to other people what having this melanoma risk means.”
Defensive versus accepting responses to the risk information and prevention recommendations were assessed with three measures. Derogation of risk information was assessed as the average of two items, “The information I received about my risk of getting melanoma seems accurate” (reverse-scored) and “My risk estimate was missing some important information about me or my family” (rpost=.400, p <.001; r1month=.277, p =.006). Based on a literature review (McQueen et al., 2013; Witte, 1994), eight items were included to assess responses to the prevention and screening recommendations. These items were subjected to an exploratory factor analysis with maximum likelihood extraction and oblique rotation, which yielded the following two factors: personal applicability of prevention recommendations, assessed as the average of six items (αpost=0.93, α1month=0.92) indicating the extent to which the sun-protection and screening recommendations provided during the counseling session were “important,” “applied to me,” and “applied to my family,” and perceived exaggeration of prevention recommendations, assessed as the average of two ratings of the extent to which the “information about reducing sun exposure [melanoma screening] was exaggerated” (rpost=.80, p <.001; r1month=.66, p <.001; Witte, 1994).
Overview of Analyses
Repeated-measures analyses of variance (ANOVAs) tested the impact of genetic testing versus family history-based counseling alone on melanoma coherence, understanding of risk, and the three measures of defensive versus accepting responses to the risk information and prevention recommendations. In all primary analyses, participants from the two kinds of families were stratified into groups based on p16 mutation status, with Group (p16 carriers, p16 noncarriers, no-test controls) as a between-participants variable. Structural equation modeling (SEM) in Mplus (Muthén & Muthén, 1998) was used to test cross-lagged models of the relations among understanding of risk and measures of defensive versus accepting responses to the risk information and prevention recommendations immediately and one month after counseling. A statistical significance criterion of p< .05 was used for all analyses.
Results
Participant Characteristics and Correlations among Study Variables
Approximately half of the participants were male (52.1%) and nearly all were White (99.0%). The average age was 37.0 years (SD=13.5, range=16–69, only two participants were minors). Mean education was “some college” or 14 years (SD=2.1), and median household income was $60,000–69,999. Participants reported an average of 0.85 (SD=1.00, range: 0–3) first-degree relatives and 1.15 second-degree relatives with melanoma (SD=1.3, range: 0–6). Participant groups did not significantly differ in number of self-reported first-degree relatives or second-degree relatives with melanoma, age, gender, education, or household income (all ps > 0.120). Because there were no significant demographic differences among the groups, these measures were not covaried in the primary analyses. There were only two instances, noted below, in which results differed when these demographic factors were statistically controlled.
Correlations among study outcomes are presented in Table 1A. As predicted, ratings of melanoma coherence, understanding of melanoma risk, and multiple aspects of defensive responses were significantly intercorrelated at both the immediate and 1-month follow-up assessments. Table 1B presents the correlations of demographic factors and baseline measures of melanoma coherence and understanding of melanoma risk with responses to test reporting and/or counseling at both assessments.
Table 1A.
Correlations among understanding of melanoma and one’s risk for it, derogation of the risk information presented, and acceptance of the behavioral management recommendations immediately following counseling (above the diagonal) and at 1-month follow-up (below the diagonal)
| 1. Melanoma coherence | 2. Understanding of melanoma risk | 3. Derogation of risk information | 4. Personal applicability of prevention recommendations | 5. Perceived exaggeration of prevention recommendations | |
|---|---|---|---|---|---|
| 1. | -- | .51** | −.33** | .25* | −.24* |
| 2. | .47** | -- | −.43** | .48** | −.45** |
| 3. | −.40** | −.55** | -- | −.57** | .62** |
| 4. | .33** | .57** | −.61** | -- | −.68** |
| 5. | −.38** | −.41** | .51** | −.56** | -- |
p<.01;
p<.05;
Table 1B.
