Abstract
Castrate resistant prostate cancer (CRPC) is the fatal-form of prostate cancer and remains androgen dependent. The reactivation of the androgen axis occurs due to adaptive intratumoral androgen biosynthesis which can be driven by adrenal androgens and /or by changes in the androgen receptor (AR) including AR gene amplification. These mechanisms are targeted with P450c17 inhibitors e.g. Abiraterone acetate and AR super-antagonists e.g. Enzalutamide. Clinical experience indicates that with either agent an initial response is followed by drug resistance and the patient clinically progresses on these agents. This article reviews the mechanisms of intrinsic and acquired drug resistance that target the androgen axis and how this might be surmounted.
Keywords: prostate cancer, steroidogenesis, androgen receptor, therapeutics
Graphical Abstract
1. Introduction
1. 1. Castration Resistant Prostate Cancer
Prostate cancer is the most common cancer in man and the second leading cause of cancer death in men per year in the US. Approximately 180,000 new cases are diagnosed and approximately 30,000 deaths occur annually [1]. Advanced localized prostate cancer is often treated with androgen deprivation therapy (ADT) which consists of leuprolide (a LH-RH agonist) [2], which is the equivalent to a chemical castration, often combined with an androgen receptor (AR) antagonist e.g. R-bicalutamide (Casodex), and or a dual type 1 and type 2 5α-reductase inhibitor (dutasteride). Following this treatment there is a period of remission, however, the cancer invariably returns and is accompanied by an increase in serum prostatic-specific antigen (PSA). PSA is an androgen dependent gene and because this increase occurs in the presence of castrate levels of circulating androgens this from of the disease is referred to as castration resistant prostate cancer (CRPC). CRPC is uniformly fatal and occurs due to adaptive responses in the tumor that reactivate the androgen axis [3, 4]. The androgen axis is defined as activation of androgen receptor (AR) signaling that leads to androgen dependent gene expression. Reactivation of the axis can occur due to adaptive intratumoral androgen biosynthesis [5-9] or by changes in the AR (e.g. AR gene amplification and mutation) [10-16]. Both mechanisms are targeted by new drugs that have been approved by the US FDA e.g. abiraterone acetate (AA) [17-20], a P450c17 (17α-hydroxylase/17,20-lyase) inhibitor which prevents the formation of adrenal androgens e.g. dehydroepiandrosterone-sulphate (DHEA-SO4), DHEA and Δ4-androstene-3,17-dione (Δ4-AD); and enzalutamide (ENZ) [12, 21], a AR super-antagonist. When used to treat metastatic CRPC (mCRPC), patients on these agents have an increased median survival of only 3-4 months before drug resistance occurs [22, 23].
1. 2. Experience with Abiraterone
AA is a P450c17 inhibitor that blocks both the 17α-hydroxylase and 17,20-lyase activity of the enzyme. Inhibition of 17α-hydroxylase prevents the production of cortisol in the adrenal and in turn blocks feed-back inhibition of the adrenal-pituitary axis so that the gland is hyperstimulated with ACTH. In an attempt to make cortisol in response to ACTH, desoxycorticosterone increases which is a potent mineralocorticoid which can lead to life-threatening hypertension. This side-effect is countered by the co-administration of prednisone, a synthetic glucocorticoid. Phase 1 and phase 3 clinical trials with AA showed significant benefit in terms of median time to PSA progression, median radiologic progression free survival and improved symptoms [12, 20, 24]. These trials also reported a > 90% decline in circulating DHEA providing a biochemical assessment of drug efficacy [24, 25].
In a neoadjuvant clinical trial we compared the effect of Leuprolide alone (12 weeks) followed by Leuprolide plus AA (12 weeks)-Arm 1, with patients that received Leuprolide plus AA (for all 24 weeks) [26]. Once patients were on AA they also received prednisone for the reasons stated above. We measured serum androgens at entry into the trial, at 12 weeks and 24 weeks using a novel validated stable isotope-dilution liquid chromatography tandem mass spectrometric (LC-MS/MS) assay, Table 1. We found that leuprolide alone decreased circulating testosterone (T) levels to castrate levels but adrenal androgens (DHEA-SO4, DHEA, Δ4-AD) were affected. Once AA was administered the adrenal androgens were decreased by > 90%. However, the amount of circulating DHEA-SO4 that remains (20,000 ng/dL) exceeds the castrate levels of circulating testosterone (5 ng/dL) by more than 4,000-fold, suggesting that a significant reservoir of circulating DHEA-SO4 remains to fuel intratumoral androgen biosynthesis. A significant difference was also observed in the serum DHT levels at 12 weeks versus the value at 24 weeks (p<0.001) in the first arm which may favor DHT formation via the backdoor pathway (see later) in the transition period from Leup to Leup + AA. The reservoir of DHEA-SO4 that remains provides a mechanism for the clinical failure of all P450c17 inhibitors which could be exacerbated if the tumor has either intrinsic or acquired resistance phenotypes (see Section 2 for definitions).
Table 1. Serum Androgen Measurements-Neoadjuvant Study Luperolide vs Luperolide plus Abiraterone Acetate.
| Serum Androgen Levels in Abi + Leuprolide Neoadjuvant Clinical Trial | |||||||
|---|---|---|---|---|---|---|---|
| 24 weeks Leuprolide/ 12 weeks AA + Prednisone |
24 weeks Leuprolide/ 24 weeks AA + Prednisone |
p Value | |||||
| Median serum androgen (ng/dL) |
Baseline (n= 28) |
Week 12 (n =28) |
Week 24 (n = 27) |
Baseline (n =27) |
Week 12 (n =29) |
Week 24 (n =27) |
|
| Testosterone | 429 | 17 | 5 | 425 | 7 | 16 | 0.0003 |
| DHT | 29 | 13 | 17 | 40 | 13 | 6 | 0.0176 |
| DHEA | 242 | 201 | 19 | 176 | 26 | 30 | < 0.0001 |
| DHEA-glucuronide | 1901 | 1508 | 515 | 1522 | 415 | 292 | 0.0003 |
| DHEA-sulfate | 231K | 200Ka | 22Kb | 195Ka | 16Kb | 17Kb | <0.0001 a vs b |
| Androsterone | 11 | 5 | 0.6 | 9 | 1 | 0.8 | 0.003 |
| Δ4-Androstene-3, 17-dione | 76 | 52 | 7 | 59 | 9 | 8 | <0.001 |
Taken from Ref: [26]
a and b superscripts refer to the groups that were compared to reject the null hypothesis. Other comparisons are at 12 weeks versus baseline based on log-transformed data
This observation provides a mechanism for the clinical failure of all P450c17 inhibitors which could be exacerbated if the tumor has either intrinsic or acquired resistance phenotypes (see Section 2 for definitions).
