Abstract
Sleep has been shown to improve the retention of newly learned words. However, most methodologies have used artificial or foreign language stimuli, with learning limited to word/novel word or word/image pairs. Such stimuli differ from many word-learning scenarios in which definition strings are learned with novel words. Thus, we examined sleep's benefit on learning new words within a native language by using very low frequency words. Participants learned 45 low-frequency English words and, at subsequent recall, attempted to recall the words when given the corresponding definitions. Participants either learned in the morning with recall in the evening (wake group), or learned in the evening with recall the following morning (sleep group). Performance change across the delay was significantly better in the sleep than the wake group. Additionally, the Levenshtein Distance, a measure of correctness of the typed word compared to the target word, became significantly worse following wake, whereas sleep protected correctness of recall. Polysomnographic data from a subsample of participants suggested that REM sleep may be particularly important for this benefit. These results lend further support for sleep's function on semantic learning even for word/definition pairs within a native language.
Keywords: Language, consolidation, sleep, declarative memory
Introduction
Learning the meaning of new words is an important aspect of human language. For children and adolescents, the ability to remember the definition of newly learned words is one of the greatest determinants of academic achievement (Marzano, 2004). Word learning is also important for achievement in adults. For instance, understanding scientific literature requires a rapidly expanding lexicon (Hayes, 1992). Gaining a better understanding of how novel words are learned and how this skill can be improved could enhance performance in a range of disciplines.
Sleep has been shown to benefit memory for declarative information. Following learning of semantically related (Plihal & Born, 2007) or unrelated (Wilson, Baran, Pace-Schott, Ivry & Spencer, 2012) word-pairs, for example, memory after a delay including sleep is maintained to a greater extent than memory following an equivalent period of wake. This performance benefit from sleep is thought to reflect the stabilization of labile memories and the transfer of information from short-term hippocampal stores to more long-term cortical storage, termed sleep-dependent memory consolidation (see reviews: Spencer, 2013; Marshall & Born, 2007).
There is evidence to suggest that sleep may play a role in novel word learning. Native English speaking high school students, with no prior knowledge of the German language, were better able to learn English-German vocabulary word pairs when sleep occurred within a few hours of encoding. Comparatively, when more than 14 hours of daytime wake separated learning and recall, performance was significantly reduced (Gais, Lucas & Born, 2006). Likewise, following a nap, preschool children were better able to identify novel objects named as nonsense words in a children's book. Importantly, this nap-related improvement in word retention was still present seven days later, demonstrating the long-term benefits of sleep (Williams & Horst, 2014).
In adults, nocturnal sleep has been shown to improve the perceptual learning of synthetic speech. Fenn and colleagues (2003) used naturalistic, computer-generated speech stimuli. Participants listened to a list of these monosyllabic consonant-vowel-consonant words while observing the printed version of the word on screen. Following training, participants were tested on their ability to recognize a new list of words, demonstrating generalization of phonological categories. Following a 12-hour period that included sleep, individuals had significantly greater accuracy during the post-test compared to a 12-hour period of wake. Importantly, sleep may play a specific role in the generalization of phonological categories that is distinct from the role of sleep on veridical learning. Fifteen-month old children exposed to an artificial language showed no significant differences on veridical recall following a nap or following subsequent wake. However, following a nap, children showed greater abstraction and generalization of language rules to novel stimuli when compared to wake (Gómez, Bootzin & Nadel, 2006).
Conversely, Dumay and Gaskell (2007) showed that sleep not only contributes to veridical recall of artificial words, but also to the incorporation of the newly learned words into long-term lexical memories. Using fictitious words that shared syllables with known English words, inhibitory lexical competition could be assessed. For example, if the fictitious word “cathedruke” became part of an individual's lexicon, it should compete with, and slow the recognition of, the known word “cathedral,” its phonological counterpart. Lexical competition only occurred following a period with overnight sleep, suggesting sleep is necessary to incorporate newly learned words into the lexicon in long-term cortical storage. This effect was replicated using functional magnetic resonance imaging (fMRI) to assess the cortical underpinnings of the lexical integration of novel words (Davis, Di Betta, Macdonald & Gaskell, 2009). Increased hippocampal activation during the learning of fictitious words correlated with post-learning retention. Additionally, following a period of sleep, cortical activation associated with fictitious word recall was not significantly different from activation associated with the English words already a part of the lexicon. Comparatively, additional training through repeated exposure to novel words, without subsequent sleep, had little effect on the cortical activation generated by the word presentations. This suggests that a two-stage process of declarative learning is at play: Newly learned words are first stored in the hippocampus and are subsequently transferred to long-term storage in the neocortex, where they are incorporated into the lexicon. This shift in storage is enhanced during periods of sleep.
