Abstract
Reliable, non-invasive methods for diagnosing and prognosing sinusoidal obstruction syndrome (SOS) early after hematopoietic cell transplantation (HCT) are needed. We used a quantitative mass spectrometry-based proteomics approach to identify candidate biomarkers of SOS by comparing plasma pooled from 20 patients with and 20 patients without SOS. Of 494 proteins quantified, we selected six proteins [L-Ficolin, vascular-cell-adhesion-molecule-1 (VCAM1), tissue-inhibitor of metalloproteinase-1, von Willebrand factor, intercellular-adhesion-molecule-1, and CD97] based on a differential heavy/light isotope ratio of at least 2 fold, information from the literature, and immunoassay availability. Next, we evaluated the diagnostic potential of these six proteins and five selected from the literature [suppression of tumorigenicity-2 (ST2), angiopoietin-2 (ANG2), hyaluronic acid (HA), thrombomodulin, and plasminogen activator inhibitor-1] in samples from 80 patients. The results demonstrate that together ST2, ANG2, L-Ficolin, HA, and VCAM1 compose a biomarker panel for diagnosis of SOS. L-Ficolin, HA, and VCAM1 also stratified patients at risk for SOS as early as the day of HCT. Prognostic Bayesian modeling for SOS onset based on L-Ficolin, HA, and VCAM1 levels on the day of HCT and clinical characteristics showed >80% correct prognosis of SOS onset. These biomarkers may provide opportunities for preemptive intervention to minimize SOS incidence and/or severity.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is a potentially life-saving treatment for many patients with inherited disorders and hematologic malignancies. However, its practical use is impeded by the risk of serious adverse events, including sinusoidal obstruction syndrome (SOS, the now preferred name for veno-occlusive disease occuring after HCT or chemotherapy). Although the overall incidence and severity after allogeneic HCT have decreased in recent years, SOS is still a life-threatening liver injury complication with >80% mortality in severe cases, and SOS affects up to 20% of allogeneic HCT recipients in some centers [1–5]. SOS can also occur after intense chemotherapy when either the chemotherapy or radiation induces both systemic inflammation and tissue damage particularly to the sinusoidal endothelial cells of the hepatic acinus [6–8]. In addition, SOS can occur after the use of drugs such as gemtuzumab ozogamicin, inotuzumab ozogamicin, and the combination of tacrolimus and sirolimus under certain circumstances [9–12].
SOS typically occurs between the first and third weeks after HCT, but may occur later, and is often clinically indistinguishable from other causes of weight gain and respiratory distress particularly in children (e.g., cytokine storm syndrome and idiopathic pneumonia syndrome) or other causes of abdominal pain and jaundice (e.g., graft-versus-host disease of the gastrointestinal tract or liver) [4]. Diagnosis of SOS is made according to two clinical criteria scales (Baltimore [13] and Seattle [6]) that measure different degrees of liver dysfunction and weight gain, and abdominal ultrasound showing a reversal of the sinusoidal flow is commonly used to confirm the diagnosis. However, these clinical criteria and reversal of the sinusoidal flow are late events in the pathology of the disease. The investigational drug defibrotide (Gentium/Jazz Pharmceutical, Palo Alto, CA) has shown the most promising results in several clinical trials [5, 14]. However, treatment with defibrotide therapy carries significant risks, particularly of severe hemorrhage, when given late in the disease course. Therefore, a noninvasive method for early and accurate diagnosis of SOS is urgently needed [15].
Although a few potential biomarkers for SOS have been identified based on hypothesis-driven testing, there is still no validated blood test for SOS. Therefore, in the present study, we applied a quantitative mass spectrometry (MS)-based proteomics discovery approach to identify potential biomarkers for SOS and then used immunoassays to test the diagnostic value of 11 candidate biomarkers. These analyses led to the identification of a reliable biomarker panel specific for SOS that can be used in the diagnosis and management of patients with this disorder. Most importantly, given the high mortality rate associated with severe SOS and the lack of a therapeutic measure with 100% efficacy for this life-threatening disease, we next focused on prognostic markers that will afford opportunities for early preventative care. Therefore, this study focused on both diagnostic and prognostic markers, and although potentially interesting, markers predictive of disease severity, response to treatment, and non-relapse mortality are beyond the scope of this study.
MATERIALS AND METHODS
Patients and samples
Three cohorts of HCT patients were included in this study (discovery, training, and independent verification cohorts). Patients were treated at the University of Michigan, at Indiana University, and at the University of Barcelona. All patients or their legal guardians provided written informed consent, and the collection of samples for studying post-HCT complications was approved by the institutional review boards of the University of Michigan, Indiana University, and Hospital Clinic of the University of Barcelona. Heparinized blood samples were collected pre-HCT or on the day of HCT, then weekly for 2 to 4 weeks after allogeneic HCT, and, in some centers, at the time of the onset of symptoms consistent with SOS.