1.3. Experience with Enzalutamide
ENZ is a potent AR antagonist that prevents nuclear localization of the receptor and promotes receptor degradation [21]. Side-effects seen with ENZ include CNS seizures mediated by an off-target effect by inhibiting GABAA currents, and gynecomastia and fatigue [27]. The CNS side-effects can be overcome by substituting the second generation AR super-antagonist ARN-509 [28]. In the phase 1-2 study with ENZ the drug decreased PSA by > 50% or more in 56% of the patients, 22% of the patients showed reduced soft-tissue disease, 56% of the patients had stabilized bone disease and 49% of patients showed a favorable circulating tumor count. PET imaging of the AR by 18F-DHT showed a 20-100% reduction in AR occupancy, and the median time to progression was 47 weeks [29]. In the AFFIRM phase 3 clinical trial ENZ was found to increase survival in prostate cancer patients who received prior chemotherapy from 13.6 months to 18.4 months versus the placebo control group[30]. In the PREVAIL phase 3 clinical trial in which patients had not received prior chemotherapy ENZ showed significant benefit with respect to all secondary end points, including the time until the initiation of cytotoxic chemotherapy (hazard ratio, 0.35), the time until the first skeletal-related event (hazard ratio, 0.72), a complete or partial soft-tissue response (59% vs. 5%), the time until prostate-specific antigen (PSA) progression (hazard ratio, 0.17), and a rate of decline of at least 50% in PSA (78% vs. 3%) where p<0.001 for all the comparisons made [22].
2. Mechanisms of Drug Resistance
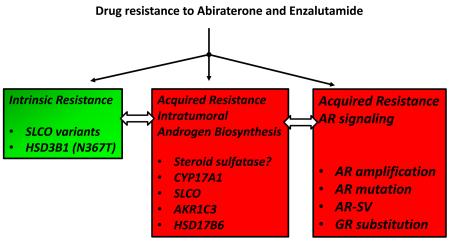
Multiple mechanisms exist to account for resistance to AA and ENZ observed clinically in mCRPC patients. These mechanisms may involve intrinsic or acquired resistance. Intrinsic resistance refers to a subset of individuals that will do poorly on these drugs due to germ-line or inherited mutations. Acquired resistance refers to either somatic mutations or changes in gene expression profiles that will surmount the effect of the drug. Acquired resistance can include adaptive changes in intratumoral androgen biosynthesis and /or AR signaling. These mechanisms are not mutually exclusive and may synergize with each other.
2.1. Intrinsic Drug Resistance
2. 1. 1. Inherited Mutations in Organic Anion Transporters
DHEA-SO4 is the major source of adrenal androgens that can fuel intratumoral androgen biosynthesis even after AA treatment. For this conjugate to become bioavailable it must be transported by the solute carrier organic anion encoded membrane transporters (SLCO), also known as organic anion transporters (OATs). The members of this family involved in steroid uptake in the prostate include SLCO1A2 and SLCO2B1 [31]. The impact of SLCO genetic variation on steroid sequestration has been evaluated using SLCO2B1 variants in prostate cancer cells. In cells transfected with the SLCO2B1 SNP variant rs12422149 (935GA; Arg312Gln), the 935A variant exhibited higher maximal DHEA-SO4 uptake when compared with either the wild type 935G allele or with mock-transfected cells [32]. In 538 patients with metastatic hormone-sensitive prostate cancer the median time to progression for patients with each of 3 SLCO2B1 alleles: rs12422149 [935G>A; Arg312Gln, minor allelic frequency 21%]; rs1789693 [A/T intron variant with minor allelic frequency of 48%]; and rs1077858 [A/G intron variant with minor allelic frequency of 43%] was 10, 7, and 12 months shorter, respectively than when the wild type allele was present [32]. Data such as these indicate that SLCO gene variants will impact prostate cancer progression and response to ADT therapy.
The HSD3B1 (N367T) mutation is the first to be identified in a steroidogenic enzyme that may affect drug response to AA [33]. HSD3B1 is the principal enzyme in the prostate involved in either the conversion of DHEA to Δ4-androstene-3,17-dione (Δ4-AD) or the conversion of Δ5-androstene-3β17β-diol (Δ5-Adiol) to T via its dual 3β-hydroxysteroid dehydrogenase/ketosteroid isomerase activities. The germline N367T mutation makes the enzyme more stable and CRPC patients harboring this mutation will be more prone to evade ADT by being able to convert residual DHEA into potent androgens more readily. Tumors bearing the HSD3B1 N367T mutation more rapidly progress to CRPC in xenograft models than in tumors bearing the wild-type allele [33]. The population frequency of the mutated allele is about 22%. It is likely that inherited SNPs in other steroidgenic enzymes may also impact drug response and hence mediate intrinsic resistance phenotypes to AA or ENZ but these have yet to be documented.
2. 2. Acquired Drug Resistance
2. 2. 1. Denovo synthesis of Androgens
Nelson and colleagues have made the case that prostate tumors catalyze denovo synthesis of active androgens [7, 8]. The conversion of [14C]-acetate into DHT was observed ex vivo in the LNCaP xenograft model and formation of cholesterol and cholesteryl esters was also observed. This mechanism could be an adaptive response to first and second line ADT. However, such studies do not take into account the large excess of DHEA-SO4 that remains after AA treatment in patients. De novo synthesis is unlikely to make a significant contribution to intratumoral androgen biosynthesis, when such a large depot of DHEA-SO4 exists in the circulation after AA treatment. Nevertheless increases in CYP11A (side-chain cleavage enzyme) and CYP17A1 transcripts have been observed in response to AA treatment and possibly contribute to acquired drug resistance [5].