Correlations of baseline measures and demographic factors with responses to genetic test reporting and/or counseling immediately following counseling (top half) and at 1-month follow-up (bottom half)
| 1. Melanoma coherence | 2. Understanding of melanoma risk | 3. Derogation of risk information | 4. Personal applicability of prevention recommendations | 5. Perceived exaggeration of prevention recommendations | |
|---|---|---|---|---|---|
| Correlation with post-counseling assessment | |||||
| First-degree relatives with melanoma | .21* | .08 | .07 | .03 | .11 |
| Age | .01 | −.03 | −.12 | .15 | −.13 |
| Gendera | −.01 | −.15 | .13 | −.10 | .15 |
| Education | .22* | .27** | −.07 | .15 | .02 |
| Melanoma coherence, Baseline | .48** | .23* | −.17 | .09 | −.01 |
| Understanding of melanoma risk, Baseline | .28** | .29** | −.10 | .27** | −.16 |
| Correlation with 1-month assessment | |||||
| First-degree relatives with melanoma | .06 | −.03 | −.15 | .03 | −.04 |
| Age | −.17 | .01 | −.20^ | .17^ | −.19^ |
| Gendera | −.04 | −.25* | .19^ | −.15 | .19^ |
| Education | .15 | .33** | −.08 | .11 | −.07 |
| Melanoma coherence, Baseline | .54** | .35** | −.31** | .16 | −.22* |
| Understanding of melanoma risk, Baseline | .37** | .35** | −.29** | .22* | −.28** |
1=male, 0=Female;
p<.01;
p<.05;
p<.10
Impact of Genetic Test Reporting and Counseling versus Family-history Based Counseling
Melanoma coherence
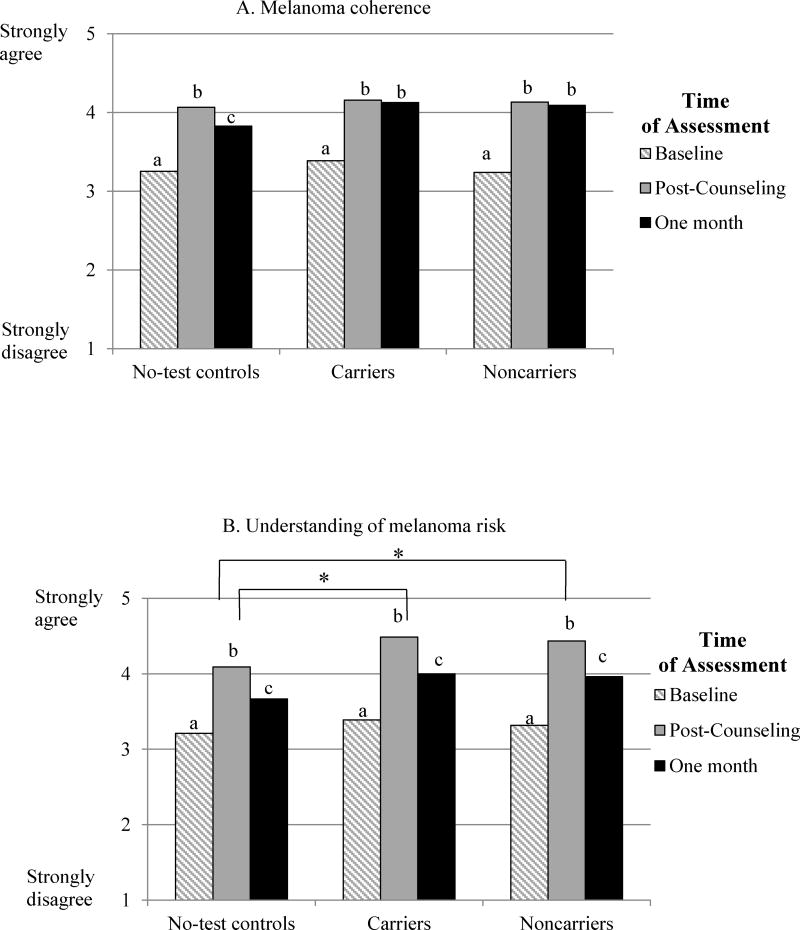
A repeated-measures ANOVA indicated that all groups reported comparable increases in melanoma coherence (Linear effect of Time: F(2,92)=57.82, p<.001), showing a curvilinear pattern (Quadratic effect of Time: (F(1,93)= 71.24, p<.001). Specifically, as shown in Figure 3A, melanoma coherence significantly increased from an average of 3.28 at baseline to 4.12 at post-counseling (p<.001), then decreased to 4.01 at one month (p=.047), remaining significantly elevated from baseline (p<.001). There were no significant differences among groups (main effect of Group: F(2,93)=0.57, p=.565) or different patterns of change over time (Group by Time interaction: F(4,184)=1.21, p=.307).