Proteomics analysis
The methods used for sample preparation, protein fractionation, MS analysis, protein identification, and quantitative analysis of protein concentrations during the intact protein analysis system have been previously reported [16–18].
Immunoassays
Suppression of tumorigenicity-2 (ST2), angiopoietin2 (ANG2), L-Ficolin, hyaluronic acid (HA), vascular cell adhesion molecule-1 (VCAM1), tissue inhibitor of metalloproteinase-1 (TIMP1), thrombomodulin (sCD141), intercellular adhesion molecule-1 (ICAM1), plasminogen activator inhibitor-1 (PAI-1), von Willebrand factor (vWF), and CD97 concentrations were measured by enzyme-linked immunosorbent assays (ELISAs). The antibodies pairs used for these ELISAs were as follows: anti-ST2 (R&D Systems, Minneapolis, MN), anti-ANG2 (R&D Systems), anti-L-Ficolin (Hycult Biotech, Plymouth Meeting, PA), anti-HA (Corgenix, Broomfield, CO), anti-VCAM1 (R&D Systems), anti-TIMP1 (R&D Systems), anti-thrombomodulin (Diaclone, Besancon, France), anti-ICAM1 (R&D Systems), anti-PAI-1 (eBioscience, San Diego, CA), anti-vWF (American Diagnostica, Stamford, CT), and anti-CD97 (R&D Systems).
For analysis, plasma samples were thawed and centrifuged at 12,000 rpm for 10 min to separate the clots at the bottom and lipids on top from the plasma. Then 150-μl aliquots of each undiluted plasma sample were transferred to individual wells of 96-well V-bottom plates. The plates were wrapped in parafilm and kept in a humid chamber at 4°C throughout the entire process, which did not exceed 96 h. Capture antibodies were reconstituted and diluted per manufacturers’ specifications or pre-coated plates were used as recommended by the manufacturer. Then 50 μl of diluted antibodies were added to wells of 96-well high-binding half-well plates, which were then sealed and incubated overnight. The next day the test plates containing the capture antibodies were washed and blocked with specific manufacturer’s recommended blocking buffer. After additional wash steps, 50-μl or 100-μl aliquots of plasma samples (dilutions listed in Supplemental Table 1) were added in duplicate to the ELISA test plates. In addition, 50-μl or 100-μl aliquots of reconstituted standard at different concentrations (see Supplemental Table 1) were added in duplicate for the preparation of 8-point standard curves per the manufacturers’ protocols. After addition of samples and standard solutions, the plates were sealed and incubated for 2 hours at room temperature on a plate rotator at 300 rpm. The ELISAs were completed by adding biotinylated detection antibodies specific for each target followed by the enzyme horseradish peroxidase (HRP) and HRP substrate. The optical density of each well was read using a plate reader set to 450–570 nm. For ELISA kits with precoated plates, the manufacturers’ protocols were applied. The ELISAs were performed in duplicate and sequentially as previously reported [18–22].
Statistical analysis
The statistical methods used for the IPAS were previously described [16–18]. Differences in characteristics between patient groups were assessed with Kruskal-Wallis tests for continuous values and χ2 tests of association for categorical values. Protein concentrations from individual samples in the training and independent sets were compared using unpaired t tests. Receiver operating characteristic (ROC) areas under the curves (AUCs) were estimated nonparametrically. Differences in median pre-HCT, day 0, +7, and +14 biomarker levels between SOS− and SOS+ patients were assessed using a Wilcoxon rank sum test. Additionally, we examined the differences in biomarkers trajectories over time using a modeling approach (see supplementary methods).
Prognostic Bayesian modeling
The plasma concentrations of three proteomic biomarkers (L-Ficolin, HA, and VCAM1) on the day of HCT were used to evaluate their prognostic performance for future occurrence of SOS onset. The clinical characteristics also included in the analysis were: age, gender, donor type (related or unrelated), donor match (matched or mismatched), transplant period (before or in 2005 or after 2005), transplant number (1 or >1), conditioning regimen (chemotherapy only or combined with irradiation), busulfan (16 mg/kg) use in the conditioning (yes or no), and cyclophosphamide use in the conditioning (yes or no). Plasma protein concentrations and clinical characteristics were used as attributes for the prognosis of SOS onset. The naïve Bayes classifier was selected for SOS onset prognosis due to its simplicity and high classification performance. Ten-fold cross-validation was used to avoid over training, bias, and/or artifacts (see supplemental methods). This naïve Bayes classifier was developed with Waikato Environment for Knowledge Analysis (WEKA) software v3.6.10 [23].