2. 2. 2. Bioavailability of DHEA-SO4
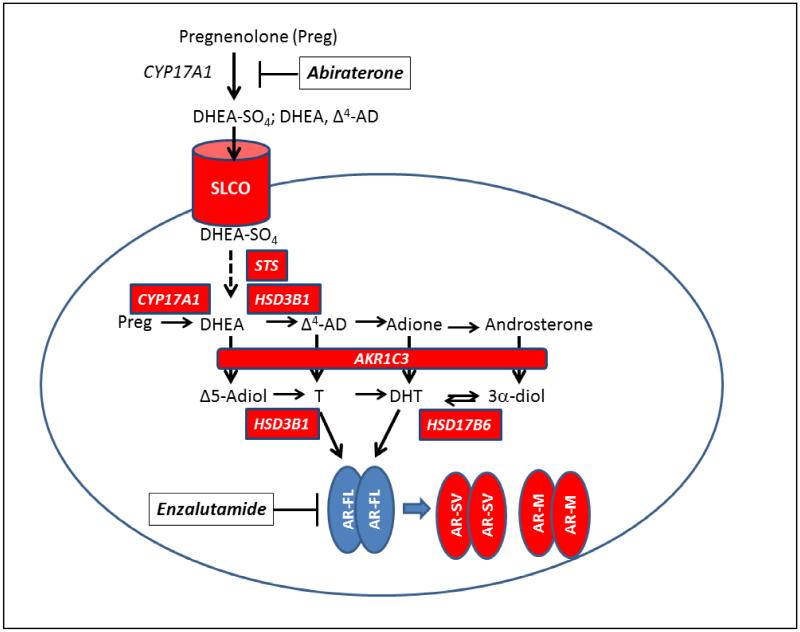
The presence of high circulating DHEA-SO4 after P450c17 inhibition can be exploited by prostate cancer tumors if there is high expression of SLCOs and steroid sulfatase (STS) to liberate free DHEA, Fig .1. SLCO1A2 is implicated in DHEA-SO4-induced prostate cancer cell growth in androgen-depleted media. SLCO1A2-transfected LNCaP and 22RV1 cells showed increased DHEA-SO4 stimulated growth when compared to SLCO1A2-knockdown cells, which were insensitive to DHEA-SO4 stimulated growth [34] In addition SLCO1A2 mRNA expression was higher in PCa cell lines grown under androgen-depleted conditions (and was suppressed by addition of DHT). These data suggest that expression of SLCO1A2 is up-regulated by androgen deprivation and may lead to acquired drug resistance, by providing more residual DHEA-SO4 for intratumoral androgen biosynthesis. Less is known about adaptive responses in STS expression following ADT.
Figure 1.
Composite Mechanisms of Drug Resistance in Castration Resistant Prostate Cancer. Mechanisms indicated in red boxes; arrow between AR-FL and AR-SV and AR-SM indicates selection pressure to produce new AR subtypes; where AR-FL, androgen receptor full length; AR-SV, androgen receptor splice variant; AR-M, mutated androgen receptor. italics = gene names; AKR1C3, aldo-keto reductase 1C3; AR-SV, = androgen receptor splice variant; AR-M, = mutated androgen receptor; CYP17A1, DHEA = dehydroepiandrosterone; Δ4-AD, 4-androstene-3,17-dione; Adione, = 5α-androstane-3,17-dione; Δ5-Adiol, 5-androstene-3β,17β-diol; DHT, 5α-dihydrotestosterone; 3α-Adiol, 5α-androstane-3α,17β-diol; HSD3B1; 3β-hydroxysteroid dehydrogenase type 1; P450c17 (17α-hydroxylase,17/20-lyase); T =testosterone; SLCO; = solute carrier organic anion transporter;
2. 2. 3. Overexpression of Type 5 17β-HSD or AKR1C3
AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase) plays an essential role in the formation of T and DHT in the prostate irrespective of the pathway used [35-38]. In the canonical pathway: DHEA→Δ4-AD→T→DHT, AKR1C3 catalyzes the reduction of Δ4-AD→T. In the alternative pathway: DHEA→Δ4-AD→5α-androstane-3,17-adione (Adione)→DHT, AKR1C3 catalyzes the reduction of Adione to DHT; in the backdoor pathway: Progesterone→5α-dihydroprogesterone→allopregnanolone→androsterone→5α-androstane-3β,17β-diol (3α-diol)→DHT, AKR1C3 catalyzes the conversion of androsterone to 3α-diol; and in the Δ5-Adiol pathway: DHEA →Δ5-Adiol →T→DHT, AKR1C3 catalyzes the conversion of DHEA to Δ5-Adiol. Thus overexpression of AKR1C3 in CPRC would provide a mechanism to divert trace androgens that remain after ADT to potent androgens via these three pathways within the tumor. Studies have shown that AKR1C3 is overexpressed in prostate cancer cell lines 10-16 fold and up to 3-fold in androgen responsive and androgen independent prostate cancer cell xenografts upon androgen deprivation when measured by qRT-PCR, Table 2 [6, 39-43]. By contrast AKR1C3 expression is repressed by androgens e.g. R1881 [44]. These studies suggest that AKR1C3 upregulation is an adaptive response to ADT, and could contribute to drug resistance observed with AA or ENZ. Investigation of the mechanism by which AKR1C3 is upregulated by ADT identified ERG as binding to the AKR1C3 gene promoter. TMPRSS2-ERG is a fusion protein, a biomarker of advanced prostate cancer and is co-expressed with AKR1C3 in advanced disease. Since ERG is induced by androgens, intratumoral production of DHT by AKR1C3 would increase ERG expression and create a feed-forward mechanism to sustain AKR1C3 expression and androgen biosynthesis. In this manner TMPRSS2-ERG overrides the repressive effect of the AR on the AKR1C3 gene promoter. TMPRSS2-ERG k/d in VCaP cells led to a reduction in AKR1C3 expression and a decrease in the ability to convert Adione to DHT supporting this mechanism [45]. It is noteworthy that analysis of gene expression in 25 mCRPC tumors obtained from the Gene Expression Omnibus revealed a significant correlation (p <0.0001 r = 0.69) between AKR1C3 and ERG co-expression where AKR1C3 increased expression ranged from 0- 100-fold and ERG expression ranged from 0-250-fold over non-expressing tumors [45].