Figure 3.
Figure 3A. Increases from baseline in melanoma coherence immediately following counseling and at the 1-month follow-up among no-test controls, p16 carriers, and p16 noncarriers
Figure 3B. Increases from baseline in understanding of melanoma risk immediately following counseling and at the 1-month follow-up among no-test controls, p16 carriers, and p16 noncarriers
Note: Bars with different superscripts indicate significant differences across Time of Assessment in each group, p<.05
* Denotes significant differences between groups, p<.05
Understanding of melanoma risk
A repeated-measures ANOVA yielded significant main effects of both Time and Group. Specifically, as shown in Figure 3B, participants in all groups reported increased understanding of melanoma risk overall following counseling (Linear effect of Time: F(2,92)=108.82, p<.001), with a significant curvilinear pattern (Quadratic effect of Time: (F(1,93)=216.44, p<.001). Across groups, understanding of risk increased from 3.30 at baseline to 4.34 at post-counseling (p<.001), then decreased to 3.88 at one month (p<.001), remaining significantly elevated from baseline (p<.001); as shown in Figure 3B, this pattern was present within each group as well. Consistent with predictions, a main effect of Group indicated that both groups of individuals who received test results (carriers: M=3.96, p=.006; noncarriers: M=3.90, p=.013) reported greater understanding of their melanoma risk than no-test controls (M=3.66; F(2,93)=4.84, p=.010). Further inspection revealed that these differences were present at both follow-up assessments but not at baseline; however, the Group by Time interaction was not significant (F(4,184)=0.56, p=.694).
Defensive versus accepting responses to risk information and prevention recommendations
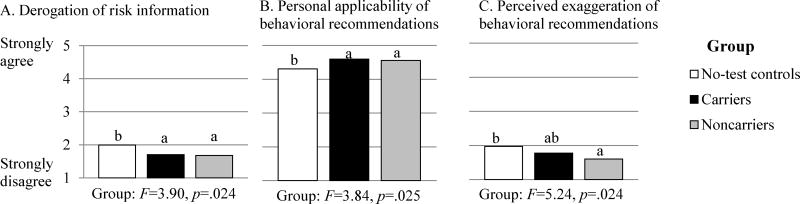
As shown in Figure 4, participants in all groups generally reported low levels of derogation of the risk information and high acceptance of prevention recommendations. However, there were significant group differences for all three outcomes, illustrated in Figure 4. Compared to no-test controls, carriers reported significantly lower derogation of risk information (Mcarriers =1.70, Mno-test =1.99, p = .031; Figure 4A) and greater perceived personal applicability of prevention recommendations (Mcarriers =4.60, Mno-test =4.31, p = .023; Figure 4B). Carriers and no-test controls did not differ in perceived exaggeration of prevention recommendations (Figure 4C). Notably, carriers did not respond more defensively than noncarriers (who were not expected to respond defensively, given the low risk estimates they received) on any index, whereas no-test controls responded more defensively than noncarriers on all three indices. The Group by Time interaction was not significant for any measure.
Figure 4.
Derogation of risk information and acceptance of prevention recommendations as a function of counseling and test reporting (p16 carriers and noncarriers) versus counseling based on family history alone (no-test controls), averaged across time of assessment (post-counseling and 1-month follow-up)
Notes: Degrees of freedom for main effect of Group are 2,94. Bars with different superscripts indicate significant differences between groups, p<.05
Two significant main effects of Time are not shown in Figure 4. Both derogation of risk information (Mpost=1.73, M1month =1.84; F(1,93)=4.11, p=.045) and perceived exaggeration of prevention recommendations (Mpost =1.69, M1month=1.85; F(1,93) =5.24, p=.024) increased from post-counseling to one month. However, neither effect was significant when four demographic factors (i.e., first-degree relatives with melanoma, age, gender, education) were simultaneously statistically controlled. These instances represent the only two analyses in which effects differed from the primary analyses reported above when these covariates were included.