Although these studies provide reason to believe that consolidation of novel word learning may be sleep-dependent, studies to date have relied on non-native language stimuli (e.g., artificial language, foreign language). Using a non-native language for novel word learning paradigms may be unique from learning within one's language. First, non-native language stimuli do not allow for the individual to use their native syntax or phonology in the process. Second, while second-language learning recruits a similar core of neural structures (see review: Perani & Abutalebi, 2005), it draws on an additional unique neural network. Using fMRI, non-native German speakers were shown to use regions of the frontotemporal language network differently from native speakers during a German sentence comprehension task (Rüschemeyer, Fiebach, Kempe & Friederici, 2005). Even in bilingual individuals, the neurological responses to the second learned language are influenced by the strategies of phonological processing of their native language. This was demonstrated in Chinese-English bilinguals, in which the two languages differ dramatically in both phonology and orthography making this an inefficient strategy (Tan et al., 2003).
Artificial languages are often used in an attempt to control for varying linguistic properties of different natural languages. For example, Fenn and colleagues (2003) designed a list of computer-generated words to approximate the distribution of phonemes within the English language in order to make the synthetic speech stimuli more representative of natural language. In the Gaskell studies, the artificial words share syllables with true English words (Davis et al., 2009; Dumay & Gaskell, 2007). However, these artificial words are not given a definition or a context in which the meaning of the words can be derived. Therefore, these paradigms do not approximate the way in which novel words and their meanings are learned within one's language. Given that the intention of language is to communicate meaning, it has been suggested that lexical entry without meaning may qualitatively differ from that with meaning (Leach & Samuel, 2007; Tamminen et al., 2010; Lindsay & Gaskell, 2013; Tamminen & Gaskell, 2013). For example, Leach and Samuel (2007) demonstrate that learning novel artificial words within the context of a story or alongside a pictorial representation produces stronger lexical engagement (the ways in which a lexical entry interacts with the existing lexicon) than those from a phoneme-monitoring paradigm.
A recent study attempted to examine novel word learning in a more naturalistic setting in children (ages 5-9 years; Henderson, Weighall & Gaskell, 2013). Henderson and colleagues taught children real but novel scientific spoken words either with semantic information (e.g., “A hippocampus is a part of your brain that helps you remember things.”) or in a form-only condition (e.g., “Hippocampus has three Ps in it”). Regardless of whether semantic information was provided, both explicit recall of the words and lexical competition effects were observed 24 hours after initial training. Improvement was not affected by practice or repeated exposure to the stimuli. Thus, the authors suggest that the effects were likely due to offline consolidation, although they did not explicitly test for this. In addition, when semantic information was provided with the stimuli, explicit memory for the newly learned words was significantly better than the form-only condition one week later. This study indicated that a naturalistic method of providing semantic information during the learning of real novel words is important for long-term retention, and thus may be a valuable methodology.
The aim of this study was to examine the role of sleep in learning written novel English word/definition pairs in native English speaking adults. We used English words that are low frequency in studies of word usage (Balota et al., 2007; see Bell & Parfetti, 1994; Parfetti, 2007). Similar to previous work on the role of sleep on novel word learning (Fenn, Nusbaum and Margoliash & 2003; Dumay & Gaskell, 2007), performance was assessed following a 12-hour period of wake or a 12-hour period that contained overnight sleep. Individuals recalled the correct word when presented with the definition. This procedure further mimics the way in which newly learned words would be recalled naturally, in an attempt to convey a particular thought or opinion. It was predicted that sleep would improve the retention of novel English word-definition pairs, and that an equivalent period of wake would lead to forgetting.
Material and methods
Participants
Participants were 59 (41 female) young adults ranging from 18 to 30 yrs (M = 20.67 ± 2.37 yrs). Participants were required to be native English speakers who lack proficiency in any other language, operationally defined by having completed no more than 2 college-level foreign language courses. Participants were also required to have no history of neurological or sleep disorder, not be taking medication that could interfere with sleep, and sleep 6 or more hours per night on average. Participants received course credit for participation.
Stimuli
Stimuli were drawn from the English Lexicon Project database (Balota et al., 2007). Words were selected to be 2 standard deviations below the mean of the English Lexicon Project's standardized measure of word frequency (Hyperspace Analogue to Language (HAL) index; Lund & Burgess, 1996). The HAL Index norms are based on approximately 131 million words from over 3,000 sources collected in 1995. A low HAL index is indicative of low likelihood of the word co-occurring with others in the English language, in other words, infrequent use. From 60 selected words with a low HAL Index, informal surveys amongst undergraduate students narrowed the list to the least recognized within the target undergraduate student population. The final list was composed of 45 words (Mean HAL Index = 0.909; Mean Log HAL Index = 0.226), and word frequencies were all under 0.1 usages per million words according to the Corpus of Global Web-Based English (GloWbE; see Table 1). These words were matched with their respective definitions taken from the New Oxford American Dictionary (McKean, 2005). All definitions ranged in length from 3-14 words (median = 8 words). All words had 1-3 morphemes, and were either verbs (n=23) or nouns (n=22).