RESULTS
Proteomic biomarker discovery
We first performed discovery proteomic analysis comparing plasma pooled from 20 patients with SOS to plasma pooled from 20 patients without SOS. The clinical characteristics of patients in this discovery cohort are provided in Table 1. Of 494 proteins identified and quantified, 151 proteins showed at least a 2-fold increase in the heavy/light isotope ratio, and 77 proteins showed a heavy-light isotope ratio of 0.5 or less (see Supplemental Table 2 for complete summary). From the identified proteins, we selected six proteins for further analysis: L-Ficolin, VCAM1, TIMP1, vWF, ICAM1, and CD97. These proteins were selected based on the observation of at least a 2-fold increase or decrease in the heavy/light isotope ratio, their involvement in relevant pathway networks and other information from the published literature indicating they may be involved in the pathogenesis of SOS, and the availability of a sandwich ELISA. In addition, five endothelial markers (ST2, ANG2, HA, thrombomodulin, and PAI-1) were measured based on previous demonstrations of their involvement in SOS [8, 24, 25] or in refractory GVHD [22, 26].
Table 1.
Patients Clinical Characteristics
| Discovery Cohort | Training Cohort | Independent Verification Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SOS− (n = 20) | SOS+ (n = 20) | P-value | SOS− (n = 13) | SOS+ (n = 32) | P-value | SOS− (n = 22) | SOS+ (n = 13) | P-value | |
| Age, years | |||||||||
| median | 43 | 43 | ns | 45 | 16 | 0.02 | 29 | 8 | 0.06 |
| range | 3–56 | 1–58 | 3–55 | 1–58 | 1–66 | 1–48 | |||
| Disease, n (%) | |||||||||
| Malignant* | 19 (95) | 18 (90) | ns | 12 (92) | 27 (84) | ns | 22 (100) | 13 (100) | ns |
| Non-malignant§ | 1 (5) | 2 (10) | 1 (8) | 5 (16) | 0 (0) | 0 (0) | |||
| Donor type, n (%) | |||||||||
| Related | 18 (90) | 17 (85) | ns | 12 (92) | 17 (53) | 0.02 | 14 (64) | 3 (33) | 0.02 |
| Unrelated | 2 (10) | 3 (15) | 1 (8) | 5 (16) | 8 (36) | 10 (77) | |||
| Donor match, n (%) | |||||||||
| Matched | 20 (100) | 20 (100) | ns | 13 (100) | 25 (78) | 0.08 | 18 (82) | 7 (54) | ns |
| Mismatched | 0 (0) | 0 (0) | 0 (0) | 7 (22) | 4 (18) | 6 (46) | |||
| Conditioning regimen intensity, n (%)‡ | |||||||||
| Full | 20 (100) | 20 (100) | ns | 13 (100) | 32 (100) | ns | 16 (73) | 13 (100) | ns |
| With Busulfan (16 mg/kg, 4 days) | 14 (74) | 17 (90) | 9 (69) | 26 (81) | 1 (5) | 3 (23) | |||
| With TBI | 2 (10) | 1 (5) | 2 (15) | 4 (12) | 8 (36) | 6 (46) | |||
| GVHD prophylaxis regimen, n (%) | |||||||||
| Tacro or CsA/MTX | 19 (95) | 18 (90) | ns | 12 (92) | 23 (72) | ns | 5 (23) | 5 (38) | ns |
| With rapamycin | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 6 (27) | 1 (8) | |||
| With MMF | 0 (0) | 0 (0) | 0 (0) | 7 (22) | 4 (18) | 4 (31) | |||
| Other# | 1 (5) | 2 (10) | 1 (8) | 1 (3) | 1 (5) | 0 (0) | |||
| NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (27) | 3 (23) | |||
| Time post-HCT to SOS onset, days | |||||||||
| median | na | 14 | na | na | 11 | na | na | 9 | na |
| range | na | 4–37 | na | 4–63 | na | 5–23 | |||
| Time post-HCT to SOS sample acquisition, days | |||||||||
| median | 14 | 14 | ns | 14 | 11 | ns | 14 | 11 | ns |
| range | 7–41 | 4–37 | 7–41 | 4–63 | 7–14 | 5–23 | |||
| Future acute GVHD 2–4, n (%) | |||||||||
| yes | 0 (0) | 0 (0) | ns | 0 (0) | 14 (44) | 0.004 | 0 (0) | 6 (46) | 0.0005 |
| no | 20 (100) | 20 (100) | 13 (100) | 18 (56) | 22 (100) | 7 (54) | |||
| Time post-HCT to GVHD onset, days | |||||||||
| median | na | na | na | na | 33 | na | na | 21 | na |
| range | 14–75 | (11–46) | |||||||
na: not applicable, ns: not significant.