Table 2. Overexpression of AKR1C3 in Prostate Cancer Patients and Prostate Cancer Cell Lines.
| Patient Samples | Cell Lines | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Affymetrix Microarray |
qRT-PCR | IHC | LNCaP | DuCaP | VCaP | 22Rv1 | PC-3 | DU-145 | |
| Stanbrough et al. [46] |
32 CRPC patients 5.3 fold increase vs primary tumors |
10-fold higher in 32 CRPC patients vs primary tumors |
11/19 CRPC recurrent prostate cancer & soft tissue mets vs 1/18 primary tumors |
||||||
| Hofland et al [40] |
10-fold hgher in CRPC (n=10; p< 0.001) vs normal prostate |
Up regulated in CS-FBS 9-fold by qRT-PCR |
Up regulated in CS-FBS 5-fold by qRT-PCR |
||||||
| Pfeiffer et al., [42] |
CRPC : low grade (n=21:20) 2.3 fold |
Up regulated in CS-FBS 5-fold by qRT-PCR |
|||||||
| Jernberg et al, [47] |
5-fold higher in bone mets vs hormone naïve patients (n=51; p< 0.01) |
Bone mets. n =51 (rs with RNA 0.73; p< 0.000001) |
|||||||
| Hamid et al., [39] |
3-fold Higher AKR1C3 (n =41) than in BPH (n= 9 samples p< 0.001) |
41 samples IHC staining (Rs with RNA 0.39; <0.0001) |
−ive IB | + ive IB | +++ ive IB | ++ ive IB AR7V |
+ ive IB | −ive IB | |
| Tian et al., [43] | 3-fold higher in 40 needle biopsy GS=6, GS=7, GS=8, GS= 9 vs low grade GS (n=10 group; p <0.05) .Positive correlation with Gleason Score |
||||||||
| Mitsiades et al., [44] |
6/19 mets upregulated |
Upregulated 3- fold in 30% mCRPC (n =19) vs normal prostate (n=19) and in 5 patients CTCs |
AKR1C3 o/exp upon ADT CS- FBS (16- fold) or MDV3100 (6-fold) by qRT- PCR Repressed by R1881 (16-fold) by qRT- PCR |
||||||
| Fankhauser et al. [79] |
20 samples: NS Enrichment of HSD17B genes as a group q value <0.1 |
2-fold higher in hormone refractory cancer vs BPH (n=4 p<0.012) Taylor cohort mCRPC 153% increase vs BPH (n= 41 p <0.001) |
|||||||
| Powell et al. [42]. |
25 mCRPC tumors by microarray show high correlation between AKR1C3 expression and ERG p < 0.0001 rs = 0.69 |
Up to 100-fold increase in AKR1C3 and 250-fold increase in ERG expression |
|||||||
CS-FBS = charcoal stripped FBS; BPH= benign prostatic hyperplasia; GS = Gleason Grade; IB = immunoblot (+ to +++ intensity of staining within the same experiment);
AKR1C3 is also one of the most overexpressed steroidogenic genes in CRPC patients [46]. A 5-10 fold increase in expression levels over primary tumors was observed using Affymetrix microarray data and validated by qRT-PCR. AKR1C3 was also overexpressed in 11/19 soft-tissue metastasis from mCRPC patients by IHC [44, 46][46] and in bone metastasis by qRT-PCR and IHC [47]. Analysis of several studies suggest that AKR1C3 is upregulated in subset of mCRPC patients where estimates are that it is overexpressed at the RNA and protein level in at least one third of all mCRPC patients [39, 43, 44, 47]. These estimates are likely to be conservative since whether patients had received prior second-line ADT therapy was not reported when measuring transcript levels. Data obtained from the Oncomine database for 363 prostate cancer tumor specimens when used to construct Kaplan-Meier survival plots showed that the median survival time for patients with tumors that were low AKR1C3 expressors and low TMPRSS2-ERG expressors was 12-13 years, but patients whose tumors were high AKR1C3 expressors and high TMPRSS2-ERG expressors had median survival times of only 7-10 years [45]. In this study high and low expressors fell either side of the median value, however, it is not clear as to whether the prostate cancer specimens were derived from radical prostatectomy samples, needle biopsies, or transurethral prostate tissue specimens. Others have since confirmed the high level of AKR1C3 transcripts in bone metastasis of CRPC patients and found that this was due to overexpression rather than by increased copy number variation [47].
2. 2. 4. Overexpression of HSD17B6
HSD17B6 is the major 3α-hydroxysteroid dehydrogenase in human prostate that catalyzes the back conversion of 3α-diol to DHT [48-50]. Long-term culture of LNCaP or VCaP cells in androgen-deprived media increased transcriptomic expression HSD17B6. In 42 prostate cancer patients undergoing androgen deprivation therapy (ADT) HSD17B6 was about 2-fold higher than that in tissues of 100 untreated individuals. In men receiving ADT, patients showing biochemical progression had a higher HSD17B6 score than those without progression [51]. These results suggest that 3α/β-diol also represent potential precursors of DHT, and the back conversion to DHT from androgen derivatives catalyzed by HSD17B6 may be a promising target to counter ADT resistance. HSD17B6 is also upregulated by TMPRSS2-ERG and when this transcription factor is silenced in VCaP cells there is a dramatic reduction in HSD17B6 expression but only a modest effect on the conversion of 3α-diol to DHT suggesting that AKR1C3 may be the major source of potent androgens in these cells [45].
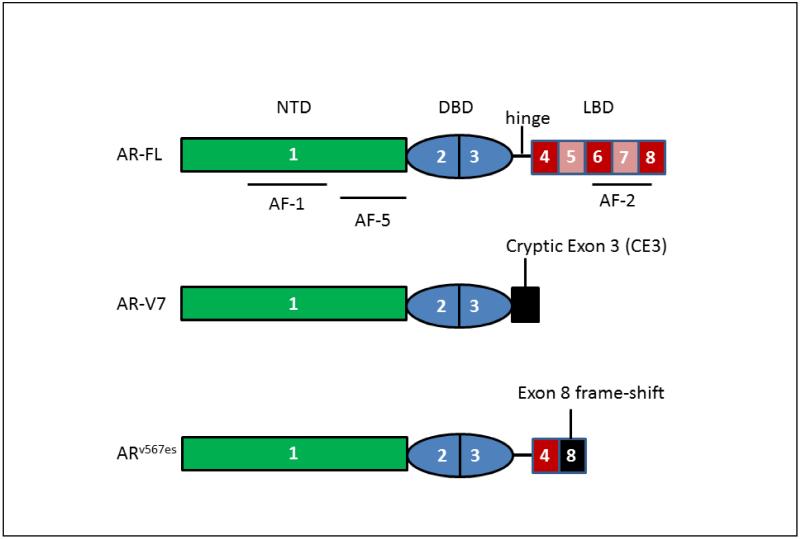
2. 2. 5. Androgen Receptor Splice Variants
Resistance to AA and ENZ, is also associated with increased expression of androgen receptor splice variants (AR-SVs) [52]. The two most common AR-SV’s are AR-V7 and ARes567 (the exome skipping variant), Fig. 2. These variants have lost their ligand-binding domain and are constitutively active without ligand [53, 54]. Originally it was felt that they would heterodimerize with full-length AR (AR-FL), but it now appears that AR-SVs are transcriptionally active in their own right [55]. Microarray analysis shows that the AR-SV transcriptionally activates a gene set that differs from AR-FL and which may make the tumor more aggressive [56]. AR-V7 may be the most important splice variant and is implicated in AA and ENZ resistance and is detected in circulating tumor cells (CTCs) of CRPC patients [57]. In this study 39% of the patients resistant to ENZ and 31% of the patients resistant to AA had detectable AR-V7. By contrast in AA resistant xenografts of VCaP cells AR-V7 mRNA was expressed as < 1% of the mRNA for AR-FL. AR-V7 is clearly associated with disease progression and the drug resistant phenotype but its exact contribution to resistance mechanisms still needs to be established [58]. The exact mechanism by which AA and ENZ result in AR-SVs is unknown, but could involve genomic rearrangement of the AR gene and/or alternative splicing of the AR pre-messenger RNA (mRNA).