Table 1.
Stimulus words and definitions.
| Word | Frequency | Definition |
|---|---|---|
| abnegate (V) | 0.01 | Renounce or reject (something desired or valuable) |
| afflatus (N) | 0.05 | A divine creative impulse or inspiration |
| almoner (N) | 0.01 | An official distributor of alms |
| anchorite (N) | 0.04 | A religious recluse |
| animadvert (V) | <0.00 | Pass criticism or censure on; speak out against |
| assiduity (N) | 0.04 | Constant or close attention to what one is doing |
| bandbox (N) | 0.02 | Term for a lover of books |
| besmear (V) | 0.01 | Smear or cover with a greasy or sticky substance |
| bethink (V) | 0.04 | Think on reflection; come to think |
| calends (N) | 0.01 | The first day of the month in the ancient Roman calendar |
| calumniate (V) | 0.02 | Make false and defamatory statements about |
| cheroot (N) | 0.03 | A cigar with both ends open and untapered |
| contumacy (N) | 0.02 | Stubborn refusal to obey or comply with authority, especially a court order or summons |
| corbel (N) | 0.04 | A projection jutting out from a wall to support a structure above it |
| deliquesce (V) | <0.00 | (Of organic matter) become liquid, typically during decomposition |
| discommode (V) | <0.00 | Cause (someone) trouble or inconvenience |
| dissever (V) | <0.00 | Divide or sever (something) |
| excogitate (V) | 0.01 | Think out, plan, or devise |
| fructify (V) | 0.04 | Make (something) fruitful or productive |
| gimcrack (N) | 0.02 | A cheap and showy ornament; a knickknack |
| hawser (N) | 0.07 | A thick rope or cable for mooring or towing a ship |
| hearthrug (N) | 0.01 | A rug laid in front of a fireplace to protect the carpet or floor |
| immure (V) | 0.01 | Enclose or confine (someone) against their will |
| imprecate (V) | 0.01 | Utter (a curse) or invoke (evil) against someone or something |
| intermit (V) | 0.01 | Suspend or discontinue (an action or practice) for a time |
| layette (N) | 0.01 | A complete outfit of clothing and equipment for a newborn infant |
| manumit (V) | 0.02 | Release from slavery; set free |
| milksop (N) | 0.02 | A person who is indecisive and lacks courage |
| mullion (N) | 0.03 | A vertical bar between the panes of glass in a window |
| notecase (N) | <0.00 | A cardboard box, typically circular, for carrying hats |
| outface (V) | <0.00 | Disconcert or defeat (an opponent) by bold confrontation |
| packhorse (N) | 0.02 | A horse used to carry loads |
| pannikin (N) | 0.02 | A small metal drinking cup |
| prorogue (V) | 0.10 | Discontinue a session of (a parliament or other legislative assembly) without dissolving it |
| rehouse (V) | 0.05 | Provide (someone) with new housing |
| rollick (V) | 0.01 | Act or behave in a jovial and exuberant fashion |
| stropped (V) | 0.01 | Sharpen on or with a strop |
| suppurate (V) | <0.00 | Undergo the formation of pus; fester |
| tapster (N) | 0.01 | A person who draws and serves alcoholic drinks at a bar |
| throve (V) | 0.02 | (Of a child, animal, or plant) grow or develop well or vigorously |
| tintype (N) | 0.02 | A photograph taken as a positive on a thin tin plate |
| ululate (V) | 0.01 | Howl or wail as an expression of strong emotion, typically grief |
| unhorse (V) | 0.01 | Cause to fall from a horse |
| usurer (N) | 0.05 | A person who lends money at unreasonably high rates of interest |
| wineskin (N) | 0.04 | An animal skin sewn up and used to hold wine |
(N) = Noun, (V) = Verb, Frequency = per million words
Task
The task consisted of four phases: passive encoding, active encoding, immediate recall, and delayed recall. All stimuli were displayed on a computer screen and input was via keyboard responses. During passive encoding, participants were shown 45 word-definition pairs, one at a time, for 20 seconds. Immediately following, in the active encoding phase, participants were presented with a definition from the set, one at a time, and were asked to type in the associated word. If the entered word was incorrect, the correct word was displayed for 1 second in order to provide feedback. Participants continued to enter words until they reached a criterion of 62% correct responses or until they went through the stimuli list 4 times. During immediate and delayed recall, participants were again presented with the definition, and asked to recall its paired word. In these phases, however, no feedback was provided, and participants only went through the word-definition pairs once.