Malignant disease included acute leukemia/MDS (n = 69), lymphoma (n = 18), multiple myeloma (n = 2), chronic leukemia (n = 13), myelofibrosis (n = 2), PNH (n = 2), neuroblastoma (n = 3), rhabdoid tumor (n =1) and carcinoid tumor (n = 1);
Non-malignant disease included severe aplastic anemia (n = 2), thalassemia (n = 3), sickle cell disease ( n = 2), chronic granulomatous disease (n = 1), and familial lymphohistiocytosis (n = 1)
Full-intensity conditioning regimens included CVB (n = 7), BuCy (n = 35), BAC (busulfan (16 mg/kg), cytarabine (8,000 mg/m2), and cyclophosphamide (120 mg/kg) (n = 31), CyTBI (n = 21), Flu or Clo + Bu (16 mg/kg) (n = 6), Busulfan/Melphalan (n = 1), Fludarabine/Melphalan (n = 1), Carboplatin/Etoposide/Melphalan (n = 4), Carboplatin/Thiotepa (n = 2), CyFlu (n =4), and CyThiotepa (n =2)
other GVHD prophyaxis: Tacro/Corticosteroids (n = 3), MTX/Corticosteroids (n = 2), Tacro/MTX/Corticosteroids (n = 1)
Development of a biomarker panel for SOS diagnosis
Using sequential ELISAs [20], levels of the 11 identified candidate biomarkers were measured in plasma from a training cohort of 45 patients: 32 SOS patients with active disease at onset (days +14 to +21 post-HCT) and 13 time-matched controls. We used diagnosis samples from SOS+ patients that were taken at the time of SOS onset, and we selected samples from SOS− patients so that both groups of samples were balanced according to time of acquisition. The clinical characteristics of patients in this training cohort are described in Table 1. The SOS− and SOS+ groups were balanced for age, primary disease, donor type (related versus unrelated), donor match, and intensity of the conditioning regimen [all full intensity with most receiving 16 mg/kg busulfan for 4 days or total body irradiation (TBI)]. More than 90% of patients received GVHD prophylaxis of methotrexate and tacrolimus (or cyclosporine) of standard duration. We tested the value of these proteins as diagnostic biomarkers of SOS using unpaired t tests and by calculating the AUCs of the ROCs, which represent the false positive and true positive rates for every possible level of a marker. Among the 11 proteins tested, 8 were found to be diagnostic biomarkers of SOS with p-values ranging from <0.001 to 0.04 and with AUCs between 0.91 and 0.70 (Figure 1). The composite ROC of the five best diagnostic markers (ST2, ANG2, L-Ficolin, HA, and VCAM1) had an AUC of 0.98 in this selected case/control training cohort (Supplemental Figure 1). Addition of TIMP1, thrombomodulin, and ICAM1 to the biomarker panel did not improve this AUC value (data not shown). Because ST2 has been shown to correlate with the development of acute GVHD [22], we evaluated its prognostic value in the training and independent cohorts. In these two cohorts, approximately 45% of SOS patients later developed GVHD (median number of days to onset of 33 and 21 vs. 11 and 9 for SOS in the training and independent cohorts, respectively). ST2 plasma concentrations at day 14 post-HCT (when almost all SOS patients have already developed clinical signs of SOS) did not differ between the SOS+GVHD− and SOS+GVHD+ groups, meaning that for SOS cases, ST2 is a diagnostic marker of SOS and this is more important than its prognostic value for future GVHD.
Figure 1. Diagnostic biomarkers of SOS according to the highest AUCs (0.91–0.70) in the training cohort.
Plasma biomarker concentrations measured by ELISA in patients with SOS (SOS+) and without SOS (SOS−) (A–H). The data are shown as mean ± standard error of the mean (SEM). Unpaired t test, significant at p < 0.05. The numbers beneath the SOS categories are the AUC percentages for each marker.
Prognostic biomarker panel for risk-stratification prior to clinical signs of SOS
With the same training cohort, we next tested the prognostic significance of these biomarkers using protein levels measured in samples taken before presentation of the clinical signs (days 0 and +7 post-HCT). Three diagnostic biomarkers were also prognostic before clinical signs were apparent (L-Ficolin, HA, and VCAM1), and the corresponding AUC values for biomarker values on the day of HCT were between 0.84 and 0.70 (Figure 2). Modeling of these biomarkers’ trajectories showed significant differences between the SOS− and SOS+ groups (Supplemental Figure 2).
Figure 2. Prognostic biomarkers of SOS prior the clinical signs in the training cohort.
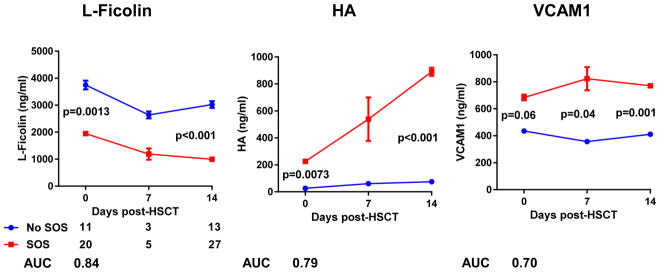
Plasma biomarker concentrations measured by ELISA in patients with SOS (SOS+) and without SOS (SOS−) at different days post-HCT (0, +7, +14). The data are shown as mean ± standard error of the mean (SEM). Median differences assessed with Wilcoxon rank sum test, significant at p < 0.05. The numbers beneath the SOS categories are the AUC percentages for each marker and days post-HCT when significant.