Figure 2.
Domains of AR-SVs. Numbers refer to AR gene exons.
2. 2. 6. Androgen Receptor Mutation and Substitute Steroid Receptors
Clinical experience with AR-antagonists such as R-bicalutamide showed that overtime the AR acquired somatic mutations that enabled the antagonist to work as an agonist and also make the AR ligand permissive [11, 15]. One of the most common mutations is T877A. This now appears to be the clinical experience with AA and ENZ as well [57, 59]. The T877A mutant can use among other steroids progesterone. Missense AR exon 8 mutations were detected in 11/62 patients (18%), including the first reported case of an F876L mutation in an ENZ resistant patient [60]. In addition, the H874Y and T877A mutations were detected in 7 AA-resistant patients. In patients (n=30) that switched from AA onto ENZ, AR gene amplification and or exon 8 mutations in CTCs predicted adverse outcomes including lower rates of PSA decline ≥ 30% (P=0.013, χ2) and shorter time to radiographic/clinical progression (p < 0.010) [60]. The ability of DHT to enhance CRPC cell growth regardless of whether the AR is expressed has been reported in a mechanism that involves activation of STAT-5 via the hijacking of the glucocorticoid receptor (GR) [61]. Preclinical prostate cancer models also point to the possibility that ENZ resistance may be mediated by the GR [62, 63]. These results are provocative since AA is often co-administered with prednisone. However, the immediate benefit of this drug combination in CRPC may not support this assertion, [64]. It is conceivable that the long term administration of a prednisone could make the tumor more dependent on the GR or act as ligand for the mutated AR.
3. 0. Biomarkers of Drug Resistance
Non-invasive biomarkers of AA and ENZ drug resistance would be ideal for precision therapy of CRPC. Thus far attention has focused on CTCs as a source of biomarkers. While CTCs report the expression of AR-SVs [57], they have not been routinely examined for expression changes in steroidogenic enzymes that may contribute to drug resistance. As yet the ability of CTC derived biomarkers to have sufficient specificity and sensitivity to predict prognostic outcome has yet to be demonstrated. Molecular profiling of tumor biopsies for transcript or protein levels of SLCO, SULT, HSD3B1, AKR1C3, HSD17B6, AR-SV could interrogate pathways in the androgen axis expected to cause drug resistance which would be actionable if therapeutics existed to act on each of these targets.
4. 0. Approaches to Counter Drug Resistance
4. 1. Combination Trials
Patients that progress on AA or ENZ could be switched to the other drug to prolong survival. However, patient experience with sequential therapy of this type has shown limited benefit and likely results from overlapping mechanisms of resistance shared by each agent [65]. An alternative would be to give both AA and ENZ concurrently and clinical trials are ongoing to determine whether there is additional benefit from such a combination. However, drug resistance is still anticipated to emerge. Thus there is a need for better approaches to counter the drug resistance phenotype.
4. 2. Sulfatase Inhibitors
If DHEA-SO4 contributes to the drug resistance phenotype this could be surmounted by the administration of a STS inhibitor 667 COUMATE (Irosustat) or STX213 [66]. Additionally, EM-1913 has been found effective in blocking 80% of the growth of the rat ventral prostate when stimulated with DHEA-SO4 [67]. Although this approach appears promising, incomplete inhibition of P450c17 still results in a significant amount of free DHEA and Δ4-AD that would be available for the synthesis of potent androgens.
4. 3. Bifunctional P450c 17 inhibitors and AR Antagonists/Degraders
New second generation inhibitors of P450c17 e.g. galaterone inhibit the 17/20-lyase activity and also antagonize and degrade the AR T877A mutant [68]. By targeting both P45017c and AR these agents could supersede combination AA and ENZ therapy and target resistance to either therapy and shows promise.
4.4. AKR1C3 Inhibitors
Based on the central role of AKR1C3 in intratumoral androgen biosynthesis and its up-regulation by ADT a series of AKR1C3 inhibitors have been developed based on repurposing non-steroidal anti-inflammatory drugs (NSAIDs) [69-72]. Three series of compounds have been reported and include: N-phenylaminobenzoates (which are fenamate derivatives): N-naphthylaminobenzoates; and N-benzoyl-indoles (which are derived from indomethacin). Lead compounds in each library no longer inhibit the NSAID target COX-1 or COX-2. Each of the leads have nanomolar potency for AKR1C3 and are effective in cell-based models in blocking the conversion of Δ4-AD to T in LNCaP-AKR1C3 cells, and block the production of PSA expression driven by the AKR1C3 substrate Δ4-AD in VCaP cells [4]. The N-naphthylaminobenzoates have the added advantage that they also act as AR antagonists and represent “first-in-class” bifunctional AKR1C3 inhibitors and AR antagonists [73]. Recently, CD-2B cells, a subline of LNCaP cells were grown in the presence of ENZ to generate a ENZ resistant cell line (C4-2B MDVR). This cell line over expressed AKR1C3 40-fold when compared to the control cell line, and this difference was observed at the mRNA and protein levels. In addition the C4-2B MDVR cells produced large amounts of T (131 pg per 5 × 106 cells) and DHT (18 pg per 5 × 106 cells) versus the parental cells which produced (0.15 pg T per 5 × 106 cells; and 0 pg DHT per 5 × 106 cells); and grew in the presence of ENZ. Indomethacin an AKR1C3 inhibitor was able to arrest the growth of these cells in cologenic assays. Xenografts of CW22Rv1 cells which are also ENZ resistant and express AKR1C3 gave rise to tumors in castrate SCID mice which were not responsive to ENZ but could be prevented from growing in the presence of indomethacin. These experiments provide important in vivo “proof-of principle” to use an AKR1C3 inhibitor to surmount ENZ drug resistance [41].