Cross-lagged Model of the Relations between Understanding of Risk, Derogation of Risk Information, and Personal Applicability of Prevention Recommendations over Time
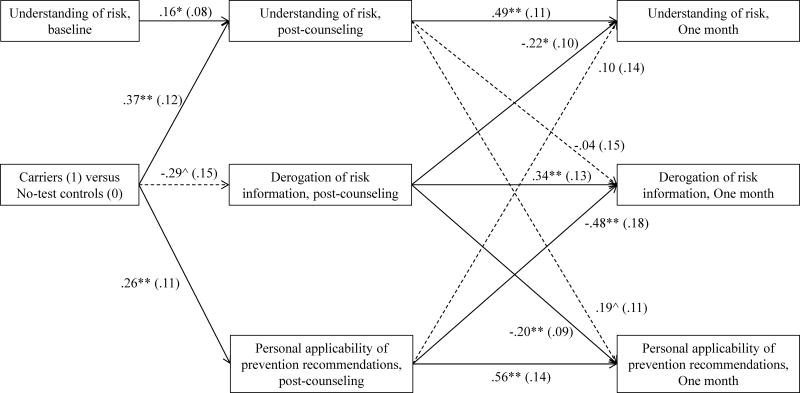
We tested whether the enhanced understanding of melanoma risk, decreased derogation of risk, and increased perceived personal applicability of prevention recommendations reported by carriers compared to no-test controls immediately following counseling predicted further changes in these outcomes one month later, when their respective post-counseling outcomes and understanding of risk at baseline were statistically controlled (Figure 5). Only carriers and no-test controls were retained in these analyses in order to pinpoint differences following receipt of genetic versus family history-based information indicating highly elevated melanoma risk. As shown in Figure 5, SEM was used to test cross-lagged effects from post-counseling to one month. Although the χ2 statistic indicated that these data differed significantly from the model (χ2(8) = 16.09, p < .05), the overall model fit was good using a fit index that is robust in small samples (CFI = .96; values >.90 indicate good model fit, Bentler, 1990). Further, the model shown in Figure 5 yielded a better fit to these data than a model excluding the cross-lagged effects (χ2 difference test: χ2 (6) = 27.52, p < .05). Consistent with prior analyses, carriers reported greater understanding of risk, somewhat lower derogation of the risk information, and greater perceived applicability of the prevention recommendations immediately following counseling.
Figure 5.
Model of the cross-lagged relations among understanding of risk, derogation of risk information, and personal applicability of behavioral recommendations immediately following and one month after genetic counseling plus test reporting (p16 carriers) or counseling based on family history alone (no-test controls).
Note: Correlations among measures within visits were included in the model, but omitted from the figure for ease of presentation. All but one such association were significant at p<.05: understanding of risk information and perceived applicability of prevention recommendations were not significantly correlated at one month. Significant pathways are indicated by solid lines and nonsignificant pathways are indicated by dashed lines. Path coefficients are unstandardized beta coefficients with standard errors in parentheses.
**p<.01, *p<.05, ^p<.10
Path coefficients from post-counseling to 1-month outcomes indicated significant stability in all three counseling outcomes, but also three significant cross-lagged effects. First, greater derogation of risk information immediately following counseling predicted further decreases in understanding of risk (β= −.22, SE=.10, p=.018) and perceived applicability of the prevention recommendations (β= −.20, SE=.09, p=.031) one month later. Additionally, greater post-counseling perceptions that the prevention recommendations were personally applicable predicted further decreases in derogation of the risk information one month later (β= −.48, SE=.18, p=.007). Finally, greater understanding of melanoma risk was not a significant predictor of further increases in the acceptance of risk information or management recommendations. Thus, the model suggests that carriers’ decreased tendency to derogate the risk information immediately following the counseling session compared to no-test controls may predict further increases in understanding of melanoma risk and acceptance of management recommendations one month later. Similarly, carriers’ increased perceptions of personal applicability of management recommendations predicted subsequent decreases in derogation of risk information.