Independent cohort for SOS biomarker analysis
We also tested the diagnostic and prognostic values of the biomarkers in an independent cohort of 35 patients (13 patients with SOS; 22 patients without SOS). The clinical characteristics of patients in this independent cohort are presented in Table 1 and were similar to the clinical characteristics of the training cohort. Despite the small sample size, the results further validated L-Ficolin, HA, and VCAM1 as diagnostic markers (AUC: 0.88, 0.81, and 0.81, respectively). We next tested L-Ficolin, HA, and VCAM1 as prognostic markers of SOS with samples taken prior to the appearance of clinical signs of SOS. L-Ficolin and HA also stratified patients at risk for SOS as early as the day of HCT in this independent cohort (Figure 3). Modeling of these biomarkers trajectories showed significant differences between the SOS+ and SOS− groups for L-Ficolin and HA but not for VCAM1 (Supplemental Figure 3). Notably, for most patients in this cohort, in addition to the day 0 and day 7 samples, samples collected before the conditioning were included, and plasma levels of L-Ficolin, and HA measured pre-transplantation did not differ between the SOS− and SOS+ groups. Therefore, these results strongly suggest that levels of these biomarkers are altered during the conditioning regimen and prior to the appearance of clinical signs of SOS, as they can be detected as early as the day of HCT.
Figure 3. Prognostic biomarkers prior the clinical signs of SOS in the independent cohort.

Plasma biomarker concentrations measured by ELISA in patients with SOS (SOS+) and without SOS (SOS−) at different days post-HCT (−7, 0, +7, +14). The data are shown as mean ± standard error of the mean (SEM). Median differences assessed with Wilcoxon rank sum test, significant at p < 0.05. The numbers beneath the SOS categories are the AUC percentages for each marker and days post-HCT when significant.
Prognostic Bayesian modeling
Three data subsets were evaluated for model building. Subset one was an imbalanced dataset (8 SOS− vs 20 SOS+) that included some missing day 0 biomarker information, subset two was a balanced dataset (11 SOS− vs 13 SOS+) that included complete clinical and biomarker information, and subset three was a balanced dataset (21 SOS− vs 20 SOS+) that included some missing day 0 biomarker information. The balanced subset two with no missing attribute information was selected to build the prognostic model. This selection was based on results comparing the correct prognosis between the three subsets tested and their corresponding ROC AUCs (Supplemental Table 3). The clinical characteristics of patients in this set are presented in Supplemental Table 4. The model was evaluated using plasma concentrations of biomarkers on day 0 with and without the addition of the clinical characteristics. Table 2 shows the results (correct prognosis and false negatives and positives) of the model building using the selected data subset. Correct prognosis was achieved in 83.3% of patients using the day 0 plasma biomarker concentrations in addition to clinical attributes (ROC AUC = 0.90).
Table 2.
Naïve Bayes classifier results stratified by 10-fold cross-validation
| Clinical characteristics + Biomarkers | Biomarkers | Clinical characteristics | |
|---|---|---|---|
| Correct Prognosis | 83.3 % | 70.8 % | 58.3 % |
| ROC AUC (Yes) | 0.90 | 0.83 | 0.61 |
| False Positive | 1 | 1 | 4 |
| False Negative | 3 | 6 | 6 |
DISCUSSION
Here we present the first use of a MS-based proteomics discovery approach to identify biomarkers of SOS in plasma samples from patients undergoing allogeneic HCT. In addition to identifying a panel of biomarkers that can be used for SOS diagnosis, we identified three biomarkers that can be used to evaluate the risk of developing SOS before clinical signs appear, even as early as the day of HCT. Earlier hypothesis-driven studies focused on markers of hemostasis and coagulation, because microthrombus formation in the hepatic sinusoid is one of the prominent clinical features of SOS. The most extensively studied biomarker for SOS is PAI-1. Elevated concentrations of PAI-1 have been shown to precede an increase in bilirubin and to have diagnostic and prognostic value [24]. Interestingly, in our analyses, with an AUC of only 0.68, PAI-1 trended toward significance as a biomarker of SOS, but the corresponding p-value did not reach significance in this patient set. Importantly, the diagnostic value of PAI-1 was less than that of eight other markers.