Procedure
All procedures were approved by the University of Massachusetts Institutional Review Board and written informed consent was obtained before the study commenced. The four experimental phases were spread across 2 sessions, with passive encoding, active encoding and immediate recall occurring during Session 1, and delayed recall occurring during Session 2. Participants were subdivided into two groups (Figure 1). The wake group started Session 1 in the morning (between 8-10 a.m.) with Session 2 occurring 12 hours later in the evening. The sleep group started in the evening (between 8-10 p.m.) with Session 2 occurring 12 hours later, the following morning.
Figure 1.
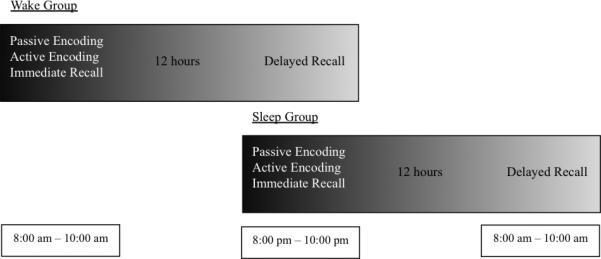
Procedural design demonstrating the difference between the sleep and wake groups.
Polysomnography (PSG) was recorded for 10 participants in the sleep group with the Aura PSG ambulatory system (Grass Technologies). The PSG montage included EOG (right and left ocular canthus), two chin EMG, and 6 cortical leads (F3, F4, C3, C4, O1, and O2) referenced to the left and right mastoids (A1 and A2). The PSG montage was applied approximately one hour before the participant's typical bedtime in the participant's place of residence.
All participants also completed a set of standardized questionnaires to assess sleep habits and chronotype. The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman & Kupfer, 1989) assessed habitual sleep habits over the last 30 days. The Epworth Sleepiness Scale (ESS; Johns, 1991) provided a measure of the participant's typical level of daytime sleepiness (Johns, 1992), whereas the Stanford Sleepiness Scale (SSS; Hoddes, Zarcone, Smythe, Phillips & Dement, 1973) provided a measure of the participant's level of sleepiness at the time of the experimental session. The Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976) assessed participant's chronotype. Lastly, a sleep survey provided information about the participant's sleep the night prior to the experimental day, as well as any potential daytime activities that could affect sleep (e.g. caffeine intake, exercise).
At the end of the second experimental session, participants were given the list of the 45 word stimuli and were asked to circle any words that they would have recognized prior to the experiment.
Analysis
Words that had been identified by participants as being familiar prior to the experiment were excluded from analyses. This was to prevent prior knowledge of the words from influencing performance on this task, and to ensure that we were, in fact, assessing novel word learning.
In order to determine the correctness of each response, a matching percentage based on the Levenshtein Distance between the correct word and the typed word was calculated. The Levenshtein Distance is used to determine the difference between two sequences through an insertion, deletion, or substitution (Levenshtein, 1966). For example, the distance between the words “bitten” and “sitting” would include two substitutions (“s” for “b”, and “i” for “e”), as well as one insertion (“g”). As such, the Levenshtein Distance number would be 3. This number is then used in an equation (LD = 100 – ((Levenshtein Distance number)*100)/MaxLength), which controls for the number of letters in the longer of the two words (e.g.: MaxLength = 7 for “sitting”), and turned into a matching percentage (57%; as opposed to the distance percentage of 43%). The matching percentage therefore represents the level of correctness of the typed word. Words with a matching percentage of 100% represent a Levenshtein Distance number of zero. Only words with a 100% Levenshtein Distance matching percentage (LD) were considered to be “correct”. The percentage of correct words remembered at each recall phase was calculated. Independent samples t-tests were used to determine whether there were any baseline differences in learning across the sleep and wake groups. Given that the primary interest of this study was the change in memory performance, a difference score was calculated by subtracting the immediate recall performance from the delayed recall performance. Independent-samples t-tests were used to compare the change in memory performance during the offline period between the sleep and the wake group.
We used LD for all items, regardless of whether they were correct or incorrect at immediate recall, to examine the change in memory accuracy over the delay. A difference score was then calculated, subtracting the LD of each word at immediate recall from the corresponding LD at delayed recall. A smaller number on this measure therefore reflects that the word recall has become more correct across the delay. For example, if an item was considered close (80%) at immediate recall, and then became completely correct (100%) across the delay, the resulting difference score would be −20, whereas a close (80%) word becoming completely incorrect (0%) across the delay would have a difference score of 80. Independent samples t-tests were used to compare the change in closeness ranking across the delay between the sleep and wake groups.