Discussion
Genetic counseling, whether accompanied by genetic test reporting or not, led to increased understanding of melanoma (illness coherence) among members of cancer-prone families. However, consistent with hypotheses, genetic test reporting had several unique benefits compared to counseling based on family history: it enhanced understanding of melanoma risk, decreased derogation of the accuracy of the risk information, and increased perceived personal applicability of recommendations for vitally important, but potentially burdensome prevention and detection behaviors. Based on prior research on defensive processing of health-risk information (Croyle et al., 2006; Jemmott et al., 1986; Liberman & Chaiken, 1992; Reed & Aspinwall, 1998), participants receiving the highest risk estimates—in the present study, p16 carriers (70x population risk) and no-test controls (30–70x) —would have been expected to respond more defensively than noncarriers who received dramatically lower risk estimates (2x). However, across three measures of defensiveness, we found that no-test controls—but not p16 carriers—responded more defensively than p16 noncarriers. Thus, although both high-risk groups reported generally high acceptance of both risk information and accompanying management recommendations, the provision of a melanoma genetic test result seems to confer benefit by attenuating defensive responding to both risk information and management recommendations.
The results of our cross-lagged panel model suggest that these benefits of genetic test reporting may be consequential. Specifically, decreased derogation of risk immediately following the counseling session predicted greater subsequent understanding of risk and perceived personal applicability of management recommendations one month later. Further, greater perceived personal applicability of prevention recommendations immediately after counseling predicted subsequent decreases in derogation of the risk information one month later. Interestingly, enhanced understanding of risk following counseling predicted only marginal further improvements in the acceptance of prevention recommendations at one month. These findings suggest that it may be important to track the interrelations of various health cognitions concerning risk and its management over time following the provision of highly elevated risk information, and to identify specific health cognitions (i.e., derogation of risk information) that might be targeted as a way to increase subsequent understanding of risk information.
Taken together, these data suggest that when no genetic test is available, patient educators may need to spend additional time helping members of high-risk families understand risk information and how it applies to them on an individualized level. These patients’ attitudes should be followed over time to determine whether understanding and acceptance of risk and prevention recommendations are maintained and to determine whether and/or how these beliefs influence their behavior in the long-term. Our results may also extend to other diseases in which it is crucial to understand the processes that promote acceptance of prevention recommendations as personally applicable among people at high risk based on their family history.
Limitations of the Present Study
The primary limitation of the present study is our use of a nonexperimental control group design rather than an experimental design. Random assignment to receipt of a genetic test result presents multiple ethical and logistic challenges. Because families with p16 mutations are rare, randomization of at-risk members of these families to testing and non-testing arms is not ideal for two reasons: randomization would divide the sample of participants eligible for p16 testing in half, and participants assigned to a no-test control group could be influenced by the experiences of their relatives who received test results in the other arm of the study. For these reasons, we offered genetic test reporting to all eligible members of p16 families and recruited members of families known not to carry a p16 mutation as no-test controls. This procedure allowed us to provide comparable risk estimates and identical management recommendations to participants from the two kinds of melanoma-prone families without denying or delaying test reporting to any eligible participants. However, this nonexperimental approach leaves open the possibility that other differences between participants from p16 families and no-test control families may account for the findings reported here. However, participant groups did not differ in age, gender, education, income, or self-reported number of first-degree relatives or second-degree relatives with melanoma, and there was no evidence of differential recruitment to the study or differential attrition as recruitment and retention were high in both groups.
Although we have interpreted the findings as representing the presence versus absence of a genetic test result, the risk estimates also differed in whether they were a point versus a range (i.e., 70x vs. 30–70x population risk), which may have contributed to the differences reported here. The greater precision of a point estimate made possible by genetic testing may reduce ambiguity (Han et al., 2011), thereby contributing to greater understanding of one’s risk. As such, it is unclear whether no-test control participants’ greater derogation of the risk information was in fact defensiveness, or a more reasoned response to receiving less precise information. Further, p16 participants were given preliminary risk estimates for members of high-risk families during the pre-testing education during their first visit (after completion of baseline measures), whereas no-test controls did not receive risk estimates until their second visit. p16 participants’ greater amount of time to think about the risk information and the repetition of risk estimates could therefore also have contributed to the greater understanding of risk reported by p16 respondents immediately following counseling. However, both of these aspects (the point estimate and the provision of pre-test counseling) are inherent in the provision of genetic test results and would also differ between genetic test reporting and counseling based on family-history alone in a clinic setting not part of a research study.