More recent studies have focused on markers of endothelial injury because another important and earlier mechanism of injury in SOS is thought to be conditioning-related injury to the hepatic sinusoidal endothelium [27]. Elevated levels of vWF, thrombomodulin (or sCD141), and ICAM1 before and early after transplantation were shown to be useful in prognosticating SOS in patients receiving sirolimus [25]. Among these markers, both thrombomodulin and ICAM1 were identified as having diagnostic value in our study, although the levels of these proteins were not found to be elevated in the early period following HCT. vWF was identified in our proteomic analysis with a heavy/light isotope ratio of 2.3, but vWF levels were not significantly different in patients with and without SOS in our training set. One possible reason for this discrepancy between our results and those of study from the Dana Farber Cancer Institute is that we did not include patients treated with sirolimus and the mechanism of injury induced by sirolimus to endothelium may be different from that induced by the conditioning regimen, as proposed in their study [25].
Known risk factors for SOS development are specific conditioning agents (particularly busulfan at 16 mg/kg (4 days), and to a lesser degree cyclophosphamide) or TBI as well as more than one HCT, allogeneic HCT, unrelated donor HCT, pre-existing liver disease or radiation to the abdomen, use of the combination of tacrolimus/rapamicine for GVHD prophylaxis, or the use of gemtuzumab ozogamicin or inotuzumab ozogamicin [9–12]. In our different cohorts, only one allo-HCT patient had a known pre-existing liver condition, three patients had abdominal irradiation, and two patients received tacrolimus and rapamycin as GVHD prophylaxis. These small sample sizes did not allow for subanalyses. Thus, we used the following clinical criteria in our analysis: age, gender, donor type (related or unrelated), donor match (matched or mismatched), transplant period (before or in 2005 or after 2005), transplant number (1 or >1), conditioning regimen (chemotherapy only or combined with irradiation), busulfan (16 mg/kg) use in the conditioning (yes or no), and cyclophosphamide use in the conditioning (yes or no), and showed that the prognostic panel of biomarkers (L-Ficolin, HA, and VCAM1) measured at day 0 of HCT significantly improved risk stratification over these known clinical characteristics, which alone provided a ROC AUC of 0.61, but in combination with the prognostic biomarker panel provided a ROC AUC of 0.90 (Table 2).
HA is mainly produced by cells of mesodermal lineage, and homeostatic HA levels are maintained by an efficient receptor-dependent removal mechanism present in the sinusoidal endothelial cells of the liver. Systemic HA levels are thus regarded as a direct marker of hepatic sinusoidal endothelial cell function. Therefore, HA is a potentially relevant marker of SOS as recently shown for SOS secondary to oxaliplatin-based chemotherapy [8]. We validated HA as a diagnosis marker of SOS with high specificity and sensitivity as well as a prognostic factor at day 0 of HCT. Notably, using the same ELISA kit, we observed HA concentrations post-HCT that were 10-fold greater than those observed post-chemotherapy.
VCAM1 is a cell surface sialoglycoprotein expressed by cytokine-activated endothelium that mediates leukocyte-endothelial cell adhesion, and a role for VCAM1 in the development of SOS has been suggested previously [3]. We also hypothesized that two other markers of endothelial injury, ST2 and ANG2, that have been shown to be elevated in refractory GVHD [22, 26] may also be elevated in SOS, which has a more prominent endothelium component than GVHD. Indeed, ST2 and ANG2 were found to be reliable biomarkers for SOS diagnosis. However, they were not prognostic of the occurrence of SOS in the early post-HCT period. These results suggest that the mechanisms by which ST2 and ANG2 levels are elevated after HCT, although activated 14 days prior to the onset of GVHD and relatively early in the course of GVHD, are later events in SOS, occurring near the time of SOS onset.
Interestingly, our proteomics analysis revealed an entirely novel marker of SOS with strong diagnostic and prognostic abilities: L-Ficolin. Furthermore, L-Ficolin’s mechanism of action seems to implicate pathways in SOS other than those related to hemostasis and endothelial injury. L-Ficolin is a complement-activating pattern-recognition lectin involved in the innate immune response and has recently been shown to be involved in homeostatic clearance of mitochondria in the liver [28]. In SOS patients, the concentrations of L-Ficolin were decreased, suggesting that this homeostatic clearance no longer happens efficiently. The identification of L-Ficolin demonstrates that the pathogenesis of SOS is multifactorial as previously hypothesized. Overall, the SOS biomarker panel assembled in the present study includes molecules involved in inflammation, innate immune response and homeostatic clearance of mitochondria, endothelial injury, and hemostatis. Furthermore, our findings suggest that activation of all of these pathways precede clinical onset of SOS by at least several days to as much as weeks.