Both GTx-therapeutics and Astellas have developed AKR1C3 inhibitors as potential therapeutics for CRPC. GTX-560, a potent competitive inhibitor of AKR1C3, revealed for the first time that this compound could block the hitherto unrecognized AR co-activator function of AKR1C3 suggesting that the compound would have added benefit in the treatment of CRPC patients [74]. GTx-560 was also found to have antitumor activity in prostate cancer cell xenograft models of CRPC. Astellas took their lead compound ASP9521 into man following favorable preclinical development, including absorption, distribution, metabolism, elimination and toxicological properties, and in vivo proof-of-principle studies to block tumor growth in prostate cancer cell xenograft models [75]. The compound was found to be orally bioavailable and well tolerated but without efficacy where the clinical endpoint was a decrease in serum PSA in mCPRC patients [76]. However, the drug was inadequately tested, 6/13 mCRPC patients withdrew from the trial and patients were not preselected for AKR1C3 status and there was no measurement of intratumoral androgens. The authors also concluded that the lack of effect might have been due to the possibility that AKR1C3 was not expressed at a higher enough level to see a beneficial effect since the patients had not received second line ADT.
4.5. Coactivator Competitive peptides
An entirely novel approach would be to annotate the co-regulators that interact with the AR-SVs in CRPC and then develop peptido-mimetics that could compete for the co-regulatory domain of the AR-SV. Progress in this area has been limited to date [77], but some “proof -of-principle” using peptides to disrupt the interaction between p160 and AR in castration-resistant AR expressing prostate cancer cells has been reported [78].
Conclusions
Drug resistance to the standard treatment-of-care for mCRPC involving AA or ENZ represents a a major clinical problem. Co-administration of both drugs is likely to prolong survival but drug resistance will prevail. Intrinsic drug resistance results from allelic variants in SLCO and HSD3B1. Acquired drug resistance involves adaptive changes in the androgen axis and several mechanisms may combine to exacerbate drug resistance e.g. the incomplete inhibition of P450c17 coupled with the overexpression of AKR1C3 may create a “perfect-storm” so that intratumoral androgen biosynthesis can occur. There is an urgent need for new agents that do not target P450c17 or the AR with a focus on targeting the mechanisms of therapeutic drug resistance. Promising approaches include the development of HSD3B1 and AKR1C3 inhibitors and peptido-mimetics that could disrupt the interaction between AR-FL, AR-SV’s with their steroid receptor co-regulators.
Publication Highlights.
Abiraterone and enzalutamide in castration resistant prostate cancer leads to drug resistance
Intrinsic drug resistance arises due to allelic variants in organic anion transporters and HSD3B1
Acquired drug resistance arises due to intratumoral androgen biosynthesis and changes in the AR
Acquired resistance can involve the induction of AKR1C3 and emergence of AR-splice variants
AKR1C3 inhibitors or peptidomimetics that block AR-splice variants may surmount drug resistance
Acknowledgements
This article was written with support from P30-ES013508 and P01-CA163227-03 awarded to TMP.
Abbreviations
- ADMET
absorption distribution, metabolism: elimination and toxicology
- Δ4-AD
4-androstene-3,17-dione
- Δ5-Adiol
5-androstene-3β,17β-diol
- 3α-Adiol
5α-androstane-3α,17β-diol
- Adione
5α-androstane-3,17-dione
- ADT
androgen deprivation therapy (ADT)
- AR
androgen receptor
- AR-FL
androgen receptor full length
- AR-SV
androgen receptor splice variant
- CRPC
castration resistant prostate cancer
- DHEA
dehydroepiandrosterone
- DHT
5α-dihydrotestosterone
- 3α-diol
5α-androstane-3α,17β-diol
- HSD
hydroxysteroid dehydrogenase
- SLCO
solute carrier organic anion transporter
- STS
steroid sulfatase
- T
testosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].SEER, Surveilance, Epidemiology, and End Results Program. 2014 http://seer.cancer.gov/statfacts/html/prost.html.