With respect to external validity, the genetic test reporting and counseling procedure used in the present study represented standard-of-care. Only the involvement in a research study, including multiple clinic visits to complete follow-up surveys and provision of a total body skin examination at baseline, distinguished participants’ involvement in the present study from the current standard-of-care for receiving genetic information in a clinical setting.
Implications for Clinical Genetic Testing for Familial Melanoma
These data, along with continued examination of the impact of melanoma genetic test reporting on prevention and screening behavior, may inform discussions of the clinical utility of genetic test reporting in the management of familial melanoma (Gerstenblith et al., 2007). A key issue has been whether genetic testing is necessary, as test results do not alter management recommendations concerning sun-protection and skin screening for members of melanoma-prone families. Improvements in sun-protection and screening behavior following receipt of genetic test results have been reported up to two years later by unaffected carriers in prior studies (Aspinwall, Taber, Leaf, et al., 2013, 2014; see also Glanz et al. 2013; Kasparian et al., 2009), but because all of these participants received both genetic counseling and test results, these benefits cannot be uniquely attributed to receipt of a positive genetic test result. The benefits of genetic test reporting compared to family history-based counseling identified in the present study – including greater understanding of risk and enhanced acceptance of risk and prevention recommendations – may ultimately lead to increased prevention and detection behaviors. Of note, we assessed defensive processing specific to the risk information and management recommendations, but it is possible that participants engaged in other kinds of defensive processing (e.g., downplaying the severity of melanoma). Additionally, some researchers have noted that defensive responses may lead to improved health behaviors if they successfully control negative emotion (van’t Riet & Ruiter, 2013; Wiebe & Korbel, 2003) or motivate people to accept behavioral recommendations as one strategy for reducing the fear of getting a disease. Therefore, important next steps in this ongoing study are to examine whether understanding and processing of risk information and prevention recommendations predict adherence to recommended sun-protection and screening behaviors. These data may also have relevance to concerns about the impact of providing negative test results to noncarrier members of high-risk families. We note that noncarriers did not interpret the prevention recommendations as exaggerated or less personally applicable because they were given low risk estimates, suggesting that negative test results may not necessarily undermine adherence.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Supplementary Material
Acknowledgments
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01 CA158322. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Support was also received from the Huntsman Cancer Foundation (HCF), the Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment, the Pedigree and Population Resource of Huntsman Cancer Institute, and the Utah Population Database. This research was supported by the Utah Cancer Registry, which is funded by contract N01-PC-35141 from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, with additional support from the Utah State Department of Health and the University of Utah. The authors acknowledge the use of the Genetic Counseling and Health Measurement and Survey Methods core facilities supported by the National Institutes of Health through National Cancer Institute Cancer Center Support Grant 5P30CA420-14 awarded to Huntsman Cancer Institute and additional support from the HCF.
The authors gratefully acknowledge the generous participation of all the family members in this study, without whom this project would not have been possible. We thank also Pam Cassidy, Taylor Haskell, Sandie Edwards, Roger Edwards, Rebecca Stoffel, Dixie Thompson, Lisa Reynolds, Tami Calder, Michelle Allred, Melissa Shepherd, Teresa Stone, Jason Hawkes, Matt Haskell, and Janice Mathews for their contributions to the conduct of the study.
Footnotes
Conflict of Interest:
Dr. Taber, Ms. Stump, Ms. Kohlmann, and Ms. Champine declare that they have no conflict of interest. Dr. Aspinwall’s work is funded by the NIH. Dr. Leachman’s work is funded by the NIH. Dr. Leachman serves on a Medical and Scientific Advisory Board for Myriad Genetics Laboratory, for which she has received an honorarium. She has collaborated with Myriad on a project to validate an assay that is unrelated to the research reported here.