The goal of the modeling was to assess whether the biomarkers (L-Ficolin, HA, and VCAM1) can prognosticate the possible future onset of SOS. The naïve Bayes algorithm implemented in the data mining software WEKA is a suitable choice to address questions regarding future occurrence of an event. In consideration of the actionable information that would be available to clinicians for a patient at risk for SOS, the model was built using the plasma concentrations of biomarkers on day 0 and was tested with and without the addition of clinical characteristics. The use of day 0 plasma biomarker concentrations yielded a 83.3% correct prognosis (ROC AUC = 0.90), suggesting that the plasma concentrations of L-Ficolin, HA, and VCAM1 at day 0 post conditioning could be used for prognosis of SOS onset. The clinical attributes alone were not useful, with only 58.3% correct prognosis (ROC AUC = 0.61). Combining the day 0 plasma biomarker concentrations with clinical characteristics resulted in over 80% correct prognosis with a ROC AUC > 0.9. This observation points to the added benefit of individual attribute probability for the overall outcome prognosis.
The high sensitivity and specificity of the biomarkers identified in the present study make them useful for real-time clinical testing and early clinical intervention. However, our different patient sets were selected for case controls of SOS. Thus, our results need to be confirmed in a large, independent prospective verification cohort, ideally across multiple institutions, to establish clinically useful cutoffs for their future use in clinical trials [29]. The ultimate goal of such trials is to find a reliable biomarker panel that identifies patients at high risk for SOS who will benefit from a preemptive intervention using agents that target endothelial injury and have been proven to be effective for treating SOS [5, 14].
In conclusion, our results demonstrate that SOS potentially can be diagnosed based on a panel of biomarkers in plasma as well as prognosticated as early as the day of HSC infusion in patients. The naïve Bayes algorithm showed that the L-Ficolin, HA, and VCAM1 plasma concentrations on day 0 post-conditioning therapy are prognostic of SOS onset and can potentially be used as prognostic proteomic biomarkers for this disease. The identified markers represent several pathways, including previously suspected pathways involved in hemostasis and endothelial injury as well as novel pathways related to innate immunity and homeostatic clearance of mitochondria. Once further validated in a clinical trial, these biomarkers could provide opportunities for preemptive intervention to minimize the incidence and severity of SOS.
Supplementary Material
Figure S1. Composite ROC curve compared to the individual ROC curves for the five best markers for SOS diagnosis (ST2, ANG2, L-Ficolin, HA, and VCAM1)
Figure S2. The trajectories of L-Ficolin, HA, and VCAM1 in the training set as modeled by population mixed effects approach. Shown is the population median for each biomarker with the p-value comparing the trajectories of the two groups.
Figure S3. The trajectories of L-Ficolin, HA, and VCAM1 in the independent set as modeled by population mixed effects approach. Shown is the population median for each biomarker with the p-value comparing the trajectories of the two groups.
Table S1. ELISA parameters for the 11 tested proteins.
Table S2. Complete list of genes identified by MS-based proteomics in pooled plasma from SOS patients.
Highlights.
ST2, ANG2, L-Ficolin, HA, and VCAM1 represent a biomarker panel for SOS diagnosis.
L-Ficolin, HA, and VCAM1 are prognostic biomarkers of SOS before symptoms appear.
These biomarkers can stratify patients at risk for SOS as soon as the day of HCT.
L-Ficolin has a mechanism of action unrelated to hemostasis and endothelial injury.
Acknowledgments
The authors would like to thank the clinicians of the University of Michigan Blood and Marrow Transplant program and at the Indiana University Blood and Marrow Transplant program, and the Barcelona Endothelium Team and Stem Cell Transplantation Unit at the Hospital Clinic of Barcelona. The Indiana University BMT data managers Lindsey Elmore and Rose Case, and all the members of the Paczesny’s laboratory. We would like to acknowledge our funding sources, including the National Institutes of Health (U54 HD071598 – Indiana University Center for Pediatric Pharmacology, RC1 HL101102, R01 CA168814), and the Leukemia & Lymphoma Society Scholar Award (129315), the Lilly Physician Scientist Initiative Award.
Footnotes
Financial Disclosure
S.P. is an inventor on a patent on “Methods of detection of graft-versus-host disease” (US- 13/573,766). E.C. has a grant from Gentium/Jazz Pharmaceutical to study the mechanism of action of defibrotide. Otherwise, the authors have no other relevant conflicts of interest to declare.