- [2].Sharifi R, Bruskewitz RC, Gittleman MC, Graham SD, Jr., Hudson PB, Stein B. Leuprolide acetate 22.5 mg 12-week depot formulation in the treatment of patients with advanced prostate cancer. Clin. Ther. 1996;18:647–657. doi: 10.1016/s0149-2918(96)80215-3. [DOI] [PubMed] [Google Scholar]
- [3].Knudsen K, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 2010;21:315–324. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin. Cancer. Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- [5].Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Chen S, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Knuuttila M, Yatkin E, Kallio J, Savolainen S, Laajala TD, Aittokallio T, Oksala R, Häkkinen M, Keski-Rahkonen P, Auriola S, Poutanen M, Mäkelä S. Castration induces up-regulation of intratumoral androgen biosynthesis and androgen receptor expression in an orthotopic VCaP human prostate cancer xenograft model. Am. J. Pathol. 2014;184:2163–2173. doi: 10.1016/j.ajpath.2014.04.010. [DOI] [PubMed] [Google Scholar]
- [7].Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, Nelson CC, Guns ES, Wasan KM. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- [8].Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- [9].Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higian CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fenton MA, Shuster TD, Fertig AM, Taplin ME, Kolvenbag G, Bubley GJ, Balk SP. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin. Cancer Res. 1997;3:1383–1388. [PubMed] [Google Scholar]
- [11].Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of the androgen receptor: a possible mechanism of bicalutamide withdrawal. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- [12].Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium., Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shi X-B, Ma A-H, Xia L, Kung H-J, de Vere White RW. Mutational analysis of 44 mutant androgen receptors from human prostate cancer. Cancer Res. 2002;62:1496–1502. [PubMed] [Google Scholar]
- [14].Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- [15].Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- [16].Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinänen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- [17].Attard G, Reid AHM, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martinins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase 1 clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confrims that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 2008;28:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- [18].Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2009;27:3742–37482. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- [20].Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beer TM, Armstrong AJ, Rathkopf DE, Loroit Y, Sternberg CN, Higano CS, Iverson P, Bhattacharya S, Carles J, Chowdury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim C-S, Kimura G, Mainwairng P, Mansbach H, Miller K, Nonnberg SB, Perabo F, Phung D, Sadd F, Scher HI, Taplin M-E, Venner PM, Tombal B, PREVAIL Investigators Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl. J. Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fizazi K, Scher I, Malina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, Mianwairing P, Harland S, Goodman OB, Jr., Sternberg CN, Li JH, Kehoh T, Haqq CM, de Bono JS, COU-AA-301 Investigators Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- [24].Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaus F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J. Clin. Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ryan CJ, Molina A, Li J, Khoh T, Small EJ, Haqq CM, Grant RP, de Bono JS, Scher HI. Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: Results from an analysis of a randomized Phase III trial. J. Clin. Oncol. 2013;31:2791–2798. doi: 10.1200/JCO.2012.45.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, Sanda MG, Davis JW, Loda M, True LD, Troncoso P, Ye H, Lis RT, Marck BT, Matsumoto AM, Balk SP, Mostaghel EA, Penning TM, Nelson PS, Xie W, Jiang Z, Haqq CM, Tamae D, Tran N, Peng W, Kheoh T, Molina A, Kantoff PW. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, Arezzo JC, Powlin SS, Dinchuk JE, Balog A, Salvati ME, Attar RM, Gottardis MM. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71:480–488. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- [28].Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, Aparicio A, Dorow S, Arora V, Shao G, Qian J, Zhao H, Yang G, Cao C, Sensintaffar J, Wasielewska T, Herbert MR, Bonnefous C, Darimont B, Scher HI, Smith-Jones P, Klang M, Smith ND, De Stanchina E, Wu N, Ouerfelli O, Rix PJ, Heyman RA, Jung ME, Sawyers CL, Hager JH. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- [31].Cho E, Montgomery RB, Mostaghel EA. Minireview: SLCO and ABC transporters: A role for steroid transport in prostate cancer progression. Endocrinology. 2014;155:4124–4132. doi: 10.1210/en.2014-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, Pomerantz M, Freedman M, Ross R, Regan M, Sharifi N, Figg WD, Balk S, Brown M, Taplin ME, Oh WK, Lee GS, Kantoff PW. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2011;29:2565–2573. doi: 10.1200/JCO.2010.31.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chang K-H, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson R, Kapur P, Guo X, Mirzaei H, Auchus RJ, Sharifi N. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, Namiki M, Kawai K, Tamai I. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem. Pharmacol. 2012;84:1070–1077. doi: 10.1016/j.bcp.2012.07.026. [DOI] [PubMed] [Google Scholar]
- [35].Fung K-M, Shea-Samara EH, Wong C, Krin R, Jones AM, Bane B, Liu CZ, Yang JT, Pitha JV, Culkin DJ, Koop BP, Penning TM, Lin H-K. Increased expression of type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with the androgen receptor in prostate carcinoma. Endocr. Related Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- [36].Lin H-K, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol. Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- [37].Penning TM, Burczynski ME, Jez JM, Hung C-F, Lin H-K, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Peehl DM, Fung K-M, Lin HK. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol. Cell. Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [39].Hamid AR, Pfeiffer MJ, Verhaegh GW, Schaafsma E, Brandt A, Sweep FC, Sedelaar JP, Schalken JA. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol. Med. 2012;18:1449–1455. doi: 10.2119/molmed.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hofland J, van Weerden WM, Dits NFJ, Steenbergen J, van Leenders GJLH, Jenster G, Schroder FH, de Jomg FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- [41].Liu C, Lou W, Zhu Y, Yang JC, Natiminty N, Gaikwad N, Evans CP, Gao AC. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015 Feb 3;:pii. doi: 10.1158/0008-5472.CAN-14-3080. canres.3080.2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pfeiffer MJ, Smit FP, Sedelaar JP, Schalken JA. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol Med. 2011;17:657–664. doi: 10.2119/molmed.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tian Y, Zhao L, Zhang H, Liu X, Zhao L, Zhao X, Li J. AKR1C3 overexpression may serve as a promising biomarker for prostate cancer progression. Diagnostic Pathol. 2014;9:42–48. doi: 10.1186/1746-1596-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, Fleisher M, Sander C, Sawyers CL, Scher HI. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–6152. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Powell K, Semann L, LaComb MK, Asangani I, Wu Y-M, Ginsburg KB, Williams J, Squire JA, Maddipati KR, Cher ML, Chinni SR. ERG/AKR1C3/AR constitutes a feed-forward loop for AR signaling in prostate cancer cells. Clinical Cancer Res. 2015 Mar 9; doi: 10.1158/1078-0432.CCR-14-2352. DOI: 10.1158/1078-0432. CCR-14-2352 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- [47].Jernberg E, Thysell E, Ylitalo EB, Rudolfsson S, Crnalic S, Widmark A, Bergh A, Wikstrom P. Characterization of the prostate cancer bone metastases according to expression levels of steroidogenic enzymes and androgen receptor splice variants. PLOS One. 2013;8:e77407. doi: 10.1371/journal.pone.0077407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone. A potential therapeutic target for androgen dependent disease. Mol. Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- [49].Mohler JL, Titus MA, Bai S, Kennerley BJ, Lih FB, Tomer KB, Wilson EM. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res. 2011;71:1486–1496. doi: 10.1158/0008-5472.CAN-10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mohler JL, Titus MA, Wilson EM. Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone. Clin. Cancer Res. 2011;17:5844–5849. doi: 10.1158/1078-0432.CCR-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ishizaki F, Nishiyama T, Kawasaki T, Miyashiro Y, Hara N, Takizawa I, Naito M, Takahashi K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013;3:1528. doi: 10.1038/srep01528. doi: 1510.1038/srep01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castrate-resistance prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sprenger CCT, Plymate SR. The link between androgen receptor splice variants and castration-resistant prostate cancer. Hormones & Cancer. 2014;5:207–2014. doi: 10.1007/s12672-014-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cao B, Qi Y, Zhang G, Xu D, Zhan Y, Alvarez X, Guo Z, Fu X, Plymate SR, Sartor O, Zhang H, Dong Y. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014;5:1646–1656. doi: 10.18632/oncotarget.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, Edwards J, Isaacs WB, Nelson PS, Bluemn E, Plymate SR, Luo J. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, Cai C, Balk SP. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, Monahan JE, Stegmeier F, Roberts TM, Sellers WR, Zhou W, Zhu P. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Disc. 2013;3:1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- [60].Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, Anderson S, McConeghy B, Shukin R, Bazov J, Youngren J, Paris PL, Thomas GV, Small EJ, Wang Y, Gleave ME, Collins CC, Chi KN, clincanres.2666.2014. [Epub ahead of print] Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2015 Feb 23;:pii. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- [61].Song C, Kim Y, Min GE, Ahn H. Dihydrotestosterone enhances castration-resistant prostate cancer cell proliferation through STAT5 activation via glucocorticoid receptor pathway. Prostate. 2014;74:1240–1248. doi: 10.1002/pros.22841. [DOI] [PubMed] [Google Scholar]
- [62].Aora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Sah N, Cai L, Efstathiou E, Logothetis C, Zhang D, Sawyers CL. Glucocorticoid receptor confers resistance to anti-androgens by by-passing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sharifi N. Steroid receptors aplenty in prostate cancer. N. Engl. J. Med. 2014;370:970–971. doi: 10.1056/NEJMcibr1315706. [DOI] [PubMed] [Google Scholar]
- [64].Sartor O, Parker CC, de Bono J. Reappraisal of glucocorticoids in castrate-resistant prostate cancer. Asian J. Androl. 2014;16 doi: 10.4103/1008-682X.133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cheng HH, Gulati R, Azad A, Nadal R, Twardowski P, Vaishampayan UN, Agarwal N, Heath EI, Pal SK, Rehman HT, Leiter A, Batten JA, Montgomery RB, Galsky MD, Antonarakis ES, Chi KN, Yu EY. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015 Jan 20; doi: 10.1038/pcan.2014.53. doi: 10.1038/pcan.2014.53. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Purohit A, Foster PA. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. Journal of Endocrinology. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]
- [67].Roy J, Lefebvre J, Maltais R, Poirier D. Inhibition of dehydroepiandosterone sulfate action in androgen-sensitive tissues by EM-1913, an inhibitor of steroid sulfatase. Mol. Cell. Endocrinol. 2013;376:148–155. doi: 10.1016/j.mce.2013.06.022. [DOI] [PubMed] [Google Scholar]
- [68].Yu Z, Cai C, Gao S, Simon NI, Shen HC, Balk SP. Galeterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptor. Clin. Cancer Res. 2014;20:4075–4085. doi: 10.1158/1078-0432.CCR-14-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Adeniji AO, Twenter BM, Byrns MC, Jin Y, Winkler JD, Penning TM. Discovery of substituted 3-(phenylamino)benzoic acids as potent and selective inhibitors of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) Bioorg. Med. Chem. Lett. 2011;21:1464–1468. doi: 10.1016/j.bmcl.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Adeniji AO, Twenter BM, Byrns MC, Jin Y, Chen M, Winkler JD, Penning TM. Development of potent and selective inhibitors of aldo-keto reductase 1C3 (type 5 17β-hydroxysteroid dehydrogenase) based on N-phenyl-aminobenzoates and their structure-activity relationships. J. Med. Chem. 2012;55:2311–2323. doi: 10.1021/jm201547v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue N-(4-chlorobenzoyl)melatonin is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3α-HSD, type 5 17β-HSD and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem. Pharmacol. 2008;75:484–493. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, Marnett LJ, Penning TM. Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem. 2013;56:2429–2446. doi: 10.1021/jm3017656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen M, Adeniji AO, Twenter BM, Winkler JD, Christianson DW, Penning TM. Crystal structures of AKR1C3 containing an N-(aryl)amino-benzoate inhibitor and a bifunctional AKR1C3 inhibitor and androgen receptor antagonist. Therapeutic leads for castrate resistant prostate cancer. Bioorg. Med. Chem. Lett. 2012;22:3492–3497. doi: 10.1016/j.bmcl.2012.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yepuru M, Wu Z, Kyulkarni A, Yin F, Barrett CM, Kim J, Steiner MS, Miller DD, Dalton JT, Narayanan R. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin. Cancer Res. 2013;19:5613–5625. doi: 10.1158/1078-0432.CCR-13-1151. [DOI] [PubMed] [Google Scholar]
- [75].Kikuchi A, Furutani T, Azami H, Watanbe K, Nimi T, Kamiyama Y, Kuromitsu S, Bakin-Bey E, Heeringa M, Uoatas T, Enjo K. In vitro and in vivo characterization of ASP9521: a novel selective, orally bioavailable inhibitor of 17β-hydroxysteroid dehydrogenase type 5 (17β-HSD5; AKR1C3) Invest. New, Drugs. 2014;32:860–870. doi: 10.1007/s10637-014-0130-5. [DOI] [PubMed] [Google Scholar]
- [76].Loriot Y, Fizazi K, Jones RJ, Brand Van den J., Molife RL, Omlin A, James ND, Baskin-Bey E, Heeringa M, Baron B, Holtkamp GM, Ouatas T, de Bono JS. Safety, tolerability and anti-tumor activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest New Drugs. 2014;32:995–1004. doi: 10.1007/s10637-014-0101-x. [DOI] [PubMed] [Google Scholar]
- [77].Mostaghel EA, Plymate SR, Montgomery B. Molecular pathways: targeting resistance in the androgen receptor for therapeutic benefit. Clin. Cancer Res. 2014;20:791–798. doi: 10.1158/1078-0432.CCR-12-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nakka M, Agoulnik IU, Weigel NL. Targeted disruption of the p160 coactivator interface of androgen receptor (AR) selectively inhibits AR activity in both androgen-dependent and castration-resistant AR-expressing prostate cancer cells. Int J Biochem Cell Biol. 2013;45:763–772. doi: 10.1016/j.biocel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fankhauser M, Tan Y, Macintyre G, Haviv I, Hong MKH, Nguyen A, Pederson JS, Costello AJ, Hovens CM, Corcoran NM. Canoncial androstenedione reduction is the predominat source of signaling androgens in hormone-refractory prostate cancer. Clin. Cancer Res. 2014;20:5547–5557. doi: 10.1158/1078-0432.CCR-13-3483. [DOI] [PubMed] [Google Scholar]