References
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genetics in Medicine. 2014;16:846–853. doi: 10.1038/gim.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Perceived risk following melanoma genetic testing: A 2-year prospective study distinguishing subjective estimates from recall. Journal of Genetic Counseling. 2014;23(3):421–437. doi: 10.1007/s10897-013-9676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiology, Biomarkers, & Prevention. 2013;22(10):1687–1697. doi: 10.1158/1055-9965.EPI-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Daily routine sun-protection improves 2 years following melanoma genetic test reporting. Genetics in Medicine. 2014 doi: 10.1038/gim.2014.37. Published online April 24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C, Orlow I, Hummer A, Armstrong B, Kricker A, Marrett L, Berwick M. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. Journal of the National Cancer Institute. 2005;97(20):1507–1515. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bishop D, Demenais F, Goldstein A, Bergman W, Bishop J, Bressac-de Paillerets B, Tucker MA. Geographical variation in the penetrance of CDKN2A mutations for melanoma. Journal of the National Cancer Institute. 2002;94(12):894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- Claes E, Evers-Kiebooms G, Denayer L, Decruyenaere M, Boogaerts A, Philippe K, et al. Predictive genetic testing for hereditary breast and ovarian cancer: Psychological distress and illness representations 1 year following disclosure. Journal of Genetic Counseling. 2005;14(5):349–363. doi: 10.1007/s10897-005-1371-4. [DOI] [PubMed] [Google Scholar]
- Croyle R, Loftus E, Barger S, Sun Y, Hart M, Gettig J. How well do people recall risk factor test results? Accuracy and bias among cholesterol screening participants. Health Psychology. 2006;25(3):425–432. doi: 10.1037/0278-6133.25.3.425. [DOI] [PubMed] [Google Scholar]
- de Hoog N, Stroebe W, de Wit JF. The impact of vulnerability to and severity of a health risk on processing and acceptance of fear-arousing communications: A meta-analysis. Review of General Psychology. 2007;11(3):258–285. doi: 10.1037/1089-2680.11.3.258. [DOI] [Google Scholar]
- Dutton-Regester K, Hayward N. Reviewing the somatic genetics of melanoma: from current to future analytical approaches. Pigment Cell & Melanoma Research. 2012;25(2):144–154. doi: 10.1111/j.1755-148X.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- Geller A, Emmons K, Brooks D, Zhang Z, Powers C, Koh H, Gilchrest B. Skin cancer prevention and detection practices among siblings of patients with melanoma. Journal of the American Academy of Dermatology. 2003;49(4):631–638. doi: 10.1067/S0190-9622(03)02126-1. [DOI] [PubMed] [Google Scholar]
- Gerstenblith MR, Goldstein AM, Tucker MA, Fraser MC. Genetic testing for melanoma predisposition: current challenges. Cancer Nursing. 2007;30(6):452–459. doi: 10.1097/01.NCC.0000300165.98391.e7. [DOI] [PubMed] [Google Scholar]
- Glanz K, Volpicelli K, Kanetsky PA, Ming ME, Schuchter LM, Jepson C, et al. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:607–614. doi: 10.1158/1055-9965.EPI-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A, Abraham C. Measuring defensive responses to threatening messages: a meta-analysis of measures. Health Psychology Review. 2007;1(2):208–229. doi: 10.1080/17437190802280889. [DOI] [Google Scholar]
- Gould RV, Brown SL, Bramwell R. Psychological adjustment to gynaecological cancer: Patients’ illness representations, coping strategies and mood disturbance. Psychology & Health. 2010;25(5):633–646. doi: 10.1080/08870440902811163. [DOI] [PubMed] [Google Scholar]
- Han PJ, Klein WP, Arora NK. Varieties of uncertainty in health care: A conceptual taxonomy. Medical Decision Making. 2011;31:828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmott JB, Ditto PH, Croyle RT. Judging health status: Effects of perceived prevalence and personal relevance. Journal of Personality and Social Psychology. 1986;50(5):899–905. doi: 10.1037/0022-3514.50.5.899. [DOI] [PubMed] [Google Scholar]