Authorship statement
A.A. conceived and planned the prognostic modeling, performed clinical computing statistical analysis, interpreted the data, and wrote the manuscript; Q.Z. and S.H. designed, performed and analyzed proteomics data; C.L.M., N.R., and J.Y. performed ELISA experiments, and participated in research discussions; K.R., J.S.,M.D-R., E.C. contributed to patient accrual, clinical data collection and quality assurance, and research discussion; L.S.H., and J.R. planned the study design, and interpreted the data; R.B. conceived and planned the statistical study for the prognostic modeling, interpreted the data, and wrote the manuscript, and S.P. conceived and planned the study design, supervised the experiments, interpreted the data, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:157–68. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreras E, Diaz-Beya M, Rosinol L, Martinez C, Fernandez-Aviles F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1713–20. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Chao N. How I treat sinusoidal obstruction syndrome. Blood. 2014;123:4023–6. doi: 10.1182/blood-2014-03-551630. [DOI] [PubMed] [Google Scholar]
- 5.Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone marrow transplantation. 2015 doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 7.Dix SP, Wingard JR, Mullins RE. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transpl. 1996;17:225–30. [PubMed] [Google Scholar]
- 8.van den Broek MA, Vreuls CP, Winstanley A, Jansen RL, van Bijnen AA, Dello SA, et al. Hyaluronic acid as a marker of hepatic sinusoidal obstruction syndrome secondary to oxaliplatin-based chemotherapy in patients with colorectal liver metastases. Annals of surgical oncology. 2013;20:1462–9. doi: 10.1245/s10434-013-2915-8. [DOI] [PubMed] [Google Scholar]
- 9.Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578–82. doi: 10.1182/blood-2003-01-0255. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–31. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kebriaei P, Wilhelm K, Ravandi F, Brandt M, de Lima M, Ciurea S, et al. Feasibility of allografting in patients with advanced acute lymphoblastic leukemia after salvage therapy with inotuzumab ozogamicin. Clinical lymphoma, myeloma & leukemia. 2013;13:296–301. doi: 10.1016/j.clml.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmona A, Diaz-Ricart M, Palomo M, Molina P, Pino M, Rovira M, et al. Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus-sirolimus on endothelial cells: protective effect of defibrotide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1439–45. doi: 10.1016/j.bbmt.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 15.Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. British journal of haematology. 2015;168:481–91. doi: 10.1111/bjh.13215. [DOI] [PubMed] [Google Scholar]
- 16.Faca V, Coram M, Phanstiel D, Glukhova V, Zhang Q, Fitzgibbon M, et al. Quantitative analysis of acrylamide labeled serum proteins by LC-MS/MS. Journal of proteome research. 2006;5:2009–18. doi: 10.1021/pr060102+. [DOI] [PubMed] [Google Scholar]
- 17.Faca V, Pitteri SJ, Newcomb L, Glukhova V, Phanstiel D, Krasnoselsky A, et al. Contribution of protein fractionation to depth of analysis of the serum and plasma proteomes. Journal of proteome research. 2007;6:3558–65. doi: 10.1021/pr070233q. [DOI] [PubMed] [Google Scholar]
- 18.Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Science translational medicine. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–8. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiema B, Harris AC, Gomez A, Pongtornpipat P, Lamiman K, Vander Lugt MT, et al. High throughput sequential ELISA for validation of biomarkers of acute graft-versus-host disease. Journal of visualized experiments : JoVE. 2012 doi: 10.3791/4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–8. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. The New England journal of medicine. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witten I, Frank E, Hall M. Data Mining: Practical Machine Learning Tools and Techniques. 3. Burlington, MA: Morgan Kaufmann; 2011. [Google Scholar]
- 24.Salat C, Holler E, Kolb HJ, Reinhardt B, Pihusch R, Wilmanns W, et al. Plasminogen activator inhibitor-1 confirms the diagnosis of hepatic veno-occlusive disease in patients with hyperbilirubinemia after bone marrow transplantation. Blood. 1997;89:2184–8. [PubMed] [Google Scholar]
- 25.Cutler C, Kim HT, Ayanian S, Bradwin G, Revta C, Aldridge J, et al. Prediction of veno-occlusive disease using biomarkers of endothelial injury. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:1180–5. doi: 10.1016/j.bbmt.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–92. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 27.Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:985–93. doi: 10.1016/j.bbmt.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Brinkmann CR, Jensen L, Dagnaes-Hansen F, Holm IE, Endo Y, Fujita T, et al. Mitochondria and the lectin pathway of complement. The Journal of biological chemistry. 2013;288:8016–27. doi: 10.1074/jbc.M112.430249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paczesny S, Hakim FT, Pidala J, Cooke K, Lathrop J, Griffith LM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Composite ROC curve compared to the individual ROC curves for the five best markers for SOS diagnosis (ST2, ANG2, L-Ficolin, HA, and VCAM1)
Figure S2. The trajectories of L-Ficolin, HA, and VCAM1 in the training set as modeled by population mixed effects approach. Shown is the population median for each biomarker with the p-value comparing the trajectories of the two groups.
Figure S3. The trajectories of L-Ficolin, HA, and VCAM1 in the independent set as modeled by population mixed effects approach. Shown is the population median for each biomarker with the p-value comparing the trajectories of the two groups.
Table S1. ELISA parameters for the 11 tested proteins.
Table S2. Complete list of genes identified by MS-based proteomics in pooled plasma from SOS patients.