PSG data were analyzed using AASM recommended sleep staging procedures (Iber, Ancoli-Israel, Chesson & Quan, 2007). The PSG data of interest were the percent of the total sleep time spent in each sleep stage including rapid eye movement (REM) and non-REM (nREM) sleep stages 1-3 (with stage 3 termed slow wave sleep, SWS). Additionally, spectral analyses of EEG data were run using BrainVision Analyzer 2 software (Ver 2.4; Brain Products), with specific interest in delta and sigma power. Data were segmented into the sleep stage of interest (SWS for delta power, and nREM2 for sigma power). Data were then segmented into 4-second epochs for artifact rejection on individual channels, and a fast-Fourier transform was applied using a Hanning window with 10% overlap and utilizing covariance. A spectral power range of 0.5-4 Hz was used for delta power analyses, and a range of 12-16 Hz was used for nREM2 sigma power. Spectral analyses are reported in power density (μV2/Hz). The measures of sleep physiology, including percent time in each sleep stage, and delta and sigma power, were correlated with changes in memory performance across the sleep group using Pearson's correlations.
Results
Of the 59 recruited participants, 5 did not complete the experiment due to scheduling or technical issues, and one was excluded as a result of not fitting the criteria for foreign language exposure. Additionally, to ensure that information was adequately encoded, only individuals who achieved a score at immediate recall that was greater than or equal to one standard deviation below the mean (≥47.3%) were included, excluding 8 participants. As a result, 45 participants (35 female; wake group: n=19; sleep group: n=26) were included in the analyses.
The sleep and wake groups did not differ on measures related to sleep quality or sleep habits. There were no significant differences in chronotype (MEQ; t(39) = 0.023, p = 0.982), daytime sleepiness (ESS; t(39) = 0.519, p = 0.606), or global PSQI scores (t(39) = 0.16, p = 0.987). Additionally, there were no differences in self-reported overnight sleep time the night prior to the experiment (t(32) = −0.792, p = 0.434), nor in their level of sleepiness at the time of the experiment for either the first (SSS; t(39) = 1.379, p = 0.176) or second session (t(39) = 1.393, p = 0.172).
On average, 3.89 (SD = 4.84) words were removed from the analysis due to participant-reported familiarity with the words prior to the experiment. Importantly, the number of familiar words removed did not differ between the sleep group (M = 4.06, SD = 5.02) and the wake group (M = 3.62, SD = 4.67; t(51) = 0.323, p = 0.748).
Importantly, accuracy (percentage correct) at immediate recall did not differ for the sleep (62.65% ± 1.90) and wake groups (66.79% ± 1.95; t(43) = 1.44, p = 0.144), indicating that performance of the groups was equivalent at baseline. Of interest in the present study was the change in performance over intervals with sleep and wake.1 The change in performance across the delay, using the difference score described above, elucidates this relationship. There was a significant difference between the sleep and wake groups on this measure (t(43) = 5.061, p < 0.001; Figure 2). The wake group forgot a significant percentage of the words across the delay (M = −5.73, SD = 5.41), whereas there was no forgetting evident in the performance of the sleep group (M = 1.20, SD =3.78).
Figure 2.
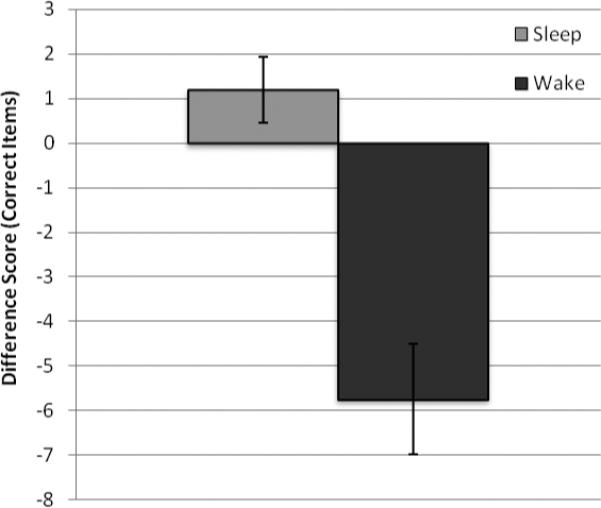
Group difference scores for correct items. The difference score was calculated as the immediate recall score subtracted from the delayed recall score. Negative scores indicate forgetting compared to immediate recall. Across the delay period, the sleep group performed significantly better than the wake group (p < 0.001). Error bars reflect the standard error.
Additionally, we examined whether accuracy of the typed responses improved over the delay period using all LD scores. The sleep and wake groups significantly differed on their change in LD across the delay (t(43) = −3.410, p = 0.001; Figure 3). Items became less close to the correct items over a 12-hour period of wake; across sleep, items maintained their LDs.
Figure 3.