- Kaptein AA, van Korlaar IM, Cameron LD, Vossen CY, van der Meer FM, Rosendaal FR. Using the common-sense model to predict risk perception and disease-related worry in individuals at increased risk for venous thrombosis. Health Psychology. 2007;26(6):807–812. doi: 10.1037/0278-6133.26.6.807. [DOI] [PubMed] [Google Scholar]
- Kasparian N, Meiser B, Butow P, Simpson J, Mann G. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genetics in Medicine. 2009;11(4):265–278. doi: 10.1097/GIM.0b013e3181993175. [DOI] [PubMed] [Google Scholar]
- Kefford R, Newton Bishop J, Bergman W, Tucker M. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: A consensus statement of the Melanoma Genetics Consortium. Journal of Clinical Oncology. 1999;17(10):3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York, NY, US: Routledge; 2003. pp. 42–65. [Google Scholar]
- Liberman A, Chaiken S. Defensive processing of personally relevant health messages. Personality and Social Psychology Bulletin. 1992;18(6):669–679. doi: 10.1177/0146167292186002. [DOI] [Google Scholar]
- Lipkus I, McBride C, Pollak K, Lyna P, Bepler G. Interpretation of genetic risk feedback among African American smokers with low socioeconomic status. Health Psychology. 2004;23(2):178–188. doi: 10.1037/0278-6133.23.2.178. [DOI] [PubMed] [Google Scholar]
- Manne S, Fasanella N, Connors J, Floyd B, Wang H, Lessin S. Sun protection and skin surveillance practices among relatives of patients with malignant melanoma: prevalence and predictors. Preventive Medicine. 2004;39(1):36–47. doi: 10.1016/j.ypmed.2004.02.028. [DOI] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Swank PR. Construct definition and scale development for defensive information processing: An application to colorectal cancer screening. Health Psychology. 2013;32(2):190–202. doi: 10.1037/a0027311. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychology & Health. 2002;17(1):1–16. doi: 10.1080/08870440290001494. [DOI] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide [Computer software manual] Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- Read CY, Perry DJ, Duffy ME. Design and Psychometric Evaluation of the Psychological Adaptation to Genetic Information Scale. Journal of Nursing Scholarship. 2005;37(3):203–208. doi: 10.1111/j.1547-5069.2005.00036.x. [DOI] [PubMed] [Google Scholar]
- Reed MB, Aspinwall LG. Self-affirmation reduces biased processing of health-risk information. Motivation and Emotion. 1998;22:99–132. doi: 10.1023/A:1021463221281. [DOI] [Google Scholar]
- Rothman AJ, Kiviniemi M. “Treating people with health information”: analysis and review of approaches to communicating health risk information. Journal of the National Cancer Institute Monographs. 1999;25:44–51. doi: 10.1093/oxfordjournals.jncimonographs.a024207. [DOI] [PubMed] [Google Scholar]
- Scherer LD, Ubel PA, McClure J, Greene SM, Alford S, Holtzman L, Fagerlin A. Belief in numbers: When and why women disbelieve tailored breast cancer risk statistics. Patient Education and Counseling. 2013;92(2):253–259. doi: 10.1016/j.pec.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, Bröcker-Vriends AT, van Asperen CJ, Sijmons RH, Tibben A. The common sense model of self-regulation and psychological adjustment to predictive genetic testing: A prospective study. Psycho-Oncology. 2007;16(12):1121–1129. doi: 10.1002/pon.1178. [DOI] [PubMed] [Google Scholar]
- van’t Riet J, Ruiter RC. Defensive reactions to health-promoting information: An overview and implications for future research. Health Psychology Review. 2013;7(Suppl 1):S104–S136. doi: 10.1080/17437199.2011.606782. [DOI] [Google Scholar]
- Wiebe DJ, Korbel C. Defensive denial, affect, and the self-regulation of health threats. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York, NY, US: Routledge; 2003. pp. 184–203. [Google Scholar]
- Witte K. Fear control and danger control: A test of the extended parallel process model (EPPM) Communication Monographs. 1994;61(2):113–134. doi: 10.1080/03637759409376328. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.