Group difference scores for LD. The difference scores were calculated as the immediate recall score subtracted from the delayed recall score. Negative scores reflect becoming more close to the correct word. Across the delay period, the sleep group performed significantly better than the wake group (p = 0.001). Error bars reflect the standard error.
Lastly, difference scores for both the change in percent correct and the mean change in LD were correlated with sleep physiology measures. The change in performance in either measure over the sleep interval was not associated with any particular sleep parameter (Table 2). There were also no significant correlations between change in LD and sleep parameters, however a trend was observed: the percentage of REM sleep trended towards an association with change in LD (r = −0.564, p = 0.09; Figure 4). As more negative LD change scores reflect becoming more similar to the correct word, greater REM sleep is associated with improving recall of words. It is important to note however that there was a very small sample (n=10) in which sleep physiology was collected. Therefore, these results should be considered preliminary.
Table 2.
Measures of sleep physiology.
| Mean (SD) | p (correct) | p (LD) | |
|---|---|---|---|
| TST (min) | 380.55 (92.25) | .202 | .335 |
| nREM1(%) | 9.18 (1.71) | .402 | .569 |
| nREM2 (%) | 54.06 (11.83) | .871 | .147 |
| SWS (%) | 20.18 (6.61) | .989 | .400 |
| REM (%) | 16.57 (6.21) | .941 | .090# |
| Delta on frontal band (F3; μV2/Hz) | 168.84 (69.83) | .312 | .804 |
| Delta on central band (C3; μV2/Hz) | 152.44 (107.35) | .109 | .848 |
| Sigma on frontal band (F3; μV2/Hz) | 3.66 (1.57) | .800 | .482 |
| Sigma on central band (C3; μV2/Hz) | 2.57 (0.78) | .701 | .250 |
TST, total sleep time; nREM, non-rapid eye movement sleep, SWS, slow wave sleep; REM, rapid eye movement. The p values correspond to Pearson's bivariate correlations with difference scores for the correct items (p(correct)) and for the LD (p(LD)).
trend value.
Figure 4.
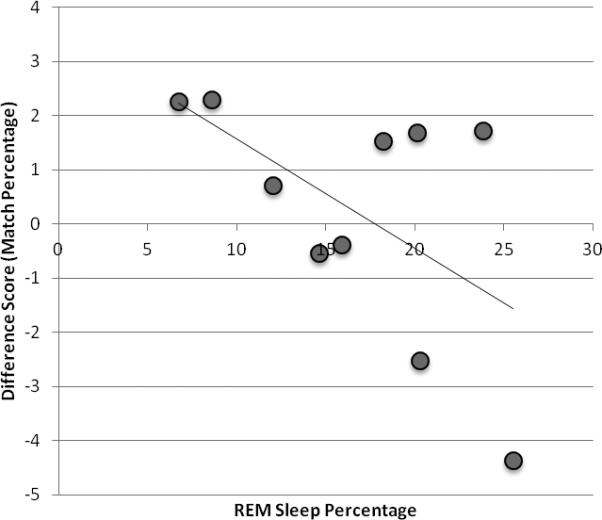
Correlations between difference score of LD and the percentage of REM sleep. The difference scores were calculated as the immediate recall score subtracted from the delayed recall score. Negative scores reflect becoming more close to the correct word. Across sleep, more improved word recall was associated with greater percentage of REM sleep (p = 0.09).
Discussion
The results of this study demonstrate that sleep is beneficial for retention of novel words. Across a 12-hour delay that included sleep, individuals were significantly better at recalling novel words than when the 12-hour delay was spent awake. This suggests that sleep is important for the retention of newly learned word/definition pairs, whereas across wake, forgetting occurs. Additionally, using the LD measure, we found that sleep was associated with protection of the accuracy of recalled word, whereas waking lead to a reduction in word accuracy.
We considered the possibility that factors other than sleep led to the change in accuracy across the delay period. Circadian differences in learning could be a factor considering that recall occurred either in the morning (sleep group) or in the evening (wake group). Perhaps recall was optimal when performed in the morning. However, counter to this alternative, there were no baseline differences in accuracy. Alternatively, it could be that the wake group is more fatigued at recall than the sleep group; however, there were no differences between groups in self-reported levels of sleepiness at either learning or recall, and there were no significant differences across groups for morning or evening chronotypes.
It is also possible that sleep played only a passive role in word retention, with the significant effects driven by daytime waking interference causing forgetting for the wake group. This possibility has been examined in multiple research paradigms (see review: Ellenbogen, Payne & Stickgold, 2006). As there was a relationship, albeit only trending, between REM sleep and behavioral outcomes on this task, our results favor an active process of sleep-dependent memory consolidation. It is also worth noting that participants in the sleep group slept for only half of the 12-hour intersession interval; consequently, there was a large opportunity for waking interference in the sleep group as well. Therefore, this does not seem a viable explanation for the observed results.
Previous literature indicates an association between correctly remembered declarative memories and SWS (Gais & Born, 2004; Peigneux et al., 2004; Plihal & Born, 2007; Rasch, Büchel, Gais & Born, 2007) as well as delta power (Wamsley, Tucker, Payne & Stickgold, 2010). However, we found a trending relationship with REM sleep percentage and performance on this task, specifically, improvement in LD. While generally agreed upon that SWS consolidates explicit representations of daytime declarative activities (Ackermann & Rasch, 2014), REM sleep is thought to play a role in abstraction or generalization (Spencer, 2013). While the change in LD does not represent generalization per se, it may reflect a similar process. During sleep, memory traces that are “close” to correct may be compared to other similar words in the individual's lexicon. Abstracting common phonological themes or patterns in native syntax, these close words could be shaped into more correct forms. Importantly, however, this result must be taken with caution given the small sample size.
A previous study demonstrated the role of sleep spindle activity on novel word lexical integration (Tamminen et al., 2010). Sigma power largely represents sleep spindles which occur at this frequency, and sleep spindles are thought to reflect hippocampal-neocortical dialogue (Fogel & Smith, 2011) due to their relationship to sharp-wave ripple activity (Siapas & Wilson, 1998). Sleep spindles have also been associated with declarative memory consolidation. Gais and colleagues (2002) found that both central and frontal spindle density during nREM2 sleep was positively correlated with declarative recall performance. Similarly, increased spindle activity was associated with declarative memory recall, even when the percentage of nREM2 was controlled for (Schabus et al., 2004). However we failed to observe a relationship between sigma power and performance changes on this task. This could be due to low power as a result of our small sample of individuals with recorded sleep physiology.
This study adds to the current body of literature supporting the role of sleep in novel word learning. The procedural design mimics the way vocabulary is learned in a naturalistic context. English speakers were taught novel English words, thereby preventing unique phonology from altering the linguistic processing or the neural representation of the newly learned words (e.g. Tan et al., 2003; Rüschemeyer, Fiebach, Kempe & Friederici, 2005). In addition, encoding and recall of the novel words were done in conjunction with a definition. Previous work has suggested that the incorporation of novel words into the lexicon without meaning or semantic context may qualitatively differ from those with meaning (Leach & Samuel, 2007; Tamminen et al., 2010; Lindsay & Gaskell, 2013; Tamminen & Gaskell, 2013). While we relied on participant's accurate post-hoc reporting that words were indeed novel, inaccuracies in these reports are not likely to systematically influence one group (sleep versus wake) over the other. Thus, this study supports that when learned within the context of semantic information, sleep is still beneficial for word learning. Lastly, in addition to strict accuracy, this study uses a unique measure (LD) to examine how sleep affects the quality of the memory for newly learned words. This study has demonstrated that sleep can protect the level of correctness of novel words whereas wake promoted a significant decline in the correctness of the recalled items.
Conclusion
Novel word learning is integral to human language; improving learning and retention of novel words is an important aspect of academic success. We demonstrated that sleep not only benefits retention of newly learned word-definition pairs, but it facilitates the improvement of items that were inaccurate but close to correct. REM sleep may play a role in the correction of these inexact memory traces, possibly through abstraction from other words in the lexicon or through other phonological rules. Future research should build on this relationship to examine how sleep supports the correction of inaccurately learned material, and whether this can be enhanced.
Acknowledgements
The authors wish to recognize the role of Brian Long in conducting this experiment. This work was supported in part by NIH R01 HL111695 and NIH R01 AG040133.
Footnotes
A 2X2 ANOVA revealed a significant main effect of session (F(1,43) = 10.974, p = 0.002) indicating a change in performance during the offline periods. While there was no significant main effect of group (F(1, 43) = 0.06, p = 0.808; delayed recall sleep = 63.85% ± 2.00, delayed recall wake = 61.05% ± 1.90, there was a significant group by session interaction (F(1,43) = 25.615, p < 0.001).
Conflict of interest
We have no conflicts of interest to disclose.
References
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, Treiman R. The English lexicon project. Behavior Research Methods. 2007;39(3):445–459. doi: 10.3758/bf03193014. doi:10.3758/BF03193014. [DOI] [PubMed] [Google Scholar]
- Bell LC, Perfetti CA. Reading skill: Some adult comparisons. Journal of Educational Psychology. 1994;86(2):244–255. doi:10.1037/0022-0663.86.2.244. [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. doi:10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Davis MH, Di Betta AM, Macdonald MJE, Gaskell MG. Learning and consolidation of novel spoken words. Journal of Cognitive Neuroscience. 2008;21(4):803–820. doi: 10.1162/jocn.2009.21059. doi:10.1162/jocn.2009.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychological Science. 2007;18(1):35–39. doi: 10.1111/j.1467-9280.2007.01845.x. doi:10.1111/j.1467-9280.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Current Opinion in Neurobiology. 2006;16(6):716–722. doi: 10.1016/j.conb.2006.10.006. doi:10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425(6958):614–616. doi: 10.1038/nature01951. doi:10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience and Biobehavioral Reviews. 2011;35(5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. doi:10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Gais S, Born J. Declarative memory consolidation: Mechanisms acting during human sleep. Learning & Memory. 2004;11(6):679–685. doi: 10.1101/lm.80504. doi:10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learning & Memory. 2006;13(3):259–262. doi: 10.1101/lm.132106. doi:10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. Journal of Neuroscience. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. doi:20026697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychological Science. 2006;17(8):670–674. doi: 10.1111/j.1467-9280.2006.01764.x. Doi:10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Hayes DP. The growing inaccessibility of science. Nature. 1992;356(6372):739. doi:10.1038/356739a0. [Google Scholar]
- Henderson L, Weighall A, Gaskell G. Learning new vocabulary during childhood: effects of semantic training on lexical consolidation and integration. Journal of Experimental Child Psychology. 2013;116(3):572–592. doi: 10.1016/j.jecp.2013.07.004. doi:10.1016/j.jecp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. PubMed: 1027738. [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. 1 Ed. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. PubMed: 1798888. [DOI] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. PubMed: 1519015. [DOI] [PubMed] [Google Scholar]
- Leach L, Samuel AG. Lexical configuration and lexical engagement: When adults learn new words. Cognitive Psychology. 2007;55(4):306–353. doi: 10.1016/j.cogpsych.2007.01.001. doi:10.1016/j.cogpsych.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenshtein VI. Binary codes capable of correcting deletions, insertions, and reversals. Soviet physics doklady. 1966;10(8):707–710. [Google Scholar]
- Lindsay S, Gaskell MG. Lexical integration of novel words without sleep. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2013;39(2):608–622. doi: 10.1037/a0029243. doi:10.1037/a0029243. [DOI] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments, & Computers. 1996;28(2):203–208. [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences. 2007;11(10):442–450. doi: 10.1016/j.tics.2007.09.001. doi:10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Marzano RJ, Marzano RJ. Building background knowledge for academic achievement. Association of Supervision and Curriculum Development; Alexandria, VA: 2004. Building academic background knowledge through direct vocabulary instruction. pp. 62–90. [Google Scholar]
- McKean E. The New Oxford American Dictionary. 2 Eds. Oxford University Press, Inc.; Oxford: 2005. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi: 10.1016/j.neuron.2004.10.007. doi:10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J. The neural basis of first and second language processing. Current Opinion in Neurobiology. 2005;15:202–206. doi: 10.1016/j.conb.2005.03.007. doi:10.1016/j.conb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Perfetti C. Reading ability: Lexical quality to comprehension. Scientific Studies of Reading. 2007;11(4):357–383. doi:10.1080/10888430701530730. [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. doi:10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. doi:10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Rüschemeyer S-A, Fiebach CJ, Kempe V, Friederici AD. Processing lexical semantic and syntactic information in first and second language: fMRI evidence from German and Russian. Human Brain Mapping. 2005;25(2):266–286. doi: 10.1002/hbm.20098. doi:10.1002/hbm.20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. PubMed: 15683137. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/s0896-6273(00)80629-7. doi: 10.1016/S0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Spencer RMC. Neurophysiological basis of sleep’s function on memory and cognition. ISRN Physiology. 2013:e619319. doi: 10.1155/2013/619319. doi:10.1155/2013/619319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Gaskell MG. Novel word integration in the mental lexicon: Evidence from unmasked and masked semantic priming. The Quarterly Journal of Experimental Psychology. 2012;66(5):1001–1025. doi: 10.1080/17470218.2012.724694. doi:10.1080/17470218.2012.724694. [DOI] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. The Journal of Neuroscience. 2010;30(43):14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. doi:10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng C-M, Siok WT, Perfetti CA, Xiong J, Gao J-H. Neural systems of second language reading are shaped by native language. Human Brain Mapping. 2003;18(3):158–166. doi: 10.1002/hbm.10089. doi:10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nature Reviews Neuroscience. 2010;11(3):218. doi: 10.1038/nrn2762-c1. doi:10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Payne JD, Stickgold R. A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learning & Memory. 2010;17(7):332–336. doi: 10.1101/lm.1828310. doi:10.1101/lm.1828310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Horst JS. Goodnight book: sleep consolidation improves word learning via storybooks. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00184. doi:10.3389/fpsyg.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging. 2012;33(5):991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. doi:10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


